The Influence of UV Varnishes on the Content of Cysteine and Methionine in Women Nail Plates—Chromatographic Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Acidic Hydrolysis of Nail Plate Samples
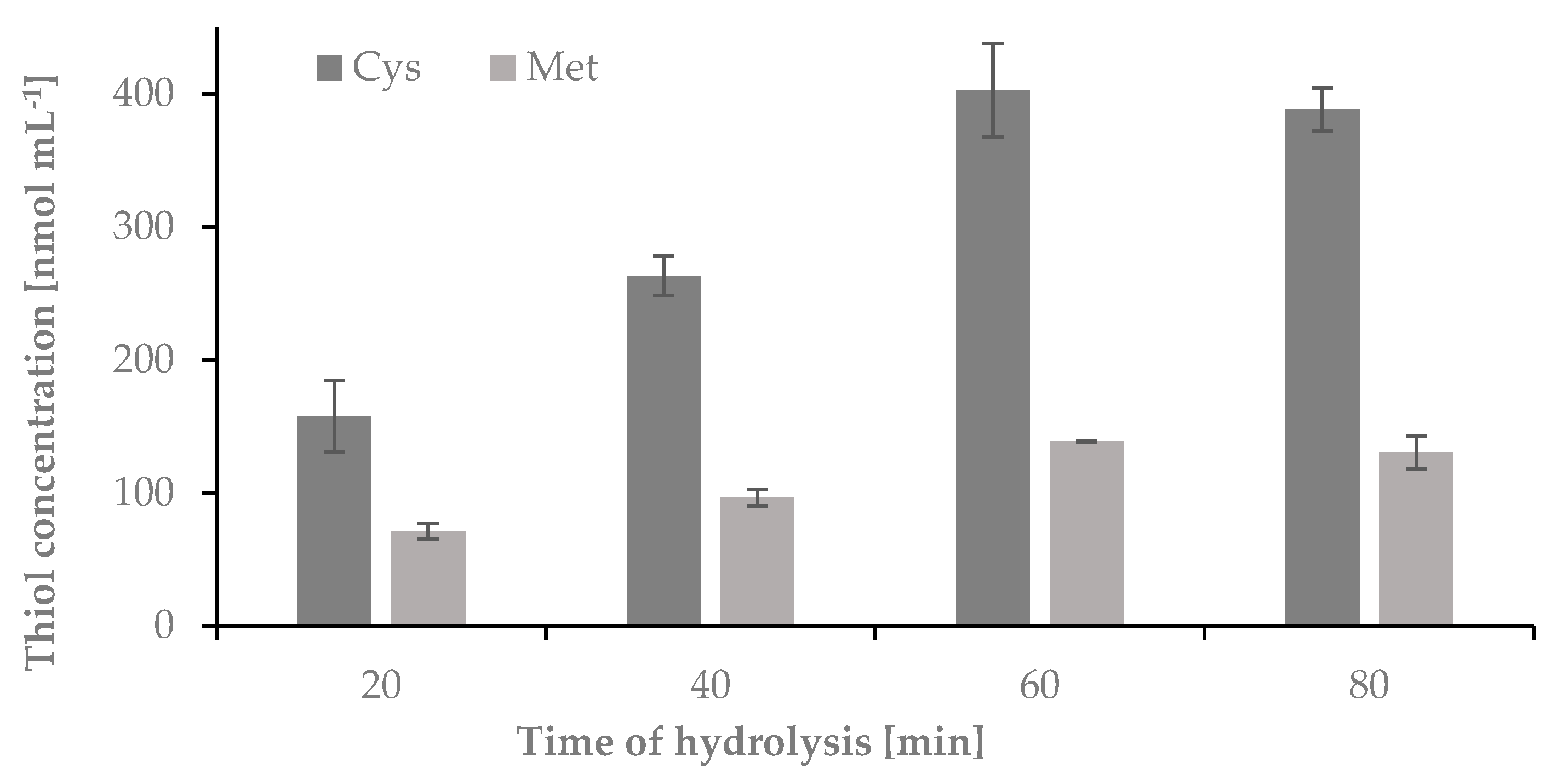
2.1.1. The Influence of Time on the Hydrolysis Yield of Nail Plate Samples
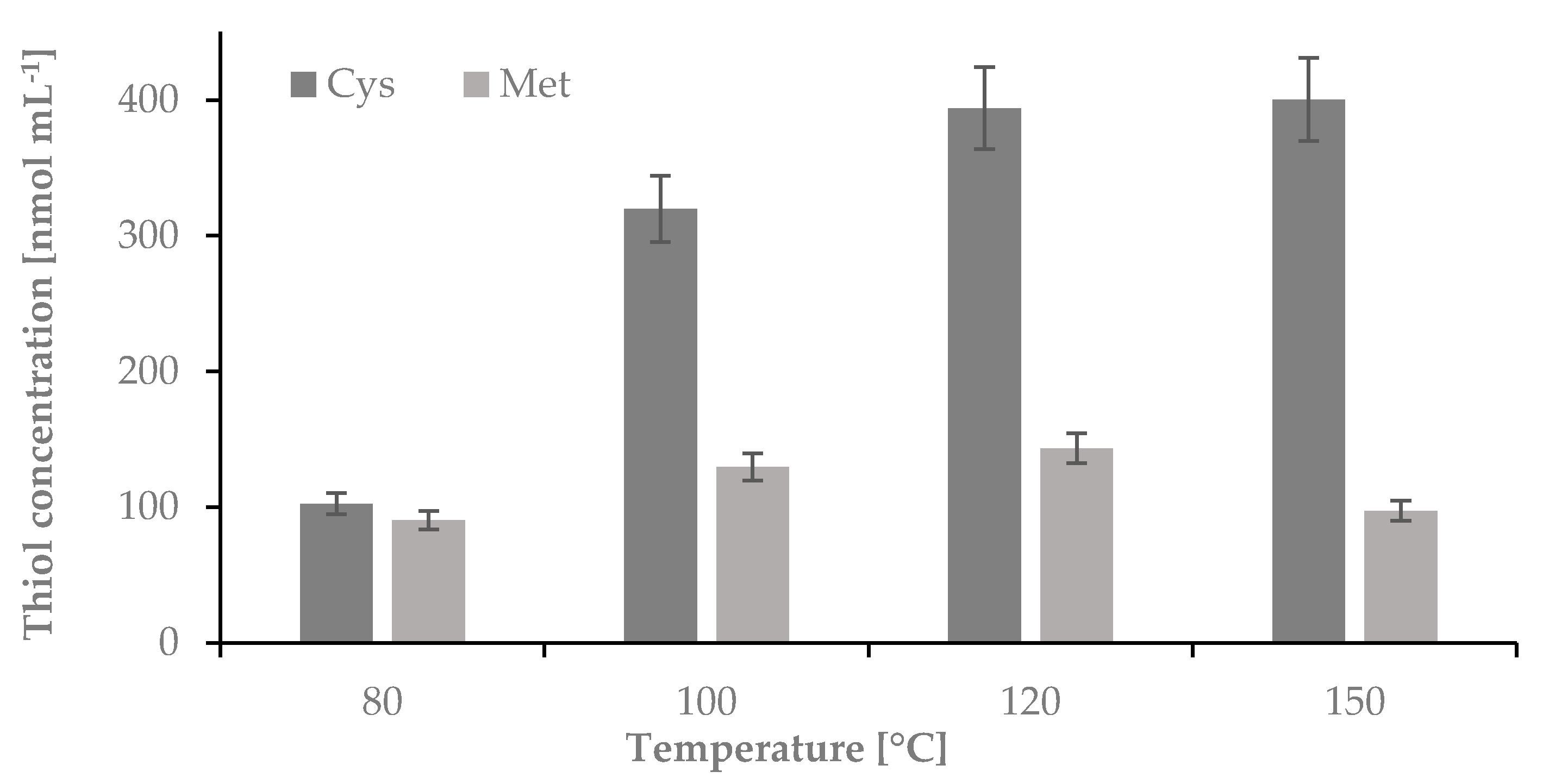
2.1.2. The Influence of Temperature on the Hydrolysis Yield of Nail Plate Samples
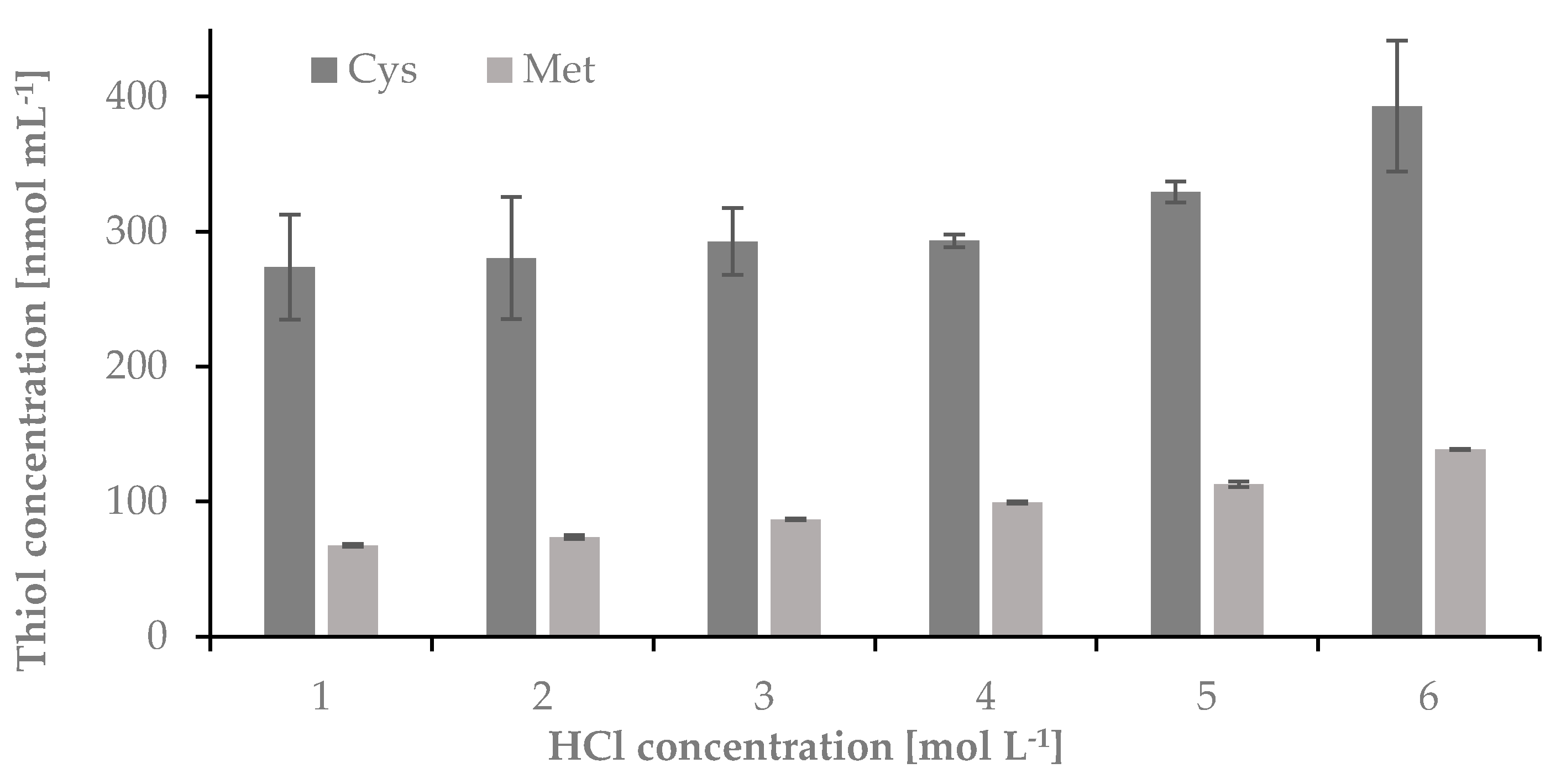
2.1.3. The Influence of Concentration of HCl on the Hydrolysis Yield of Nail Plate Samples
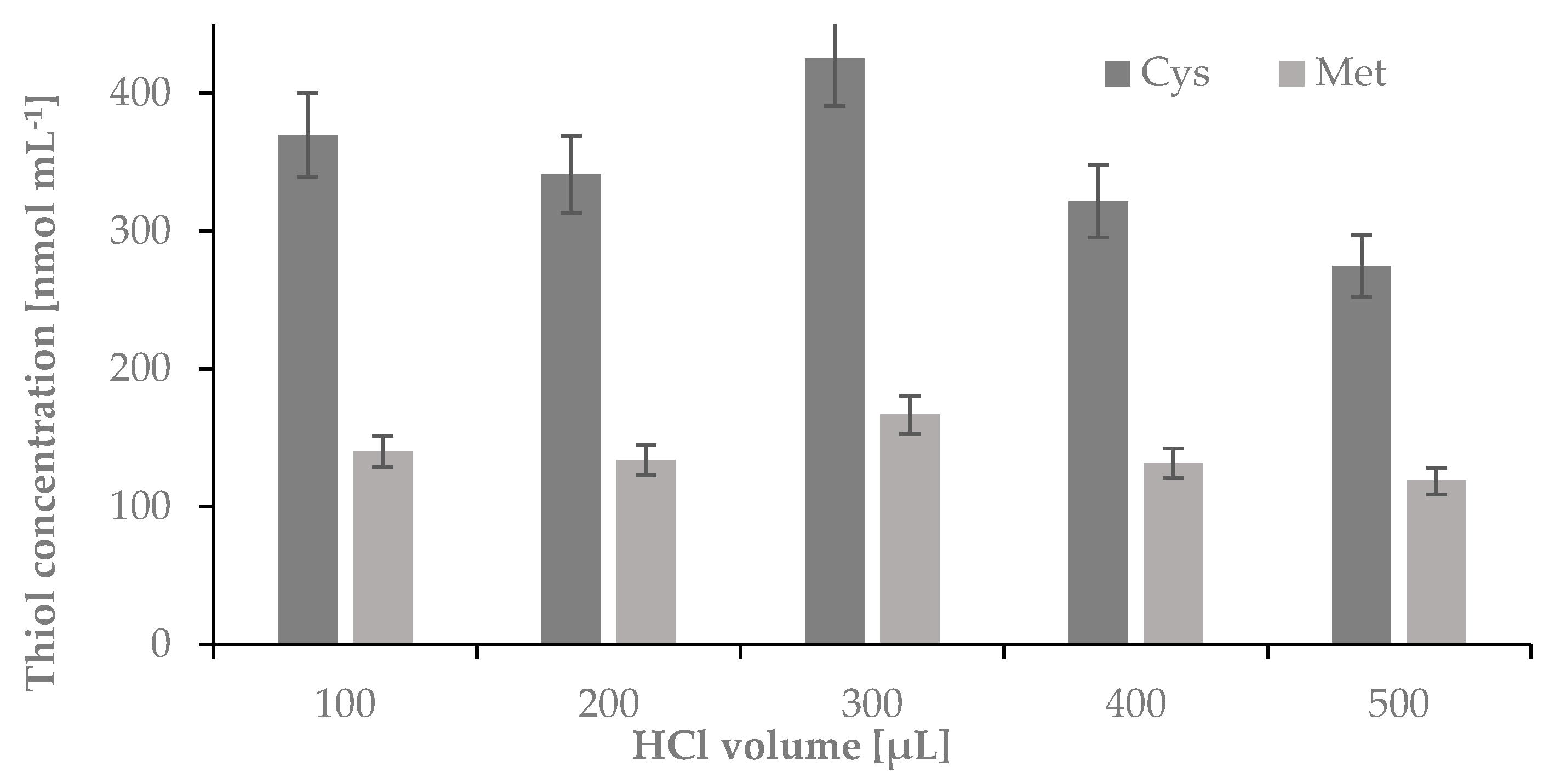
2.1.4. The Influence of a Volume of HCl on the Hydrolysis Yield of Nail Plate Samples
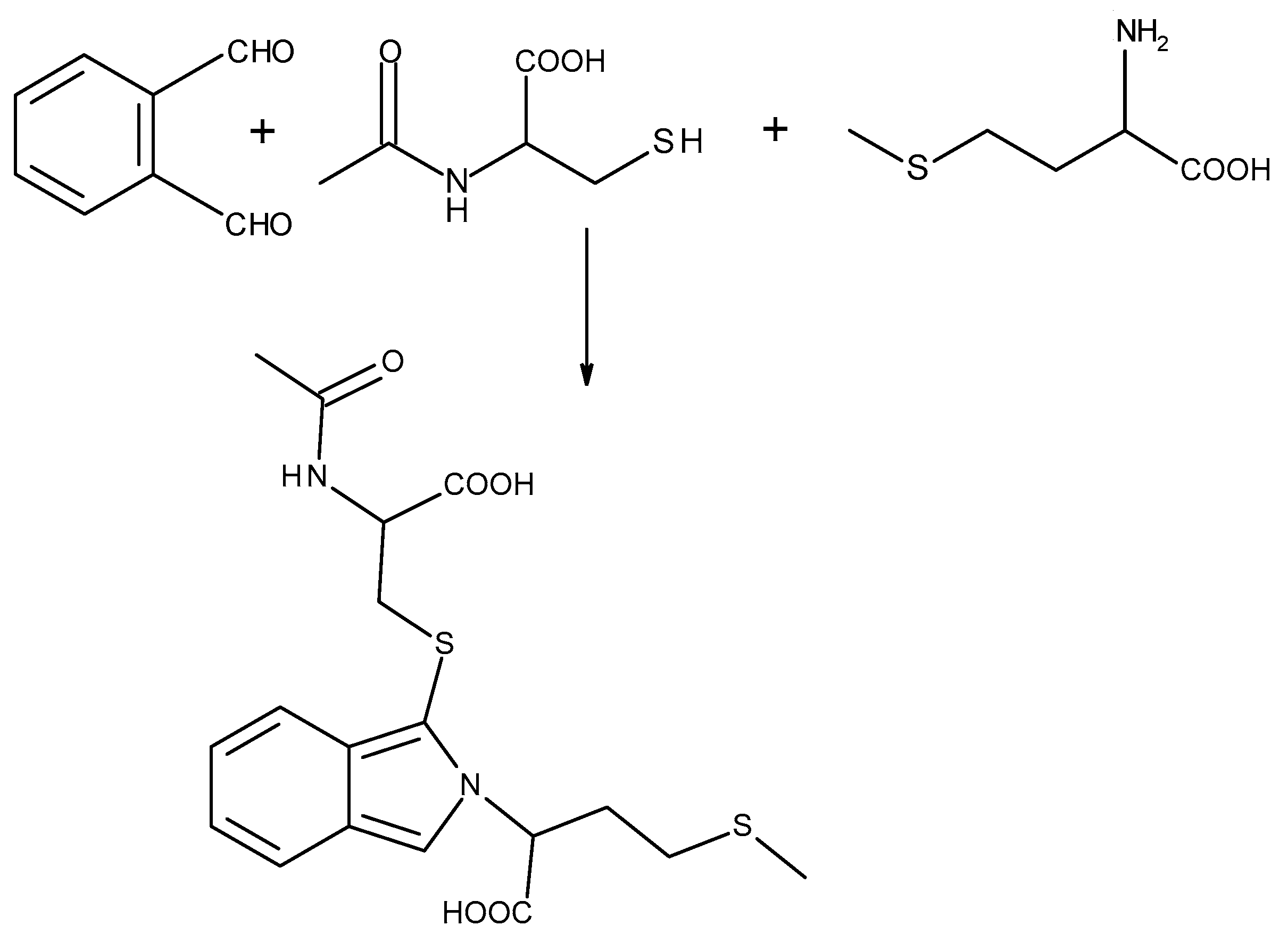
2.2. Reduction and Derivatization
2.2.1. Reduction of the Disulfide Bonds
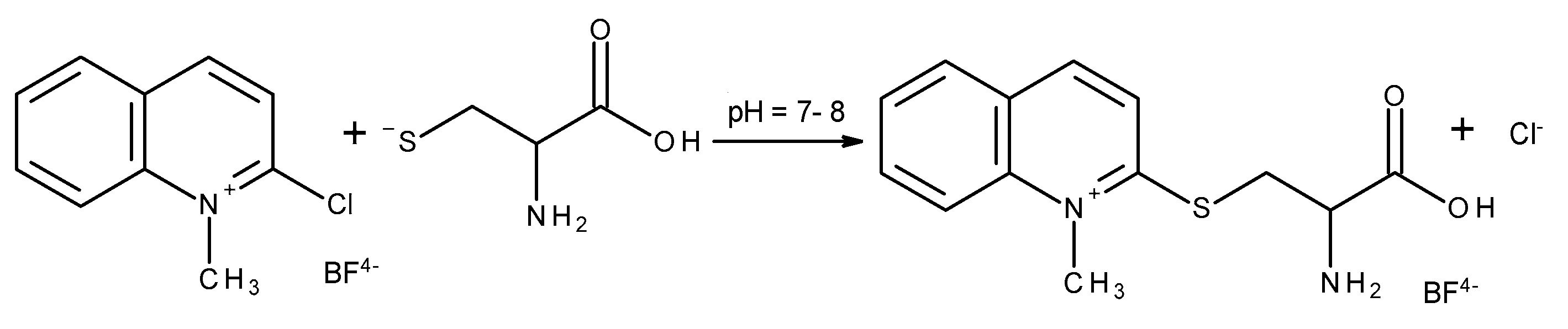
2.2.2. Derivatization of a Thiol Group
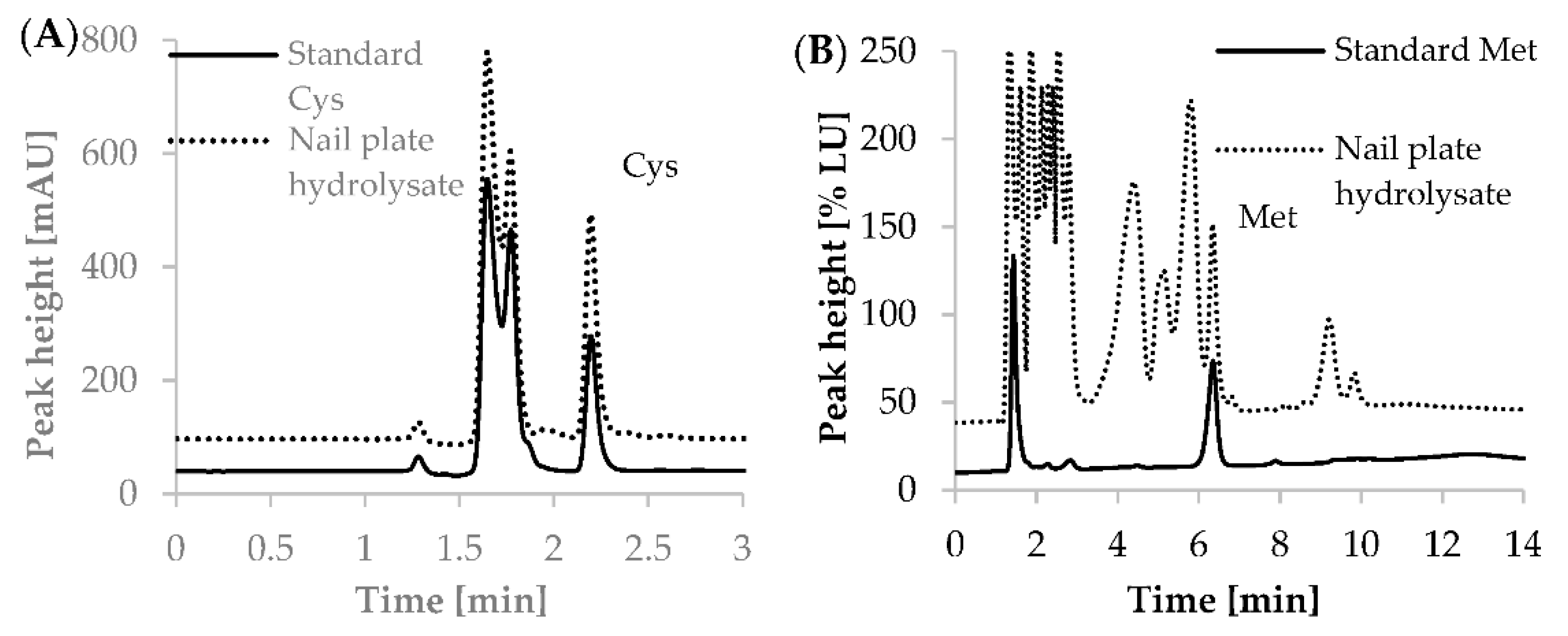
2.2.3. Selectivity and Specificity
2.2.4. Matrix Effects
2.2.5. Method Calibrations
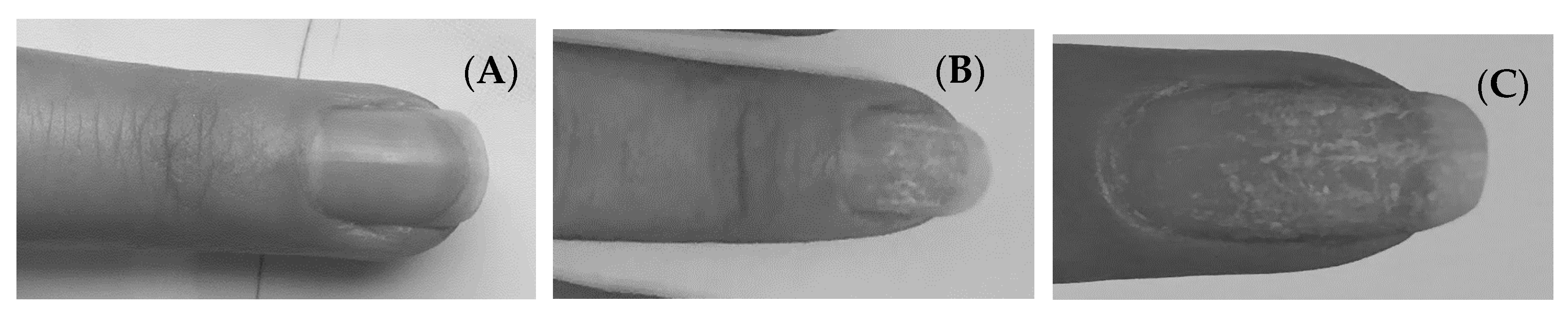
2.3. Cys and Met in Nail Plates before and after Applying a Hybrid Manicure
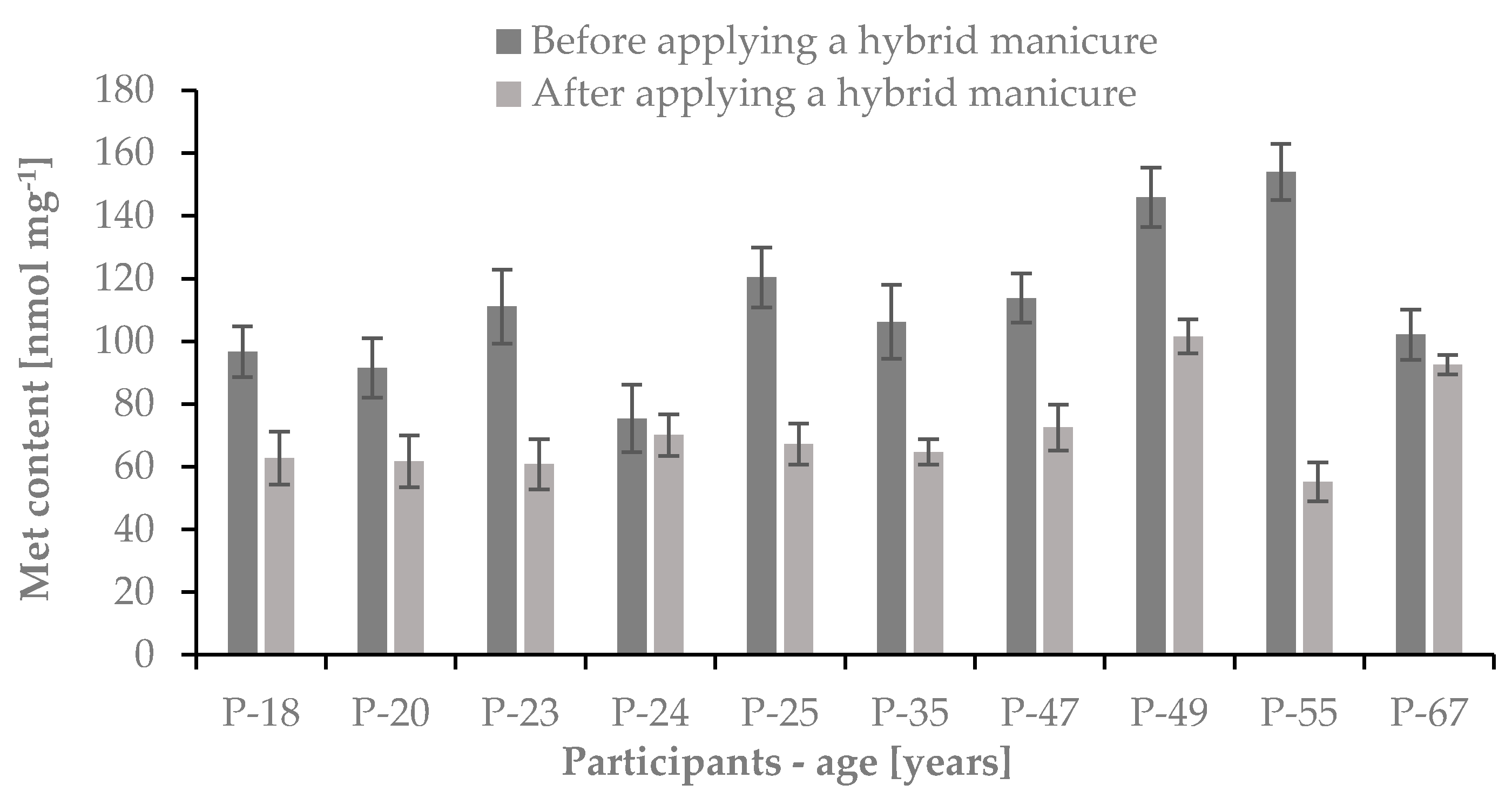
2.3.1. The Content of Cys and Met in Nail Plates
2.3.2. Measurement of Nail Plate Thickness
3. Materials and Methods
3.1. Materials
3.1.1. Chemicals
3.1.2. Instrumentation
3.1.3. Stock Solutions
3.2. Sample Protocols
3.2.1. Human Nail Plate Samples
3.2.2. Hydrolysis of Nail Plate Samples
3.2.3. Sample Preparation for Determination of Cys
3.2.4. Sample Preparation for Determination of Met
3.2.5. HPLC Conditions for Determination of Cys
3.2.6. HPLC Conditions for Determination of Met
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saleah, S.A.; Kim, P.; Seong, D.; Wijesinghe, R.E.; Jeon, M.; Kim, J. A preliminary study of post-progressive nail-art effects on in vivo nail plate using optical coherence tomography-based intensity profiling assessment. Sci. Rep. 2021, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.F.; Chimento, S.M.; Hu, S.; Sanchez, M.; Zaiac, M.; Tosti, A. Nail damage from gel polish manicure. J. Cosmet. Dermatol. 2012, 11, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, W.; McKittrick, J.; Meyers, M.A. Keratin: Structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Prog. Mater. Sci. 2016, 76, 229–318. [Google Scholar] [CrossRef] [Green Version]
- de Berker, D.A.R.; Andre, J.; Baran, R. Nail biology and nail science. Int. J. Cosmet. Sci. 2007, 29, 241–275. [Google Scholar] [CrossRef]
- Barba, C.; Méndez, S.; Martí, M.; Parra, L.; Coderch, L. Water content of hair and nails. Thermochim. Acta 2009, 494, 136–140. [Google Scholar] [CrossRef]
- Shipp, L.R.; Warner, C.A.; Rueggeberg, F.A. Further investigation into the risk of skin cancer associated with the use of UV nail lamps. JAMA Dermatol. 2014, 150, 775–777. [Google Scholar] [CrossRef] [Green Version]
- Batory, M.; Wołowiec-Korecka, E.; Rotsztejn, H. The effect of various primers improving adhesiveness of gel polish hybrids on pH, TOWL and overall nail plates condition. J. Cosmet. Dermatol. 2019, 18, 1529–1538. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136, 1636–1640. [Google Scholar] [CrossRef] [Green Version]
- Baran, R.; Schoon, D. Nail beauty. J. Cosmet. Dermatol. 2004, 3, 167–170. [Google Scholar] [CrossRef]
- Iorizzo, M.; Piraccini, B.M.; Tosti, A. Nail cosmetics in nail disorders. J. Cosmet. Dermatol. 2007, 6, 53–58. [Google Scholar] [CrossRef]
- Shah, K.N.; Rubin, A.I. Nail disorders as signs of pediatric systemic disease. Curr. Probl. Pediatr. Adolesc. Health Care 2012, 42, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Koppel, K.; Adhikari, K. Sensory factors affecting female consumers acceptability of nail polish. Int. J. Cosmet. Sci. 2015, 37, 642–650. [Google Scholar] [CrossRef]
- Singal, A.; Arora, R. Nail as a window of systemic diseases. Indian Dermatol. Online J. 2015, 6, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Borowczyk, K.; Wróblewski, J.; Suliburska, J.; Akahoshi, N.; Ishii, I.; Jakubowski, H. Mutations in homocysteine metabolism genes increase keratin, N-homocysteinylation and damage in mice. Int. J. Genom. 2018, 2018, 7570850. [Google Scholar] [CrossRef] [PubMed]
- Borowczyk, K.; Suliburska, J.; Jakubowski, H. Demethylation of methionine and keratin damage in human hair. Amino Acids 2018, 50, 537–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butz, L.W.; Du Vigneaud, V. The formation of a homologue of cystine by the decomposition of methionine with sulfuric acid. J. Biol. Chem. 1932, 99, 135–141. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Chwatko, G.; Głowacki, R.; Kubalczyk, P.; Bald, E. Ultraviolet derivatization of low-molecular-mass thiols for high performance liquid chromatography and capillary electrophoresis analysis. J. Chromatogr. B 2011, 879, 1290–1307. [Google Scholar] [CrossRef]
- Borowczyk, K.; Krawczyk, M.; Kubalczyk, P.; Chwatko, G. Determination of lipoic acid in biological samples. Bioanalysis 2015, 7, 1785–1798. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Chwatko, G.; Głowacki, R.; Bald, E. Determination of endogenous thiols and thiol drugs in urine by HPLC with ultraviolet detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 3300–3308. [Google Scholar] [CrossRef]
- Borowczyk, K.; Olejarz, P.; Chwatko, G.; Szylberg, M.; Głowacki, R. A simplified method for simultaneous determination of α-lipoic acid and low-molecular-mass thiols in human plasma. Int. J. Mol. Sci. 2020, 21, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bald, E.; Głowacki, R.; Drzewoski, J. Determination by liquid chromatography of free and total cysteine in human urine in the form of its S-quinolinium derivative. J. Chromatogr. A 2001, 913, 319–329. [Google Scholar] [CrossRef]
- Borowczyk, K.; Wyszczelska-Rokiel, M.; Kubalczyk, P.; Głowacki, R. Simultaneous determination of albumin and low-molecular-mass thiols in plasma by HPLC with UV detection. J. Chromatogr. B 2015, 981, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, A.; Głowacka, I.E.; Pasternak, B.; Głowacki, R.; Chwatko, G. The first method for determination of lipoyllysine in human urine after oral lipoic acid supplementation. Bioanalysis 2019, 11, 1359–1373. [Google Scholar] [CrossRef] [PubMed]
- Borowczyk, K.; Chwatko, G.; Kubalczyk, P.; Jakubowski, H.; Kubalska, J.; Głowacki, R. Simultaneous determination of methionine and homocysteine by on column derivatization with o-phtaldialdehyde. Talanta 2016, 161, 917–924. [Google Scholar] [CrossRef]
- European Medicines Agency. Committee for Medicinal Products for Human Use, Guideline on Bioanalytical Method Validation. Available online: https://www.ema.europa.eu (accessed on 1 June 2020).
- FDA Guidance for Industry Bioanalytical Method Validation. Available online: https://www.fda.gov (accessed on 19 June 2020).
- Motswaledi, M.H.; Mayayise, M.C. Nail changes in systemic diseases. SA Fam. Pract. 2010, 52, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Bald, E.; Głowacki, R. 2-Chloro-1-methylquinolinium tetrafluoroborate as an effective and thiol specific UV-tagging reagent for liquid chromatography. J. Liq. Chrom. Relat. Technol. 2001, 24, 1323–1339. [Google Scholar] [CrossRef]


| Linear Range [nmol mL−1] | Regression Equation | R2 | Imprecision [%] | Recovery [%] | |||
|---|---|---|---|---|---|---|---|
| Min. | Max. | Min. | Max. | ||||
| Cys | 50.0–600.0 | y = 0.131x + 60.39 | 0.999 | 0.4 | 2.6 | 100.9 | 122.2 |
| Met | 60.0–250.0 | y = 1.008x + 74.4 | 0.998 | 0.1 | 11.7 | 95.0 | 109.6 |
| Concentrations | Precision [%] | Accuracy [%] | |||
|---|---|---|---|---|---|
| Intra-Day | Inter-Day | Intra-Day | Inter-Day | ||
| Cys [nmol mL−1] | 50.0 | 4.1 | 5.6 | 100.3 | 112.2 |
| 300.0 | 4.7 | 9.2 | 110.4 | 101.6 | |
| 600.0 | 2.3 | 8.1 | 93.2 | 98.1 | |
| Met [nmol mL−1] | 60.0 | 3.7 | 3.6 | 99.3 | 115.6 |
| 160.0 | 3.3 | 6.8 | 91.7 | 109.4 | |
| 250.0 | 5.3 | 5.6 | 99.8 | 104.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowczyk, K.; Głowacki, R. The Influence of UV Varnishes on the Content of Cysteine and Methionine in Women Nail Plates—Chromatographic Studies. Int. J. Mol. Sci. 2021, 22, 12447. https://doi.org/10.3390/ijms222212447
Borowczyk K, Głowacki R. The Influence of UV Varnishes on the Content of Cysteine and Methionine in Women Nail Plates—Chromatographic Studies. International Journal of Molecular Sciences. 2021; 22(22):12447. https://doi.org/10.3390/ijms222212447
Chicago/Turabian StyleBorowczyk, Kamila, and Rafał Głowacki. 2021. "The Influence of UV Varnishes on the Content of Cysteine and Methionine in Women Nail Plates—Chromatographic Studies" International Journal of Molecular Sciences 22, no. 22: 12447. https://doi.org/10.3390/ijms222212447






