Detection of Mycoplasma Contamination in Transplanted Retinal Cells by Rapid and Sensitive Polymerase Chain Reaction Test
Abstract
:1. Introduction
2. Results
2.1. PCR Sensitivity in the Mycoplasma PCR Test
2.2. Specificity of the Mycoplasma PCR Test
2.3. Representative Data of Mycoplasma-Positive Cells in Laboratory Cultures
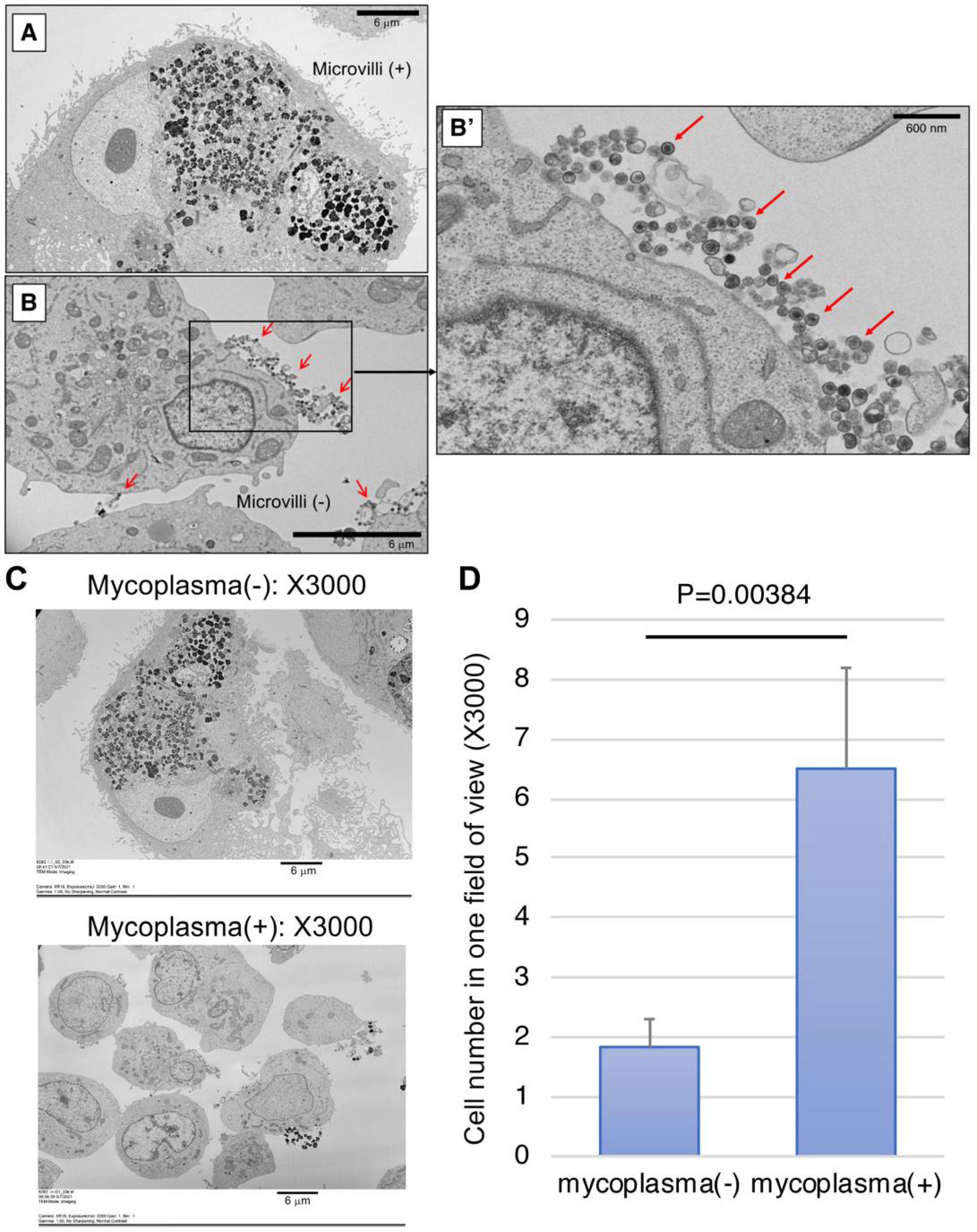
2.4. Detection of Mycoplasma by SEM
3. Discussion
4. Materials and Methods
4.1. Cells and DNA Extraction
4.2. Mycoplasma Strains
4.3. CFU and GC Measurements
4.4. PCR
4.5. Scanning Electron Microscope
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | colony-forming unit |
| Cp | crossing point |
| GC | genomic copy |
| iPSC | induced pluripotent stem cells |
| PCR | polymerase chain reaction |
| qPCR | quantitative real-time PCR |
| RPE | retinal pigment epithelium |
| rDNA | ribosomal DNA |
| SEM | scanning electron microscope |
References
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Sugita, S.; Mandai, M.; Hirami, Y.; Takagi, S.; Maeda, T.; Fujihara, M.; Matsuzaki, M.; Yamamoto, M.; Iseki, K.; Hayashi, N.; et al. HLA-Matched Allogeneic iPS Cells-Derived RPE Transplantation for Macular Degeneration. J. Clin. Med. 2020, 9, 2217. [Google Scholar] [CrossRef] [PubMed]
- Teyssou, R.; Poutiers, F.; Saillard, C.; Grau, O.; Laigret, F.; Bové, J.M.; Bébéar, C. Detection of mollicute contamination in cell cultures by 16S rDNA amplification. Mol. Cell Probes 1993, 7, 209–216. [Google Scholar] [CrossRef]
- Uphoff, C.C.; Drexler, H.G. Comparative PCR analysis for detection of mycoplasma infections in continuous cell lines. In Vitro Cell Dev. Biol. Anim. 2002, 38, 79–85. [Google Scholar] [CrossRef]
- Drexler, H.G.; Uphoff, C.C. Mycoplasma contamination of cell cultures: Incidence, sources, effects, detection, elimination, prevention. Cytotechnology 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Kong, F.; James, G.; Gordon, S.; Zelynski, A.; Gilbert, G.L. Species-specific PCR for identification of common contaminant mollicutes in cell culture. Appl. Environ. Microbiol. 2001, 67, 3195–3200. [Google Scholar] [CrossRef] [Green Version]
- Van Kuppeveld, F.J.; Johansson, K.E.; Galama, J.M.; Kissing, J.; Bölske, G.; van der Logt, J.T.; Melchers, W.J. Detection of mycoplasma contamination in cell cultures by a mycoplasma group-specific PCR. Appl. Environ. Microbiol. 1994, 60, 149–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uphoff, C.C.; Drexler, H.G. Detection of mycoplasma contaminations in cell cultures by PCR analysis. Hum. Cell 1999, 12, 229–236. [Google Scholar]
- Uphoff, C.C.; Drexler, H.G. Detection of Mycoplasma contamination in cell cultures. Curr. Protoc. Mol. Biol. 2014, 106, 28.4.1–28.4.14. [Google Scholar] [CrossRef] [PubMed]
- Young, L.; Sung, J.; Stacey, G.; Masters, J.R. Detection of Mycoplasma in cell cultures. Nat. Protoc. 2010, 5, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Salling, H.K.; Bang-Christensen, S.R. Multi-primer qPCR assay capable of highly efficient and specific detection of the vast majority of all known Mycoplasma. Biologicals 2016, 44, 129–138. [Google Scholar] [CrossRef]
- Shahhosseiny, M.H.; Hosseiny, Z.; Khoramkhorshid, H.R.; Azari, S.; Shokrgozar, M.A. Rapid and sensitive detection of Mollicutes in cell culture by polymerase chain reaction. J. Basic Microbiol. 2010, 50, 171–178. [Google Scholar] [CrossRef]
- Jean, A.; Tardy, F.; Allatif, O.; Grosjean, I.; Blanquier, B.; Gerlier, D. Assessing mycoplasma contamination of cell cultures by qPCR using a set of universal primer pairs targeting a 1.5 kb fragment of 16S rRNA genes. PLoS ONE 2017, 12, e0172358. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, M.; Sugita, S.; Shimizu, N.; Watanabe, K.; Nakagawa, I.; Mochizuki, M. Broad-range real-time PCR assay for detection of bacterial DNA in ocular samples from infectious endophthalmitis. Jpn J. Ophthalmol. 2012, 56, 529–535. [Google Scholar] [CrossRef]
- Makabe, K.; Sugita, S.; Hono, A.; Kamao, H.; Takahashi, M. Mycoplasma Ocular Infection in Subretinal Graft Transplantation of iPS Cells-Derived Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1298–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavan-Langston, D. Mycoplasmal anterior and posterior uveitis. I. Clinical manifestations of the experimental disease. Arch. Ophthalmol. 1969, 82, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Pavan-Langston, D., II. Histopathologic manifestations of mycoplasmal uveitis. Arch. Ophthalmol. 1969, 82, 253–258. [Google Scholar] [PubMed]
- Liu, E.M.; Janigian, R.H. Mycoplasma pneumoniae: The other masquerader. JAMA Ophthalmol. 2013, 131, 251–253. [Google Scholar] [CrossRef] [Green Version]
- Matsou, A.; Riga, P.; Samouilidou, M.; Dimitrakos, S.; Anastasopoulos, E. Bilateral intermediate uveitis with appearance of frosted branch angiitis and association with Mycoplasma pneumoniae infection: Case report and review of the literature. J. AAPOS 2016, 20, 358–361. [Google Scholar] [CrossRef]
- Yashar, S.S.; Yashar, B.; Epstein, E.; Viani, R.M. Uveitis associated with Mycoplasma pneumoniae meningitis. Acta Ophthalmol. Scand. 2001, 79, 100–101. [Google Scholar] [CrossRef]
- Sugita, S.; Iwasaki, Y.; Makabe, K.; Kamao, H.; Mandai, M.; Shiina, T.; Ogasawara, K.; Hirami, Y.; Kurimoto, Y.; Takahashi, M. Successful Transplantation of Retinal Pigment Epithelial Cells from MHC Homozygote iPSCs in MHC-Matched Models. Stem Cell Rep. 2016, 7, 635–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugita, S.; Makabe, K.; Fujii, S.; Iwasaki, Y.; Kamao, H.; Shiina, T.; Ogasawara, K.; Takahashi, M. Detection of Retinal Pigment Epithelium-Specific Antibody in iPSC-Derived Retinal Pigment Epithelium Transplantation Models. Stem Cell Rep. 2017, 9, 1501–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, S.; Sugita, S.; Futatsugi, Y.; Ishida, M.; Edo, A.; Makabe, K.; Kamao, H.; Iwasaki, Y.; Sakaguchi, H.; Hirami, Y.; et al. A Strategy for Personalized Treatment of iPS-Retinal Immune Rejections Assessed in Cynomolgus Monkey Models. Int. J. Mol. Sci. 2020, 21, 3077. [Google Scholar] [CrossRef]
- Sugita, S.; Futatsugi, Y.; Ishida, M.; Edo, A.; Takahashi, M. Retinal Pigment Epithelial Cells Derived from Induced Pluripotent Stem (iPS) Cells Suppress or Activate T Cells via Costimulatory Signals. Int. J. Mol. Sci. 2020, 21, 6507. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Mandai, M.; Kamao, H.; Takahashi, M. Immunological aspects of RPE cell transplantation. Prog. Retin. Eye Res. 2021, 84, 100950. [Google Scholar] [CrossRef]
- Sugita, S.; Kamao, H.; Iwasaki, Y.; Okamoto, S.; Hashiguchi, T.; Iseki, K.; Hayashi, N.; Mandai, M.; Takahashi, M. Inhibition of T-cell activation by retinal pigment epithelial cells derived from induced pluripotent stem cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Iwasaki, Y.; Makabe, K.; Kimura, T.; Futagami, T.; Suegami, S.; Takahashi, M. Lack of T Cell Response to iPSC-Derived Retinal Pigment Epithelial Cells from HLA Homozygous Donors. Stem Cell Rep. 2016, 7, 619–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolezel, J.; Bartos, J.; Voglmayr, H.; Greilhuber, J. Nuclear DNA content and genome size of trout and human. Cytom. A 2003, 51, 127–128. [Google Scholar]




| Mollicutes Species | Strains | CFU/mL | GC/mL * | GC/CFU Ratios |
|---|---|---|---|---|
| Mycoplasma arginini | ATCC 23838 | 5 × 108 | 1.36 × 109 | 2.72 |
| Mycoplasma hyorhinis | NBRC 14858 | 8.83 × 108 | 1.9 × 109 | 2.15 |
| Mycoplasma pneumoniae | NBRC 14401 | 5.1 × 107 | 1.56 × 109 | 30.59 |
| Acholeplasma laidlawii | NBRC 14400 | 1.07 × 108 | 1.06 × 109 | 9.91 |
| Mycoplasma fermentans | NBRC 14854 | 1.04 × 109 | 3.76 × 109 | 3.62 |
| Mycoplasma salivarium | NBRC 14478 | 1.41 × 109 | 2.1 × 109 | 1.49 |
| Mycoplasma orale | NBRC 14477 | 6.37 × 108 | 2.32 × 109 | 3.64 |
| Mycoplasma hominis | NBRC 14850 | 4 × 107 | 1.76 × 109 | 4.55 |
| Ureaplasma urealyticum | ATCC 27618 | 2.03 × 108 | 7.2 × 108 | 3.54 |
| A. laidlawii | ||
| Spike (CFU/mL) | Run | Positive rate (%) |
| 100 | 4/4 * | 100 |
| 10 | 4/4 | 100 |
| 1 | 1/4 | 25 |
| M. arginini | ||
| Spike (CFU/mL) | Run | Positive rate (%) |
| 100 | 4/4 | 100 |
| 10 | 4/4 | 100 |
| 1 | 2/4 | 50 |
| M. hyorhinis | ||
| Spike (CFU/mL) | Run | Positive rate (%) |
| 100 | 4/4 | 100 |
| 10 | 4/4 | 100 |
| 1 | 0/4 | 0 |
| M. pneumoniae | ||
| Spike (CFU/mL) | Run | Positive rate (%) |
| 100 | 4/4 | 100 |
| 10 | 4/4 | 100 |
| 1 | 3/4 | 75 |
| M. orale | ||
| Spike (CFU/mL) | Run | Positive rate (%) |
| 100 | 4/4 | 100 |
| 10 | 4/4 | 100 |
| 1 | 3/4 | 75 |
| M. fermentans | ||
| Spike (CFU/mL) | Run | Positive rate (%) |
| 100 | 4/4 | 100 |
| 10 | 4/4 | 100 |
| 1 | 3/4 | 75 |
| M. salivarium | ||
| Spike (CFU/mL) | Run | Positive rate (%) |
| 100 | 4/4 | 100 |
| 10 | 4/4 | 100 |
| 1 | 1/4 | 25 |
| M. hominis | ||
| Spike (CFU/mL) | Run | Positive rate (%) |
| 100 | 4/4 | 100 |
| 10 | 4/4 | 100 |
| 1 | 4/4 | 100 |
| U. urealyticum | ||
| Spike (CFU/mL) | Run | Positive rate (%) |
| 100 | 4/4 | 100 |
| 10 | 1/4 | 25 |
| 1 | 0/4 | 0 |
| Strains | Clone No. | Number of Copies (Copies/mL) | PCR Results |
|---|---|---|---|
| Gram-positive strains | |||
| Staphylococcus aureus | NBRC12732 | 1.0 × 104 | negative |
| MRSA | JCM8702 | 1.0 × 104 | negative |
| Staphylococcus epidermidis | JCM2414 | 1.0 × 104 | negative |
| Streptococcus pyogenes | RIMD 3123004 | 1.0 × 104 | negative |
| Streptococcus sanguinis | JCM5708 | 1.0 × 104 | negative |
| Streptococcus pneumoniae | NBRC102642 | 1.0 × 104 | negative |
| Enterococcus faecalis | JCM20313 | 1.0 × 104 | negative |
| Corynebacterium diphtheriae | JCM1310 | 1.0 × 104 | negative |
| Bacillus cereus | JCM20266 | 1.0 × 104 | negative |
| Clostridium perfringens | JCM1290 | 1.0 × 104 | negative |
| Propionibacterium acnes | JCM6425 | 1.0 × 104 | negative |
| Nocardia asteroides | NBRC14403 | 1.0 × 104 | negative |
| Gram-negative strains | |||
| Escherichia coli | JCM20135 | 1.0 × 104 | negative |
| Klebsiella pneumoniae | JCM1662 | 1.0 × 104 | negative |
| Pseudomonas aeruginosa | JCM6425 | 1.0 × 104 | negative |
| Moraxella lacunata | JCM20914 | 1.0 × 104 | negative |
| Myco Finder PCR | DNA Staining | Nested PCR | Real-Time PCR ** | |
|---|---|---|---|---|
| Method | Semi-quantitative PCR | Staining | Qualitative PCR | Quantitative PCR |
| Testing | Simplified | Experienced | Experienced | Experienced |
| Specimens | Cells (≤1 × 106) | Culture supernatant | Culture supernatant | Cells (≤5 × 106) |
| Sensitivity | 10 CFU/mL | 100 CFU/mL | 100 CFU/mL | 10 CFU/mL |
| Testing time * | 140 min | 6 days | 6 h | 4~5 h |
| JapanesePharmacopoeia | 17th revision | 16th revision | 16th revision | 17th revision |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugita, S.; Hono, A.; Fujino, S.; Futatsugi, Y.; Yunomae, Y.; Shimizu, N.; Takahashi, M. Detection of Mycoplasma Contamination in Transplanted Retinal Cells by Rapid and Sensitive Polymerase Chain Reaction Test. Int. J. Mol. Sci. 2021, 22, 12555. https://doi.org/10.3390/ijms222212555
Sugita S, Hono A, Fujino S, Futatsugi Y, Yunomae Y, Shimizu N, Takahashi M. Detection of Mycoplasma Contamination in Transplanted Retinal Cells by Rapid and Sensitive Polymerase Chain Reaction Test. International Journal of Molecular Sciences. 2021; 22(22):12555. https://doi.org/10.3390/ijms222212555
Chicago/Turabian StyleSugita, Sunao, Ayumi Hono, Shoko Fujino, Yoko Futatsugi, Yuta Yunomae, Norio Shimizu, and Masayo Takahashi. 2021. "Detection of Mycoplasma Contamination in Transplanted Retinal Cells by Rapid and Sensitive Polymerase Chain Reaction Test" International Journal of Molecular Sciences 22, no. 22: 12555. https://doi.org/10.3390/ijms222212555
APA StyleSugita, S., Hono, A., Fujino, S., Futatsugi, Y., Yunomae, Y., Shimizu, N., & Takahashi, M. (2021). Detection of Mycoplasma Contamination in Transplanted Retinal Cells by Rapid and Sensitive Polymerase Chain Reaction Test. International Journal of Molecular Sciences, 22(22), 12555. https://doi.org/10.3390/ijms222212555