Exploring the Impact of the Microbiome on Neuroactive Steroid Levels in Germ-Free Animals
Abstract
:1. Introduction
2. Results
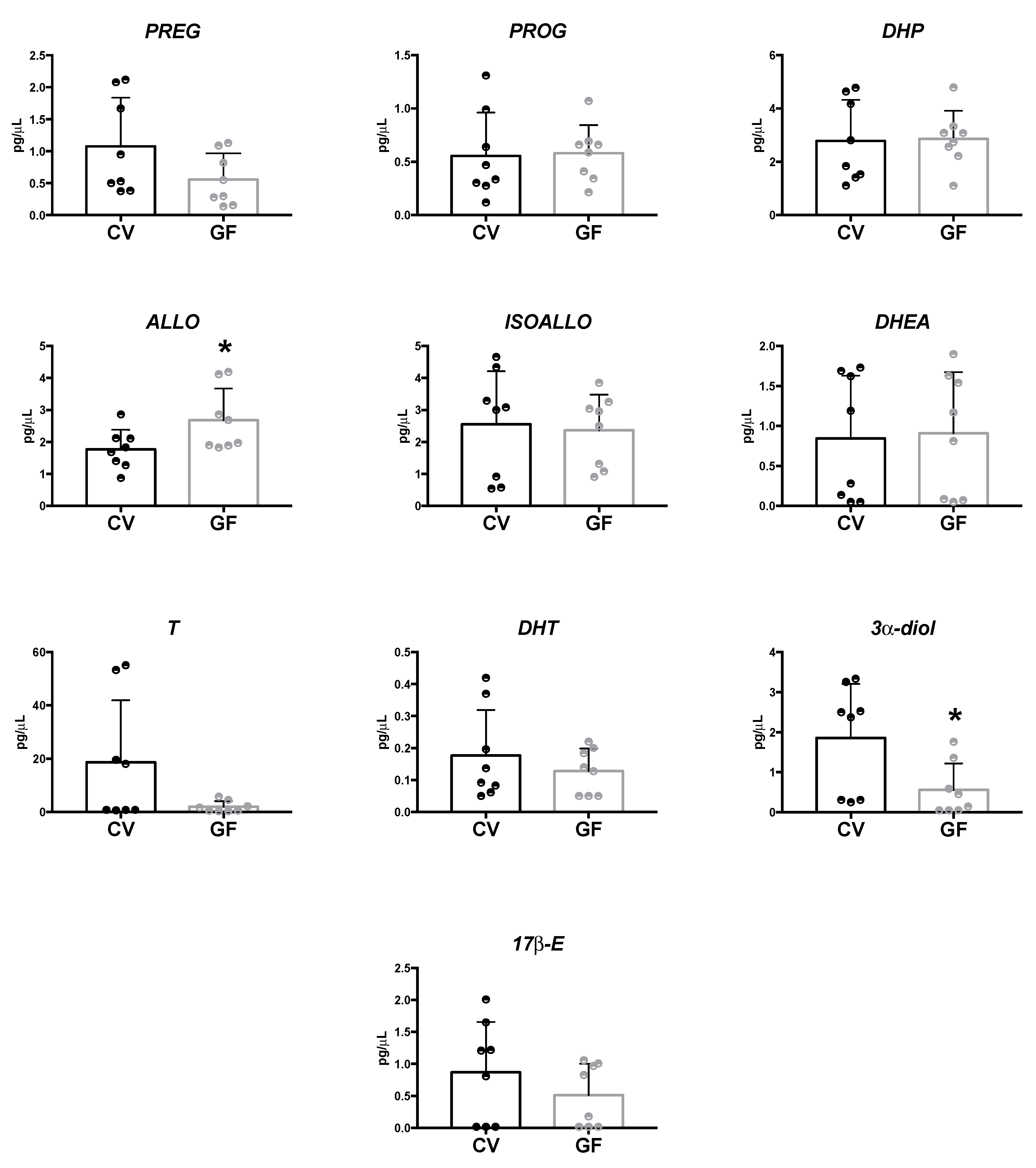
2.1. Assessment of Neuroactive Steroid Levels in Plasma
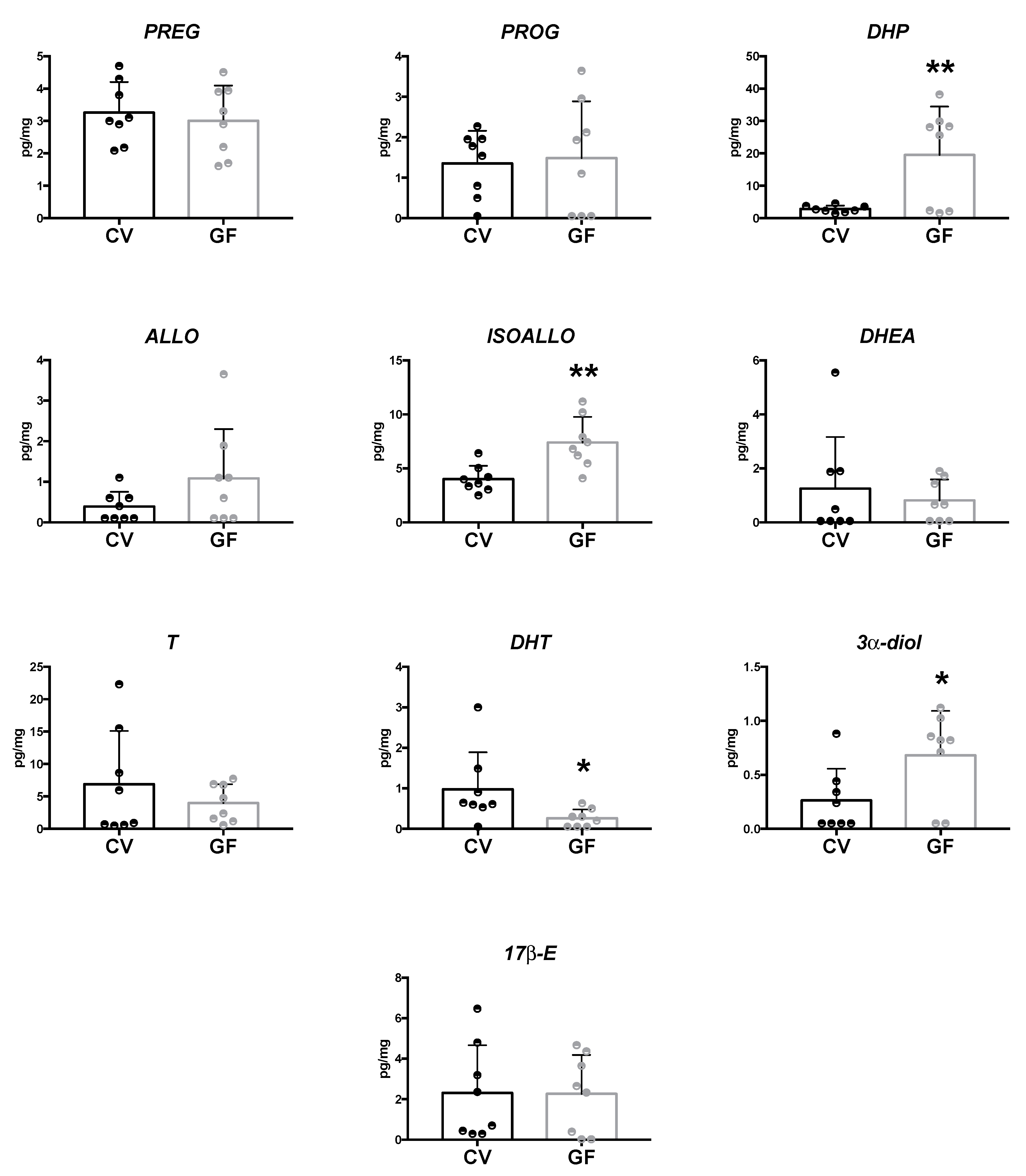
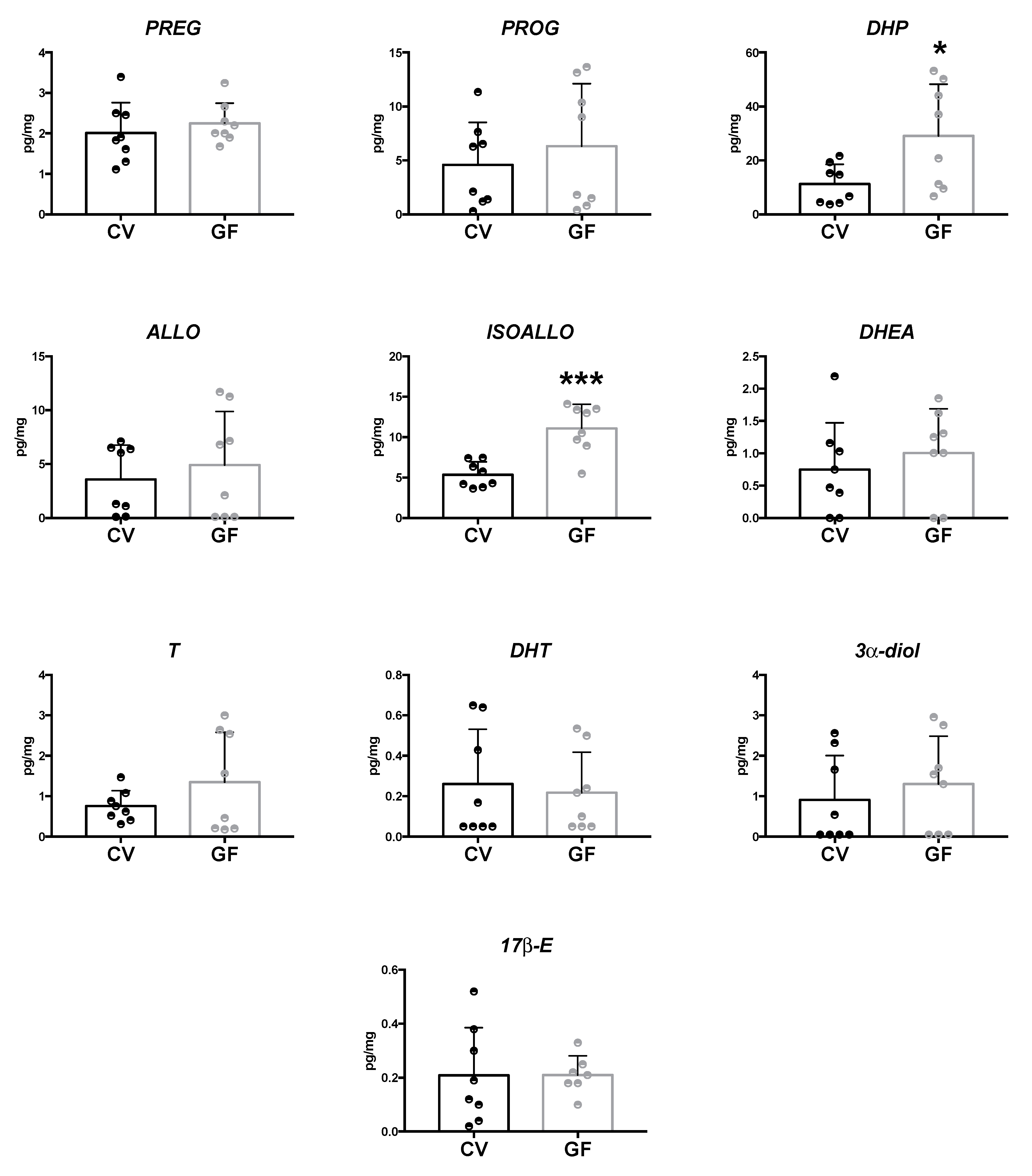
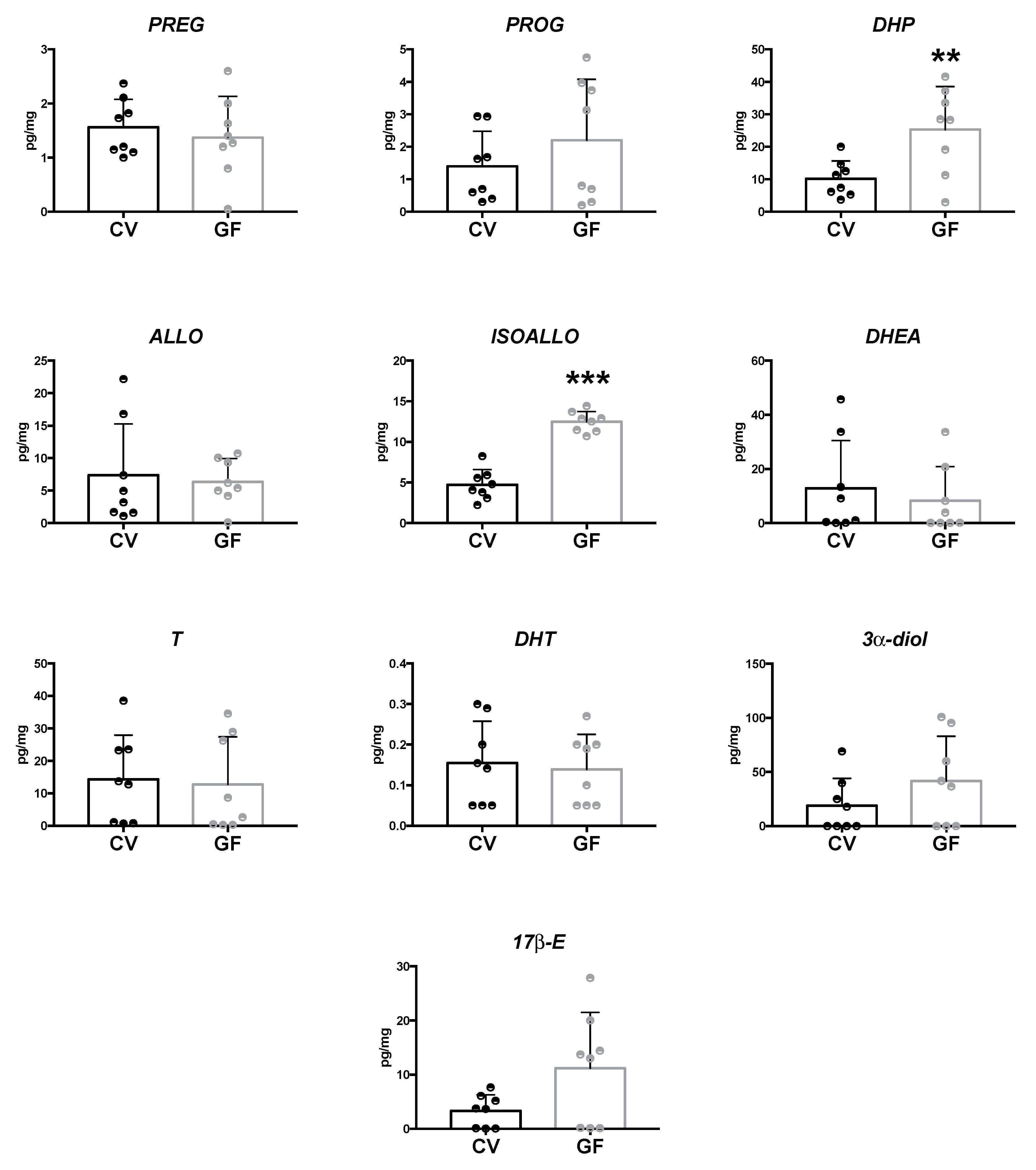
2.2. Assessment of Neuroactive Steroid Levels in Brain Areas
2.2.1. Hippocampus
2.2.2. Cerebellum and Cerebral Cortex
2.2.3. Hypothalamus
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tissue Collection
4.3. Reagents and Chemicals
4.4. Liquid Chromatography–Tandem Mass Spectrometry Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Lai, Y.; Liu, C.W.; Yang, Y.; Hsiao, Y.C.; Ru, H.; Lu, K. High-coverage metabolomics uncovers microbiota-driven biochemical landscape of interorgan transport and gut-brain communication in mice. Nat. Commun. 2021, 12, 6000. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front. Neuroendocr. 2018, 51, 80–101. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, A.; Kobiita, A.; Ye, T.; Chambon, P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 2013, 153, 812–827. [Google Scholar] [CrossRef] [Green Version]
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 2016, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jasarevic, E.; Morrison, K.E.; Bale, T.L. Sex differences in the gut microbiome-brain axis across the lifespan. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150122. [Google Scholar] [CrossRef] [Green Version]
- Fields, C.T.; Chassaing, B.; Paul, M.J.; Gewirtz, A.T.; de Vries, G.J. Vasopressin deletion is associated with sex-specific shifts in the gut microbiome. Gut Microbes 2017, 9, 13–25. [Google Scholar] [CrossRef]
- Yurkovetskiy, L.; Burrows, M.; Khan, A.A.; Graham, L.; Volchkov, P.; Becker, L.; Antonopoulos, D.; Umesaki, Y.; Chervonsky, A.V. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013, 39, 400–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Indias, I.; Sanchez-Alcoholado, L.; Sanchez-Garrido, M.A.; Martin-Nunez, G.M.; Perez-Jimenez, F.; Tena-Sempere, M.; Tinahones, F.J.; Queipo-Ortuno, M.I. Neonatal androgen exposure causes persistent gut microbiota dysbiosis related to metabolic disease in adult female rats. Endocrinology 2016, 157, en20161317. [Google Scholar] [CrossRef] [Green Version]
- Menon, R.; Watson, S.E.; Thomas, L.N.; Allred, C.D.; Dabney, A.; Azcarate-Peril, M.A.; Sturino, J.M. Diet complexity and estrogen receptor beta status affect the composition of the murine intestinal microbiota. Appl. Environ. Microbiol. 2013, 79, 5763–5773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Marcos, J.A.; Barroso, A.; Rangel-Zuniga, O.A.; Perdices-Lopez, C.; Haro, C.; Sanchez-Garrido, M.A.; Molina-Abril, H.; Ohlsson, C.; Perez-Martinez, P.; Poutanen, M.; et al. Interplay between gonadal hormones and postnatal overfeeding in defining sex-dependent differences in gut microbiota architecture. Aging 2020, 12, 19979–20000. [Google Scholar] [CrossRef]
- Tramullas, M.; Collins, J.M.; Fitzgerald, P.; Dinan, T.G.; SM, O.M.; Cryan, J.F. Estrous cycle and ovariectomy-induced changes in visceral pain are microbiota-dependent. iScience 2021, 24, 102850. [Google Scholar] [CrossRef]
- Mueller, S.; Saunier, K.; Hanisch, C.; Norin, E.; Alm, L.; Midtvedt, T.; Cresci, A.; Silvi, S.; Orpianesi, C.; Verdenelli, M.C.; et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: A cross-sectional study. Appl. Environ. Microbiol. 2006, 72, 1027–1033. [Google Scholar] [CrossRef] [Green Version]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haro, C.; Rangel-Zuniga, O.A.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Perez-Martinez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortes, J.A.; Tena-Sempere, M.; et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, 11, e0154090. [Google Scholar] [CrossRef] [Green Version]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef] [Green Version]
- Fuhrman, B.J.; Feigelson, H.S.; Flores, R.; Gail, M.H.; Xu, X.; Ravel, J.; Goedert, J.J. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J. Clin. Endocrinol. Metab. 2014, 99, 4632–4640. [Google Scholar] [CrossRef]
- Goedert, J.J.; Jones, G.; Hua, X.; Xu, X.; Yu, G.; Flores, R.; Falk, R.T.; Gail, M.H.; Shi, J.; Ravel, J.; et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: A population-based case-control pilot study. J. Natl. Cancer Inst. 2015, 107, djv147. [Google Scholar] [CrossRef] [PubMed]
- Markle, J.G.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, D.; Pesaresi, M.; Abbiati, F.; Calabrese, D.; Giatti, S.; Garcia-Segura, L.M.; Melcangi, R.C. Comparison of plasma and cerebrospinal fluid levels of neuroactive steroids with their brain, spinal cord and peripheral nerve levels in male and female rats. Psychoneuroendocrinology 2013, 38, 2278–2290. [Google Scholar] [CrossRef] [PubMed]
- Giatti, S.; Diviccaro, S.; Serafini, M.M.; Caruso, D.; Garcia-Segura, L.M.; Viviani, B.; Melcangi, R.C. Sex differences in steroid levels and steroidogenesis in the nervous system: Physiopathological role. Front. Neuroendocrinol. 2019, 56, 100804. [Google Scholar] [CrossRef] [PubMed]
- Melcangi, R.C.; Garcia-Segura, L.M.; Mensah-Nyagan, A.G. Neuroactive steroids: State of the art and new perspectives. Cell Mol. Life Sci. 2008, 65, 777–797. [Google Scholar] [CrossRef] [Green Version]
- Giatti, S.; Diviccaro, S.; Falvo, E.; Garcia-Segura, L.M.; Melcangi, R.C. Physiopathological role of the enzymatic complex 5alpha-reductase and 3alpha/beta-hydroxysteroid oxidoreductase in the generation of progesterone and testosterone neuroactive metabolites. Front. Neuroendocrinol. 2020, 57, 100836. [Google Scholar] [CrossRef]
- Balleza, D.; Sacchi, M.; Vena, G.; Galloni, D.; Puia, G.; Facci, P.; Alessandrini, A. Effects of neurosteroids on a model membrane including cholesterol: A micropipette aspiration study. Biochim. Biophys. Acta 2015, 1848, 1268–1276. [Google Scholar] [CrossRef] [Green Version]
- Sacchi, M.; Balleza, D.; Vena, G.; Puia, G.; Facci, P.; Alessandrini, A. Effect of neurosteroids on a model lipid bilayer including cholesterol: An Atomic Force Microscopy study. Biochim. Biophys. Acta 2015, 1848, 1258–1267. [Google Scholar] [CrossRef] [Green Version]
- Brandt, N.; Vierk, R.; Fester, L.; Anstotz, M.; Zhou, L.; Heilmann, L.F.; Kind, S.; Steffen, P.; Rune, G.M. Sex-specific Difference of Hippocampal Synaptic Plasticity in Response to Sex Neurosteroids. Cereb. Cortex 2020, 30, 2627–2641. [Google Scholar] [CrossRef]
- Islam, M.N.; Sakimoto, Y.; Jahan, M.R.; Ishida, M.; Tarif, A.M.M.; Nozaki, K.; Masumoto, K.H.; Yanai, A.; Mitsushima, D.; Shinoda, K. Androgen Affects the Dynamics of Intrinsic Plasticity of Pyramidal Neurons in the CA1 Hippocampal Subfield in Adolescent Male Rats. Neuroscience 2020, 440, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Frye, C.A. Some rewarding effects of androgens may be mediated by actions of its 5alpha-reduced metabolite 3alpha-androstanediol. Pharm. Biochem. Behav. 2007, 86, 354–367. [Google Scholar] [CrossRef] [Green Version]
- Rosellini, R.A.; Svare, B.B.; Rhodes, M.E.; Frye, C.A. The testosterone metabolite and neurosteroid 3alpha-androstanediol may mediate the effects of testosterone on conditioned place preference. Brain Res. Brain Res. Rev. 2001, 37, 162–171. [Google Scholar] [CrossRef]
- Compagnone, N.A.; Mellon, S.H. Dehydroepiandrosterone: A potential signalling molecule for neocortical organization during development. Proc. Natl. Acad. Sci. USA 1998, 95, 4678–4683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karishma, K.K.; Herbert, J. Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression. Eur. J. Neurosci. 2002, 16, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, B.; Ma, W.; Barker, J.L.; Chang, Y.H.; Zhao, W.; Rubinow, D.R. Dehydroepiandrosterone (DHEA) and its sulfated derivative (DHEAS) regulate apoptosis during neurogenesis by triggering the Akt signaling pathway in opposing ways. Brain Res. Mol. Brain Res. 2002, 98, 58–66. [Google Scholar] [CrossRef]
- Perez-Neri, I.; Montes, S.; Ojeda-Lopez, C.; Ramirez-Bermudez, J.; Rios, C. Modulation of neurotransmitter systems by dehydroepiandrosterone and dehydroepiandrosterone sulfate: Mechanism of action and relevance to psychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1118–1130. [Google Scholar] [CrossRef]
- Chu, L.; Huang, Y.; Xu, Y.; Wang, L.K.; Lu, Q. An LC-APCI(+)-MS/MS-based method for determining the concentration of neurosteroids in the brain of male mice with different gut microbiota. J. Neurosci. Methods 2021, 360, 109268. [Google Scholar] [CrossRef]
- Scott, G.A.; Terstege, D.J.; Vu, A.P.; Law, S.; Evans, A.; Epp, J.R. Disrupted Neurogenesis in Germ-Free Mice: Effects of Age and Sex. Front. Cell Dev. Biol. 2020, 8, 407. [Google Scholar] [CrossRef]
- Hoban, A.E.; Stilling, R.M.G.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Microbial regulation of microRNA expression in the amygdala and prefrontal cortex. Microbiome 2017, 5, 102. [Google Scholar] [CrossRef] [Green Version]
- Neufeld, K.A.; Kang, N.; Bienenstock, J.; Foster, J.A. Effects of intestinal microbiota on anxiety-like behavior. Commun. Integr. Biol. 2011, 4, 492–494. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Giatti, S.; Calabrese, D.; Pesaresi, M.; Cermenati, G.; Mitro, N.; Viviani, B.; Garcia-Segura, L.M.; Caruso, D. Levels and actions of progesterone and its metabolites in the nervous system during physiological and pathological conditions. Prog. Neurobiol. 2014, 113, 56–69. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Giatti, S.; Garcia-Segura, L.M. Levels and actions of neuroactive steroids in the nervous system under physiological and pathological conditions: Sex-specific features. Neurosci. Biobehav. Rev. 2016, 67, 25–40. [Google Scholar] [CrossRef]
- Lambert, J.J.; Belelli, D.; Peden, D.R.; Vardy, A.W.; Peters, J.A. Neurosteroid modulation of GABAA receptors. Prog. Neurobiol. 2003, 71, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Belelli, D.; Lambert, J.J. Neurosteroids: Endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 2005, 6, 565–575. [Google Scholar] [CrossRef]
- Bitran, D.; Hilvers, R.J.; Kellogg, C.K. Anxiolytic effects of 3 alpha-hydroxy-5 alpha[beta]-pregnan-20-one: Endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991, 561, 157–161. [Google Scholar] [CrossRef]
- Wang, M.; He, Y.; Eisenman, L.N.; Fields, C.; Zeng, C.M.; Mathews, J.; Benz, A.; Fu, T.; Zorumski, E.; Steinbach, J.H.; et al. 3beta -hydroxypregnane steroids are pregnenolone sulfate-like GABA(A) receptor antagonists. J. Neurosci. 2002, 22, 3366–3375. [Google Scholar] [CrossRef] [Green Version]
- Backstrom, T.; Wahlstrom, G.; Wahlstrom, K.; Zhu, D.; Wang, M.D. Isoallopregnanolone; an antagonist to the anaesthetic effect of allopregnanolone in male rats. Eur. J. Pharm. 2005, 512, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Monnet, F.P.; Mahe, V.; Robel, P.; Baulieu, E.E. Neurosteroids, via sigma receptors, modulate the [3H] norepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc. Natl. Acad. Sci. USA 1995, 92, 3774–3778. [Google Scholar] [CrossRef] [Green Version]
- Demirgoren, S.; Majewska, M.D.; Spivak, C.E.; London, E.D. Receptor binding and electrophysiological effects of dehydroepiandrosterone sulfate, an antagonist of the GABAA receptor. Neuroscience 1991, 45, 127–135. [Google Scholar] [CrossRef]
- Bergeron, R.; de Montigny, C.; Debonnel, G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: Effects mediated via sigma receptors. J. Neurosci. 1996, 16, 1193–1202. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.K.; Ticku, M.K. An update on GABAA receptors. Brain Res. Brain Res. Rev. 1999, 29, 196–217. [Google Scholar] [CrossRef]
- Mo, Q.; Lu, S.; Garippa, C.; Brownstein, M.J.; Simon, N.G. Genome-wide analysis of DHEA- and DHT-induced gene expression in mouse hypothalamus and hippocampus. J. Steroid. Biochem. Mol. Biol. 2009, 114, 135–143. [Google Scholar] [CrossRef]
- Mo, Q.; Lu, S.F.; Simon, N.G. Dehydroepiandrosterone and its metabolites: Differential effects on androgen receptor trafficking and transcriptional activity. J. Steroid Biochem. Mol. Biol. 2006, 99, 50–58. [Google Scholar] [CrossRef]
- Mo, Q.; Lu, S.F.; Hu, S.; Simon, N.G. DHEA and DHEA sulfate differentially regulate neural androgen receptor and its transcriptional activity. Brain Res. Mol. Brain Res. 2004, 126, 165–172. [Google Scholar] [CrossRef]
- Lu, S.F.; Mo, Q.; Hu, S.; Garippa, C.; Simon, N.G. Dehydroepiandrosterone upregulates neural androgen receptor level and transcriptional activity. J. Neurobiol. 2003, 57, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhou, H.; Zhang, S.; Yuan, J.; Wu, H. Effects of gut microbiota disturbance induced in early life on the expression of extrasynaptic GABA-A receptor alpha5 and delta subunits in the hippocampus of adult rats. Brain Res. Bull. 2017, 135, 113–119. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maqsood, R.; Stone, T.W. The Gut-Brain Axis, BDNF, NMDA and CNS Disorders. Neurochem. Res. 2016, 41, 2819–2835. [Google Scholar] [CrossRef]
- Diviccaro, S.; Giatti, S.; Borgo, F.; Barcella, M.; Borghi, E.; Trejo, J.L.; Garcia-Segura, L.M.; Melcangi, R.C. Treatment of male rats with finasteride, an inhibitor of 5alpha-reductase enzyme, induces long-lasting effects on depressive-like behavior, hippocampal neurogenesis, neuroinflammation and gut microbiota composition. Psychoneuroendocrinology 2019, 99, 206–215. [Google Scholar] [CrossRef]
- Borgo, F.; Macandog, A.D.; Diviccaro, S.; Falvo, E.; Giatti, S.; Cavaletti, G.; Melcangi, R.C. Alterations of gut microbiota composition in post-finasteride patients: A pilot study. J. Endocrinol. Investig. 2020, 44, 1263–1273. [Google Scholar] [CrossRef]
- Giatti, S.; Foglio, B.; Romano, S.; Pesaresi, M.; Panzica, G.; Garcia-Segura, L.M.; Caruso, D.; Melcangi, R.C. Effects of Subchronic Finasteride Treatment and Withdrawal on Neuroactive Steroid Levels and their Receptors in the Male Rat Brain. Neuroendocrinology 2016, 103, 746–757. [Google Scholar] [CrossRef]
- Pesaresi, M.; Maschi, O.; Giatti, S.; Garcia-Segura, L.M.; Caruso, D.; Melcangi, R.C. Sex differences in neuroactive steroid levels in the nervous system of diabetic and non-diabetic rats. Horm. Behav. 2010, 57, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Scurati, S.; Maschi, O.; De Angelis, L.; Roglio, I.; Giatti, S.; Garcia-Segura, L.M.; Melcangi, R.C. Evaluation of neuroactive steroid levels by liquid chromatography-tandem mass spectrometry in central and peripheral nervous system: Effect of diabetes. Neurochem. Int. 2008, 52, 560–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, D.; Pesaresi, M.; Maschi, O.; Giatti, S.; Garcia-Segura, L.M.; Melcangi, R.C. Effect of short-and long-term gonadectomy on neuroactive steroid levels in the central and peripheral nervous system of male and female rats. J. Neuroendocrinol. 2010, 22, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
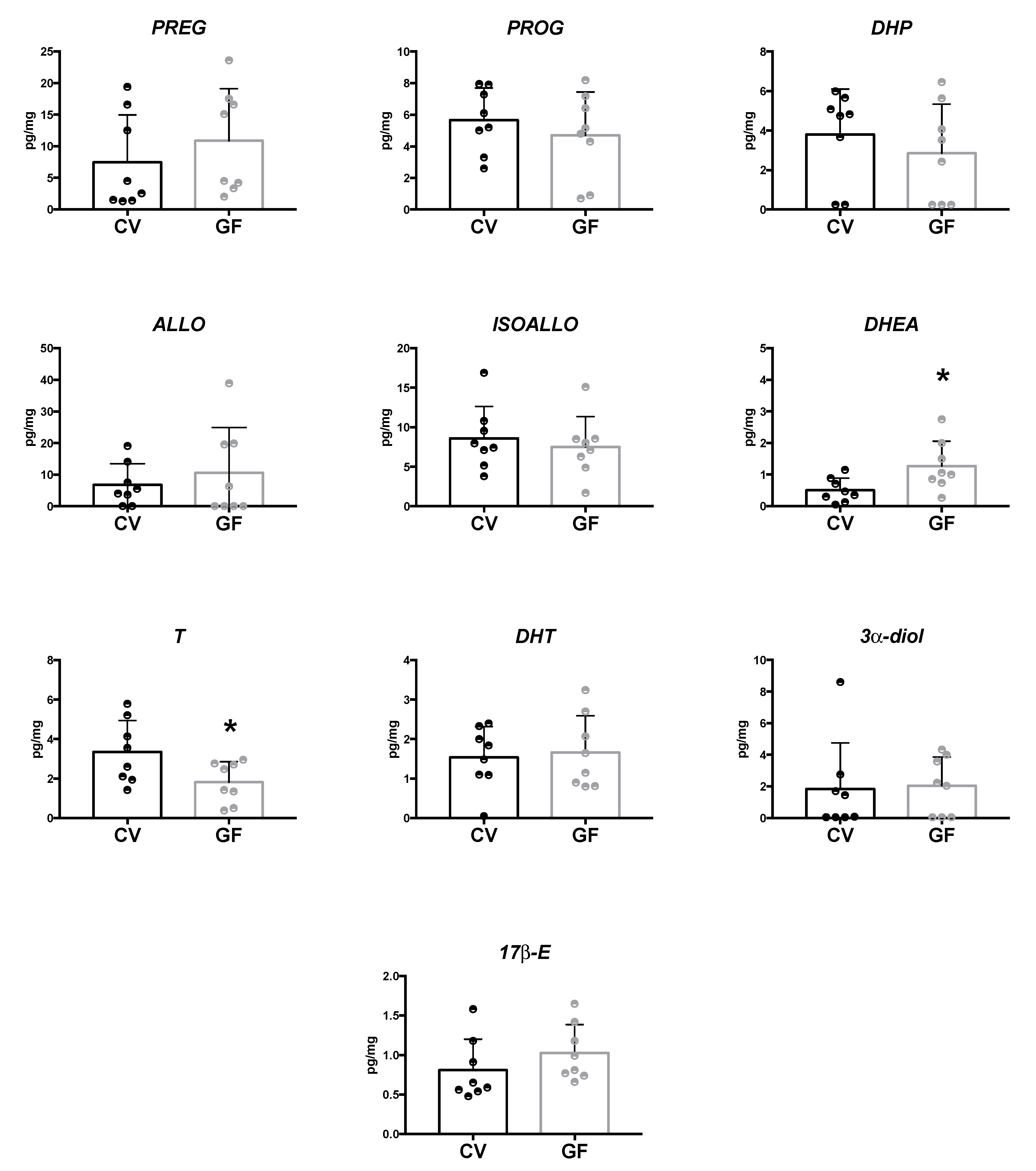
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diviccaro, S.; Caputi, V.; Cioffi, L.; Giatti, S.; Lyte, J.M.; Caruso, D.; O’Mahony, S.M.; Melcangi, R.C. Exploring the Impact of the Microbiome on Neuroactive Steroid Levels in Germ-Free Animals. Int. J. Mol. Sci. 2021, 22, 12551. https://doi.org/10.3390/ijms222212551
Diviccaro S, Caputi V, Cioffi L, Giatti S, Lyte JM, Caruso D, O’Mahony SM, Melcangi RC. Exploring the Impact of the Microbiome on Neuroactive Steroid Levels in Germ-Free Animals. International Journal of Molecular Sciences. 2021; 22(22):12551. https://doi.org/10.3390/ijms222212551
Chicago/Turabian StyleDiviccaro, Silvia, Valentina Caputi, Lucia Cioffi, Silvia Giatti, Joshua M. Lyte, Donatella Caruso, Siobhain M. O’Mahony, and Roberto Cosimo Melcangi. 2021. "Exploring the Impact of the Microbiome on Neuroactive Steroid Levels in Germ-Free Animals" International Journal of Molecular Sciences 22, no. 22: 12551. https://doi.org/10.3390/ijms222212551





