3D Printed Multiphasic Scaffolds for Osteochondral Repair: Challenges and Opportunities
Abstract
:1. Introduction
2. Osteochondral Tissue: Anatomy, Pathology and Treatments
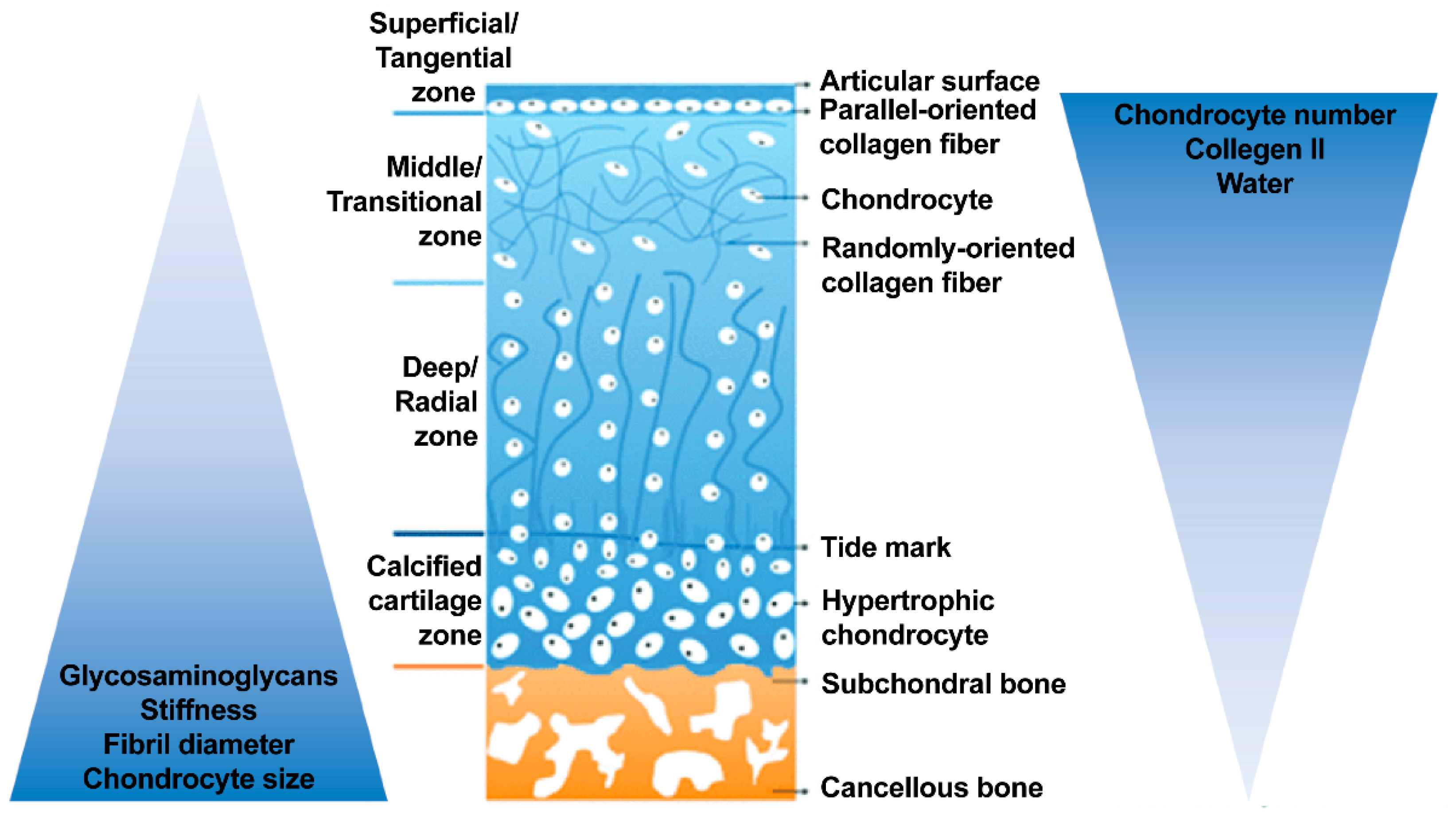
2.1. Structure of Osteochondral Tissue
2.1.1. Articular Cartilage
2.1.2. Calcified Zone and Tidemark: The Transition/Interface
2.1.3. Subchondral Bone
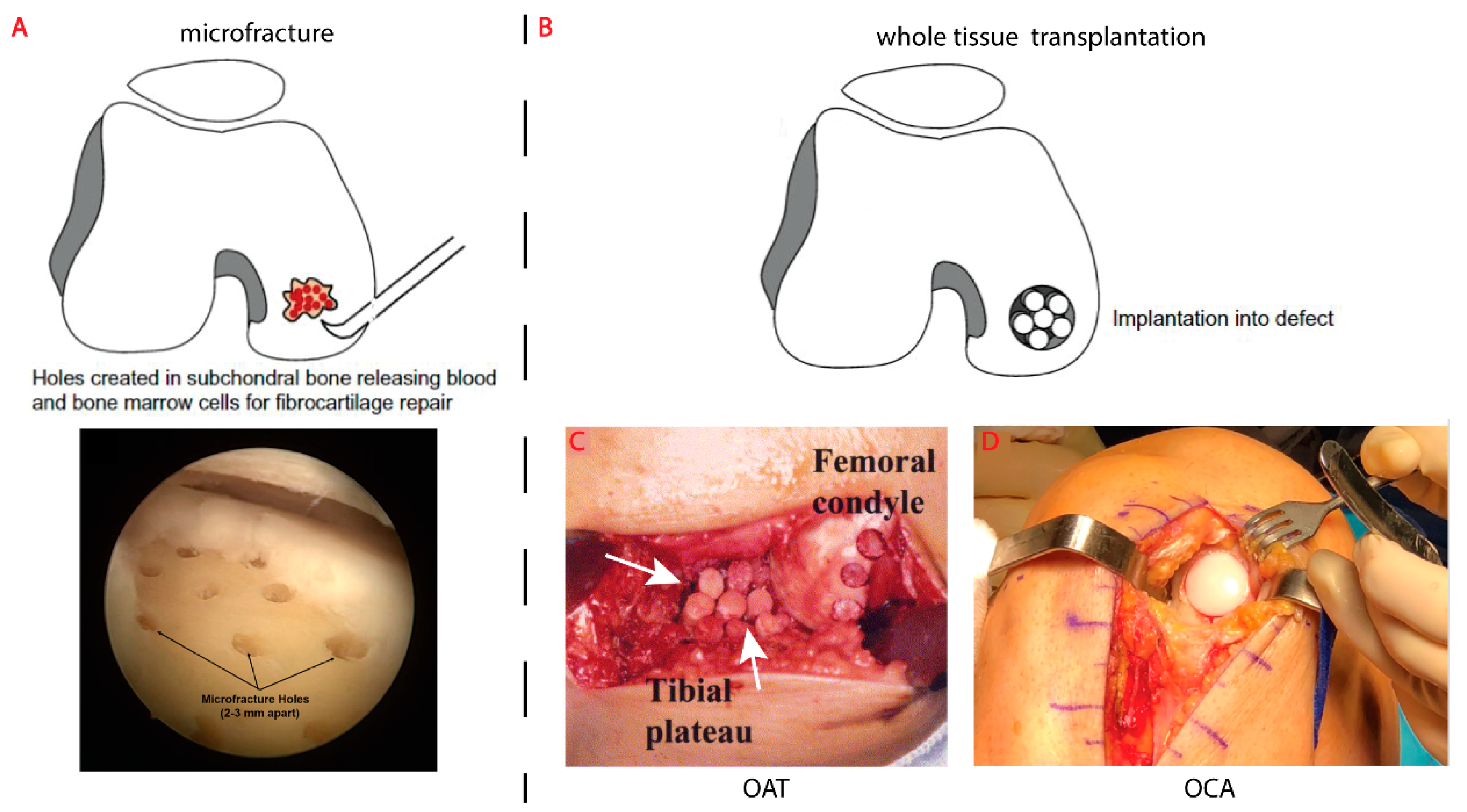
2.2. Existing Surgical Treatments for Osteochondral Defects
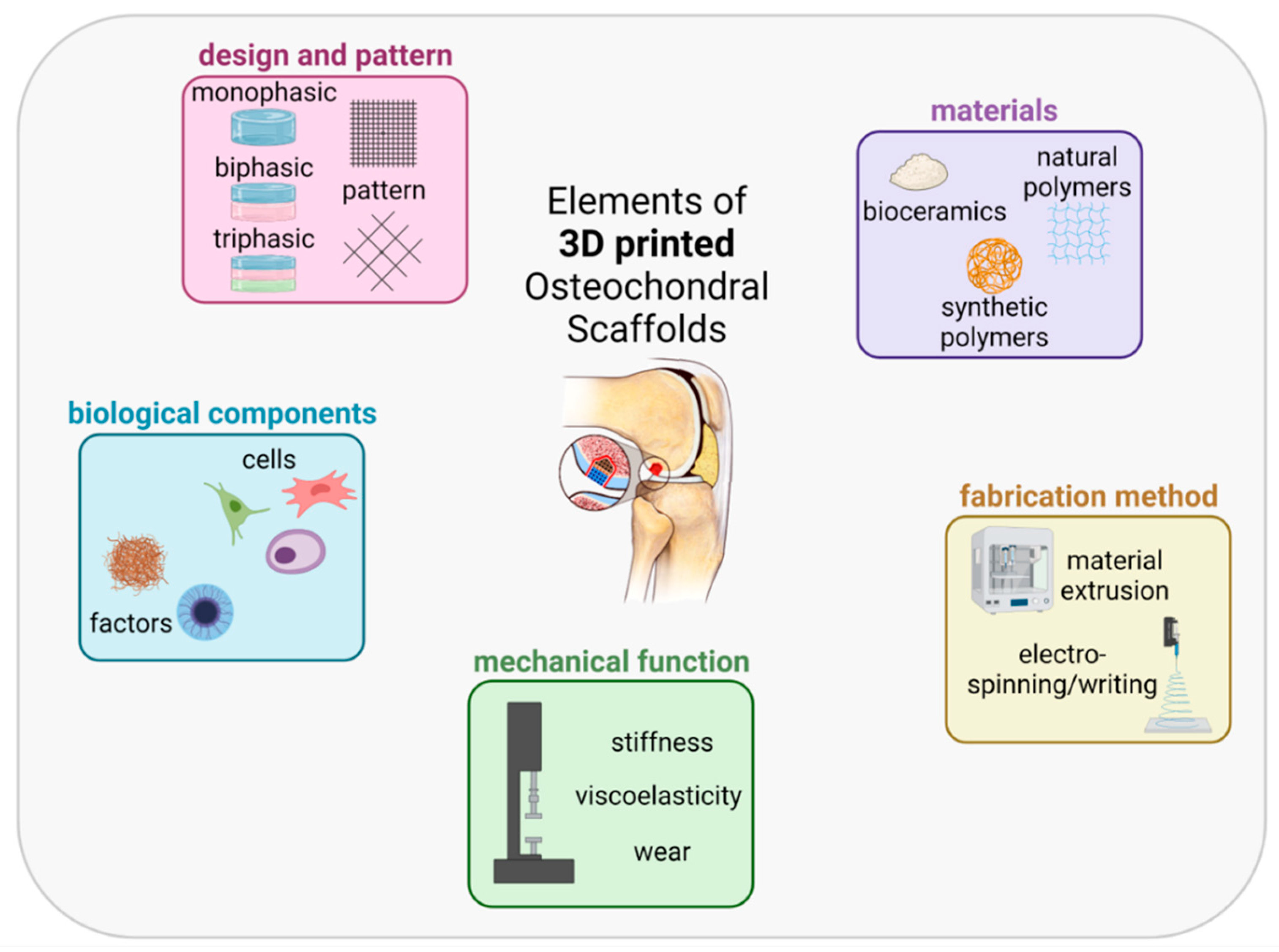
3. Engineering New Osteochondral Tissue
3.1. Elements of an OC Scaffold: Materials
3.1.1. Natural Polymers
3.1.2. Synthetic Polymers
3.1.3. Bioceramics
3.2. Elements of an OC Scaffold: Fabrication Method
3.2.1. Material Extrusion
3.2.2. Melt Electro-Writing and Electrospinning
3.2.3. Stereolithography and Digital Light Processing

3.3. Elements of an OC Scaffold: Mechanical Function

3.4. Elements of an OC Scaffold: Biological Components
3.4.1. Considerations of Cell Type
3.4.2. Culture Conditions and Growth Factors
3.5. Elements of an OC Scaffold: Design
3.5.1. Monophasic Scaffold
3.5.2. Biphasic Scaffold
3.5.3. Triphasic Scaffold
3.5.4. Triphasic Scaffold
4. Functional Evaluation: In Vitro and In Vivo
5. Discussion and Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OC | Osteochondral |
| OAT | Osteochondral Autograft Transfer |
| OCA | Osteochondral Allograft Transplant |
| PLA | Polylactic Acid |
| PCL | Polycaprolactone |
| PLGA | Poly(L-lactic-co-Glycolic Acid) |
| PEG | Polyethylene Glycol |
| PVA | Polyvinyl Alcohol |
| ECM | Extracellular Matrix |
| HA | Hydroxyapatite |
| TCP | Tricalcium Phosphate |
| ME | Material Extrusion |
| MEW | Melt Electro-Writing |
| ES | Electro-Spinning |
| SLA | Stereolithography |
| DLP | Digital Light Processing |
| MSC | Mesenchymal Stem/Stromal Cell |
| bmMSC | Bone Marrow derived Mesenchymal Stem/Stromal Cell |
| ACPC | Articular Cartilage Progenitor Cell |
| IGF | Insulin-like Growth Factor |
| BMP | Bone Morphogenetic Proteins |
| FGF | Fibroblast Growth Factor |
| TGF | Transforming Growth Factor |
References
- Hall, S. ISE EBook Online Access for Basic Biomechanics; McGraw-Hill Higher Education: New York, NY, USA, 2021; ISBN 9781264363674. [Google Scholar]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Gomoll, A.H.; Minas, T. The quality of healing: Articular cartilage. Wound Repair Regen. 2014, 22, 30–38. [Google Scholar] [CrossRef]
- Giffin, J.R.; Annunziata, C.C.; Vogrin, T.M.; Harner, C.D. Primary repair of osteochondral and chondral injury. Oper. Tech. Orthop. 2001, 11, 83–89. [Google Scholar] [CrossRef]
- Steinert, A.F.; Nöth, U.; Tuan, R.S. Concepts in gene therapy for cartilage repair. Injury 2008, 39, 97–113. [Google Scholar] [CrossRef] [Green Version]
- Ebihara, G.; Sato, M.; Yamato, M.; Mitani, G.; Kutsuna, T.; Nagai, T.; Ito, S.; Ukai, T.; Kobayashi, M.; Kokubo, M.; et al. Cartilage repair in transplanted scaffold-free chondrocyte sheets using a minipig model. Biomaterials 2012, 33, 3846–3851. [Google Scholar] [CrossRef]
- Hunziker, E.B. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef] [Green Version]
- Armiento, A.R.; Alini, M.; Stoddart, M.J. Articular fibrocartilage-Why does hyaline cartilage fail to repair? Adv. Drug Deliv. Rev. 2019, 146, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Outerbridge, R.E. The etiology of chondromalacia patellae. J. Bone Joint Surg. Br. 1961, 43, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, E.S.; Bajaj, S.; Ghodadra, N.S.; Cole, B.J. Basic science and surgical treatment options for articular cartilage injuries of the knee. J. Orthop. Sports Phys. Ther. 2012, 42, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widuchowski, W.; Widuchowski, J.; Trzaska, T. Articular cartilage defects: Study of 25,124 knee arthroscopies. Knee 2007, 14, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E.; Rose, M.B.; Wille, A.J.; Wiedrick, J.; Crawford, D.C. Arthroscopic Mechanical Chondroplasty of the Knee Is Beneficial for Treatment of Focal Cartilage Lesions in the Absence of Concurrent Pathology. Orthop. J. Sport. Med. 2017, 5, 5. [Google Scholar] [CrossRef]
- Curl, W.W.; Krome, J.; Gordon, E.S.; Rushing, J.; Smith, B.P.; Poehling, G.G. Cartilage injuries: A review of 31,516 knee arthroscopies. Arthrosc. J. Arthrosc. Relat. Surg. 1997, 13, 456–460. [Google Scholar] [CrossRef]
- Beris, A.E.; Lykissas, M.G.; Papageorgiou, C.D.; Georgoulis, A.D. Advances in articular cartilage repair. Injury 2005, 36 (Suppl. S4), S14–S23. [Google Scholar] [CrossRef]
- Guettler, J.H.; Demetropoulos, C.K.; Yang, K.H.; Jurist, K.A. Osteochondral defects in the human knee: Influence of defect size on cartilage rim stress and load redistribution to surrounding cartilage. Am. J. Sports Med. 2004, 32, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, G.M.; Niemeyer, P.; Hochrein, A.; Stoddart, M.J.; Angele, P. Articular Cartilage Repair of the Knee in Children and Adolescents. Orthop. J. Sport. Med. 2018, 6, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, T.C.; Giuliani, J.R.; Svoboda, S.J.; Owens, B.D. Knee Cartilage Tibio-Femoral Injuries. Tech. Orthop. 2010, 25, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Lepage, S.I.M.; Robson, N.; Gilmore, H.; Davis, O.; Hooper, A.; St John, S.; Kamesan, V.; Gelis, P.; Carvajal, D.; Hurtig, M.; et al. Beyond Cartilage Repair: The Role of the Osteochondral Unit in Joint Health and Disease. Tissue Eng. Part B Rev. 2019, 25, 114–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, M.; Tradati, D.; Maione, A.; Uboldi, F.M.; Usellini, E.; Berruto, M. Cell-free osteochondral scaffolds provide a substantial clinical benefit in the treatment of osteochondral defects at a minimum follow-up of 5 years. J. Exp. Orthop. 2021, 8, 62. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920. [Google Scholar] [CrossRef] [Green Version]
- Abdollahiyan, P.; Oroojalian, F.; Mokhtarzadeh, A.; de la Guardia, M. Hydrogel-Based 3D Bioprinting for Bone and Cartilage Tissue Engineering. Biotechnol. J. 2020, 15, 12. [Google Scholar] [CrossRef]
- Harley, W.S.; Li, C.C.; Toombs, J.; O’Connell, C.D.; Taylor, H.K.; Heath, D.E.; Collins, D.J. Advances in biofabrication techniques towards functional bioprinted heterogeneous engineered tissues: A comprehensive review. Bioprinting 2021, 23, e00147. [Google Scholar] [CrossRef]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.-W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 13001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viola, M.; Piluso, S.; Groll, J.; Vermonden, T.; Malda, J.; Castilho, M. The Importance of Interfaces in Multi-Material Biofabricated Tissue Structures. Adv. Healthc. Mater. 2021, 10, 2101021. [Google Scholar] [CrossRef]
- Lim, K.S.; Levato, R.; Costa, P.F.; Castilho, M.D.; Alcala-Orozco, C.R.; van Dorenmalen, K.M.A.; Melchels, F.P.W.; Gawlitta, D.; Hooper, G.J.; Malda, J.; et al. Bio-resin for high resolution lithography-based biofabrication of complex cell-laden constructs. Biofabrication 2018, 10, 34101. [Google Scholar] [CrossRef] [PubMed]
- Abdulghani, S.; Morouço, P.G. Biofabrication for osteochondral tissue regeneration: Bioink printability requirements. J. Mater. Sci. Mater. Med. 2019, 30, 20. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athanasiou, K.A.; Darling, E.M.; Hu, J.C. Articular Cartilage Tissue Engineering; Morgan & Claypool Publishers: San Rafael, CA, USA, 2009; ISBN 9781598298758. [Google Scholar]
- Lees, D.; Partington, P. Articular cartilage. Orthop. Trauma 2016, 30, 265–272. [Google Scholar] [CrossRef]
- Correia, C.R.; Reis, R.L.; Mano, J.F. Multiphasic, Multistructured and Hierarchical Strategies for Cartilage Regeneration. In Engineering Mineralized and Load Bearing Tissues; Bertassoni, L.E., Coelho, P.G., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 143–160. ISBN 978-3-319-22345-2. [Google Scholar]
- Vyas, C.; Poologasundarampillai, G.; Hoyland, J.; Bartolo, P. 12-3D printing of biocomposites for osteochondral tissue engineering. In Biomedical Composites; Woodhead Publishing: Sawston, UK, 2017; pp. 261–302. ISBN 978-0-08-100752-5. [Google Scholar]
- Kabir, W.; Di Bella, C.; Choong, P.F.M.; O’Connell, C.D. Assessment of Native Human Articular Cartilage: A Biomechanical Protocol. Cartilage 2020, 1–11. [Google Scholar] [CrossRef]
- Jurvelin, J.S.; Buschmann, M.D.; Hunziker, E.B. Mechanical anisotropy of the human knee articular cartilage in compression. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2003, 217, 215–219. [Google Scholar] [CrossRef]
- Kiviranta, P.; Lammentausta, E.; Töyräs, J.; Kiviranta, I.; Jurvelin, J.S. Indentation diagnostics of cartilage degeneration. Osteoarthr. Cartil. 2008, 16, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Antons, J.; Marascio, M.G.M.; Nohava, J.; Martin, R.; Applegate, L.A.; Bourban, P.E.; Pioletti, D.P. Zone-dependent mechanical properties of human articular cartilage obtained by indentation measurements. J. Mater. Sci. Mater. Med. 2018, 29, 57. [Google Scholar] [CrossRef] [Green Version]
- Renault, J.-B.; Carmona, M.; Tzioupis, C.; Ollivier, M.; Argenson, J.-N.; Parratte, S.; Chabrand, P. Tibial subchondral trabecular bone micromechanical and microarchitectural properties are affected by alignment and osteoarthritis stage. Sci. Rep. 2020, 10, 3975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, M.; Danielsen, C.C.; Hvid, I. Bone density does not reflect mechanical properties in early-stage arthrosis. Acta Orthop. Scand. 2001, 72, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Day, J.S.; Ding, M.; van der Linden, J.C.; Hvid, I.; Sumner, D.R.; Weinans, H. A decreased subchondral trabecular bone tissue elastic modulus is associated with pre-arthritic cartilage damage. J. Orthop. Res. 2001, 19, 914–918. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.-C.; Yan, C.H.; Chiu, K.Y.; Wei, Q.; Zhao, J.; Guo, X.E.; Leung, F.; Lu, W.W. Abnormal subchondral bone remodeling and its association with articular cartilage degradation in knees of type 2 diabetes patients. Bone Res. 2017, 5, 17034. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Quinn, T.M.; Häuselmann, H.-J. Quantitative structural organization of normal adult human articular cartilage. Osteoarthr. Cartil. 2002, 10, 564–572. [Google Scholar] [CrossRef] [Green Version]
- Mow, V.C.; Holmes, M.H.; Lai, W.M. Fluid transport and mechanical properties of articular cartilage: A review. J. Biomech. 1984, 17, 377–394. [Google Scholar] [CrossRef]
- Thonar, E.J.-M.A.; Masuda, K.; Manicourt, D.H.; Kuettner, K.E. Structure and Function of Normal Human Adult Articular Cartilage. In Osteoarthritis: Clinical and Experimental Aspects; Reginster, J.-Y., Pelletier, J.-P., Martel-Pelletier, J., Henrotin, Y., Crasborn, L., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–19. ISBN 978-3-642-60026-5. [Google Scholar]
- Mansfield, J.C.; Bell, J.S.; Winlove, C.P. The micromechanics of the superficial zone of articular cartilage. Osteoarthr. Cartil. 2015, 23, 1806–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eschweiler, J.; Horn, N.; Rath, B.; Betsch, M.; Baroncini, A.; Tingart, M.; Migliorini, F. The Biomechanics of Cartilage-An Overview. Life 2021, 11, 302. [Google Scholar] [CrossRef]
- Han, S.-K.; Federico, S.; Grillo, A.; Giaquinta, G.; Herzog, W. The Mechanical Behaviour of Chondrocytes Predicted with a Micro-structural Model of Articular Cartilage. Biomech. Model. Mechanobiol. 2007, 6, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Setton, L.A.; Elliott, D.M.; Mow, V.C. Altered mechanics of cartilage with osteoarthritis: Human osteoarthritis and an experimental model of joint degeneration. Osteoarthr. Cartil. 1999, 7, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Decker, R.S.; Koyama, E.; Pacifici, M. Articular Cartilage: Structural and Developmental Intricacies and Questions. Curr. Osteoporos. Rep. 2015, 13, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, S.J.; Blain, E.J. Chapter 4-Cartilage Mechanobiology: How Chondrocytes Respond to Mechanical Load. In Mechanobiology in Health and Disease; Verbruggen, S., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 99–126. ISBN 978-0-12-812952-4. [Google Scholar]
- Sefat, F.; Raja, T.I.; Zafar, M.S.; Khurshid, Z.; Najeeb, S.; Zohaib, S.; Ahmadi, E.D.; Rahmati, M.; Mozafari, M. Chapter 3-Nanoengineered Biomaterials for Cartilage Repair. In Nanoengineered Biomaterials for Regenerative Medicine; Mozafari, M., Rajadas, J., Kaplan, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 39–71. ISBN 978-0-12-813355-2. [Google Scholar]
- Xia, Y.; Momot, K.; Chen, Z.; Chen, C.; Kahn, D.; Badar, F. Introduction to Cartilage. In Biophysics and Biochemistry of Cartilage by NMR and MRI; Royal Society of Chemistry: London, UK, 2016; pp. 1–43. ISBN 978-1-78262-133-1. [Google Scholar]
- Fan, X.; Wu, X.; Crawford, R.; Xiao, Y.; Prasadam, I. Macro, Micro, and Molecular. Changes of the Osteochondral Interface in Osteoarthritis Development. Front. Cell Dev. Biol. 2021, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Khorshidi, S.; Karkhaneh, A. Engineering of gradient osteochondral tissue: From nature to lab. Acta Biomater. 2019, 87, 41–54. [Google Scholar] [CrossRef]
- Bhosale, A.M.; Richardson, J.B. Articular cartilage: Structure, injuries and review of management. Br. Med. Bull. 2008, 87, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.R.; Kojima, T.; Yasuda, T.; Mwale, F.; Kobayashi, M.; Laverty, S. Composition and structure of articular cartilage: A template for tissue repair. Clin. Orthop. Relat. Res. 2001, 391, S26–S33. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wu, J.P.; Kirk, T.B.; Carrino, J.A.; Xiang, C.; Xu, J. High-resolution measurements of the multilayer ultra-structure of articular cartilage and their translational potential. Arthritis Res. Ther. 2014, 16, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nukavarapu, S.P.; Dorcemus, D.L. Osteochondral tissue engineering: Current strategies and challenges. Biotechnol. Adv. 2013, 31, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.; Tan, H.; Chen, G.; Guo, L.; Yang, L. Analysis of the Mineral Composition of the Human Calcified Cartilage Zone. Int. J. Med. Sci. 2012, 9, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, H.; Kawcak, C. The Importance of Subchondral Bone in the Pathophysiology of Osteoarthritis. Front. Vet. Sci. 2018, 5, 178. [Google Scholar] [CrossRef]
- Kawcak, C.E.; McIlwraith, C.W.; Norrdin, R.W.; Park, R.D.; James, S.P. The role of subchondral bone in joint disease: A review. Equine Vet. J. 2001, 33, 120–126. [Google Scholar] [CrossRef]
- Becerra, J.; Andrades, J.A.; Guerado, E.; Zamora-Navas, P.; López-Puertas, J.M.; Reddi, A.H. Articular Cartilage: Structure and Regeneration. Tissue Eng. Part B Rev. 2010, 16, 617–627. [Google Scholar] [CrossRef]
- Bonewald, L.F. Chapter 313-Cell–Cell and Cell–Matrix Interactions in Bone. In Handbook of Cell Signaling; Bradshaw, R.A., Dennis, E.A., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 2647–2662. ISBN 978-0-12-374145-5. [Google Scholar]
- Di Luca, A.; Van Blitterswijk, C.; Moroni, L. The osteochondral interface as a gradient tissue: From development to the fabrication of gradient scaffolds for regenerative medicine. Birth Defects Res. C Embryo Today 2015, 105, 34–52. [Google Scholar] [CrossRef]
- Madry, H.; van Dijk, C.N.; Mueller-Gerbl, M. The basic science of the subchondral bone. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 419–433. [Google Scholar] [CrossRef]
- Magill, P.; Byrne, D.P.; Baker, J.F.; Mulhall, K.J. Review Article: Osteochondral Reconstruction and Grafting. J. Orthop. Surg. 2011, 19, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Gracitelli, G.C.; Moraes, V.Y.; Franciozi, C.E.; Luzo, M.V.; Belloti, J.C. Surgical interventions (microfracture, drilling, mosaicplasty, and allograft transplantation) for treating isolated cartilage defects of the knee in adults. Cochrane Database Syst. Rev. 2016, 9, CD010675. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.Z.; Swenson, R.D.; Lynch, S.A. Knee cartilage defect: Marrow stimulating techniques. Curr. Rev. Musculoskelet. Med. 2015, 8, 451–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, S.; Angele, P.; Arnold, M.; Brehme, K.; Cotic, M.; Haasper, C.; Hinterwimmer, S.; Imhoff, A.B.; Petersen, W.; Salzmann, G.; et al. Practice in rehabilitation after cartilage therapy: An expert survey. Arch. Orthop. Trauma Surg. 2013, 133, 311–320. [Google Scholar] [CrossRef]
- Steadman, J.R.; Rodkey, W.G.; Briggs, K.K. Microfracture: Its History and Experience of the Developing Surgeon. Cartilage 2010, 1, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steadman, J.R.; Rodkey, W.G.; Singleton, S.B.; Briggs, K.K. Microfracture technique forfull-thickness chondral defects: Technique and clinical results. Oper. Tech. Orthop. 1997, 7, 300–304. [Google Scholar] [CrossRef]
- Bader, S.; Miniaci, A. Mosaïcplasty. Orthopedics 2011, 34, e491–e493. [Google Scholar] [CrossRef] [PubMed]
- Robert, H. Chondral repair of the knee joint using mosaicplasty. Orthop. Traumatol. Surg. Res. 2011, 97, 418–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, P.G.; Williamson, T.; Murray, I.R.; Al-Hourani, K.; White, T.O. Sporting participation following the operative management of chondral defects of the knee at mid-term follow up: A systematic review and meta-analysis. J. Exp. Orthop. 2020, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Rowland, R.; Colello, M.; Wyland, D.J. Osteochondral Autograft Transfer Procedure: Arthroscopic Technique and Technical Pearls. Arthrosc. Tech. 2019, 8, e713–e719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shekhar, A.; Reddy, S.; Patil, S.; Tapasvi, S. Mid-term outcomes of arthroscopic osteochondral autograft transplantation for focal chondral defects of the knee. J. Arthrosc. Surg. Sport. Med. 2021, 2, 41–46. [Google Scholar] [CrossRef]
- Pareek, A.; Reardon, P.; Maak, T.; Levy, B.; Stuart, M.; Krych, A. Long-term Outcomes After Osteochondral Autograft Transfer: A Systematic Review at Mean Follow-up of 10.2 Years. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 1174–1184. [Google Scholar] [CrossRef]
- Ma, H.-L.; Hung, S.-C.; Wang, S.-T.; Chang, M.-C.; Chen, T.-H. Osteochondral autografts transfer for post-traumatic osteochondral defect of the knee-2 to 5 years follow-up. Injury 2004, 35, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Hangody, L.; Fules, P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: Ten years of experimental and clinical experience. J. Bone Jt. Surg. 2003, 85, 25–32. [Google Scholar] [CrossRef]
- Stone, A.V.; Christian, D.R.; Redondo, M.L.; Yanke, A.B.; Southworth, T.M.; Tauro, T.M.; Cole, B.J. Osteochondral Allograft Transplantation and Osteochondral Autograft Transfer. Oper. Tech. Sports Med. 2018, 26, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Richter, D.L.; Schenck, R.C.J.; Wascher, D.C.; Treme, G. Knee Articular Cartilage Repair and Restoration Techniques: A Review of the Literature. Sports Health 2016, 8, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Solheim, E.; Hegna, J.; Øyen, J.; Harlem, T.; Strand, T. Results at 10 to 14 years after osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee. Knee 2013, 20, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Trengove, A.; Di Bella, C.; O’Connor, A.J. The Challenge of Cartilage Integration: Understanding a Major Barrier to Chondral Repair. Tissue Eng. Part B. Rev. 2021. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Hall, A.C. Hyperosmolarity normalises serum-induced changes to chondrocyte properties in a model of cartilage injury. Eur. Cells Mater. 2016, 31, 205–220. [Google Scholar] [CrossRef]
- Wang, D.; Chang, B.; Coxe, F.R.; Pais, M.D.; Wickiewicz, T.L.; Warren, R.F.; Rodeo, S.A.; Williams, R.J. 3rd Clinically Meaningful Improvement After Treatment of Cartilage Defects of the Knee With Osteochondral Grafts. Am. J. Sports Med. 2019, 47, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Assenmacher, A.T.; Pareek, A.; Reardon, P.J.; Macalena, J.A.; Stuart, M.J.; Krych, A.J. Long-term Outcomes After Osteochondral Allograft: A Systematic Review at Long-term Follow-up of 12.3 Years. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Torrie, A.M.; Kesler, W.W.; Elkin, J.; Gallo, R.A. Osteochondral allograft. Curr. Rev. Musculoskelet. Med. 2015, 8, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Pearsall, A.W.; Madanagopal, S.G.; Hughey, J.T. Osteoarticular Autograft and Allograft Transplantation of the Knee: 3 year Follow-up. Orthopedics 2008, 31, 73. [Google Scholar]
- Ye, K.; Di Bella, C.; Myers, D.E.; Choong, P.F.M. The osteochondral dilemma: Review of current management and future trends. ANZ J. Surg. 2014, 84, 211–217. [Google Scholar] [CrossRef]
- Wang, K.C.; Frank, R.M.; Cotter, E.J.; Christian, D.R.; Cole, B.J. Arthroscopic Management of Isolated Tibial Plateau Defect With Microfracture and Micronized Allogeneic Cartilage–Platelet-Rich Plasma Adjunct. Arthrosc. Tech. 2017, 6, e1613–e1618. [Google Scholar] [CrossRef] [Green Version]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Téllez, D.A.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids. Polymers 2017, 9, 671. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Fuchs, S.; Shariati, K.; Ma, M. Specialty Tough Hydrogels and Their Biomedical Applications. Adv. Healthc. Mater. 2020, 9, e1901396. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xu, Z.; Liang, Q.; Li, H.; Peng, L.; Wu, M.; Zhao, X.; Cui, X.; Ruan, C.; Liu, W. Osteochondral Regeneration with 3D-Printed Biodegradable High-Strength Supramolecular Polymer Reinforced-Gelatin Hydrogel Scaffolds. Adv. Sci. 2019, 6, 1900867. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, L.; Suo, H.; Yan, M.; Yin, J.; Fu, J. 3D printing of biomimetic multi-layered GelMA/nHA scaffold for osteochondral defect repair. Mater. Des. 2019, 171, 107708. [Google Scholar] [CrossRef]
- Shim, J.-H.; Jang, K.-M.; Hahn, S.K.; Park, J.Y.; Jung, H.; Oh, K.; Park, K.M.; Yeom, J.; Park, S.H.; Kim, S.W.; et al. Three-dimensional bioprinting of multilayered constructs containing human mesenchymal stromal cells for osteochondral tissue regeneration in the rabbit knee joint. Biofabrication 2016, 8, 14102. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, S.; Liu, S.; Cui, W. Evaluation of oriented electrospun fibers for periosteal flap regeneration in biomimetic triphasic osteochondral implant. J. Biomed. Mater. Res. B. Appl. Biomater. 2014, 102, 1407–1414. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, J.-C.; Shim, J.-H.; Lee, J.-S.; Park, H.; Kim, S.W.; Doh, J.; Cho, D.-W. A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication 2014, 6, 35004. [Google Scholar] [CrossRef]
- Mancini, I.A.D.; Vindas Bolaños, R.A.; Brommer, H.; Castilho, M.; Ribeiro, A.; van Loon, J.P.A.M.; Mensinga, A.; van Rijen, M.H.P.; Malda, J.; van Weeren, R. Fixation of Hydrogel Constructs for Cartilage Repair in the Equine Model: A Challenging Issue. Tissue Eng. Part C Methods 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Liu, X.; Wei, Y.; Xuan, C.; Liu, L.; Lai, C.; Chai, M.; Zhang, Z.; Wang, L.; Shi, X. A Biomimetic Biphasic Osteochondral Scaffold with Layer-Specific Release of Stem Cell Differentiation Inducers for the Reconstruction of Osteochondral Defects. Adv. Healthc. Mater. 2020, 9, 2000076. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Li, J.; Zhang, J.; Pan, Z.; Liu, Y.; Zhou, F.; Hong, Y.; Hu, Y.; Gu, Y.; Ouyang, H.; et al. An interleukin-4-loaded bi-layer 3D printed scaffold promotes osteochondral regeneration. Acta Biomater. 2020, 117, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Kosik-Kozioł, A.; Heljak, M.; Święszkowski, W. Mechanical properties of hybrid triphasic scaffolds for osteochondral tissue engineering. Mater. Lett. 2020, 261, 126893. [Google Scholar] [CrossRef]
- Diloksumpan, P.; de Ruijter, M.; Castilho, M.; Gbureck, U.; Vermonden, T.; van Weeren, P.R.; Malda, J.; Levato, R. Combining multi-scale 3D printing technologies to engineer reinforced hydrogel-ceramic interfaces. Biofabrication 2020, 12, 025014. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.E.; Vaquette, C.; Theodoropoulos, C.; Klein, T.J.; Hutmacher, D.W. Multiphasic construct studied in an ectopic osteochondral defect model. J. R. Soc. Interface 2014, 11, 20140184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; He, X.; Xin, C.; Zhu, Y.; Liu, Z. 3D printing of an integrated triphasic MBG-alginate scaffold with enhanced interface bonding for hard tissue applications. J. Mater. Sci. Mater. Med. 2020, 31, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, H.; Hao, G.; Zhang, Y.; Ding, H.; Fan, Z.; Sun, L. 3D Printing Hydrogel Scaffolds with Nanohydroxyapatite Gradient to Effectively Repair Osteochondral Defects in Rats. Adv. Funct. Mater. 2021, 31, 2006697. [Google Scholar] [CrossRef]
- Kilian, D.; Ahlfeld, T.; Akkineni, A.R.; Bernhardt, A.; Gelinsky, M.; Lode, A. 3D Bioprinting of osteochondral tissue substitutes–in vitro-chondrogenesis in multi-layered mineralized constructs. Sci. Rep. 2020, 10, 8277. [Google Scholar] [CrossRef]
- Critchley, S.; Sheehy, E.J.; Cunniffe, G.; Diaz-Payno, P.; Carroll, S.F.; Jeon, O.; Alsberg, E.; Brama, P.A.J.; Kelly, D.J. 3D printing of fibre-reinforced cartilaginous templates for the regeneration of osteochondral defects. Acta Biomater. 2020, 113, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Pepelanova, I.; Kruppa, K.; Scheper, T.; Lavrentieva, A. Gelatin-Methacryloyl (GelMA) Hydrogels with Defined Degree of Functionalization as a Versatile Toolkit for 3D Cell Culture and Extrusion Bioprinting. Bioengineering 2018, 5, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dienes, J.; Browne, S.; Farjun, B.; Amaral Passipieri, J.; Mintz, E.L.; Killian, G.; Healy, K.E.; Christ, G.J. Semisynthetic Hyaluronic Acid-Based Hydrogel Promotes Recovery of the Injured Tibialis Anterior Skeletal Muscle Form and Function. ACS Biomater. Sci. Eng. 2021, 7, 1587–1599. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.; Liang, Q.; Liu, B.; Li, H.; Wu, Y.; Zhang, Y.; Lin, Z.; Wu, M.; Ruan, C.; et al. Direct 3D Printing of High Strength Biohybrid Gradient Hydrogel Scaffolds for Efficient Repair of Osteochondral Defect. Adv. Funct. Mater. 2018, 28, 1706644. [Google Scholar] [CrossRef]
- Nooeaid, P.; Salih, V.; Beier, J.P.; Boccaccini, A.R. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell. Mol. Med. 2012, 16, 2247–2270. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.; Henry, L.; McGennisken, E.; Onofrillo, C.; Bella, C.; Duchi, S.; O’Connell, C.; Pirogova, E. Characterization of Polycaprolactone Nanohydroxyapatite Composites with Tunable Degradability Suitable for Indirect Printing. Polymers 2021, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Guo, J.; Zhang, H.; Zhang, X.; Yin, C.; Wang, L.; Zhu, Y.; Yao, Q. 3D Molecularly Functionalized Cell-Free Biomimetic Scaffolds for Osteochondral Regeneration. Adv. Funct. Mater. 2019, 29, 1807356. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Thunsiri, K.; Pitjamit, S.; Pothacharoen, P.; Pruksakorn, D.; Nakkiew, W.; Wattanutchariya, W. The 3D-Printed Bilayer’s Bioactive-Biomaterials Scaffold for Full-Thickness Articular Cartilage Defects Treatment. Materials 2020, 13, 3417. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, A.; Longoni, A.; Criscenti, G.; Lorenzo-Moldero, I.; Klein-Gunnewiek, M.; Vancso, J.; van Blitterswijk, C.; Mota, C.; Moroni, L. Surface energy and stiffness discrete gradients in additive manufactured scaffolds for osteochondral regeneration. Biofabrication 2016, 8, 15014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, H.; Zhang, L.; Jia, S.; Liu, J.; Xiong, Z.; Sun, W. Biomimetic design and fabrication of multilayered osteochondral scaffolds by low-temperature deposition manufacturing and thermal-induced phase-separation techniques. Biofabrication 2017, 9, 25021. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.B.M.; Sivadas, V.P.; Nair, P.D.P. 3D-printed biphasic scaffolds for the simultaneous regeneration of osteochondral tissues. Biomed. Mater. 2021, 16, 054102. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lian, Q.; Li, D.; Jin, Z. Biphasic osteochondral scaffold fabrication using multi-material mask projection stereolithography. Rapid Prototyp. J. 2019, 25, 277–288. [Google Scholar] [CrossRef]
- Zhang, W.; Lian, Q.; Li, D.; Wang, K.; Hao, D.; Bian, W.; He, J.; Jin, Z. Cartilage repair and subchondral bone migration using 3D printing osteochondral composites: A one-year-period study in rabbit trochlea. Biomed Res. Int. 2014, 2014, 746138. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, P.; Chen, Y.; Li, M.; Chen, C.; Lu, H. 3D-Printed Extracellular Matrix/Polyethylene Glycol Diacrylate Hydrogel Incorporating the Anti-inflammatory Phytomolecule Honokiol for Regeneration of Osteochondral Defects. Am. J. Sports Med. 2020, 48, 2808–2818. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Guo, L.; Sun, H. Manufacture of Biomaterials. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Oxford, UK, 2019; pp. 116–134. ISBN 978-0-12-805144-3. [Google Scholar]
- Yousefi, A.-M.; Hoque, M.E.; Prasad, R.G.; Uth, N. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: A review. J. Biomed. Mater. Res. A 2015, 103, 2460–2481. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Hsieh, M.-F.; Fang, C.-H.; Jiang, C.-P.; Lin, B.; Lee, H.-M. Osteochondral Regeneration Induced by TGF-beta Loaded Photo Cross-Linked Hyaluronic Acid Hydrogel Infiltrated in Fused Deposition-Manufactured Composite Scaffold of Hydroxyapatite and Poly (Ethylene Glycol)-Block-Poly(epsilon-Caprolactone). Polymers 2017, 9, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moses, J.C.; Saha, T.; Mandal, B.B. Chondroprotective and osteogenic effects of silk-based bioinks in developing 3D bioprinted osteochondral interface. Bioprinting 2020, 17, e00067. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Cannillo, V. Hydroxyapatite and tricalcium phosphate composites with bioactive glass as second phase: State of the art and current applications. J. Biomed. Mater. Res. Part A 2016, 104, 1030–1056. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, C.; Chang, J.; Wu, C. Bioactive scaffolds for osteochondral regeneration. J. Orthop. Transl. 2019, 17, 15–25. [Google Scholar] [CrossRef]
- Nyberg, E.; Rindone, A.; Dorafshar, A.; Grayson, W.L. Comparison of 3D-Printed Poly-ɛ-Caprolactone Scaffolds Functionalized with Tricalcium Phosphate, Hydroxyapatite, Bio-Oss, or Decellularized Bone Matrix. Tissue Eng. Part A 2017, 23, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Adamkiewicz, M.; Rubinsky, B. Cryogenic 3D printing for tissue engineering. Cryobiology 2015, 71, 518–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Buchanan, F.; Mitchell, C.; Dunne, N. Printability of calcium phosphate: Calcium sulfate powders for the application of tissue engineered bone scaffolds using the 3D printing technique. Mater. Sci. Eng. C 2014, 38, 1–10. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Noh, I.; Mikos, A.G.; Zhang, S. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 2017, 137, 37–48. [Google Scholar] [CrossRef]
- Castro, N.J.; Patel, R.; Zhang, L.G. Design of a Novel 3D Printed Bioactive Nanocomposite Scaffold for Improved Osteochondral Regeneration. Cell. Mol. Bioeng. 2015, 8, 416–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowicki, M.; Zhu, W.; Sarkar, K.; Rao, R.; Zhang, L.G. 3D printing multiphasic osteochondral tissue constructs with nano to micro features via PCL based bioink. Bioprinting 2020, 17, e00066. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Choi, B.; Wu, B.; Lee, M. Customized biomimetic scaffolds created by indirect three-dimensional printing for tissue engineering. Biofabrication 2013, 5, 45003. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jia, S.; Xiong, Z.; Long, Q.; Yan, S.; Hao, F.; Liu, J.; Yuan, Z. 3D-printed scaffolds with calcified layer for osteochondral tissue engineering. J. Biosci. Bioeng. 2018, 126, 389–396. [Google Scholar] [CrossRef]
- Doyle, S.E.; Duchi, S.; Onofrillo, C.; Quigley, A.; Di Bella, C.; Pirogova, E.; O’Connell, C.D. Printing between the Lines: Intricate Biomaterial Structures Fabricated via Negative Embodied Sacrificial Template 3D (NEST3D) Printing. Adv. Mater. Technol. 2021, 6, 7. [Google Scholar] [CrossRef]
- Jiwoon, L.; Jesse, W.; Sanjay, N.; Sung, Y. Prediction of geometric characteristics in polycaprolactone (PCL) scaffolds produced by extrusion-based additive manufacturing technique for tissue engineering. Rapid Prototyp. J. 2019, 26, 238–248. [Google Scholar]
- Sodupe Ortega, E.; Sanz-Garcia, A.; Pernia-Espinoza, A.; Escobedo-Lucea, C. Efficient Fabrication of Polycaprolactone Scaffolds for Printing Hybrid Tissue-Engineered Constructs. Materials 2019, 12, 613. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, C.D.; Konate, S.; Onofrillo, C.; Kapsa, R.; Baker, C.; Duchi, S.; Eekel, T.; Yue, Z.; Beirne, S.; Barnsley, G.; et al. Free-form co-axial bioprinting of a gelatin methacryloyl bio-ink by direct in situ photo-crosslinking during extrusion. Bioprinting 2020, 19, e00087. [Google Scholar] [CrossRef]
- O’Connell, C.; Ren, J.; Pope, L.; Li, Y.; Mohandas, A.; Blanchard, R.; Duchi, S.; Onofrillo, C. Characterizing Bioinks for Extrusion Bioprinting: Printability and Rheology. Methods Mol. Biol. 2020, 2140, 111–133. [Google Scholar]
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef]
- Mikos, A.G.; Sarakinos, G.; Lyman, M.D.; Ingber, D.E.; Vacanti, J.P.; Langer, R. Prevascularization of porous biodegradable polymers. Biotechnol. Bioeng. 1993, 42, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Gupte, M.J.; Swanson, W.B.; Hu, J.; Jin, X.; Ma, H.; Zhang, Z.; Liu, Z.; Feng, K.; Feng, G.; Xiao, G.; et al. Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization. Acta Biomater. 2018, 82, 1–11. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Pan, W.; Yang, F.; Jiang, W.; Wu, X.; Kong, X.; Dai, K.; Hao, Y. In vitro and in vivo study of additive manufactured porous Ti6Al4V scaffolds for repairing bone defects. Sci. Rep. 2016, 6, 34072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginestra, P.; Pandini, S.; Ceretti, E. Hybrid multi-layered scaffolds produced via grain extrusion and electrospinning for 3D cell culture tests. Rapid Prototyp. J. 2020, 26, 593–602. [Google Scholar] [CrossRef]
- Deng, C.; Zhu, H.; Li, J.; Feng, C.; Yao, Q.; Wang, L.; Chang, J.; Wu, C. Bioactive Scaffolds for Regeneration of Cartilage and Subchondral Bone Interface. Theranostics 2018, 8, 1940–1955. [Google Scholar] [CrossRef]
- Chen, L.; Deng, C.; Li, J.; Yao, Q.; Chang, J.; Wang, L.; Wu, C. 3D printing of a lithium-calcium-silicate crystal bioscaffold with dual bioactivities for osteochondral interface reconstruction. Biomaterials 2019, 196, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Wang, X.; Li, J.; Deng, C.; Liu, Y.; Yao, Q.; Wang, L.; Chang, J.; Wu, C. 3D printing of Mo-containing scaffolds with activated anabolic responses and bi-lineage bioactivities. Theranostics 2018, 8, 4372–4392. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- de Lima, C.S.A.; Balogh, T.S.; Varca, J.P.R.O.; Varca, G.H.C.; Lugão, A.B.; Camacho-Cruz, L.A.; Bucio, E.; Kadlubowski, S.S. An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications. Pharmaceutics 2020, 12, 970. [Google Scholar] [CrossRef]
- Dalton, P.D. Melt electrowriting with additive manufacturing principles. Curr. Opin. Biomed. Eng. 2017, 2, 49–57. [Google Scholar] [CrossRef]
- Yoon, Y.I.; Park, K.E.; Lee, S.J.; Park, W.H. Fabrication of Microfibrous and Nano-/Microfibrous Scaffolds: Melt and Hybrid Electrospinning and Surface Modification of Poly(L-lactic acid) with Plasticizer. Biomed Res. Int. 2013, 2013, 309010–309048. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Direct Writing By Way of Melt Electrospinning. Adv. Mater. 2011, 23, 5651–5657. [Google Scholar] [CrossRef]
- Kade, J.C.; Dalton, P.D. Polymers for Melt Electrowriting. Adv. Healthc. Mater. 2021, 10, 2001232. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, C.D.; Bridges, O.; Everett, C.; Antill-O’Brien, N.; Onofrillo, C.; Di Bella, C. Electrostatic Distortion of Melt-Electrowritten Patterns by 3D Objects: Quantification, Modeling, and Toolpath Correction. Adv. Mater. Technol. 2021, 6, 2100345. [Google Scholar] [CrossRef]
- Hejazi, F.; Bagheri-Khoulenjani, S.; Olov, N.; Zeini, D.; Solouk, A.; Mirzadeh, H. Fabrication of nanocomposite/nanofibrous functionally graded biomimetic scaffolds for osteochondral tissue regeneration. J. Biomed. Mater. Res. Part A 2021, 109, 1657–1669. [Google Scholar] [CrossRef]
- Ho, S.T.B.; Hutmacher, D.W.; Ekaputra, A.K.; Hitendra, D.; Hui, J.H. The evaluation of a biphasic osteochondral implant coupled with an electrospun membrane in a large animal model. Tissue Eng. Part A 2010, 16, 1123–1141. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A. On the way to clean and safe electrospinning—Green electrospinning: Emulsion and suspension electrospinning. Polym. Adv. Technol. 2011, 22, 372–378. [Google Scholar] [CrossRef]
- Wortmann, M.; Frese, N.; Sabantina, L.; Petkau, R.; Kinzel, F.; Gölzhäuser, A.; Moritzer, E.; Hüsgen, B.; Ehrmann, A. New Polymers for Needleless Electrospinning from Low-Toxic Solvents. Nanomaterials 2019, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Saidy, N.T.; Shabab, T.; Bas, O.; Rojas-González, D.M.; Menne, M.; Henry, T.; Hutmacher, D.W.; Mela, P.; De-Juan-Pardo, E.M. Melt Electrowriting of Complex 3D Anatomically Relevant Scaffolds. Front. Bioeng. Biotechnol. 2020, 8, 793. [Google Scholar] [CrossRef]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Melt electrospinning today: An opportune time for an emerging polymer process. Prog. Polym. Sci. 2016, 56, 116–166. [Google Scholar] [CrossRef]
- Wunner, F.M.; Wille, M.-L.; Noonan, T.G.; Bas, O.; Dalton, P.D.; De-Juan-Pardo, E.M.; Hutmacher, D.W. Melt Electrospinning Writing of Highly Ordered Large Volume Scaffold Architectures. Adv. Mater. 2018, 30, 1706570. [Google Scholar] [CrossRef]
- Cui, Z.; Wright, L.D.; Guzzo, R.; Freeman, J.W.; Drissi, H.; Nair, L.S. Poly(d-lactide)/poly(caprolactone) nanofiber-thermogelling chitosan gel composite scaffolds for osteochondral tissue regeneration in a rat model. J. Bioact. Compat. Polym. 2013, 28, 115–125. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef] [PubMed]
- Manapat, J.Z.; Chen, Q.; Ye, P.; Advincula, R.C. 3D Printing of Polymer Nanocomposites via Stereolithography. Macromol. Mater. Eng. 2017, 302, 1600553. [Google Scholar] [CrossRef]
- Li, F.; Macdonald, N.P.; Guijt, R.M.; Breadmore, M.C. Increasing the functionalities of 3D printed microchemical devices by single material, multimaterial, and print-pause-print 3D printing. Lab Chip 2019, 19, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Fiedor, P.; Ortyl, J. A New Approach to Micromachining: High-Precision and Innovative Additive Manufacturing Solutions Based on Photopolymerization Technology. Materials 2020, 13, 2951. [Google Scholar] [CrossRef]
- Kumar, H.; Sakthivel, K.; Mohamed, M.G.A.; Boras, E.; Shin, S.R.; Kim, K. Designing Gelatin Methacryloyl (GelMA)-Based Bioinks for Visible Light Stereolithographic 3D Biofabrication. Macromol. Biosci. 2021, 21, 2000317. [Google Scholar] [CrossRef] [PubMed]
- Schoonraad, S.A.; Fischenich, K.M.; Eckstein, K.N.; Crespo-Cuevas, V.; Savard, L.M.; Muralidharan, A.; Tomaschke, A.A.; Uzcategui, A.C.; Randolph, M.A.; McLeod, R.R.; et al. Biomimetic and mechanically supportive 3D printed scaffolds for cartilage and osteochondral tissue engineering using photopolymers and digital light processing. Biofabrication 2021, 13, 44106. [Google Scholar] [CrossRef]
- Bian, W.; Li, D.; Lian, Q.; Li, X.; Zhang, W.; Wang, K.; Jin, Z. Fabrication of a bio-inspired beta-Tricalcium phosphate/collagen scaffold based on ceramic stereolithography and gel casting for osteochondral tissue engineering. Rapid Prototyp. J. 2012, 18, 68–80. [Google Scholar] [CrossRef]
- Castro, N.J.; O’Brien, J.; Zhang, L.G. Integrating biologically inspired nanomaterials and table-top stereolithography for 3D printed biomimetic osteochondral scaffolds. Nanoscale 2015, 7, 14010–14022. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Chen, Y.; Liu, J.; Wang, L.; Xu, M. 3D printing of biphasic osteochondral scaffold with sintered hydroxyapatite and polycaprolactone. J. Mater. Sci. 2021, 56, 16623–16633. [Google Scholar] [CrossRef]
- Qiao, Z.; Lian, M.; Han, Y.; Sun, B.; Zhang, X.; Jiang, W.; Li, H.; Hao, Y.; Dai, K. Bioinspired stratified electrowritten fiber-reinforced hydrogel constructs with layer-specific induction capacity for functional osteochondral regeneration. Biomaterials 2021, 266, 120385. [Google Scholar] [CrossRef]
- Hernandez, J.L.; Park, J.; Yao, S.; Blakney, A.K.; Nguyen, H.V.; Katz, B.H.; Jensen, J.T.; Woodrow, K.A. Effect of tissue microenvironment on fibrous capsule formation to biomaterial-coated implants. Biomaterials 2021, 273, 120806. [Google Scholar] [CrossRef] [PubMed]
- Carnicer-Lombarte, A.; Chen, S.-T.; Malliaras, G.G.; Barone, D.G. Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics. Front. Bioeng. Biotechnol. 2021, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. Part A 2017, 105, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, J.; Bjursten, L.M. A new and evolving paradigm for biocompatibility. J. Tissue Eng. Regen. Med. 2007, 1, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Jani, M.M.; Parker, R.D. Internal fixation devices for the treatment of unstable osteochondritis dissecans and chondral lesions. Oper. Tech. Sports Med. 2004, 12, 170–175. [Google Scholar] [CrossRef]
- Wei, P.; Xu, Y.; Zhang, H.; Wang, L. Continued sustained insulin-releasing PLGA nanoparticles modified 3D-Printed PCL composite scaffolds for osteochondral repair. Chem. Eng. J. 2021, 422, 130051. [Google Scholar] [CrossRef]
- Hu, X.; Man, Y.; Li, W.; Li, L.; Xu, J.; Parungao, R.; Wang, Y.; Zheng, S.; Nie, Y.; Liu, T.; et al. 3D Bio-Printing of CS/Gel/HA/Gr Hybrid Osteochondral Scaffolds. Polymers 2019, 11, 1601. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yue, H.; Huang, W.; Lin, X.; Xie, X.; He, Z.; He, X.; Liu, S.; Bai, L.; Lu, B.; et al. Cryogenic 3D printing of heterogeneous scaffolds with gradient mechanical strengths and spatial delivery of osteogenic peptide/TGF-β1 for osteochondral tissue regeneration. Biofabrication 2020, 12, 25030. [Google Scholar] [CrossRef]
- Bittner, S.M.; Smith, B.T.; Diaz-Gomez, L.; Hudgins, C.D.; Melchiorri, A.J.; Scott, D.W.; Fisher, J.P.; Mikos, A.G. Fabrication and mechanical characterization of 3D printed vertical uniform and gradient scaffolds for bone and osteochondral tissue engineering. Acta Biomater. 2019, 90, 37–48. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Rathan, S.; Hobbs, C.; Pitacco, P.; Freeman, F.E.; Cunniffe, G.M.; Dunne, N.J.; McCarthy, H.O.; Nicolosi, V.; O’Brien, F.J.; et al. Pore-forming bioinks to enable spatio-temporally defined gene delivery in bioprinted tissues. J. Control. Release 2019, 301, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Yonezawa, T.; Hubbell, K.; Dai, G.; Cui, X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol. J. 2015, 10, 1568–1577. [Google Scholar] [CrossRef]
- Deng, C.; Lin, R.; Zhang, M.; Qin, C.; Yao, Q.; Wang, L.; Chang, J.; Wu, C. Micro/Nanometer-Structured Scaffolds for Regeneration of Both Cartilage and Subchondral Bone. Adv. Funct. Mater. 2019, 29, 1806068. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Doberenz, F.; Kilian, D.; Vater, C.; Korn, P.; Lauer, G.; Lode, A.; Gelinsky, M. Bioprinting of mineralized constructs utilizing multichannel plotting of a self-setting calcium phosphate cement and a cell-laden bioink. Biofabrication 2018, 10, 45002. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-Y.; Kim, K.-I.; Park, S.; Im, G.-I. In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials 2014, 35, 3571–3581. [Google Scholar] [CrossRef] [PubMed]
- Castro-Viñuelas, R.; Sanjurjo-Rodríguez, C.; Piñeiro-Ramil, M.; Hermida-Gómez, T.; Fuentes-Boquete, I.M.; de Toro-Santos, F.J.; Blanco-García, F.J.; Díaz-Prado, S.M. Induced pluripotent stem cells for cartilage repair: Current status and future perspectives. Eur. Cell. Mater. 2018, 36, 96–109. [Google Scholar] [CrossRef]
- Diekman, B.O.; Christoforou, N.; Willard, V.P.; Sun, H.; Sanchez-Adams, J.; Leong, K.W.; Guilak, F. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2012, 109, 19172–19177. [Google Scholar] [CrossRef] [Green Version]
- Jha, B.S.; Farnoodian, M.; Bharti, K. Regulatory considerations for developing a phase I investigational new drug application for autologous induced pluripotent stem cells-based therapy product. Stem Cells Transl. Med. 2021, 10, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, C. Dedifferentiation: Inspiration for devising engineering strategies for regenerative medicine. NPJ Regen. Med. 2020, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Vis, M.A.M.; Ito, K.; Hofmann, S. Impact of Culture Medium on Cellular Interactions in in vitro Co-culture Systems. Front. Bioeng. Biotechnol. 2020, 8, 911. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, J.; Zhu, J.; Xiao, Z.; He, C.; Shi, H.; Li, X.; Yang, S.; Xiao, J. Research Advances in Tissue Engineering Materials for Sustained Release of Growth Factors. Biomed Res. Int. 2015, 2015, 808202. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7, 469. [Google Scholar] [CrossRef]
- Spiller, K.L.; Liu, Y.; Holloway, J.L.; Maher, S.A.; Cao, Y.; Liu, W.; Zhou, G.; Lowman, A.M. A novel method for the direct fabrication of growth factor-loaded microspheres within porous nondegradable hydrogels: Controlled release for cartilage tissue engineering. J. Control. Release 2012, 157, 39–45. [Google Scholar] [CrossRef]
- Kim, J.; Lin, B.; Kim, S.; Choi, B.; Evseenko, D.; Lee, M. TGF-β1 conjugated chitosan collagen hydrogels induce chondrogenic differentiation of human synovium-derived stem cells. J. Biol. Eng. 2015, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Holland, T.A.; Tabata, Y.; Mikos, A.G. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J. Control. Release 2005, 101, 111–125. [Google Scholar] [CrossRef]
- Blaney Davidson, E.N.; Vitters, E.L.; van Lent, P.L.E.M.; van de Loo, F.A.J.; van den Berg, W.B.; van der Kraan, P.M. Elevated extracellular matrix production and degradation upon bone morphogenetic protein-2 (BMP-2) stimulation point toward a role for BMP-2 in cartilage repair and remodeling. Arthritis Res. Ther. 2007, 9, R102. [Google Scholar] [CrossRef] [Green Version]
- Knippenberg, M.; Helder, M.N.; Zandieh Doulabi, B.; Wuisman, P.I.J.; Klein-Nulend, J. Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochem. Biophys. Res. Commun. 2006, 342, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Crecente-Campo, J.; Borrajo, E.; Vidal, A.; Garcia-Fuentes, M. New scaffolds encapsulating TGF-β3/BMP-7 combinations driving strong chondrogenic differentiation. Eur. J. Pharm. Biopharm. 2017, 114, 69–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaul, G.; Cucchiarini, M.; Arntzen, D.; Zurakowski, D.; Menger, M.D.; Kohn, D.; Trippel, S.B.; Madry, H. Local stimulation of articular cartilage repair by transplantation of encapsulated chondrocytes overexpressing human fibroblast growth factor 2 (FGF-2) in vivo. J. Gene Med. 2006, 8, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Orth, P.; Kaul, G.; Cucchiarini, M.; Zurakowski, D.; Menger, M.D.; Kohn, D.; Madry, H. Transplanted articular chondrocytes co-overexpressing IGF-I and FGF-2 stimulate cartilage repair in vivo. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [Green Version]
- Kieswetter, K.; Schwartz, Z.; Alderete, M.; Dean, D.D.; Boyan, B.D. Platelet derived growth factor stimulates chondrocyte proliferation but prevents endochondral maturation. Endocrine 1997, 6, 257–264. [Google Scholar] [CrossRef]
- Maehara, H.; Sotome, S.; Yoshii, T.; Torigoe, I.; Kawasaki, Y.; Sugata, Y.; Yuasa, M.; Hirano, M.; Mochizuki, N.; Kikuchi, M.; et al. Repair of large osteochondral defects in rabbits using porous hydroxyapatite/collagen (HAp/Col) and fibroblast growth factor-2 (FGF-2). J. Orthop. Res. 2010, 28, 677–686. [Google Scholar] [CrossRef]
- Lu, S.; Lam, J.; Trachtenberg, J.E.; Lee, E.J.; Seyednejad, H.; van den Beucken, J.J.J.P.; Tabata, Y.; Wong, M.E.; Jansen, J.A.; Mikos, A.G.; et al. Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials 2014, 35, 8829–8839. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Lyu, J.; Xing, F.; Chen, R.; Duan, X.; Xiang, Z. A biphasic, demineralized, and Decellularized allograft bone-hydrogel scaffold with a cell-based BMP-7 delivery system for osteochondral defect regeneration. J. Biomed. Mater. Res. A 2020, 108, 1909–1921. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Gan, H.; Wang, H.; Li, Q.; Wang, Z. Application of combined porous tantalum scaffolds loaded with bone morphogenetic protein 7 to repair of osteochondral defect in rabbits. Int. Orthop. 2018, 42, 1437–1448. [Google Scholar] [CrossRef]
- Caballero Aguilar, L.M.; Silva, S.M.; Moulton, S.E. Growth factor delivery: Defining the next generation platforms for tissue engineering. J. Control. Release 2019, 306, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Chung, B.; Tamaddon, M.; Carr, J.; Liu, C.; Cartmell, S.H. Osteochondral tissue coculture: An in vitro and in silico approach. Biotechnol. Bioeng. 2019, 116, 3112–3123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipriani, F.; Krueger, M.; González, I.; Sierra, L.; Alonso, M.; Kock, L.; Rodríguez-Cabello, J. Cartilage Regeneration in Preannealed Silk Elastin-Like Co-Recombinamers Injectable Hydrogel Embedded with Mature Chondrocytes in an Ex Vivo Culture Platform. Biomacromolecules 2018, 19, 4333–4347. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, Y.; Dou, C.; Dong, S. Microenvironment in subchondral bone: Predominant regulator for the treatment of osteoarthritis. Ann. Rheum. Dis. 2021, 80, 413–422. [Google Scholar] [CrossRef]
- O’Connell, C.D.; Zhang, B.; Onofrillo, C.; Duchi, S.; Blanchard, R.; Quigley, A.; Bourke, J.; Gambhir, S.; Kapsa, R.; Di Bella, C.; et al. Tailoring the mechanical properties of gelatin methacryloyl hydrogels through manipulation of the photocrosslinking conditions. Soft Matter 2018, 14, 2142–2151. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Khader, A.; Arinzeh, T.L. Biodegradable zinc oxide composite scaffolds promote osteochondral differentiation of mesenchymal stem cells. Biotechnol. Bioeng. 2020, 117, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Mancini, I.A.D.; Schmidt, S.; Brommer, H.; Pouran, B.; Schäfer, S.; Tessmar, J.; Mensinga, A.; van Rijen, M.H.P.; Groll, J.; Blunk, T.; et al. A composite hydrogel-3D printed thermoplast osteochondral anchor as example for a zonal approach to cartilage repair: In vivo performance in a long-term equine model. Biofabrication 2020, 12, 35028. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Advancing Disease Modeling in Animal-Based Research in Support of Precision Medicine: Proceedings of a Workshop; The National Academies Press: Washington, DC, USA, 2018; ISBN 978-0-309-47116-9. [Google Scholar]
- De Pieri, A.; Korman, B.D.; Jüngel, A.; Wuertz-Kozak, K. Engineering Advanced In Vitro Models of Systemic Sclerosis for Drug Discovery and Development. Adv. Biol. 2021, 5, 2000168. [Google Scholar] [CrossRef]
- Nikolakopoulou, P.; Rauti, R.; Voulgaris, D.; Shlomy, I.; Maoz, B.M.; Herland, A. Recent progress in translational engineered in vitro models of the central nervous system. Brain 2020, 143, 3181–3213. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Buss, A.; Pullig, O.; Ehlicke, F. Ex vivo osteochondral test system with control over cartilage defect depth—A pilot study to investigate the effect of oxygen tension and chondrocyte-based treatments in chondral and full thickness defects in an organ model. bioRxiv 2021, 3, 100173. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Tomaschke, A.; Kleinjan, E.; Muralidharan, A.; Pascual-Garrido, C.; McLeod, R.R.; Ferguson, V.L.; Bryant, S.J. A Stereolithography-Based 3D Printed Hybrid Scaffold for In Situ Cartilage Defect Repair. Macromol. Biosci. 2018, 18, 1700267. [Google Scholar] [CrossRef] [PubMed]
- Mouser, V.H.M.; Dautzenberg, N.M.M.; Levato, R.; van Rijen, M.H.P.; Dhert, W.J.A.; Malda, J.; Gawlitta, D. Ex vivo model unravelling cell distribution effect in hydrogels for cartilage repair. ALTEX 2018, 35, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Schmutzer, M.; Aszodi, A. Cell compaction influences the regenerative potential of passaged bovine articular chondrocytes in an ex vivo cartilage defect model. J. Biosci. Bioeng. 2017, 123, 512–522. [Google Scholar] [CrossRef]
- de Vries-van Melle, M.L.; Mandl, E.W.; Kops, N.; Koevoet, W.J.L.M.; Verhaar, J.A.N.; van Osch, G.J.V.M. An osteochondral culture model to study mechanisms involved in articular cartilage repair. Tissue Eng. Part C Methods 2012, 18, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Duchi, S.; Doyle, S.; Eekel, T.; O’Connell, C.D.; Augustine, C.; Choong, P.; Onofrillo, C.; Di Bella, C. Protocols for Culturing and Imaging a Human Ex Vivo Osteochondral Model for Cartilage Biomanufacturing Applications. Materials 2019, 12, 640. [Google Scholar] [CrossRef] [Green Version]
- Malda, J.; Benders, K.E.M.; Klein, T.J.; de Grauw, J.C.; Kik, M.J.L.; Hutmacher, D.W.; Saris, D.B.F.; van Weeren, P.R.; Dhert, W.J.A. Comparative study of depth-dependent characteristics of equine and human osteochondral tissue from the medial and lateral femoral condyles. Osteoarthr. Cartil. 2012, 20, 1147–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madry, H.; Ochi, M.; Cucchiarini, M.; Pape, D.; Seil, R. Large animal models in experimental knee sports surgery: Focus on clinical translation. J. Exp. Orthop. 2015, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.R.; Szczodry, M.; Bruno, S. Animal models for cartilage regeneration and repair. Tissue Eng. Part B Rev. 2010, 16, 105–115. [Google Scholar] [CrossRef]
- McGowan, K.B.; Stiegman, G. Regulatory Challenges for Cartilage Repair Technologies. Cartilage 2012, 4, 4–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, Y.-H.; Shen, B.-Y.; Wang, Y.-H.; Lin, B.; Lee, H.-M.; Hsieh, M.-F. Healing of Osteochondral Defects Implanted with Biomimetic Scaffolds of Poly(ε-Caprolactone)/Hydroxyapatite and Glycidyl-Methacrylate-Modified Hyaluronic Acid in a Minipig. Int. J. Mol. Sci. 2018, 19, 1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdollahi, S.; Davis, A.; Miller, J.H.; Feinberg, A.W. Expert-guided optimization for 3D printing of soft and liquid materials. PLoS ONE 2018, 13, e0194890. [Google Scholar] [CrossRef] [PubMed]
- Bawolin, N.K.; Zhang, W.J.; Chen, X.B. A Brief Review of the Modelling of the Time Dependent Mechanical Properties of Tissue Engineering Scaffolds. J. Biomimetics Biomater. Tissue Eng. 2010, 6, 19–33. [Google Scholar] [CrossRef]
- Andani, M.T.; Shayesteh Moghaddam, N.; Haberland, C.; Dean, D.; Miller, M.J.; Elahinia, M. Metals for bone implants. Part 1. Powder metallurgy and implant rendering. Acta Biomater. 2014, 10, 4058–4070. [Google Scholar] [CrossRef]
- Raut, H.K.; Das, R.; Liu, Z.; Liu, X.; Ramakrishna, S. Biocompatibility of Biomaterials for Tissue Regeneration or Replacement. Biotechnol. J. 2020, 15, 2000160. [Google Scholar] [CrossRef]
- Zhou, X.; Esworthy, T.; Lee, S.-J.; Miao, S.; Cui, H.; Plesiniak, M.; Fenniri, H.; Webster, T.; Rao, R.D.; Zhang, L.G. 3D Printed scaffolds with hierarchical biomimetic structure for osteochondral regeneration. Nanomedicine 2019, 19, 58–70. [Google Scholar] [CrossRef] [PubMed]


| OC Region | Mechanical Test | Elastic/Young’s Modulus | Ref |
|---|---|---|---|
| Articular Cartilage | Indentation | 1.03 ± 0.48 Mpa | [33] |
| Unconfined compression | 0.854 ± 0.348 MPa | [34] | |
| 0.64 ± 0.30 MPa | [35] | ||
| Calcified Cartilage | Indentation | 6.44 ± 1.02 MPa | [36] |
| Subchondral Bone | Indentation | ≈6–13 GPa | [37] |
| Unconfined compression | 297–475 MPa | [38] | |
| Unconfined compression via finite element modelling | 3–20 GPa | [39] | |
| 296 ± 107–497 ± 52 MPa | [40] |
| Cartilage Phase | Calcified Cartilage Phase | Subchondral Bone Phase | Ref | |
|---|---|---|---|---|
| Material | Methacrylated hyaluronan, isocynatoethyl acrylate−modified β−cyclodextrin, kartogenin | All materials found in cartilage and bone phase | HA, alendronate | [102] |
| Design | Homogenously casted hydrogel | 0°, 90° log pile infiltrated with homogenously casted hydrogel | 0°, 90° logpile | |
| Cells | Human bmMSCs * | bmMSCs * | Human bmMSCs * | |
| Material | Cartilage ECM, chitosan | PLGA, TCP | PLGA, TCP | [121] |
| Design | Orientated casted hydrogel | 0°, 90° log pile however ≈ 50 µm spacing between fibers so practically close to a solid disk | 0°, 90° logpile | |
| Cells | Goat bmMSCs | Acellular | Goat bmMSCs | |
| Material | Alginate, PLA | Alginate, GelMA, TCP, | PCL | [104] |
| Design | 0°, 90° log pile | 0°, 90° log pile | 0°, 90° log pile | |
| Cells | Acellular | Acellular | Acellular | |
| Material | PCL, GelMA | PCL + all materials in cartilage phase | α-TCP, nano−HA, hydrogel (either unmodified or modified poloxamer) | [105] |
| Design | 0°, 90° log pile PCL infiltrated with homogenously casted GelMA | 0°, −0°, −90°, −90° log pile (cartilage phase) and 0°, 90° log pile (bone phase) | 0°, −0°, −90°, −90° log pile | |
| Cells | Articular cartilage progenitor cells (ACPCs) | Acellular | Acellular | |
| Material | Sodium alginate | Sodium alginate, mesoporous bioactive glasses | Sodium alginate, mesoporous bioactive glasses | [107] |
| Design | 0°, 90° log pile | Dense/solid phase | 0°, 60° rotation steps | |
| Cells | Acellular | Acellular | Acellular |
| In Vitro | |||||
|---|---|---|---|---|---|
| Design | Materials | Elastic Modulus | Degradation | Outcome | Ref |
| Mono-phasic | Insulin, PLGA, polydopamine, PCL | Monophasic scaffold: 233.71 ± 7.57 MPa | N/A | Significant increase in cell number, alkaline phosphatase, glycosaminoglycan/protein and Alizarin Red after 7–14 days when MSCs and chondrocytes were seeded onto the scaffold. There was also significant increase in SOX-9, collagen I and aggrecan suggesting chondrogenic differentiation and RUNX-2, collagen II and osteocalcin suggesting osteogenic differentiation. | [190] |
| Biphasic | PLA, PCL, HA, chitosan, silk firoin | Cartilage phase: 1.01 ± 0.04 GPa Bone phase: 1.07 ± 0.16 GPa | 0.33 ± 0.09% after 30 days | Cell viability increased from 125.25 ± 9.36% to 308.28 ± 7.88% from day 1 to 14 respectively. The presence of HA and CS/SF increased cell proliferation. | [119] |
| Biphasic | P(NAGA-co-THMMA) hydrogels, β-TCP | Biphasic scaffold: 16–115 kPa | N/A | Significant increase in collagen II and aggrecan after 14 days. Significant increase in alkaline phosphatase, collagen I, osteocalcin and RUNX2 after 14 days cultured in non-osteogenic media. | [113] |
| Biphasic | PCL, HA, interleukin-4 GelMA | Biphasic scaffold: 73 ± 1 to 75 ± 3 MPa | ≈75% weight loss in 8 weeks | The cartilage scaffold was anti-inflammatory and had an increase in cell number after 5 days. Increase in RUNX2 and Alizarin Red staining in subchondral phase compared to the control. | [103] |
| Multi-phasic | PCL, PVA gelation, chitosan, nano-HA, | Multiphasic scaffold: 6.2 ± 0.5 MPa (low strain) 70 ± 29 MPa (40% strain) | ≈35% weight loss in 12 weeks | Increase in MSC cell number over 21 days. Greater cell density, proliferation, and migration in the subchondral bone phase over the cartilage. | [165] |
| In Vivo (Animals) | |||||
|---|---|---|---|---|---|
| Animal | Design | Materials | Duration | Outcome | Ref |
| Rabbit | Monophasic | Self-assembling peptide hydrogel coated PCL | 12 weeks | Coating with hydrogel reduces chondrocyte death rate, and enhanced cell growth. Highly improved hydrophilicity and biomimetic ECM structures. Promoted neobone and neocartilage regeneration. | [117] |
| Rabbit | Biphasic | mPEG-PCL, HA, glycidyl methacrylate-hyaluronic acid, TGF-β1 | 12 weeks | The empty control had neobone formation only while the scaffold group had neobone and neocarilage formation. Some scaffold remained in the defect. | [130] |
| Rat | Biphasic | P(NAGA-co-THMMA) hydrogels, β-TCP | 12 weeks | In the subchondral bone phase there was a significant increase in the total volume of tissue regenerated and bone mineral density compared to the control group and there was strong staining for osteocalcin, collagen I and toluidine blue. Neocartilage formation was present in the cartilage region with strong staining for glycosaminoglycan, collagen II and toluidine blue. | [113] |
| Rabbit | Biphasic | PCL, HA, interleukin-4 GelMA | 16 weeks | In the subchondral bone phase there was a significant increase in the total volume of tissue regenerated compared to the control group. Qualitative and quantitative Safranin O staining results were higher compared to the control. | [103] |
| Rabbit | Biphasic | β-TCP, PEG | 12 months | By 12 months there was tissue formation the entire defect. In the subchondral bone phase there was a significant increase in the total volume of tissue regenerated at 24 weeks compared to the control. | [124] |
| Mini-pigs | Biphasic | mPEG-PCL, HA, glycidyl methacrylate-hyaluronic acid, TGF-β1 | 12 months | Scaffold was still present in the subchondral bone phase while the cartilage phase was taken over by semi-mature cartilage. The subchondral bone phase also contained mixed bone and fibrotic tissue Of note: the control defect was completely filled but with fibrocartilage. | [243] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doyle, S.E.; Snow, F.; Duchi, S.; O’Connell, C.D.; Onofrillo, C.; Di Bella, C.; Pirogova, E. 3D Printed Multiphasic Scaffolds for Osteochondral Repair: Challenges and Opportunities. Int. J. Mol. Sci. 2021, 22, 12420. https://doi.org/10.3390/ijms222212420
Doyle SE, Snow F, Duchi S, O’Connell CD, Onofrillo C, Di Bella C, Pirogova E. 3D Printed Multiphasic Scaffolds for Osteochondral Repair: Challenges and Opportunities. International Journal of Molecular Sciences. 2021; 22(22):12420. https://doi.org/10.3390/ijms222212420
Chicago/Turabian StyleDoyle, Stephanie E., Finn Snow, Serena Duchi, Cathal D. O’Connell, Carmine Onofrillo, Claudia Di Bella, and Elena Pirogova. 2021. "3D Printed Multiphasic Scaffolds for Osteochondral Repair: Challenges and Opportunities" International Journal of Molecular Sciences 22, no. 22: 12420. https://doi.org/10.3390/ijms222212420
APA StyleDoyle, S. E., Snow, F., Duchi, S., O’Connell, C. D., Onofrillo, C., Di Bella, C., & Pirogova, E. (2021). 3D Printed Multiphasic Scaffolds for Osteochondral Repair: Challenges and Opportunities. International Journal of Molecular Sciences, 22(22), 12420. https://doi.org/10.3390/ijms222212420







