Evidence of G-Protein-Coupled Receptors (GPCR) in the Parasitic Protozoa Plasmodium falciparum—Sensing the Host Environment and Coupling within Its Molecular Signaling Toolkit
Abstract
:1. An Overview of GPCRs in Multicellular Eukaryotes
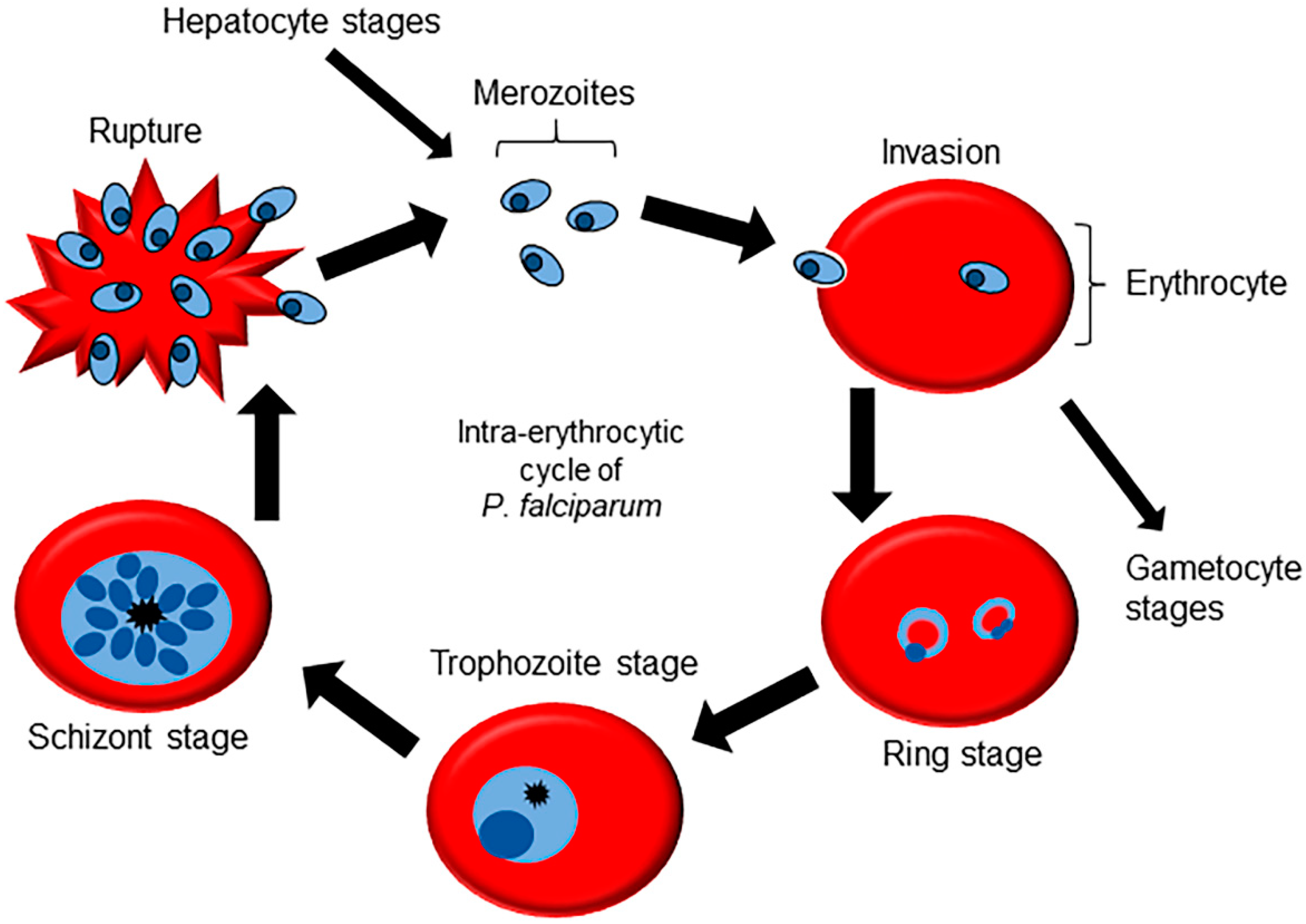
2. Molecular Machinery for Cellular Signaling in Plasmodium Genus
3. In Silico Studies for the Identification of GPCR Candidates in Plasmodium falciparum
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; Fitzhugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mombaerts, P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat. Rev. Neurosci. 2004, 5, 263–278. [Google Scholar] [CrossRef]
- Overington, J.P.; Al-Lazikani, B.; Hopkins, A. How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Rask-Andersen, M.; Masuram, S.; Schiöth, H.B. The Druggable Genome: Evaluation of Drug Targets in Clinical Trials Suggests Major Shifts in Molecular Class and Indication. Annu. Rev. Pharmacol. Toxicol. 2013, 54, 9–26. [Google Scholar] [CrossRef]
- Manglik, A.; Kobilka, B. The role of protein dynamics in GPCR function: Insights from the β2AR and rhodopsin. Curr. Opin. Cell Biol. 2014, 27, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Latorraca, N.R.; Venkatakrishnan, A.J.; Dror, R.O. GPCR Dynamics: Structures in Motion. Chem. Rev. 2016, 117, 139–155. [Google Scholar] [CrossRef]
- Ahmad, R.; Lahuna, O.; Sidibe, A.; Daulat, A.; Zhang, Q.; Luka, M.; Guillaume, J.-L.; Gallet, S.; Guillonneau, F.; Hamroune, J.; et al. GPR50-Ctail cleavage and nuclear translocation: A new signal transduction mode for G protein-coupled receptors. Cell. Mol. Life Sci. 2020, 77, 5189–5205. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, A.J.; Deupi, X.; Lebon, G.; Heydenreich, F.; Flock, T.; Miljus, T.; Balaji, S.; Bouvier, M.; Veprintsev, D.; Tate, C.G.; et al. Diverse activation pathways in class A GPCRs converge near the G-protein-coupling region. Nature 2016, 536, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T. New concepts in pharmacological efficacy at 7TM receptors: IUPHAR Review 2. Br. J. Pharmacol. 2013, 168, 554–575. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, D.M.; Rasmussen, S.G.F.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, T.M.; Makimura, H.; Mobbs, C.V. The physiological function of the agouti-related peptide gene: The control of weight and metabolic rate. Ann. Med. 2003, 35, 425–433. [Google Scholar] [CrossRef]
- Downes, G.; Gautam, N. The G Protein Subunit Gene Families. Genomics 1999, 62, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Lokits, A.D.; Indrischek, H.; Meiler, J.; Hamm, H.E.; Stadler, P.F. Tracing the evolution of the heterotrimeric G protein α subunit in Metazoa. BMC Evol. Biol. 2018, 18, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12. [Google Scholar] [CrossRef]
- Kristiansen, K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: Molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol. Ther. 2004, 103, 21–80. [Google Scholar] [CrossRef]
- Oldham, W.; Hamm, H.E. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2008, 9, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.M.; Sleno, R.; Gora, S.; Zylbergold, P.; Laverdure, J.-P.; Labbé, J.-C.; Miller, G.J.; Hébert, T.E. The Expanding Roles of Gβγ Subunits in G Protein–Coupled Receptor Signaling and Drug Action. Pharmacol. Rev. 2013, 65, 545–577. [Google Scholar] [CrossRef] [Green Version]
- Gurevich, E.V.; Tesmer, J.; Mushegian, A.; Gurevich, V. G protein-coupled receptor kinases: More than just kinases and not only for GPCRs. Pharmacol. Ther. 2012, 133, 40–69. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.-L.; Khoshouei, M.; Radjainia, M.; Zhang, Y.; Glukhova, A.; Tarrasch, J.; Thal, D.; Furness, S.; Christopoulos, G.; Coudrat, T.; et al. Phase-plate cryo-EM structure of a class B GPCR–G-protein complex. Nature 2017, 546, 118–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, B.; Feng, D.; Hu, H.; Chu, M.; Qu, Q.; Tarrasch, J.T.; Li, S.; Kobilka, T.S.; Kobilka, B.K.; et al. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 2017, 546, 248–253. [Google Scholar] [CrossRef]
- Zhou, X.E.; He, Y.; de Waal, P.; Gao, X.; Kang, Y.; Van Eps, N.; Yin, Y.; Pal, K.; Goswami, D.; White, T.A.; et al. Identification of Phosphorylation Codes for Arrestin Recruitment by G Protein-Coupled Receptors. Cell 2017, 170, 457–469.e13. [Google Scholar] [CrossRef] [Green Version]
- Cahill, T.J.; Thomsen, A.; Tarrasch, J.T.; Plouffe, B.; Nguyen, A.; Yang, F.; Huang, L.-Y.; Kahsai, A.W.; Bassoni, D.L.; Gavino, B.J.; et al. Distinct conformations of GPCR–β-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc. Natl. Acad. Sci. USA 2017, 114, 2562–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, P.; Srivastava, A.; Ghosh, E.; Ranjan, R.; Dogra, S.; Yadav, P.N.; Shukla, A.K. Core engagement with β-arrestin is dispensable for agonist-induced vasopressin receptor endocytosis and ERK activation. Mol. Biol. Cell 2017, 28, 1003–1010. [Google Scholar] [CrossRef]
- Moaven, H.; Koike, Y.; Jao, C.C.; Gurevich, V.; Langen, R.; Chen, J. Visual arrestin interaction with clathrin adaptor AP-2 regulates photoreceptor survival in the vertebrate retina. Proc. Natl. Acad. Sci. USA 2013, 110, 9463–9468. [Google Scholar] [CrossRef] [Green Version]
- Ménard, R.; Tavares, J.; Cockburn, I.; Markus, M.; Zavala, F.; Amino, R. Looking under the skin: The first steps in malarial infection and immunity. Nat. Rev. Genet. 2013, 11, 701–712. [Google Scholar] [CrossRef]
- Tavares, J.; Formaglio, P.; Thiberge, S.; Mordelet, E.; Van Rooijen, N.; Medvinsky, A.; Ménard, R.; Amino, R. Role of host cell traversal by the malaria sporozoite during liver infection. J. Exp. Med. 2013, 210, 905–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prudêncio, M.; Rodriguez, A.; Mota, M.M. The silent path to thousands of merozoites: The Plasmodium liver stage. Nat. Rev. Genet. 2006, 4, 849–856. [Google Scholar] [CrossRef]
- Grüring, C.; Heiber, A.; Kruse, F.; Ungefehr, J.; Gilberger, T.-W.; Spielmann, T. Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nat. Commun. 2011, 2, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, C.R.S.; Markus, R.; Madeira, L. Tertian and Quartan Fevers: Temporal Regulation in Malarial Infection. J. Biol. Rhythm. 2001, 16, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Molina-Cruz, A.; Brzostowski, J.; Liu, P.; Luo, Y.; Gunalan, K.; Li, Y.; Ribeiro, J.M.C.; Miller, L.H. PfCDPK1 is critical for malaria parasite gametogenesis and mosquito infection. Proc. Natl. Acad. Sci. USA 2018, 115, 774–779. [Google Scholar] [CrossRef] [Green Version]
- Graumans, W.; Tadesse, F.G.; Andolina, C.; Van Gemert, G.-J.; Teelen, K.; Lanke, K.; Gadisa, E.; Yewhalaw, D.; Van De Vegte-Bolmer, M.; Siebelink-Stoter, R.; et al. Semi-high-throughput detection of Plasmodium falciparum and Plasmodium vivax oocysts in mosquitoes using bead-beating followed by circumsporozoite ELISA and quantitative PCR. Malar. J. 2017, 16, 356. [Google Scholar] [CrossRef] [PubMed]
- Koyama, F.C.; Chakrabarti, D.; Garcia, C.R. Molecular machinery of signal transduction and cell cycle regulation in Plasmodium. Mol. Biochem. Parasitol. 2009, 165, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hotta, C.; Gazarini, M.; Beraldo, F.H.; Varotti, F.D.P.; Lopes, C.; Markus, R.; Pozzan, T.; Garcia, C. Calcium-dependent modulation by melatonin of the circadian rhythm in malarial parasites. Nature 2000, 2, 466–468. [Google Scholar] [CrossRef]
- Beraldo, F.H.; Garcia, C.R.S. Products of tryptophan catabolism induce Ca2+ release and modulate the cell cycle of Plasmodium falciparum malaria parasites. J. Pineal Res. 2005, 39, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Budu, A.; Peres, R.; Bueno, V.B.; Catalani, L.H.; Garcia, C.R.D.S. N1-acetyl-N2-formyl-5-methoxykynuramine modulates the cell cycle of malaria parasites. J. Pineal Res. 2006, 42, 261–266. [Google Scholar] [CrossRef]
- Lima, W.R.; Tessarin-Almeida, G.; Rozanski, A.; Parreira, K.S.; Moraes, M.S.; Martins, D.C.; Hashimoto, R.; Galante, P.; Garcia, C.R. Signaling transcript profile of the asexual intraerythrocytic development cycle of Plasmodium falciparum induced by melatonin and cAMP. Genes Cancer 2016, 7, 323–339. [Google Scholar] [CrossRef] [Green Version]
- Koyama, F.C.; Azevedo, M.F.; Budu, A.; Chakrabarti, D.; Garcia, C.R.S. Melatonin-Induced Temporal up-Regulation of Gene Expression Related to Ubiquitin/Proteasome System (UPS) in the Human Malaria Parasite Plasmodium falciparum. Int. J. Mol. Sci. 2014, 15, 22320–22330. [Google Scholar] [CrossRef] [Green Version]
- Lima, W.R.; Moraes, M.; Alves, E.; Azevedo, M.; Passos, D.O.; Garcia, C.R.S. The PfNF-YB transcription factor is a downstream target of melatonin and cAMP signalling in the human malaria parasite Plasmodium falciparum. J. Pineal Res. 2012, 54, 145–153. [Google Scholar] [CrossRef]
- Lima, W.R.; Martins, D.C.; Parreira, K.S.; Scarpelli, P.; De Moraes, M.S.; Topalis, P.; Hashimoto, R.; Garcia, C.R.S. Genome-wide analysis of the human malaria parasite Plasmodium falciparum transcription factor PfNF-YB shows interaction with a CCAAT motif. Oncotarget 2017, 8, 113987–114001. [Google Scholar] [CrossRef]
- Singh, M.K.; Tessarin-Almeida, G.; Dias, B.K.M.; Pereira, P.S.; Costa, F.; Przyborski, J.M.; Garcia, C.R.S. A nuclear protein, PfMORC confers melatonin dependent synchrony of the human malaria parasite P. falciparum in the asexual stage. Sci. Rep. 2021, 11, 2057. [Google Scholar] [CrossRef]
- Dorin, D.; Semblat, J.-P.; Poullet, P.; Alano, P.; Goldring, J.P.D.; Whittle, C.; Patterson, S.; Chakrabarti, D.; Doerig, C. PfPK7, an atypical MEK-related protein kinase, reflects the absence of classical three-component MAPK pathways in the human malaria parasite Plasmodium falciparum. Mol. Microbiol. 2005, 55, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Dorin-Semblat, D.; Sicard, A.; Doerig, C.; Ranford-Cartwright, L.; Doerig, C. Disruption of the Pf PK7 Gene Impairs Schizogony and Sporogony in the Human Malaria Parasite Plasmodium falciparum. Eukaryot. Cell 2008, 7, 279–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, F.; Ribeiro, R.; Garcia, J.; Mauro, F.; Chakrabarti, D.; Garcia, C. Ubiquitin Proteassome System and the atypical kinase PfPK7 are involved in melatonin signaling in Plasmodium falciparum. J. Pineal Res. 2013, 53, 147–153. [Google Scholar] [CrossRef]
- Pease, B.N.; Huttlin, E.; Jedrychowski, M.P.; Dorin-Semblat, D.; Sebastiani, D.; Segarra, D.T.; Roberts, B.F.; Chakrabarti, R.; Doerig, C.; Gygi, S.P.; et al. Characterization of Plasmodium falciparum Atypical Kinase PfPK7–Dependent Phosphoproteome. J. Proteome Res. 2018, 17, 2112–2123. [Google Scholar] [CrossRef]
- Dias, B.K.; Nakabashi, M.; Alves, M.R.R.; Portella, D.P.; Santos, B.M.; Almeida, F.C.D.S.; Ribeiro, R.Y.; Schuck, D.C.; Jordão, A.K.; Garcia, C.R. The Plasmodium falciparum eIK1 kinase (PfeIK1) is central for melatonin synchronization in the human malaria parasite. Melatotosil blocks melatonin action on parasite cell cycle. J. Pineal Res. 2020, 69, e12685. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Bartlett, P.J.; Garcia, C.R.; Thomas, A.P. Melatonin and IP3-induced Ca2+ Release from Intracellular Stores in the Malaria Parasite Plasmodium falciparum within Infected Red Blood Cells. J. Biol. Chem. 2011, 286, 5905–5912. [Google Scholar] [CrossRef] [Green Version]
- Pecenin, M.F.; Borges-Pereira, L.; Levano-Garcia, J.; Budu, A.; Alves, E.; Mikoshiba, K.; Thomas, A.; Garcia, C.R. Blocking IP 3 signal transduction pathways inhibits melatonin-induced Ca2+ signals and impairs P. falciparum development and proliferation in erythrocytes. Cell Calcium 2018, 72, 81–90. [Google Scholar] [CrossRef]
- Beraldo, F.H.; Mikoshiba, K.; Garcia, C.R.S. Human malarial parasite, Plasmodium falciparum, displays capacitative calcium entry: 2-aminoethyl diphenylborinate blocks the signal transduction pathway of melatonin action on the P. falciparum cell cycle. J. Pineal Res. 2007, 43, 360–364. [Google Scholar] [CrossRef]
- Garcia, C.R.; Alves, E.; Pereira, P.H.S.; Bartlett, P.J.; Thomas, A.P.; Mikoshiba, K.; Plattner, H.; Sibley, L.D. InsP3 Signaling in Apicomplexan Parasites. Curr. Top. Med. Chem. 2017, 17, 2158–2165. [Google Scholar] [CrossRef] [Green Version]
- Alves, E.; Nakaya, H.; Guimarães, E.; Garcia, C.R.S. Combining IP affinity chromatography and bioinformatics reveals a novel protein-IP3 binding site on Plasmodium falciparum MDR1 transporter. bioRxiv 2021. [Google Scholar] [CrossRef]
- Cruz, L.N.; Juliano, M.A.; Budu, A.; Juliano, L.; Holder, A.A.; Blackman, M.J.; Garcia, C.R. Extracellular ATP triggers proteolysis and cytosolic Ca2+ rise in Plasmodium berghei and Plasmodium yoelii malaria parasites. Malar. J. 2012, 11, 69. [Google Scholar] [CrossRef] [Green Version]
- Levano-Garcia, J.; Dluzewski, A.R.; Markus, R.P.; Garcia, C.R.S. Purinergic signalling is involved in the malaria parasite Plasmodium falciparum invasion to red blood cells. Purinergic Signal. 2010, 6, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Budu, A.; Garcia, C.R. Generation of second messengers in Plasmodium. Microbes Infect. 2012, 14, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.A.; Kelly, J.M. Purine nucleotide cyclases in the malaria parasite. Trends Parasitol. 2004, 20, 227–232. [Google Scholar] [CrossRef]
- Weber, J.H.; Vishnyakov, A.; Hambach, K.; Schultz, A.; Schultz, J.E.; Linder, J.U. Adenylyl cyclases from Plasmodium, Paramecium and Tetrahymena are novel ion channel/enzyme fusion proteins. Cell. Signal. 2003, 16, 115–125. [Google Scholar] [CrossRef]
- Ono, T.; Cabrita-Santos, L.; Leitao, R.; Bettiol, E.; Purcell, L.A.; Diaz-Pulido, O.; Andrews, L.B.; Tadakuma, T.; Bhanot, P.; Mota, M.M.; et al. Adenylyl Cyclase α and cAMP Signaling Mediate Plasmodium Sporozoite Apical Regulated Exocytosis and Hepatocyte Infection. PLoS Pathog. 2008, 4, e1000008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Alam, M.M.; Pal-Bhowmick, I.; Brzostowski, J.A.; Chitnis, C.E. Distinct External Signals Trigger Sequential Release of Apical Organelles during Erythrocyte Invasion by Malaria Parasites. PLoS Pathog. 2010, 6, e1000746. [Google Scholar] [CrossRef] [Green Version]
- Dawn, A.; Singh, S.; More, K.R.; Siddiqui, F.A.; Pachikara, N.; Ramdani, G.; Langsley, G.; Chitnis, C.E. The Central Role of cAMP in Regulating Plasmodium falciparum Merozoite Invasion of Human Erythrocytes. PLoS Pathog. 2014, 10, e1004520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thélu, J.; Bracchi, V.; Burnod, J.; Ambroise-Thomas, P. Evidence for expression of a Ras-like and a stage specific GTP binding homologous protein by Plasmodium falciparum. Cell. Signal. 1994, 6, 777–782. [Google Scholar] [CrossRef]
- Dyer, M.; Day, K. Expression of Plasmodium falciparum trimeric G proteins and their involvement in switching to sexual development. Mol. Biochem. Parasitol. 2000, 108, 67–78. [Google Scholar] [CrossRef]
- Kaiser, A.; Langer, B.; Przyborski, J.; Kersting, D.; Krüger, M. A Putative Non-Canonical Ras-Like GTPase from P. falciparum: Chemical Properties and Characterization of the Protein. PLoS ONE 2015, 10, e0140994. [Google Scholar] [CrossRef] [Green Version]
- Harrison, T.; Samuel, B.U.; Akompong, T.; Hamm, H.; Mohandas, N.; Lomasney, J.W.; Haldar, K. Erythrocyte G Protein-Coupled Receptor Signaling in Malarial Infection. Science 2003, 301, 1734–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, Y.; Ikeda, M.; Shimizu, T. Proteome-wide classification and identification of mammalian-type GPCRs by binary topology pattern. Comput. Biol. Chem. 2004, 28, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Madeira, L.; Galante, P.A.F.; Budu, A.; Azevedo, M.F.; Malnic, B.; Garcia, C.R.S. Genome-Wide Detection of Serpentine Receptor-Like Proteins in Malaria Parasites. PLoS ONE 2008, 3, e1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Wang, C.; Otto, T.D.; Oberstaller, J.; Liao, X.; Adapa, S.R.; Udenze, K.; Bronner, I.F.; Casandra, D.; Mayho, M.; et al. Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science 2018, 360. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, B.M.; Gonzaga, D.T.G.; da Silva, F.C.; Ferreira, V.F.; Garcia, C.R.S. Plasmodium falciparum Knockout for the GPCR-Like PfSR25 Receptor Displays Greater Susceptibility to 1,2,3-Triazole Compounds That Block Malaria Parasite Development. Biomolecules 2020, 10, 1197. [Google Scholar] [CrossRef]
- Tchoufack, E.J.N.; Hahnfeld, L.; Pitschelatow, G.; Bennink, S.; Pradel, G. The endoplasmic reticulum-resident serpentine receptor SR10 has important functions for asexual and sexual blood stage development of Plasmodium falciparum. Mol. Biochem. Parasitol. 2020, 239, 111315. [Google Scholar] [CrossRef]
- Marapana, D.S.; Dagley, L.F.; Sandow, J.J.; Nebl, T.; Triglia, T.; Pasternak, M.; Dickerman, B.K.; Crabb, B.S.; Gilson, P.R.; Webb, A.I.; et al. Plasmepsin V cleaves malaria effector proteins in a distinct endoplasmic reticulum translocation interactome for export to the erythrocyte. Nat. Microbiol. 2018, 3, 1010–1022. [Google Scholar] [CrossRef]
- Aurrecoechea, C.; Brestelli, J.; Brunk, B.P.; Dommer, J.; Fischer, S.; Gajria, B.; Gao, X.; Gingle, A.; Grant, G.; Harb, O.S.; et al. PlasmoDB: A functional genomic database for malaria parasites. Nucleic Acids Res. 2009, 37, D539–D543. [Google Scholar] [CrossRef] [Green Version]
- Subudhi, A.K.; O’Donnell, A.J.; Ramaprasad, A.; Abkallo, H.M.; Kaushik, A.; Ansari, H.R.; Abdel-Haleem, A.; Ben Rached, F.; Kaneko, O.; Culleton, R.; et al. Malaria parasites regulate intra-erythrocytic development duration via serpentine receptor 10 to coordinate with host rhythms. Nat. Commun. 2020, 11, 2763. [Google Scholar] [CrossRef]
- Gupta, S.; Singh, D.; Singh, S. In silico characterization of Plasmodium falciparum purinergic receptor: A novel chemotherapeutic target. Syst. Synth. Biol. 2015, 9, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Joshi, N.; Saini, M.; Singh, S. Antimalarial and Plasmodium falciparum serpentine receptor 12 targeting effect of a purinergic receptor antagonist FDA approved drug. bioRxiv 2020. [Google Scholar] [CrossRef]
- Pereira, P.H.S.; Brito, G.; Moraes, M.; Kiyan, C.L.; Avet, C.; Bouvier, M.; Garcia, C.R. BRET sensors unravel that Plasmodium falciparum serpentine receptor 12 (PfSR12) increases surface expression of mammalian GPCRs in HEK293 cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Moraes, M.S.; Budu, A.; Singh, M.; Borges-Pereira, L.; Levano-Garcia, J.; Curra, C.; Picci, L.; Pace, T.; Ponzi, M.; Pozzan, T.; et al. Plasmodium falciparum GPCR-like receptor SR25 mediates extracellular K+ sensing coupled to Ca2+ signaling and stress survival. Sci. Rep. 2017, 7, 9545. [Google Scholar] [CrossRef]
- Santos, B.M.; Dias, B.K.M.; Nakabashi, M.; Garcia, C.R.S. The Knockout for G Protein-Coupled Receptor-Like PfSR25 Increases the Susceptibility of Malaria Parasites to the Antimalarials Lumefantrine and Piperaquine but Not to Medicine for Malaria Venture Compounds. Front. Microbiol. 2021, 12, 638869. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, S.; Iwai, M.; Nishiu, J.; Itoh, M.; Tomoike, H.; Horiuchi, M.; Nakamura, Y.; Tanaka, T. Inhibition of Experimental Intimal Thickening in Mice Lacking a Novel G-Protein–Coupled Receptor. Circulation 2003, 107, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Mosienko, V.; Rasooli-Nejad, S.; Kishi, K.; De Both, M.; Jane, D.; Huentelman, M.J.; Kasparov, S.; Teschemacher, A.G. Putative Receptors Underpinning l-Lactate Signalling in Locus Coeruleus. Neuroglia 2018, 1, 25. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.A.; Garcia, C.; Chandran, V.R.; Van Rooijen, N.; Zhou, Y.; Winzeler, E.; Nussenzweig, V. Exposure of Plasmodium sporozoites to the intracellular concentration of potassium enhances infectivity and reduces cell passage activity. Mol. Biochem. Parasitol. 2007, 156, 32–40. [Google Scholar] [CrossRef]
| Protein | H. sapiens 1 | P. falciparum |
|---|---|---|
| GRK | AAA58620; AAA60175 | - |
| Gα | AAC50363; NP_002064; AAH87537 | - 2 |
| Gβ | AAA35922 | - |
| Gγ | AAC39869; AAK53385 | - |
| Adenylate cyclase | CAA84552 | PF3D7_1404600 |
| Guanylyl cyclase | NP_000848 | PF3D7_1360500 |
| Phosphodiesterase | AAA03592 | PF3D7_1321500 |
| Phospholipase C | AAA60112 | PF3D7_1013500 |
| PKC | EAW89014 | - |
| PKA | AAC41690 | PF3D7_0934800 |
| β-Arrestin | AAH03636; AAH67368 | - |
| Clathrin | NP_009029; NP_001825 | PF3D7_1435500; PF3D7_1219100 |
| MAPK | NP_002736 | PF3D7_1113900 |
| EPAC | NP_001362802 | PF3D7_1417400 |
| IP3 receptor | NP_002215 | - |
| SR1 | SR10 | SR12 | SR25 | |
|---|---|---|---|---|
| SIZE (AA) | 773 | 655 | 470 | 357 |
| N TERMINAL LENGTH | 508 | 381 | 232 | 51 |
| C TERMINAL LENGTH | 23 | 35 | 18 | 9 |
| PREDICTED CLEAVAGE SITE | - | 30–31 (SNG-QL) | 21–22 (YYL-TK) | 28–29 (VFT-AF) |
| 7 TRANSMEMBRANEREGION | 509–750 | 382–620 | 233–452 | 217–440 |
| PREDICTED CLASSIFICATION | Class C | Class A | - | Class A |
| SIGNAL PEPTIDE (SIGNALP 3.0) | no | yes | yes | yes |
| SIGNAL PEPTIDE (SIGNALP 5.0) | no | no | no | no |
| SIGNAL PEPTIDE (PHOBIUS) | no | no 1 | no 1 | yes |
| TRANSMEMBRANE DOMAINS | 7 | 8 2 | 7 | 8 2 |
| EXPRESSED MAINLY IN (STAGES) 3 | Schizont, gametocytes, ookinete | Ring, schizont, gametocytes | Trophozoite, schizont, gametocyte, ookinete | Ring, trophozoite, schizont, gametocyte |
| FOUND IN (SPECIES) 3,4 | P. falciparum, P. reichenowi | P. falciparum, P. reichenowi, P. berghei, P. chabaudi, P. gallinaceum, P. knowlesi, P. malariae, P. ovale, P. vivax | P. falciparum, P. berghei, P. chabaudi, P. cynomolgi, P. gallinaceum, P. knowlesi, P. malariae, P. ovale, P. reichenowi, P. vivax, P. yoelii | P. falciparum, P. berghei, P. gallinaceum, P. knowlesi, P. malariae, P. ovale, P. reichenowi, P. vivax |
| KO PHENOTYPE | Slow growth rate | Slow growth rate. Reduced cycle length. | Dispensable | Essential [65]/dispensable [66] |
| REFERENCES | - | [67,68,69,70] | [71,72,73] | [66,74,75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, P.H.S.; Garcia, C.R.S. Evidence of G-Protein-Coupled Receptors (GPCR) in the Parasitic Protozoa Plasmodium falciparum—Sensing the Host Environment and Coupling within Its Molecular Signaling Toolkit. Int. J. Mol. Sci. 2021, 22, 12381. https://doi.org/10.3390/ijms222212381
Pereira PHS, Garcia CRS. Evidence of G-Protein-Coupled Receptors (GPCR) in the Parasitic Protozoa Plasmodium falciparum—Sensing the Host Environment and Coupling within Its Molecular Signaling Toolkit. International Journal of Molecular Sciences. 2021; 22(22):12381. https://doi.org/10.3390/ijms222212381
Chicago/Turabian StylePereira, Pedro H. S., and Celia R. S. Garcia. 2021. "Evidence of G-Protein-Coupled Receptors (GPCR) in the Parasitic Protozoa Plasmodium falciparum—Sensing the Host Environment and Coupling within Its Molecular Signaling Toolkit" International Journal of Molecular Sciences 22, no. 22: 12381. https://doi.org/10.3390/ijms222212381






