Abstract
Abiotic stresses are increasingly harmful to crop yield and quality. Calcium and its signaling pathway play an important role in modulating plant stress tolerance. As specific Ca2+ sensors, calcineurin B-like (CBL) proteins play vital roles in plant stress response and calcium signaling. The CBL family has been identified in many plant species; however, the characterization of the CBL family and the functional study of apple MdCBL proteins in salt response have yet to be conducted in apple. In this study, 11 MdCBL genes were identified from the apple genome. The coding sequences of these MdCBL genes were cloned, and the gene structure and conserved motifs were analyzed in detail. The phylogenetic analysis indicated that these MdCBL proteins could be divided into four groups. The functional identification in Na+-sensitive yeast mutant showed that the overexpression of seven MdCBL genes could confer enhanced salt stress resistance in transgenic yeast. The function of MdCBL10.1 in regulating salt tolerance was also verified in cisgenic apple calli and apple plants. These results provided valuable insights for future research examining the function and mechanism of CBL proteins in regulating apple salt tolerance.
1. Introduction
Plants are inevitably exposed to a variety of adverse environmental conditions due to their sessile lifestyle. They have evolved a series of signal transduction mechanisms to fine-regulate the body’s adaptation to the environment. Calcium ion (Ca2+) is a nutrient element crucial for plant growth and development. It also acts as the ubiquitous intracellular secondary messenger initiating the Ca2+ signaling pathway, which is a fine regulatory mechanism responsible for the acquisition, perception, transformation, transmission, and decryption of external stimuli [1]. Ca2+ signals are an important regulator of growth, development, and biotic and abiotic stresses in plants. Refs. [2,3,4,5]. Under stress conditions, Ca2+ signals induced by the stimulus are first perceived, decoded, and transmitted by Ca2+ sensors [6].
Based on the protein structural characteristics, Ca2+ sensors are divided into two types of sensors: sensor relays and sensor responders [7,8]. Sensor relays include calmodulin (CaM)-like proteins (CMLs) and calcineurin B-like (CBL) proteins, which do not have kinase activity [8,9]. They specifically target downstream proteins to transfer the perceived calcium signals. Sensor responder proteins, such as CaMs and Ca2+-dependent protein kinases (CDPKs), have all the functions of Ca2+ sensor relay proteins as well as the kinase activity [8,10,11]. As a result, CaMs, CMLs, CDPKs, and CBLs constitute sensors in the Ca2+ signal transduction pathway [12]. CaM is a ubiquitous conserved Ca2+-binding protein found in both animals and plants. The CML family was identified as encoding proteins that contain the CaM-like EF-hand structures and share at least 16% homology with CaM in amino acid residues [13,14,15,16]. CDPK comprises a kinase domain and a CaM-like domain (four EF-hands) in a single protein; thus, it acts as not only a Ca2+ sensor but also an effector [17,18,19]. The CBL family belongs to a unique group of calcium sensors in plants. Ca2+ can bind to the elongation factor (EF) hand domains of the CBL proteins, which changes their phosphorylation status. The change in the phosphorylation status of Ca2+ sensors activates several protein kinases, which sometimes lead to a protein phosphorylation cascade [6,20,21].
Decades of research have revealed that the CBL family proteins play important roles in plant stress response and resistance regulation. For example, CBL1 functions under drought, high salt, and hyperosmotic stresses in plants [22,23]. CBL2 and CBL3 regulate ion homeostasis across the vacuolar membranes under salt stress. Studies also proved that CBLs were involved in K+ regulation, which indirectly regulated the Na+ homeostasis. For example, AtCBL1/AtCBL9 regulates plant K+ homeostasis and salt tolerance by regulating the K+ channel AKT1 and K+ transporter HAK5 [3,24,25], while CBL4 modulates the activity of the plasma membrane K+ channel AKT2 [26]. The involvement of CBL in regulating plant salt stress response has been widely reported in a CIPK-dependent manner. The first identified CBL-CIPK pathway was the salt overly sensitive (SOS) pathway in Arabidopsis. Under salt stress, SOS3 (CBL4) interacts with SOS2, and the SOS2–SOS3 complex activates the transport properties of the cell membrane-located SOS1 to promote the Na+ efflux, thereby enhancing plant salt tolerance [27,28]. Other studies also found that the SOS3 homolog SOS3-like calcium-binding protein8 (SCABP8)/CBL10 interacted with SOS2 and enhanced plant salt tolerance by activating SOS1 [28,29,30,31]. Because of the crucial roles of CBL family proteins in plant growth and stress response, this family has been identified at the genome-wide level in many plant species [1,8,32,33,34]. For example, ten CBLs are present in Arabidopsis and rice [35], eight in pineapples [32], nine in peppers [10], and five in eggplant [36]. However, no detailed identification and characterization of the apple CBL family have been reported till now.
Apple (Malus domestica) is one of the most widely grown and economically valuable fruit crops globally. Abiotic stresses, such as high salinity, severely restrict its global yield and quality. Although CBL proteins play an important role in regulating plant salt tolerance, little is known regarding the function of apple CBL proteins in salt stress response. Genome-wide identification and gene cloning of CBL family genes were performed in apples in this study. The collinearity, phylogenetic relationship, gene structure, and conserved motifs of these MdCBLs were analyzed in detail. The functional identification in the Na+-sensitive yeast mutant showed that several MdCBLs played positive roles in modulating salt response. The function of MdCBL10.1 to enhance apple salt tolerance was verified in cisgenic apple calli and apple plants. These results provided valuable insights for subsequent research on the functions and regulatory mechanisms of MdCBLs in apples.
2. Materials and Methods
2.1. Sequence Retrieval and Identification of Apple CBL Family Proteins
The apple proteome file (GDDH13_1-1_prot.fasta) was downloaded from the GDR database (Genome Database for Rosaceae; https://www.rosaceae.org/, accessed on 6 April 2021), and the protein sequences of 10 AtCBLs were downloaded from the TAIR (The Arabidopsis Information Resource) database (https://www.arabidopsis.org/, accessed on 6 April 2021). The HMM file EF-hand_7.hmm (PF13499.8) was downloaded from the Pfam database and used as a query to search the apple proteome. Phylogenetic analysis was subsequently performed with the protein sequences of the HMMER screening results and the 10 AtCBLs. Further, 11 MdCBL family proteins were identified from the phylogenetic tree (Figure S1). This result was also verified with the local BLASTp search using the 10 AtCBLs as queries, which was conducted with the BioEdit software (version 7.0.9.0).
2.2. Collinearity Analysis and Characterization of Apple CBL Family Genes
The GFF file (gene_models_20170612.gff3) that contained location data for apple CBL family genes was downloaded from the GDR database. The collinearity analysis between different apple chromosomes was performed with MCScanX software, and the results were visualized using TBtools software. The protein length, mass weight, pI (isoelectric point) values, and charge at pH 7.0 were determined with the DNAstar software (version 7.1.0). The best hits in Arabidopsis for MdCBL proteins were determined by the local BLASTp search.
2.3. Phylogenetic Relationships, Gene Structure, and Conserved Motif Analysis
Phylogenetic analyses were constructed with the MEGA-X software (version 10.0.5) using the neighbor-joining method (bootstrap method, 1000 replicates, Poisson model, pairwise deletion). The intron–exon schematic structures of MdCBL genes were drawn with the TB tools. The online MEME software (version 5.3.3; https://meme-suite.org/meme/tools/meme, accessed on 26 April 2021) was used to identify conserved motifs in the protein sequences of these MdCBLs. Detailed methods refer to previous studies [37,38].
2.4. Cis-Acting Elements in the Promoters of MdCBL Genes
The upstream regions (1500 bp) of MdCBL genes were obtained from the apple genome file (GDDH13_1-1_formatted.fasta) downloaded from the GDR database. Abiotic stress– or hormone response–related regulatory elements were identified using the PlantCARE software (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 13 May 2021).
2.5. Vector Construction, Genetic Transformation, and Stress Treatment
For gene cloning of MdCBLs, total RNA was extracted from the leaves of “Golden Delicious” apple using a plant RNA isolation kit [Wolact, Vicband Life Sciences Company (HK) Limited] and reverse transcribed using a PrimeScript First Strand cDNA Synthesis Kit (Takara, Dalian, China). Gene-specific primers were designed based on the predicted coding sequences (CDSs) of these MdCBLs obtained from the GDR database.
For the transformation of yeast mutants, CDSs of MdCBLs were cloned into the pDR196 vector. The recombinant MdCBLs–pDR196 vector was transformed into the Na+-sensitive yeast mutant Δena1-4 using the LiAc/ss carrier DNA/PEG method. After growth selection on selective medium (synthetic defined medium minus the appropriate amino acids) and PCR confirmation of transgene presence, three single colonies of each strain were selected for subsequent experiments. Detailed methods of yeast transformation and salt treatment referred to previous studies [39,40].
For the overexpression of the MdCBL10.1 gene in the apple callus, the CDSs of MdCBL10.1 were cloned into the pBI121 vector under the control of the 35S promoter. Detailed methods of genetic transformation and salt stress treatment on the apple calluses refer to previous studies [39,40].
To obtain the composite apple plants with MdCBL10.1 expression whose expression increased or was interfered in roots, the CDSs and a 300-bp fragment of MdCBL10.1 were cloned into the overexpression vector pCambia2300-GFP and the RNAi vector pK7GWIWG2D, respectively. Methods of Agrobacterium rhizogenes K599-mediated genetic transformation referred to previous studies [39,40]. For NaCl stress treatment, the plants in the treatment group were irrigated with 150 mM NaCl solution for 10 days at 5-day intervals. The same number of plants in the control group were irrigated with distilled water.
2.6. Measurement of Stress-Related Physiological Parameters
Relative electrolyte leakage (REL) and Na+ content were measured with a flame photometer (M410; Sherwood Scientific, Cambridge, UK) as described previously [39,40]. Malondialdehyde (MDA) content, H2O2 and O2− content, and plant root activity were measured using Suzhou Comin Biotechnology test kits (Suzhou Comin Biotechnology Co., Ltd., Suzhou, China).
2.7. Statistical Analysis
IBM SPSS Statistics software (version 26; SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Significant differences (p < 0.05) were determined using the Student t test or Tukey’s multiple range test.
3. Results
3.1. Identification, Characterization, and Gene Duplication of Apple CBL Family Genes
The HMM file (PF13499.8) was used as a query to search the apple proteome using the HMMER software (hmmsearch, version 3.1b2) so as to screen the CBL family proteins in apple. With default inclusion threshold, 230 protein sequences were obtained (Supplementary File S1). Then, the protein sequences of the ten Arabidopsis CBL family members were downloaded from the TAIR database. These ten AtCBL proteins, along with the 230 proteins in HMMER screening results, were used for phylogenetic analysis. Based on the phylogenetic tree (Figure S1), 11 proteins were finally identified as apple CBL family members and named based on their orthlogs in Arabidopsis (Table 1). The predicted protein sequences of these 11 MdCBLs were 187–259 amino acids (aa) in length, with predicted mass weight, isoelectric point (pI), and charge at pH 7.0 ranging from 21.38 to 29.53, 4.42 to 4.89, and –10.78 to –17.30, respectively (Table 1).

Table 1.
Characterization of the CBL family genes in apple.
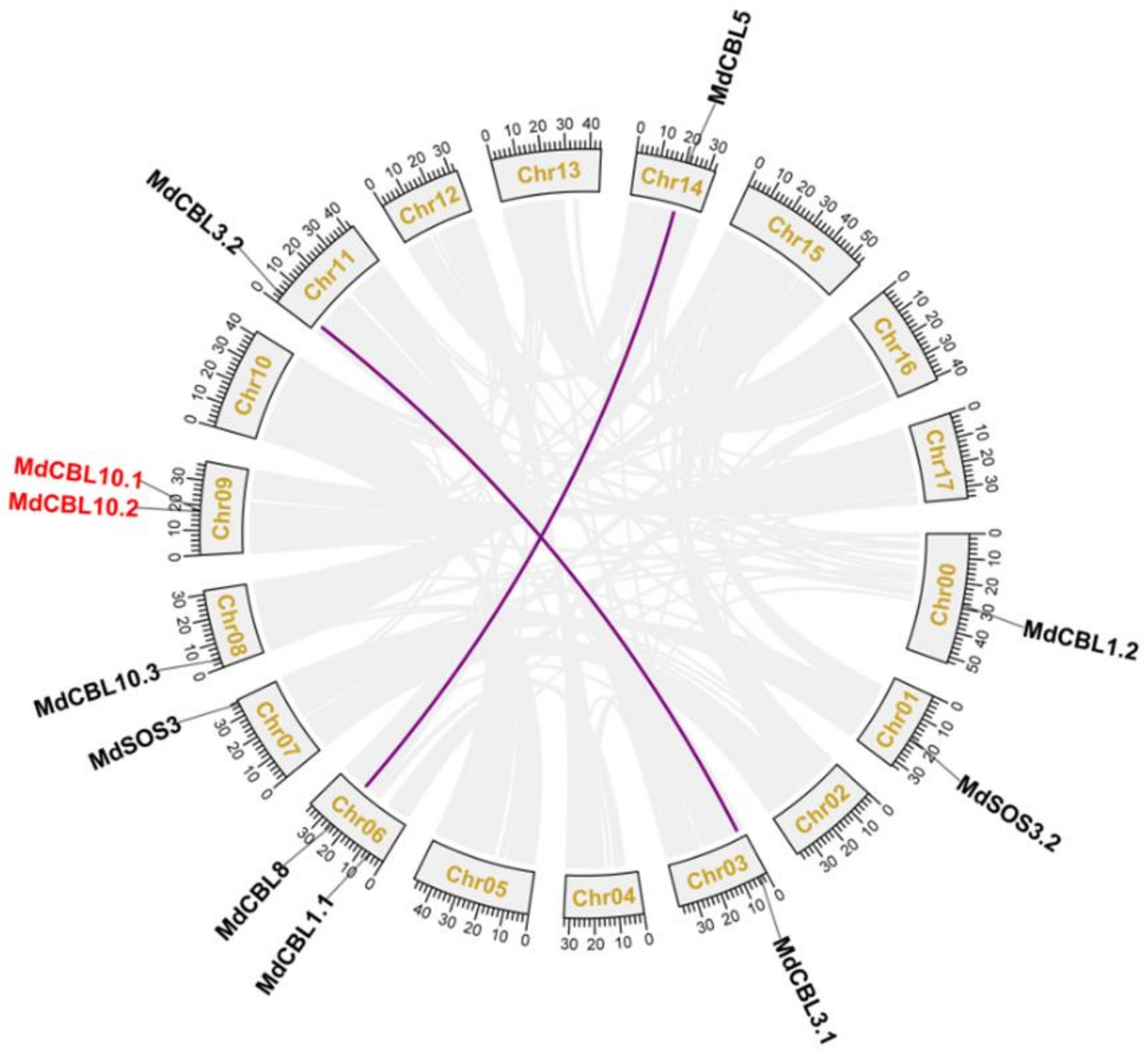
Based on the genomic location information obtained from the GDR database, 10 of the 11 MdCBL genes were randomly distributed on 8 of the 17 chromosomes of the apple, and the remaining one (MdCBL1.2) was localized to unassembled genomic scaffolds (Table 1 and Figure 1). Segmental and tandem duplications are the main causes of gene family expansion in plants. The collinear analyses between different apple chromosomes were performed with MCScanX software to investigate the gene duplication events among these MdCBL genes. The results showed complex patterns of collinearity between different chromosomes. The collinear analysis also revealed two segmental duplication events (MdCBL3.1 and MdCBL3.2, and MdCBL5 and MdCBL8) and one tandem duplication event (MdCBL10.1 and MdCBL10.2) among these MdCBL genes (Figure 1).
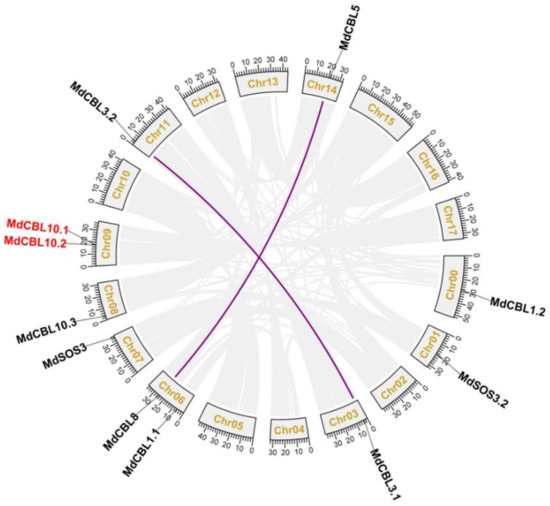
Figure 1.
Genome locations of CBL family genes in apple. Purple lines and red font indicate the segmental and tandem duplication genes, respectively. Collinear blocks are represented by grayish lines. Chr00 represents the unassembled genomic scaffolds.
3.2. Phylogenetic Analysis, Gene Structure Display, Prediction of Conserved Motifs, and Cloning of Apple CBL Family Genes
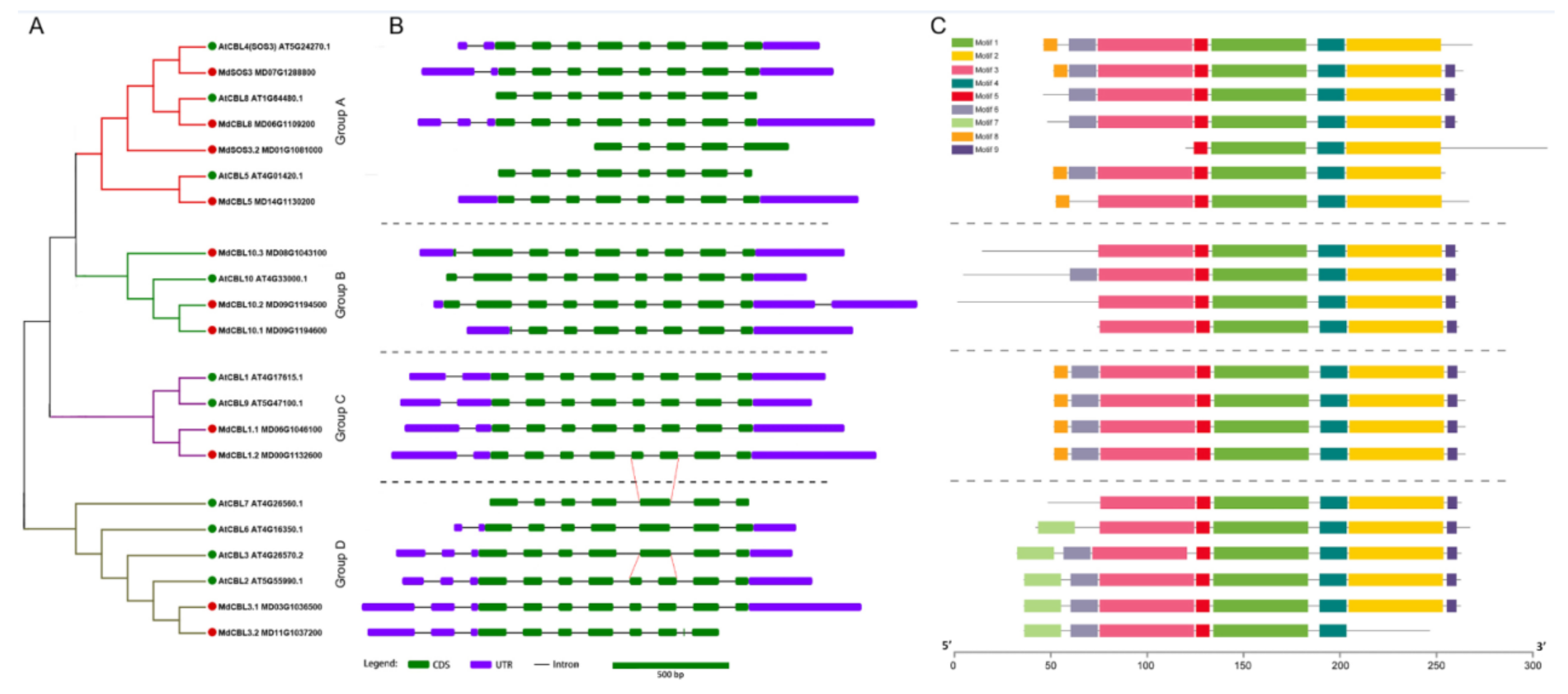
Previous studies showed that the members of the CBL family in plants could be divided into four groups. A phylogenetic analysis based on the protein sequences of the 11 MdCBLs and 10 AtCBLs was performed to investigate the relationship between these 11 CBL family members in apples. The phylogenetic tree showed that the 11 MdCBL proteins could be divided into four groups, similar to the grouping results in Arabidopsis and many other plant species (Figure 2A).
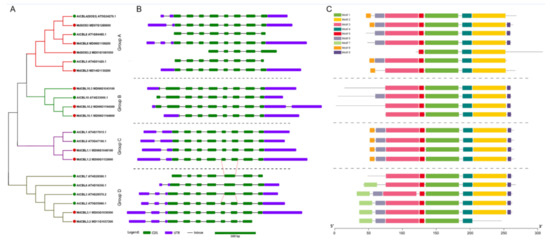
Figure 2.
Phylogenic relationships (A), gene structure (B), and conserved motifs (C) for CBL family proteins in apples and Arabidopsis. Green and red dots indicate CBL proteins in Arabidopsis and apple, respectively. Red lines show that the fifth exon of AtCBL7 and AtCBL3 could be further differentiated into two shorter exons. Introns are represented by black line segments of the same length to facilitate the comparison of the exon–intron composition patterns of these genes.
Gene structure is one of the factors that reflects the evolution of a multigene family. Thus, the exon–intron composition patterns of these MdCBL and AtCBL genes were analyzed. Although the length of introns varied significantly (Figure S2), genes that belonged to the same group exhibited the same exon–intron composition patterns, with the largest variation in the N-terminal region of these genes (Figure 2B). Based on gene structure analysis, three MdCBL genes with partial exons missing compared with other CBL genes in the same group, namely MdSOS3.2, Md10.1, and MdCBL3.2, were also identified. This result suggested that the predicted coding sequences of these three genes in apple genome (GDDH13) might be wrong. The conserved motifs of the CBL family were further explored using the online software MEME, and nine conserved motifs were found (Figure S3). The motifs 1–5 were conserved in almost all CBL proteins except MdSOS3.2 and MdCBL3.2 (Figure 2C). Besides, MdCBL10.1 was the missing part of the N-terminal compared with other proteins in group B (Figure 2C). These results further supported the presence of errors in the prediction of coding sequences of these three genes.
Gene-specific primers were designed based on the predicted sequences in the apple genome (GDDH13) and used for gene cloning to confirm the coding sequence of MdCBL genes. Using total RNA extracted from the leaves of “Golden Delicious” apple as the template, the coding sequences of these MdCBL genes were finally obtained by polymerase chain reaction (PCR) amplification, except for MdSOS3.2 (Supplementary File S2). The sequence alignment showed that the predicted protein sequences of MdCBL10.1 (MD09G1194600) and MdCBL3.2 (MD11G1037200) were missing a segment of N-terminal and C-terminal, respectively (Figure S4A). Although the coding sequence of MdSOS3.2 was not obtained, the sequence comparison between MD01G1081000 and MdSOS3 indicated an incorrect fragment deletion and an incorrect fragment insertion in the N- and C-terminals of MD01G1081000, respectively (Figure S4B).
3.3. Promoter Analysis of MdCBL Genes
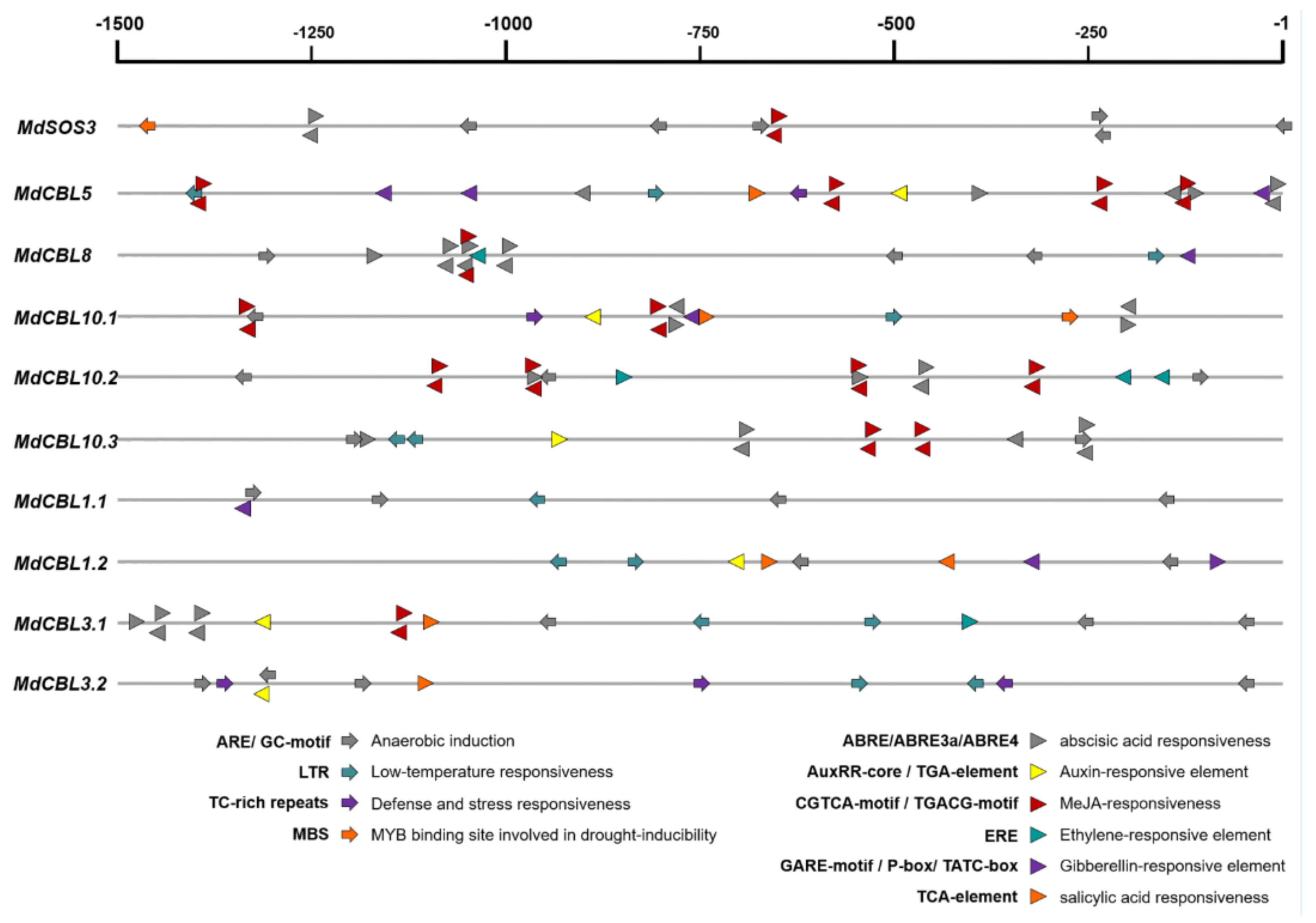
CBL family genes are involved in plant response to various environmental stresses. The promoter regions (upstream 1500 bp of the start codon ATG) of these genes were obtained from the apple genome (GDDH13) and submitted to the online software PlantCARE for cis-acting element analysis to explore the possible response of MdCBL genes to abiotic stresses. Various cis-elements related to abiotic stresses (hypoxia, cold, and drought) and plant hormone (ABA, auxin, MeJA, ethylene, GA, and SA) responsiveness were found (Figure 3 and Supplementary File S3), suggesting that these MdCBL genes played an important role in apple stress response. Many of these cis-elements appeared multiple times in the promoter region of the same gene (Figure 3). Besides, more hormone response–related cis-elements were present in the promoter regions of MdCBLs in groups A and B, especially cis-elements related to ABA and MeJA, while the cis-elements in the promoter regions of MdCBLs in groups C and D were mostly related to abiotic stress response (Figure 3). This indicated the functional differentiation of MdCBL genes between different groups.
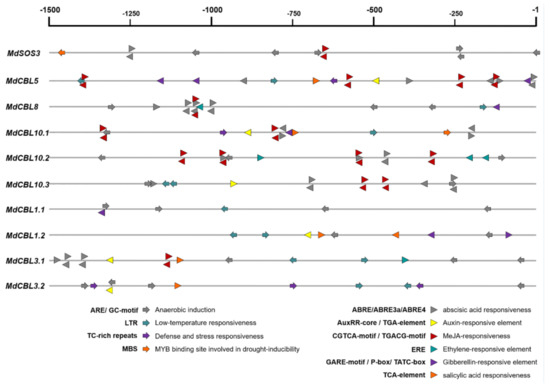
Figure 3.
Cis-element analysis of the MdCBL gene promoter regions in apple. Arrows and triangles indicate the cis-elements related to abiotic stress response and hormone response, respectively. Positive and negative directions indicate whether the motif existed in the plus or minus strand of the cis-acting elements, respectively.
3.4. Functional Identification of Mdcbls in Regulating Salt Tolerance in Yeast
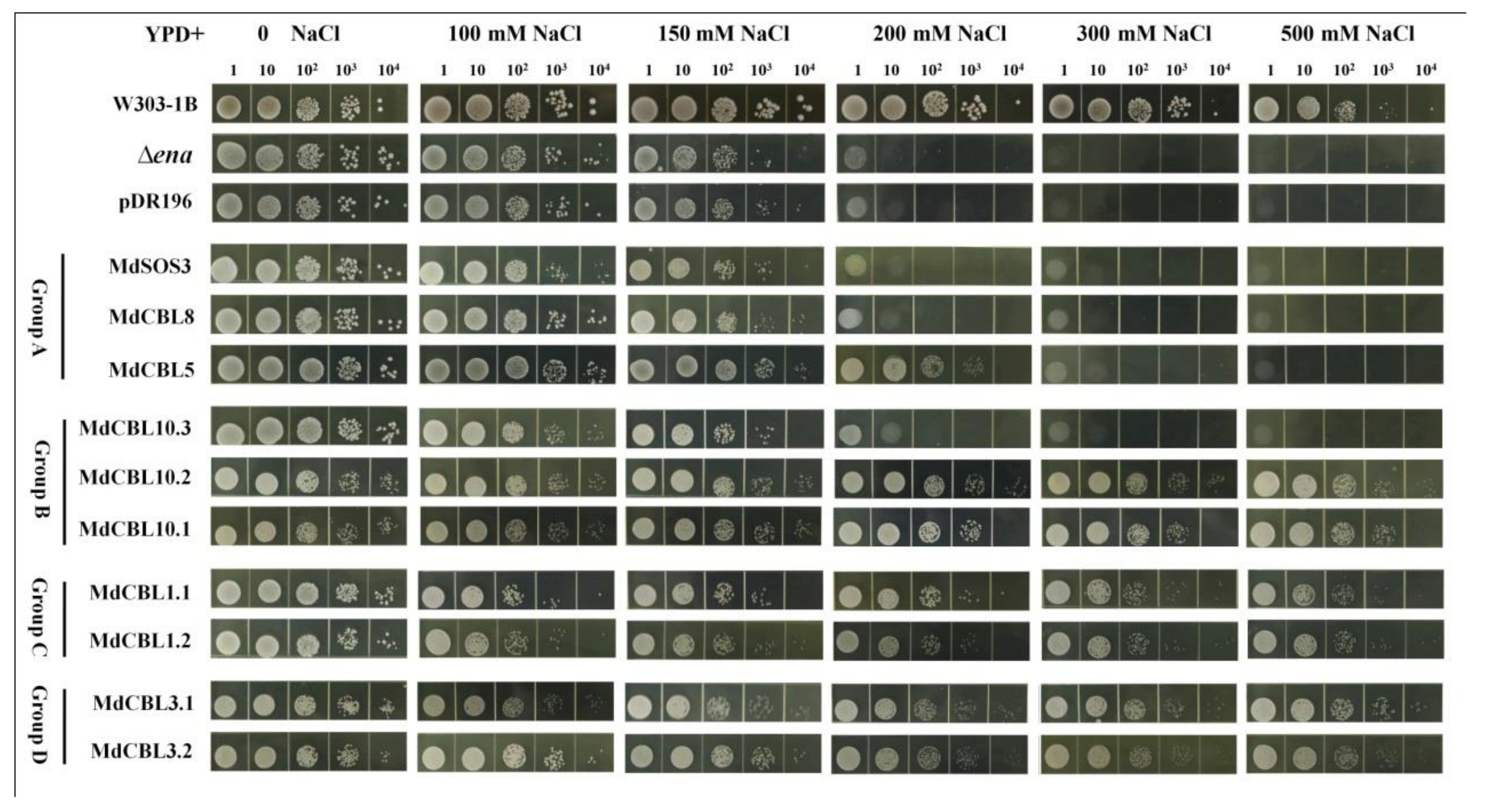
Previous studies demonstrated that CBL proteins affected plant salt tolerance through the SOS pathway. The yeast mutant strain Δena1−4 that lacked Na+-ATPase and was sensitive to high [Na+] was used to identify which CBL proteins in apples had a significant regulatory effect on salt tolerance. The full-length coding sequences of the ten MdCBL genes were cloned into the pDR196 vector and then transformed into the yeast mutant Δena1−4. The yeast strain W313-1B and positive transformants of the empty vector pDR196 were used as positive and negative controls. All of these strains were cultured in a YPD medium containing different concentrations of NaCl for three days. The growth of all strains was almost the same in a normal YPD medium (0 NaCl) (Figure 4). The addition of NaCl significantly inhibited the growth of these strains. The growth of the yeast mutant Δena1−4 was almost completely inhibited at a 200 mM NaCl concentration. The overexpression of any one of the seven MdCBL genes (MdCBL5, MdCBL10.1, MdCBL10.2, MdCBL1.1, MdCBL1.2, MdCBL3.1, and MdCBL3.2) could significantly inhibit the sensitivity of Δena1−4 to a high NaCl concentration (Figure 4). These results indicated that these seven MdCBL genes played a positive role in modulating salt tolerance in yeast. The growth of MdCBL5 transgenic yeast was also completely inhibited with the further increase in the NaCl concentration (Figure 4), indicating a stronger capacity of the other six MdCBL genes than MdCBL5 in enhancing yeast salt tolerance.
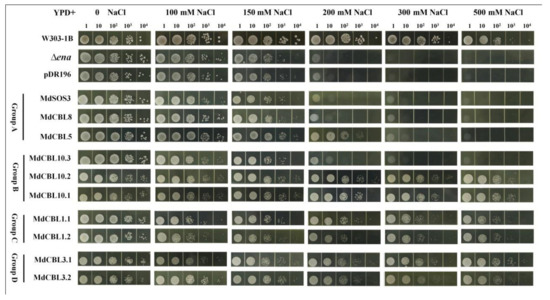
Figure 4.
Functional identification of MdCBLs in the Na+-sensitive yeast mutant. Aliquots (10 µL) of serial dilutions (100, 101, 102, 103, and 104) were dotted onto the YPD medium in the presence of 0, 100, 150, 200, 300, and 500 mM NaCl and grown for 3 days. W303-1B was the positive control. Δena1−4 and Δena1−4 transformed with the pDR196 empty vector were used as negative controls.
3.5. Overexpression of MdCBL10.1 Improved Salt Tolerance of Cisgenic Apple Calli
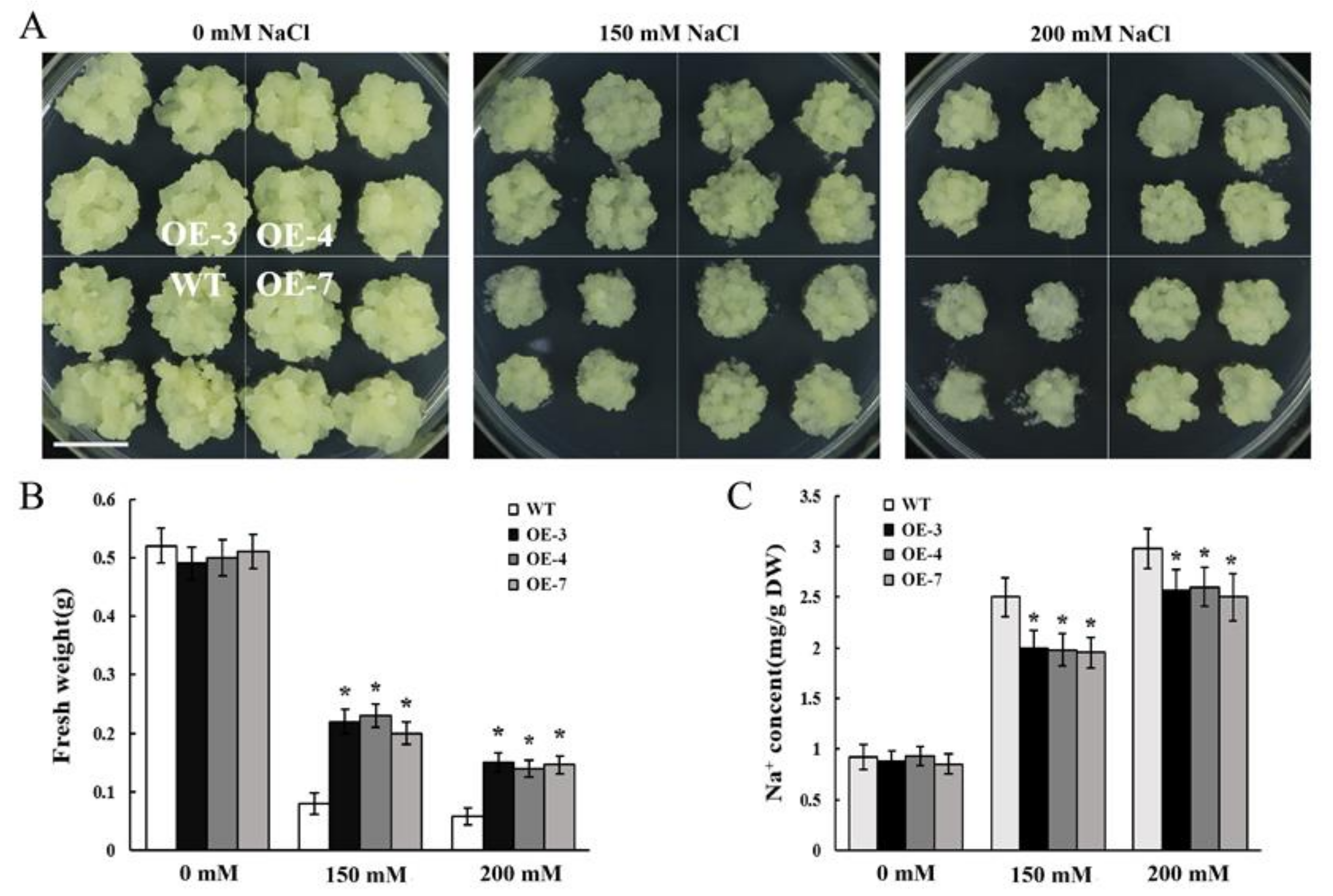
The SOS3/CBL10-SOS2-SOS1 and CBL10-CIPK8-SOS1 signaling pathways are the paramount regulatory mechanisms for facilitating Na+ extrusion and are critical to the ability of plants to adapt to high salinity [31,41]. Based on the functional identification results in yeast, MdCBL10.1 was selected for the subsequent transgene and functional identification in apple. Full-length CDS of MdCBL10.1 was cloned into the pBI121 vector and transformed into apple calli. PCR identification and quantitative reverse transcription (qRT)-PCR expression analysis demonstrated that several cisgenic lines with high MdCBL10.1 expression were obtained (Figure S5). Three lines (OE-3, OE-4, and OE-7) with high MdCBL10.1 expression levels were selected for NaCl treatment.
After 20 days of culture, no significant difference was found between OE lines and wild type (WT) on the normal MS medium. The growth of all lines was inhibited under NaCl treatment, with significantly reduced fresh weight compared with the apple calli cultured in MS medium (Figure 5A,B). However, the growth of MdCBL10.1 cisgenic lines was significantly better, and the fresh weight was significantly higher than that of the WT under salt treatment. These results suggested that the overexpression of MdCBL10.1 could significantly improve the salt tolerance of cisgenic apple calli (Figure 5A,B). In Arabidopsis, CBL10 participates in the SOS pathway and promotes the Na+ efflux [31,41]. Therefore, the Na+ content of the apple calli was measured. No significant difference was observed between different lines cultured in the normal MS medium. Under NaCl treatment, the Na+ contents of three cisgenic lines were significantly lower than that of the WT (Figure 5C), suggesting that the overexpression of MdCBL10.1 could inhibit the excessive accumulation of Na+ in cisgenic apple calli.
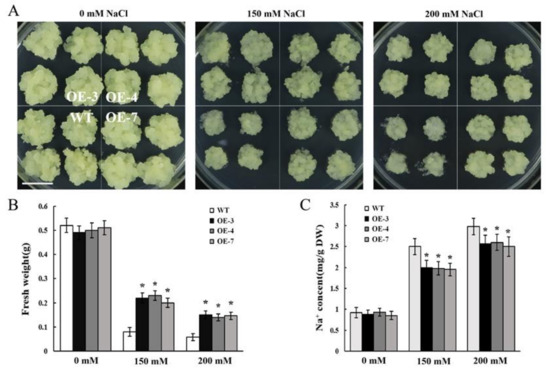
Figure 5.
Functional identification of MdCBL10.1 in cisgenic apple calli. (A) Phenotypes of the cisgenic (OE) and wild-type (WT) apple calli treated with NaCl stress. The scale bar represents 1 cm. Fresh weight (B) and Na+ content (C) in cisgenic and WT apple calli. For (B) and (C), error bars represent the SD of three independent biological replicates, with each biological repeat having at least three dishes. Bars labeled with * in each panel are significantly different from the WT (p < 0.05, Student t test).
3.6. Overexpression of MdCBL10.1 in Roots Enhanced the Salt Tolerance of Apple Plants
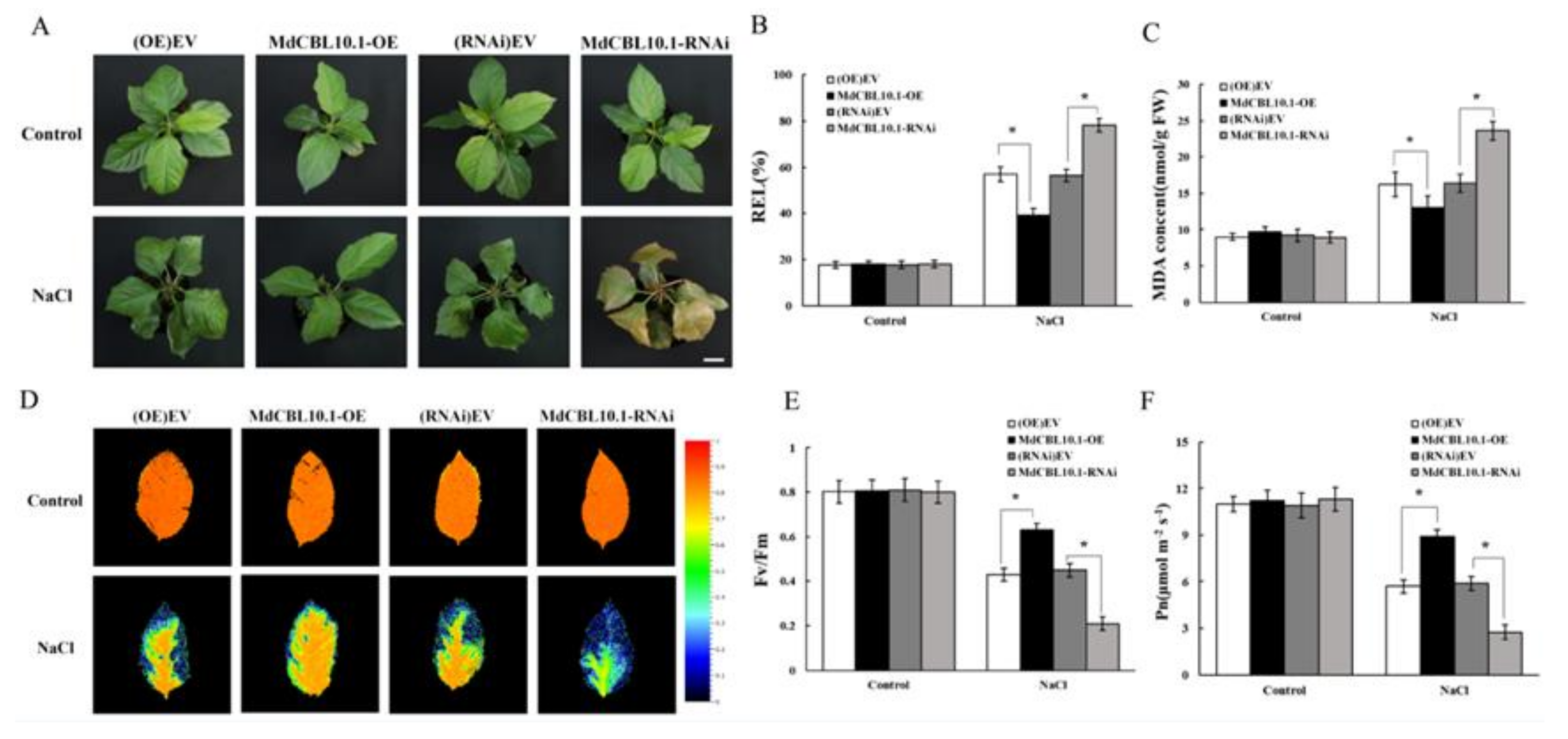
The use of A. rhizogenes K599 to obtain plants with cisgenic roots provides a convenient way for studying the function of MdCBL10.1 in apple plants because of the low genetic transformation efficiency [39,40]. Full-length CDS of MdCBL10.1 was cloned into the pCAMBIA2300 vector fused with a GFP tag, and a selected inhibitory fragment of MdCBL10.1 was cloned into the RNA-interference vector pK7GWIWG2D. Through GFP fluorescence detection and expression analysis (Figure S6), 20 plants with high MdCBL10.1 expression (MdCBL10.1-OE) and 20 plants with MdCBL10.1 expression significantly inhibited (MdCBL10.1-RNAi) were selected for subsequent salt treatment. Plants with their roots transformed with empty vectors pCAMBIA2300-GFP (OE-EV) and pK7GWIWG2D (RNAi-EV) were used as controls.
Under normal conditions (control group), no significant difference was observed between different types of cisgenic plants. After ten days of 150 mM NaCl irrigation, the RNAi plants were severely damaged by salt treatment, with their leaves showing obvious yellowing, wilting, and even death. Compared with the RNAi and control lines, the OE plants exhibited a better growth state, with their leaves still bright green and vigorous (Figure 6A). The measurements of relative ion leakage (REL) and MDA content in the leaves of these plants also indicated that the OE plants suffered less, whereas the RNAi plants suffered more stress damage caused by NaCl treatment (Figure 6B,C).
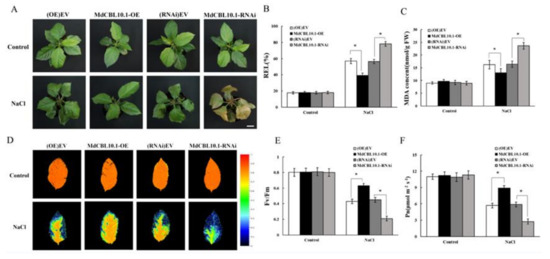
Figure 6.
Phenotypic analysis of MdCBL10.1 cisgenic apple plants under 150 mM NaCl treatment. (A) Growth phenotypes of cisgenic apple plants after NaCl treatment. Scale bars represent 3 cm. (B) Relative electrolyte leakage (REL) of apple leaves. (C) Malondialdehyde (MDA) content in apple leaves. Representative chlorophyll fluorescence images (D), Fv/Fm ratios (E), and net photosynthetic rate (Pn) (F) of cisgenic apple plants under normal and NaCl stress conditions. (OE)-EV and (RNAi)-EV represent roots transformed with the empty vector pCambia2300 and pK7GWIWG2D, respectively. The values of each index for cisgenic plants of the same type are the average values from all lines. Values are means of 20 replicates ± SD (each plant acts as a biological replicate). * in each panel denotes values significantly different from the corresponding control lines (p < 0.05, Student t test).
Besides REL and MDA content, the maximum quantum yield of PSII (Fv/Fm) is also an appropriate indicator for the early identification of the degree of damage in plants. Under normal conditions, the leaves of all lines were healthy and maintained high Fv/Fm ratios (Figure 6D,E). After NaCl treatment, the Fv/Fm of leaves of the RNAi plants decreased significantly, while that of the OE plants remained high (Figure 6D,E). The damage to photosynthetic units directly affected photosynthesis. After NaCl treatment, the net photosynthetic rate (Pn) of RNAi plants was significantly lower, while that of the OE plants was significantly higher than that of the control lines (Figure 6F). This was consistent with the performance of Fv/Fm and further supported that the overexpression of MdCBL10.1 alleviated the stress damage to apple plants caused by NaCl treatment.
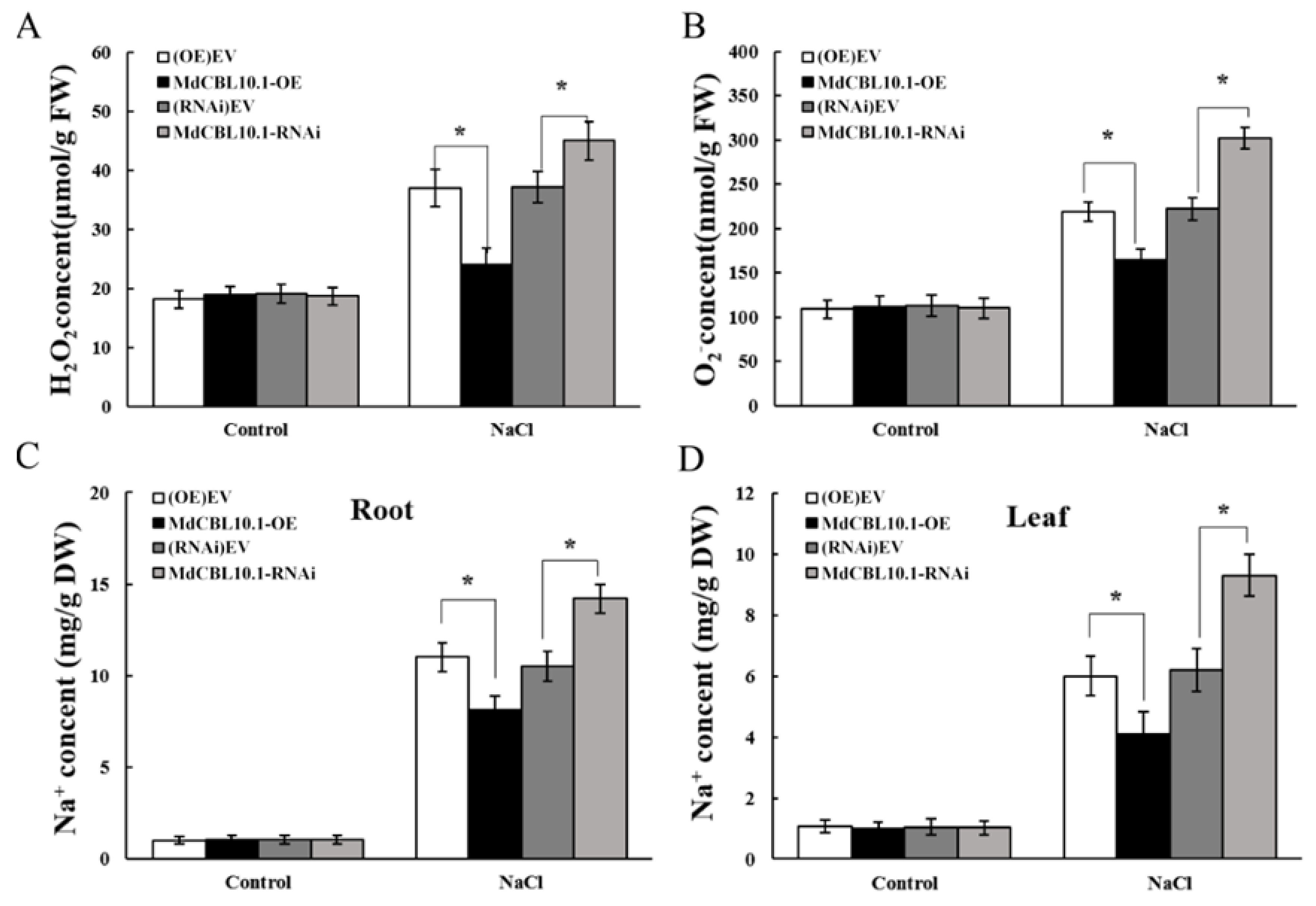
Salt stress triggers the accumulation of reactive oxygen species (ROS), which is harmful to plant growth. The accumulation of ROS in the roots of these NaCl-treated apple plants was determined to evaluate the damage caused by salt stress to root systems. The results showed that the roots of the OE lines accumulated less H2O2 and O2−, while the roots of the RNAi plants accumulated more ROS than controls (Figure 7A,B). Since MdCBL10.1 overexpression could inhibit Na+ accumulation in apple calli, the Na+ content in these apple plants after NaCl treatment was measured. As expected, the Na+ content in the roots and leaves of OE plants was significantly lower than that of the controls, while the RNAi plants showed the opposite trend (Figure 7C,D). These results indicated that MdCBL10.1 overexpression in roots could reduce the Na+ content in both roots and leaves, thereby alleviating salt stress-induced damage to apple plants.
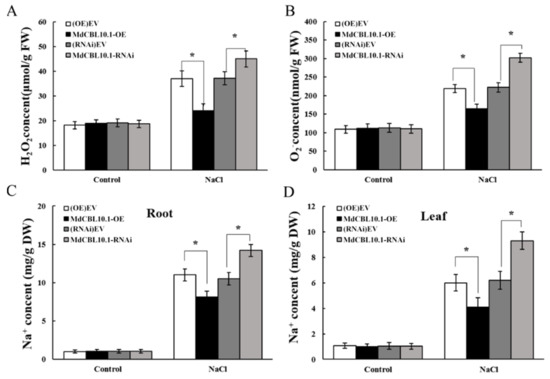
Figure 7.
Overexpression of MdCBL10.1 in apple roots inhibited the ROS and Na+ accumulation in cisgenic apple plants. (A) Hydrogen peroxide (H2O2) content in roots. (B) Superoxide anion (O2−) content in roots. (C) Na+ content in roots. (D) Na+ content in leaves. The values of each index are the average values of all lines in cisgenic plants of the same type. Values are means of 20 replicates ± SD. * in each panel denotes values significantly different from the corresponding control lines (p < 0.05, Student t test).
4. Discussion
Plants are inevitably exposed to a variety of adverse environmental conditions, such as water shortage, low temperature, high salinity, and so forth, due to the sessile lifestyle. These abiotic stresses are increasingly harmful to crop yield and quality. Calcium and its signaling pathway play an important role in plant stress response. As specific Ca2+ sensors, CBL proteins play vital roles in calcium signaling and stress resistance regulation [7]. Apple is one of the most economically important fruits in the world. Its cultivation and extension are restricted by various abiotic stresses. The study of CBL proteins is thus important for the resistance breeding of apple. To date, the CBL family proteins in many plant species have been identified at the genome-wide level. However, no detailed characterization of apple CBL family proteins has been reported, and little is known about their functions in abiotic stress response.
Ca2+ is an essential element for plant growth and survival, and Ca2+ signals are an important regulator of growth, development, and biotic and abiotic stresses in plants [7,38,42]. Studies in various fruit tree crops also have shown that Ca2+ plays an important role in regulating fruit development, ripening, quality, and storage [43,44,45,46,47]. The CBL family has been identified and systematically studied in many plant species, such as dicotyledons Arabidopsis (10 AtCBLs) [33,48], eggplant (5 SmCBLs) [36], cotton (13 GaCBLs, 13 GrCBLs, 22 GhCBLs) [1], Cassava (9 MeCBLs) [12], pepper (9 CaCBLs) [10], tea plant (7 CsCBLs) [32], and pigeon pea (9 CcCBLs) [49], as well as the monocotyledon rice (10 OsCBLs) [33,48], due to the important role of CBL proteins in Ca2+ signaling. The CBL family has also been identified in some fruit trees such as grapevine (8 VvCBLs) [8], banana (11 MaCBLs) [50], and pineapple (8 AcCBLs) [11]. Although the genomes of these species are significantly larger than that of Arabidopsis, the number of CBL genes in most of these species is not significantly higher than that in Arabidopsis [48]. In this study, 11 MdCBL genes were identified from apples (Table 1), only one more than that from Arabidopsis, suggesting that the CBL family in apples had not expanded significantly during evolution. This was different from other gene families that were previously identified in apple, such as the bHLH [37], Lhc [51], and CaCA [38] families. Tandem and segmental duplication events are fundamental mechanisms of gene family expansion. Considering the two genome-wide duplication events that occurred during apple evolution [52], collinear analysis between these MdCBLs was performed. The results showed that only two segmental duplication events occurred (Figure 1), indicating that the CBL family did not greatly expand during apple evolution. The evolutionary analysis of many plant species also suggested that the number of CBL members was independent of their genome size [48].
Although the number of CBL members varied, the phylogenetic analysis of CBL proteins in various plant species indicated that this family should be divided into four groups. In this study, the MdCBL proteins were divided into four groups (groups A to D), as in Arabidopsis (Figure 2A). This grouping result was also supported by the gene structure analysis and conserved motif prediction. The intron/exon and motif composition patterns of genes within the same group were consistent, whereas significant differences were observed between different groups (Figure 2B,C). In addition, based on the comparison of gene structure and conserved motifs of genes within the same group, three MdCBL genes (MdSOS3.2, MdCBL10.1, and MdCBL3.2) that might have errors in their predicted CDS in the apple genome were identified. The gene cloning results confirmed this speculation (Table 1 and Figure S4) and further suggested that this comparative analysis method could help people identify genes in a gene family whose coding sequences were incorrectly predicted. On the contrary, the high similarity of gene structure, conserved motifs, and cis-acting elements (Figure 2 and Figure 3) suggested functional redundancy among MdCBL genes, especially MdCBL genes in the same group. Similar results were also found in Arabidopsis, such as SOS3 and CBL10 [31].
Stress stimulation causes a transient increase in the intracellular Ca2+ concentration. CBL proteins can sense and interact with intracellular increased Ca2+. The binding of Ca2+ promotes the interaction between CBL and CIPK proteins, which is crucial for the activation of the kinase activity of CIPKs. Then, the activated CIPKs phosphorylate downstream substrates, further triggering a range of response mechanisms [7,33,42]. Decades of research have revealed extensive and complex interaction networks between CBL and CIPK family proteins. As Ca2+ sensors, each CBL has three or more EF-hand domains and Ca2+-binding sites. Besides, the CBL proteins harbor a conserved FPSF domain in their C-terminal, which is the target of phosphorylation by CIPK. Moreover, many of the CBL proteins also contain conserved MGCXXS/T motifs in their N-terminal, which contribute to the anchorage of CBLs in the membrane to transduce a Ca2+ signal [48,49,50,53]. Several studies proved that different CBLs could be localized to the plasma or vacuole membrane and could regulate plant salt tolerance by promoting the Na+ efflux or sequestration [7,42]. In this study, all MdCBL proteins contained the FPSF domain in their C-terminal (Figure 2, Figures S3 and S4), suggesting complex interactions between MdCBLs and CIPK family proteins in apple. For example, four MdCBL proteins were found to interact with the CIPK family protein MdSOS2L1 [54]. Many of these MdCBLs contained the conserved MGCXXS/T domain in their N-terminal. Further, the distribution of this domain showed obvious group specificity, which was found only in groups A and C of the MdCBL family (Figure 2, Figures S3 and S4). These results suggested that MdCBL proteins in these two groups might be more likely to function on the membrane and also indicated the functional and subcellular differentiation of CBL proteins between different groups. More studies on the subcellular localization and functional identification of apple CBL proteins are needed to confirm this hypothesis.
The CBL-CIPK model is reported to characterize many forms of abiotic stress responses. The first identified CBL-CIPK pathway was the SOS pathway, which plays vital roles in plant salt tolerance regulation [55]. It contains three key components: SOS1 (Na+/H+ antiporter), SOS2 (CIPK24), and SOS3 (CBL4). Under salt stress, the SOS2–SOS3 complex activates the transport properties of SOS1 to promote the Na+ efflux, thus enhancing plant salt tolerance. Subsequent studies found that CBL10 (SCABP8) could also interact with SOS2 and activate SOS1, suggesting the functional redundancy between SOS3 and CBL10 [31]. Studies in apples also showed that MdCBL10 could interact with MdSOS2L1, an SOS2-like protein that played positive roles in regulating plant salt tolerance [54]. In addition, a recent study found that the CBL10–CIPK8 complex regulated plant salt tolerance through its interaction with SOS1 [41]. These results, combined with the functional identification results in yeast (Figure 4), led to the selection of MdCBL10.1 for further genetic transformation and functional identification in apple. The phenotypic comparison and measurement of stress-related physiological indicators showed that MdCBL10.1 overexpression significantly enhanced the salt tolerance of cisgenic apple materials (Figure 5, Figure 6 and Figure 7). Moreover, the significantly reduced Na+ content in transgenic and cisgenic materials indicated that MdCBL10.1 overexpression could significantly inhibit Na+ accumulation under salt stress. These results suggested that MdCBL10.1 enhanced plant salt tolerance by inhibiting Na+ accumulation, and this was probably achieved through the SOS pathway.
In conclusion, 11 MdCBL genes were identified from apple. CDSs of these genes were cloned, and the gene structure and conserved motifs were analyzed. Several MdCBLs that played positive roles in salt tolerance were identified using the Na+-sensitive yeast mutant. The function of MdCBL10.1 in regulating salt tolerance was also identified in cisgenic apple materials in detail. This study provided a foundation for future research examining the function and mechanism of CBL proteins in regulating apple salt tolerance.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms222212430/s1. Figure S1: Phylogenetic analysis of proteins in HMMER screening results and CBL family members in Arabidopsis; Figure S2: Comparison of gene structure of the CBL family genes in apples and Arabidopsis; Figure S3: Putative conserved motifs identified in the sequences of apple CBL family proteins. Red dots indicate the conserved “FPSF” and “MGCxxS/T” domains; Figure S4: Sequence comparison between the predicted MdCBLs in the apple genome and the MdCBLs that were actually cloned in this study; Figure S5: Identification of cisgenic apple calli. (A) PCR identification of the MdCBL10.1 transgene based on genomic DNA extracted from apple calli. (B) Expression level of MdCBL10.1 in cisgenic apple calli. MdMDH served as an internal reference gene. Bars labeled with different letters in each panel are significantly different (p < 0.05, one-way ANOVA and Duncan’s test); Figure S6: Identification of the MdCBL10.1 transgene in the roots of apple plants. (A) GFP expression in cisgenic apple roots. (B) qRT-PCR determination of MdCBL10.1 expression in cisgenic apple roots. (OE)-EV and (RNAi)-EV represent roots transformed with the empty vector pCambia2300 and pK7GWIWG2D, respectively. MdMDH served as an internal reference gene. Bars labeled with different letters in each panel are significantly different (p < 0.05, one-way ANOVA and Duncan’s test). Supplementary File S1: HMMER screening results. Supplementary File S2: CDS and protein sequences of the cloned MdCBL genes. Supplementary File S3: Cis-elements identified in the promoter regions of MdCBL genes. Plus and minus signs indicate whether the cis-element was located in the plus or minus strand.
Author Contributions
P.C., J.Y., K.M. and F.M. conceived the project; P.C., J.Y. and K.M. designed the research plan; P.C., J.Y., Q.M., H.L. and Y.C. carried out the experiments; P.C., J.Y. and Q.M. analyzed the data; J.Y., K.M. and F.M. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Key Research and Development Program of China (2018YFD1000300/2018YFD1000301), the National Natural Science Foundation of China (31701894), the Key S&T Special Projects of Shaanxi Province (2020zdzx03–01-02), and the China Agriculture Research System of MOF and MARA (CARS-27).
Institutional Review Board Statement
No applicable.
Informed Consent Statement
No applicable.
Data Availability Statement
All data supporting the findings of this study are available within the paper and within its supplementary data published online.
Acknowledgments
We thank Huazhong Shi (Texas Tech University) and Jiafu Jiang (Nanjing Agricultural University) for providing the Na+-sensitive yeast mutants. Apple calli (‘Orin’) and the ‘Gala’ (GL-3) seedlings were kindly provided by Yujin Hao (Shandong Agricultural University) and Zhinghong Zhang (Shenyang Agricultural University), respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lu, T.T.; Zhang, G.F.; Sun, L.R.; Wang, J.; Hao, F.S. Genome-wide identification of CBL family and expression analysis of CBLs in response to potassium deficiency in cotton. PeerJ 2017, 5, e3653. [Google Scholar] [CrossRef] [Green Version]
- Luan, S.; Kudla, J.; Rodriguez-Concepcion, M.; Yalovsky, S.; Gruissem, W. Calmodulins and calcineurin B-like proteins: Calcium sensors for specific signal response coupling in plants. Plant Cell 2002, 14, S389–S400. [Google Scholar] [CrossRef] [Green Version]
- Cheong, Y.H.; Pandey, G.K.; Grant, J.J.; Batistic, O.; Li, L.; Kim, B.G.; Lee, S.C.; Kudla, J.; Luan, S. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J. Cell Mol. Biol. 2007, 52, 223–239. [Google Scholar] [CrossRef]
- Mazars, C.; Bourque, S.; Mithofer, A.; Pugin, A.; Ranjeva, R. Calcium homeostasis in plant cell nuclei. New Phytol. 2009, 181, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, S.; Bethke, P.C.; Jones, R.L. Calcium homeostasis in plants. J. Cell Sci. 1993, 106, 453–462. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, K.; Yadav, A.K.; Kanchan, K.; Baghel, M.; Kateriya, S.; Pandey, G.K. Genome-wide identification and biochemical characterization of calcineurin B-like calcium sensor proteins in Chlamydomonas reinhardtii. Biochem. J. 2020, 477, 1879–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanyal, S.K.; Pandey, A.; Pandey, G.K. The CBL-CIPK signaling module in plants: A mechanistic perspective. Physiol. Plant. 2015, 155, 89–108. [Google Scholar] [CrossRef]
- Xi, Y.; Liu, J.Y.; Dong, C.; Cheng, Z.M. The CBL and CIPK gene family in grapevine (Vitis vinifera): Genome-wide analysis and expression profiles in response to various abiotic stresses. Front. Plant Sci. 2017, 8, 978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.N.; Zhang, B.; Deng, J.W.; Chen, L.; Ullah, A.; Yang, X.Y. Genome-wide analysis of CBL and CIPK family genes in cotton: Conserved structures with divergent interactions and expression. Physiol. Mol. Biol. Plant 2021, 27, 359–368. [Google Scholar] [CrossRef]
- Ma, X.; Gai, W.X.; Qiao, Y.M.; Ali, M.; Wei, A.M.; Luo, D.X.; Li, Q.H.; Gong, Z.H. Identification of CBL and CIPK gene families and functional characterization of CaCIPK1 under Phytophthora capsici in pepper (Capsicum annuum L.). BMC Genom. 2019, 20, 775. [Google Scholar] [CrossRef]
- Aslam, M.; Fakher, B.; Jakada, B.H.; Zhao, L.H.; Cao, S.J.; Cheng, Y.; Qin, Y. Genome-wide identification and expression profiling of CBL-CIPK gene family in pineapple (Ananas comosus) and the role of AcCBL1 in abiotic and biotic stress response. Biomolecules 2019, 9, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Yan, Y.; Tie, W.W.; Ding, Z.H.; Wu, C.L.; Ding, X.P.; Wang, W.Q.; Xia, Z.Q.; Guo, J.C.; Peng, M. Genome-wide analyses of calcium sensors reveal their involvement in drought stress response and storage roots deterioration after harvest in Cassava. Genes 2018, 9, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, M.F.; Xu, C.; Cao, H.H.; Shi, Y.; Chen, J.; Chai, Y.; Li, Z.G. Tomato calmodulin-like protein SlCML37 is a calcium (Ca2+) sensor that interacts with proteasome maturation factor SlUMP1 and plays a role in tomato fruit chilling stress tolerance. J. Plant Physiol. 2021, 258, 153373. [Google Scholar] [CrossRef]
- Jung, H.; Chung, P.J.; Park, S.H.; Redillas, M.C.F.R.; Kim, Y.S.; Suh, J.W.; Kim, J.K. Overexpression of OsERF48 causes regulation of OsCML16, a calmodulin-like protein gene that enhances root growth and drought tolerance. Plant Biotechnol. J. 2017, 15, 1295–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Verde, V.; Trande, M.; D’Onofrio, M.; Dominici, P.; Astegno, A. Binding of calcium and target peptide to calmodulin-like protein CML19, the centrin 2 of Arabidopsis thaliana. Int. J. Biol. Macromol. 2018, 108, 1289–1299. [Google Scholar] [CrossRef]
- Trande, M.; Pedretti, M.; Bonza, M.C.; Di Matteo, A.; D’Onofrio, M.; Dominici, P.; Astegno, A. Cation and peptide binding properties of CML7, a calmodulin-like protein from Arabidopsis thaliana. J. Inorg. Biochem. 2019, 199, 110796. [Google Scholar] [CrossRef]
- Zhao, P.C.; Liu, Y.J.; Kong, W.Y.; Ji, J.Y.; Cai, T.Y.; Guo, Z.F. Genome-wide identification and characterization of calcium-dependent protein kinase (CDPK) and CDPK-related kinase (CRK) gene families in Medicago truncatula. Int. J. Mol. Sci. 2021, 22, 1044. [Google Scholar] [CrossRef]
- Bi, Z.Z.; Wang, Y.H.; Li, P.C.; Sun, C.; Qin, T.Y.; Bai, J.P. Evolution and expression analysis of CDPK genes under drought stress in two varieties of potato. Biotechnol. Lett. 2021, 43, 511–521. [Google Scholar] [CrossRef]
- Crizel, R.L.; Perin, E.C.; Vighi, I.L.; Woloski, R.; Seixas, A.; Pinto, L.D.; Rombaldi, C.V.; Galli, V. Genome-wide identification, and characterization of the CDPK gene family reveal their involvement in abiotic stress response in Fragaria × ananassa. Sci. Rep. 2020, 10, 11040. [Google Scholar] [CrossRef]
- Chu, L.C.; Offenborn, J.N.; Steinhorst, L.; Wu, X.N.; Xi, L.; Li, Z.; Jacquot, A.; Lejay, L.; Kudla, J.; Schulze, W.X. Plasma membrane calcineurin B-like calcium-ion sensor proteins function in regulating primary root growth and nitrate uptake by affecting global phosphorylation patterns and microdomain protein distribution. New Phytol. 2021, 229, 2223–2237. [Google Scholar] [CrossRef]
- Lu, L.; Chen, X.Y.; Zhu, L.M.; Li, M.J.; Zhang, J.B.; Yang, X.Y.; Wang, P.K.; Lu, Y.; Cheng, T.L.; Shi, J.S.; et al. NtCIPK9: A calcineurin B-like protein-interacting protein kinase from the Halophyte Nitraria tangutorum, enhances arabidopsis salt tolerance. Front. Plant Sci. 2020, 11, 1112. [Google Scholar] [CrossRef]
- Albrecht, V.; Weinl, S.; Blazevic, D.; D’Angelo, C.; Batistic, O.; Kolukisaoglu, U.; Bock, R.; Schulz, B.; Harter, K.; Kudla, J. The calcium sensor CBL1 integrates plant responses to abiotic stresses. Plant J. Cell Mol. Biol. 2003, 36, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, C.; Li, L.; Fu, D.; Zhang, Y.; Yang, P.; Zhang, T.; Wang, C. A novel role of the calcium sensor CBL1 in response to phosphate deficiency in Arabidopsis thaliana. J. Plant Physiol. 2020, 253, 153266. [Google Scholar] [CrossRef]
- Yan, Y.; He, M.; Guo, J.; Zeng, H.; Wei, Y.; Liu, G.; Hu, W.; Shi, H. The CBL1/9-CIPK23-AKT1 complex is essential for low potassium response in cassava. Plant Physiol. Biochem. PPB 2021, 167, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, H.D.; Chen, L.Q.; Wang, Y.; Liu, L.L.; He, L.; Wu, W.H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 2006, 125, 1347–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Held, K.; Pascaud, F.; Eckert, C.; Gajdanowicz, P.; Hashimoto, K.; Corratge-Faillie, C.; Offenborn, J.N.; Lacombe, B.; Dreyer, I.; Thibaud, J.B.; et al. Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res. 2011, 21, 1116–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Q.S.; Guo, Y.; Dietrich, M.A.; Schumaker, K.S.; Zhu, J.K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA 2002, 99, 8436–8441. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhu, J.K. A calcium sensor homolog required for plant salt tolerance. Science 1998, 280, 1943–1945. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.G.; Waadt, R.; Cheong, Y.H.; Pandey, G.K.; Dominguez-Solis, J.R.; Schultke, S.; Lee, S.C.; Kudla, J.; Luan, S. The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. Plant J. Cell Mol. Biol. 2007, 52, 473–484. [Google Scholar] [CrossRef]
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 2007, 19, 1415–1431. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, Y.X.; Li, H.; Teng, R.M.; Wang, Y.; Zhuang, J. Genome-wide identification and expression analysis of calcineurin B-like protein and calcineurin B-like protein-interacting protein kinase family genes in tea plant. DNA Cell Biol. 2019, 38, 824–839. [Google Scholar] [CrossRef] [PubMed]
- Kolukisaoglu, U.; Weinl, S.; Blazevic, D.; Batistic, O.; Kudla, J. Calcium sensors and their interacting protein kinases: Genomics of the Arabidopsis and rice CBL-CIPK signaling networks. Plant Physiol. 2004, 134, 43–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Yue, D.; Wei, W.; Hu, Y.; Feng, J.; Zou, Z. Characterization and functional analysis of calmodulin and calmodulin-like genes in Fragaria vesca. Front. Plant Sci. 2016, 7, 1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Hou, X.; Xia, Z.; Yan, Y.; Wei, Y.; Wang, L.; Zou, M.; Lu, C.; Wang, W.; Peng, M. Genome-wide survey and expression analysis of the calcium-dependent protein kinase gene family in cassava. Mol. Genet. Genom. MGG 2016, 291, 241–253. [Google Scholar] [CrossRef]
- Li, J.; Jiang, M.M.; Ren, L.; Liu, Y.; Chen, H.Y. Identification and characterization of CBL and CIPK gene families in eggplant (Solanum melongena L.). Mol. Genet. Genom. MGG 2016, 291, 1769–1781. [Google Scholar] [CrossRef]
- Mao, K.; Dong, Q.; Li, C.; Liu, C.; Ma, F. Genome wide identification and characterization of apple bHLH Transcription factors and expression analysis in response to drought and salt stress. Front. Plant Sci. 2017, 8, 480. [Google Scholar] [CrossRef] [Green Version]
- Mao, K.; Yang, J.; Wang, M.; Liu, H.; Guo, X.; Zhao, S.; Dong, Q.; Ma, F. Genome-wide analysis of the apple CaCA superfamily reveals that MdCAX proteins are involved in the abiotic stress response as calcium transporters. BMC Plant Biol. 2021, 21, 81. [Google Scholar] [CrossRef]
- Yang, J.; Guo, X.; Li, W.; Chen, P.; Cheng, Y.; Ma, F.; Mao, K. MdCCX2 of apple functions positively in modulation of salt tolerance. Environ. Exp. Bot. 2021, 192, 104663. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Guo, X.; Chen, P.; Cheng, Y.; Mao, K.; Ma, F. Cation/Ca2+ Exchanger 1 (MdCCX1), a plasma membrane-localized Na+ transporter, enhances plant salt tolerance by inhibiting excessive accumulation of Na+ and reactive oxygen species. Front. Plant Sci. 2021, 12, 46189. [Google Scholar] [CrossRef]
- Yin, X.; Xia, Y.; Xie, Q.; Cao, Y.; Wang, Z.; Hao, G.; Song, J.; Zhou, Y.; Jiang, X. The protein kinase complex CBL10-CIPK8-SOS1 functions in Arabidopsis to regulate salt tolerance. J. Exp. Bot. 2020, 71, 1801–1814. [Google Scholar] [CrossRef]
- Tang, R.J.; Wang, C.; Li, K.L.; Luan, S. The CBL-CIPK Calcium signaling network: Unified paradigm from 20 years of discoveries. Trends Plant Sci. 2020, 25, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Samiotaki, M.; Tsiolas, G.; Sarrou, E.; Stamatakis, G.; Ganopoulos, I.; Martens, S.; Argiriou, A.; et al. Novel insights into the calcium action in cherry fruit development revealed by high-throughput mapping. Plant Mol. Biol. 2020, 104, 597–614. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Karamanoli, K.; Lazaridou, A.; Matsi, T.; Molassiotis, A. Metabolomic and physico-chemical approach unravel dynamic regulation of calcium in sweet cherry fruit physiology. Plant Physiol. Biochim. 2017, 116, 68–79. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, M.T.; Wang, M.J.; Xu, Y.S.; Chen, W.T.; Yang, G.S. Transcriptome analysis of calcium-induced accumulation of anthocyanins in grape skin. Sci. Hortic. 2020, 260, 108871. [Google Scholar] [CrossRef]
- Martins, V.; Billet, K.; Garcia, A.; Lanoue, A.; Geros, H. Exogenous calcium deflects grape berry metabolism towards the production of more stilbenoids and less anthocyanins. Food Chem. 2020, 313, 126123. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Sarrou, E.; Stavridou, E.; Ganopoulos, I.; Karamanoli, K.; Madesis, P.; Martens, S.; Molassiotis, A. An integrated metabolomic and gene expression analysis identifies heat and calcium metabolic networks underlying postharvest sweet cherry fruit senescence. Planta 2019, 250, 2009–2022. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Mohanta, N.; Mohanta, Y.K.; Parida, P.; Bae, H.H. Genome-wide identification of Calcineurin B-Like (CBL) gene family of plants reveals novel conserved motifs and evolutionary aspects in calcium signaling events. BMC Plant Biol. 2015, 15, 189. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.H.; Dong, B.Y.; Yang, Q.; Niu, L.L.; Li, H.H.; Cao, H.Y.; Amin, R.; Meng, D.; Fu, Y.J. Screening of CBL genes in pigeon pea with focus on the functional analysis of CBL4 in abiotic stress tolerance and flavonoid biosynthesis. Environ. Exp. Bot. 2020, 177, 104102. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, R.M.; Lin, X.J.; Zhou, Y.J.; Tang, F.L.; Yao, Y.; Liu, J.; Wang, L.X.; Yin, X.M.; Liu, Y.X.; et al. Genome-wide analysis and expression tendency of banana (Musa acuminata L.) calcineurin B-like (MaCBL) genes under potassium stress. Horticulturae 2021, 7, 70. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, H.; Luo, J.; Wang, H.; Dong, Q.; Wang, Y.; Yang, K.; Mao, K.; Ma, F. Genome-wide analysis of the light-harvesting chlorophyll a/b-binding gene family in apple (Malus domestica) and functional characterization of MdLhcb4.3, which confers tolerance to drought and osmotic stress. Plant Physiol. Biochem. PPB 2020, 154, 517–529. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhao, C.L.; Zhao, M.F.; Li, Y.Z.; Wen, G.S. Phylogeny and evolution of calcineurin B-like (CBL) gene family in grass and functional analyses of rice CBLs. J. Plant Biol. 2020, 63, 117–130. [Google Scholar] [CrossRef]
- Hu, D.G.; Ma, Q.J.; Sun, C.H.; Sun, M.H.; You, C.X.; Hao, Y.J. Overexpression of MdSOS2L1, a CIPK protein kinase, increases the antioxidant metabolites to enhance salt tolerance in apple and tomato. Physiol. Plant. 2016, 156, 201–214. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).