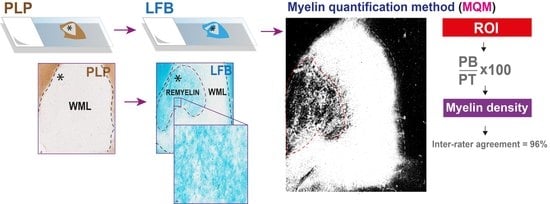
Myelin Quantification in White Matter Pathology of Progressive Multiple Sclerosis Post-Mortem Brain Samples: A New Approach for Quantifying Remyelination
Abstract
:1. Introduction
2. Results
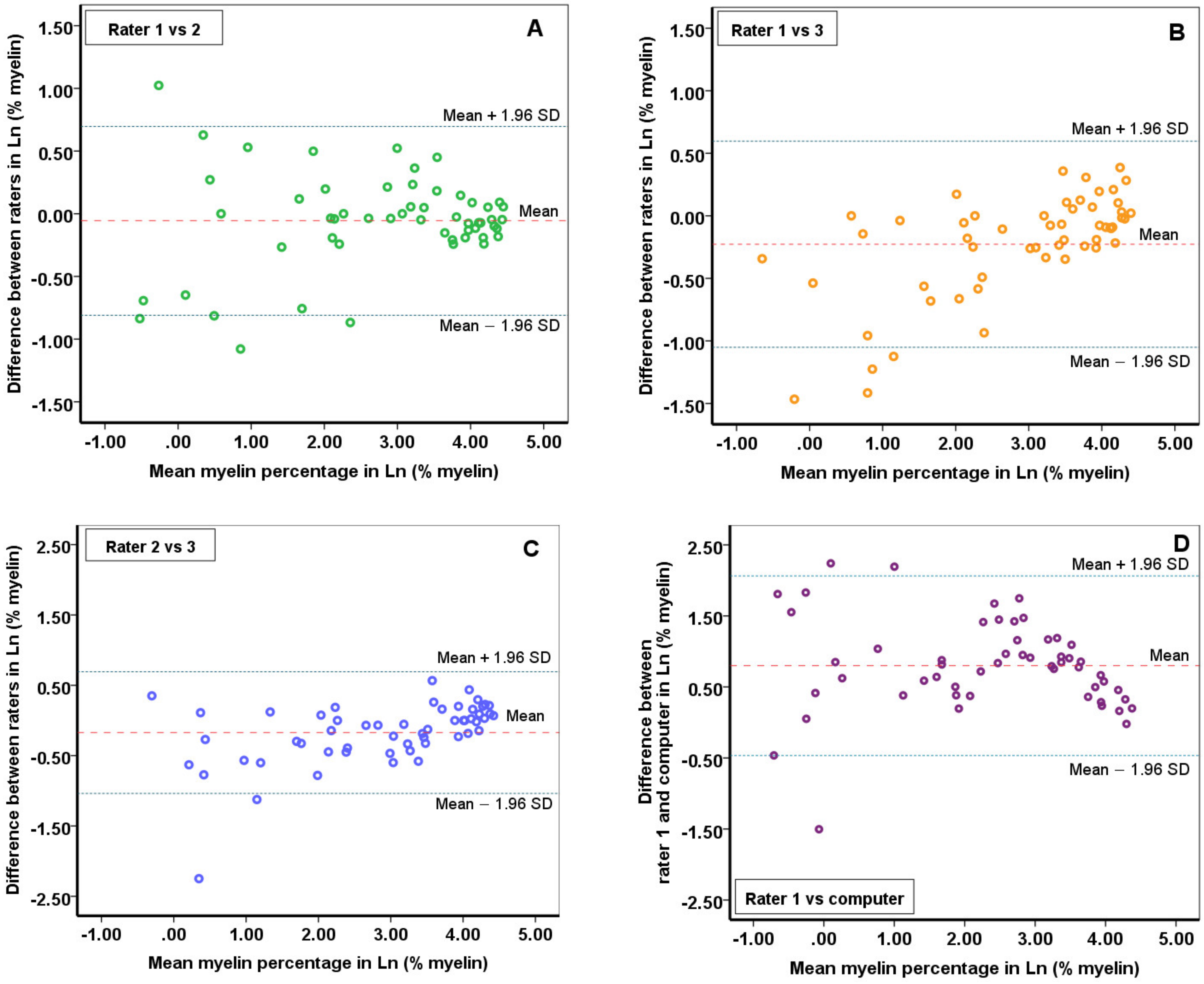
2.1. The Reliability of the Myelin Quantitative Measurements
2.2. Reliability of Myelin Quantification Using ImageJ Built-In Automatic Global Threshold Calculation
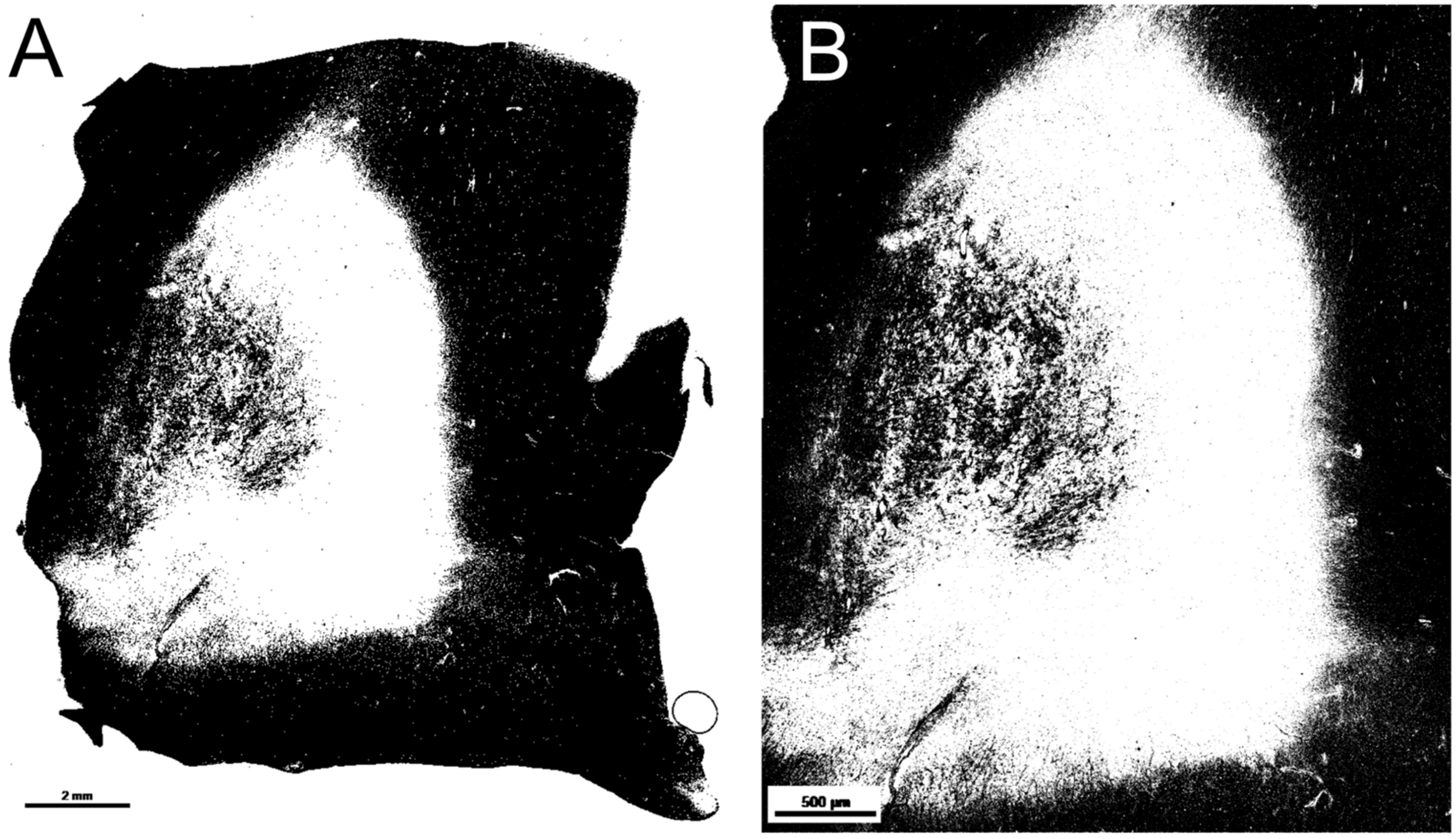
2.3. Quality Analysis—Myelin Computation with Different NAWM Magnifications
2.4. Quality Analysis—Black Pixel Ratio in Lesions with Different Image Magnifications
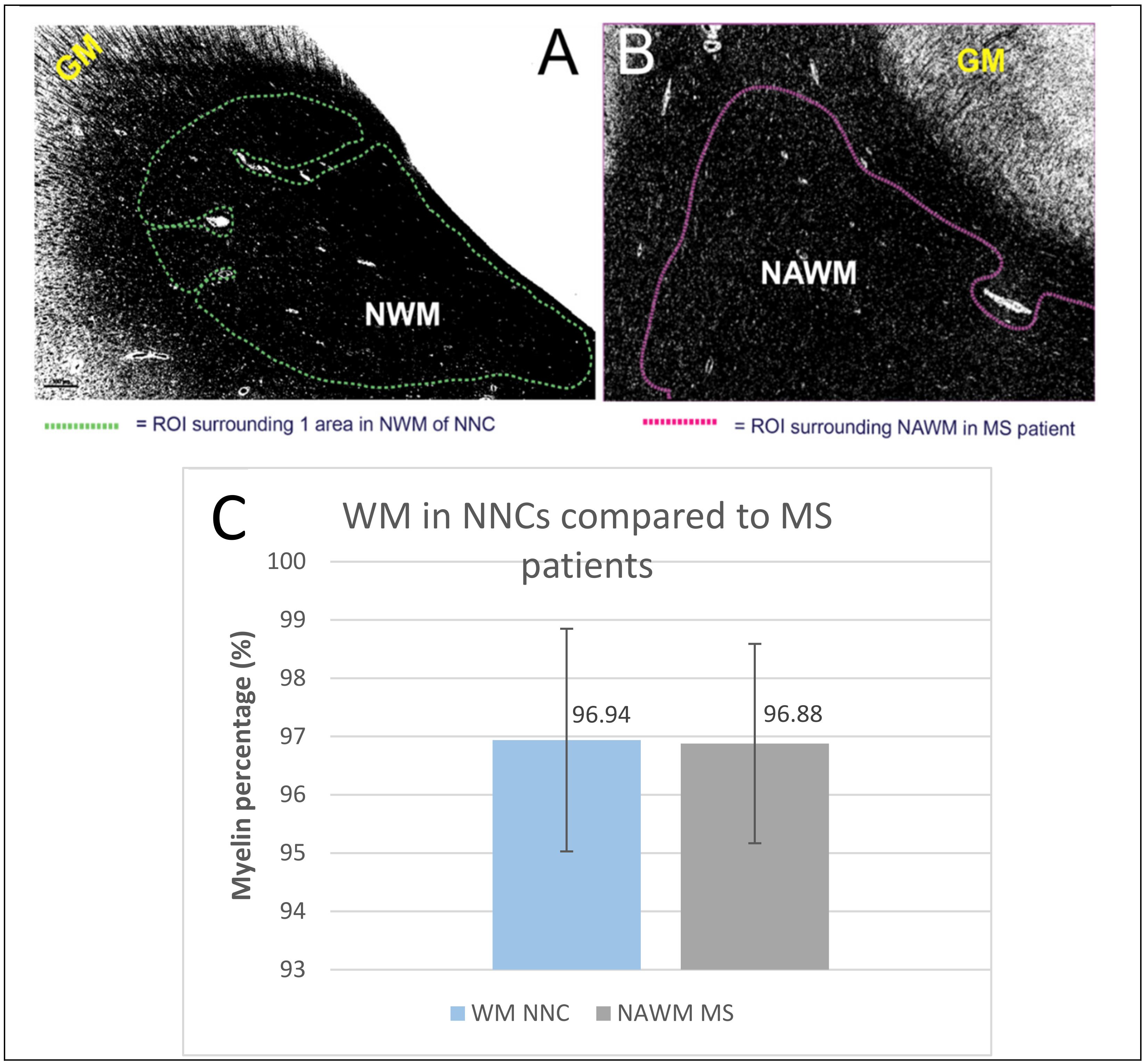
2.5. Non-Neurological Control
2.6. Sub Analyses—Myelin Swellings, DAWM and Encephalitis
3. Discussion
3.1. Non-Neurological Controls, DAWM, Encephalitis
3.2. Study Strengths and Limitations
3.3. Conclusion and Future Prospects
4. Materials and Methods
4.1. Sample Collection
4.2. Histological Processing
4.2.1. Luxol Fast Blue
4.2.2. PLP Staining
4.3. Preliminary Screening
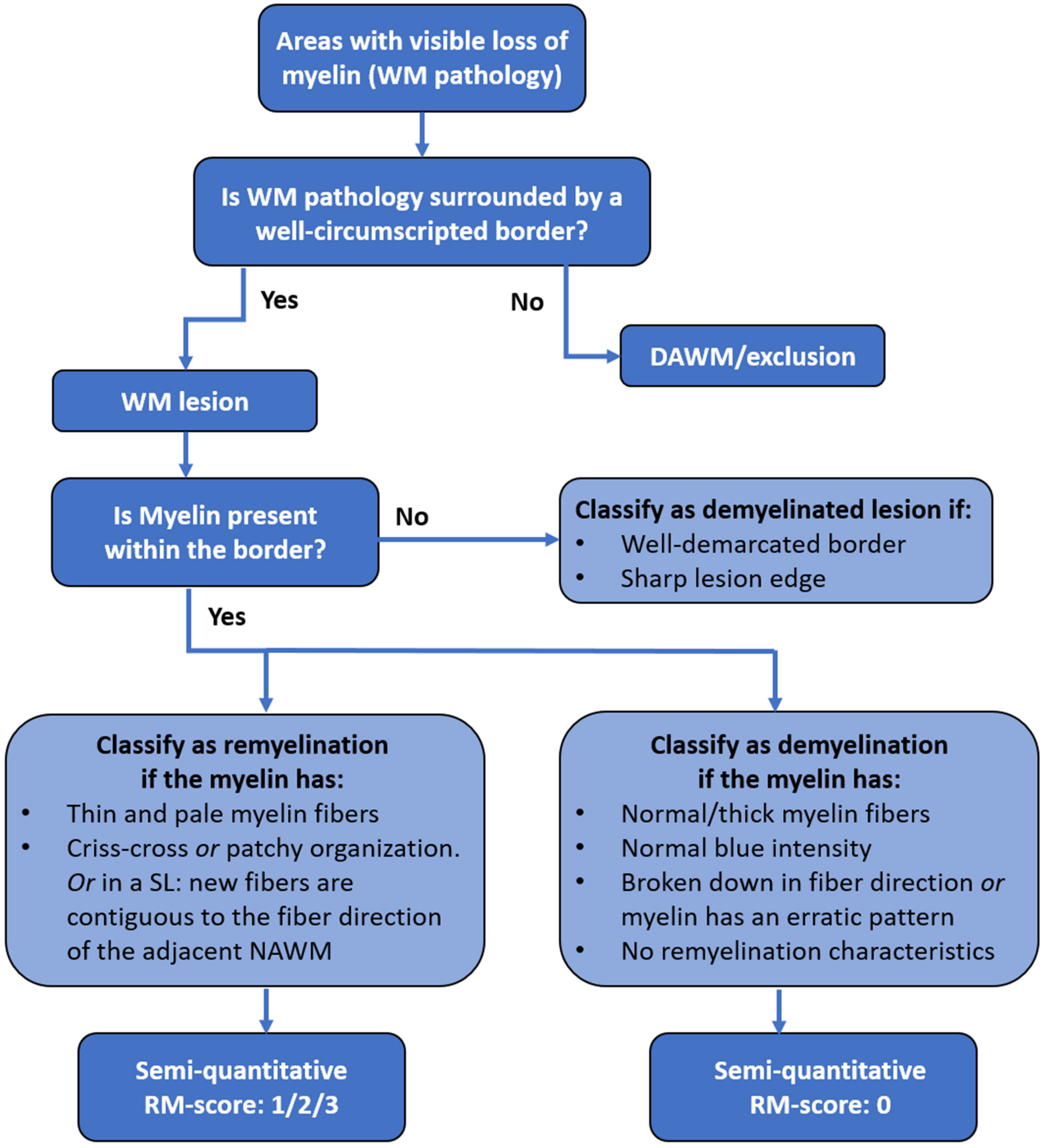
4.4. Microscopic Criteria for Demyelination, Remyelination and DAWM
4.5. Quantitative Analysis of Myelin
4.5.1. Pre-Analysis Image Alterations
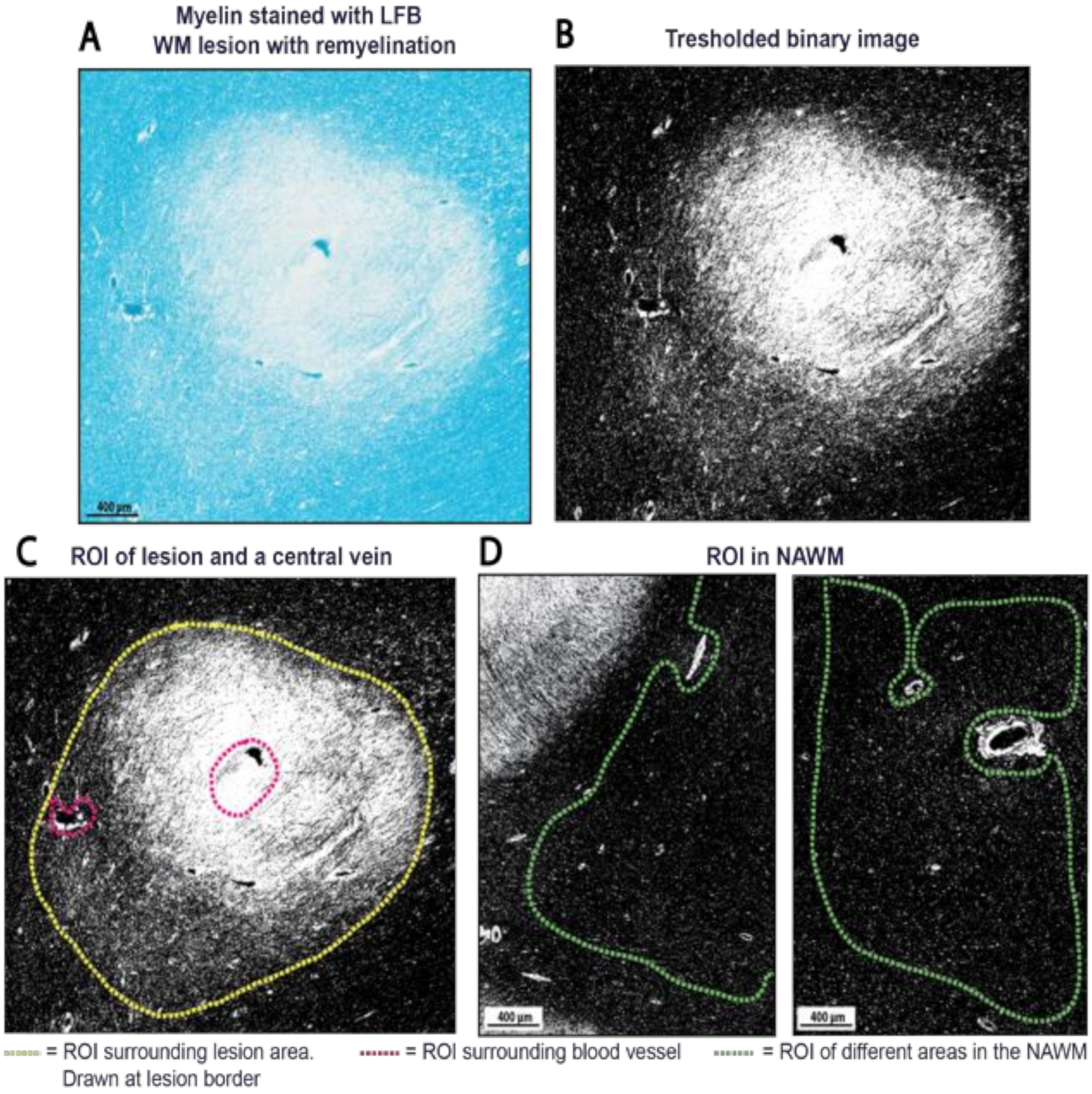
4.5.2. Selection of Regions of Interest
4.5.3. Myelin Quantity Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuhlmann, T.; Ludwin, S.; Prat, A.; Antel, J.; Brück, W.; Lassmann, H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017, 133, 13–24. [Google Scholar] [CrossRef]
- Höftberger, R.; Lassmann, H. Inflammatory Demyelinating Diseases of the Central Nervous System. Handb. Clin. Neurol. 2018, 145, 263–283. [Google Scholar] [CrossRef]
- Lassmann, H. The New Pathology of Multiple Sclerosis. Mult. Scler. 2000, 6, 409–410. [Google Scholar] [CrossRef]
- Faissner, S.; Plemel, J.R.; Gold, R.; Yong, V.W. Progressive multiple sclerosis: From pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 2019, 18, 905–922. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Querejeta, I.; Sáenz-Cuesta, M.; Muñoz-Culla, M.; Otaegui, D. Models for Studying Myelination, Demyelination and Remyelination. Neuromol. Med. 2017, 19, 181–192. [Google Scholar] [CrossRef]
- Franklin, R.J.M. Why does remyelination fail in multiple sclerosis? Nat. Rev. Neurosci. 2002, 3, 705–714. [Google Scholar] [CrossRef]
- Dulamea, A.O. The contribution of oligodendrocytes and oligodendrocyte progenitor cells to central nervous system repair in multiple sclerosis: Perspectives for remyelination therapeutic strategies. Neural Regen. Res. 2017, 12, 1939–1944. [Google Scholar] [CrossRef]
- Lubetzki, C.; Zalc, B.; Williams, A.; Stadelmann, C.; Stankoff, B. Remyelination in multiple sclerosis: From basic science to clinical translation. Lancet Neurol. 2020, 18, 678–688. [Google Scholar] [CrossRef]
- Plemel, J.R.; Liu, W.Q.; Yong, V.W. Remyelination therapies: A new direction and challenge in multiple sclerosis. Nat. Rev. Drug Discov. 2017, 16, 617–634. [Google Scholar] [CrossRef]
- Heß, K. Lesion stage-dependent causes for impaired remyelination in MS. Acta Neuropathol. 2020, 140, 359–375. [Google Scholar] [CrossRef]
- Duncan, G.J. Myelin regulatory factor drives remyelination in multiple sclerosis. Acta Neuropathol. 2017, 134, 403–422. [Google Scholar] [CrossRef] [PubMed]
- Miron, V.E. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013, 16, 1211–1218. [Google Scholar] [CrossRef] [Green Version]
- Kotter, M.R.; Li, W.W.; Zhao, C.; Franklin, R.J.M. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J. Neurosci. 2006, 26, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Kotter, M.R.; Zhao, C.; van Rooijen, N.; Franklin, R.J.M. Macrophage-depletion induced impairment of experimental CNS remyelination is associated with a reduced oligodendrocyte progenitor cell response and altered growth factor expression. Neurobiol. Dis. 2005, 18, 166–175. [Google Scholar] [CrossRef]
- Ruckh, J.M. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 2012, 10, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, A.F. Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nat. Neurosci. 2019, 22, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Mi, S. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat. Neurosci. 2005, 8, 745–751. [Google Scholar] [CrossRef]
- Starost, L. Extrinsic immune cell-derived, but not intrinsic oligodendroglial factors contribute to oligodendroglial differentiation block in multiple sclerosis. Acta Neuropathol. 2020, 140, 715–736. [Google Scholar] [CrossRef]
- Patani, R.; Balaratnam, M.; Vora, A.; Reynolds, R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol. Appl. Neurobiol. 2007, 33, 277–287. [Google Scholar] [CrossRef]
- Albert, M.; Antel, J.; Brück, W.; Stadelmann, C. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 2007, 17, 129–138. [Google Scholar] [CrossRef]
- Strijbis, E.M.M.; Kooi, E.J.; van der Valk, P.; Geurts, J.J.G. Cortical remyelination is heterogeneous in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2017, 76, 390–401. [Google Scholar] [CrossRef] [Green Version]
- Goldschmidt, T.; Antel, J.; König, F.B.; Brück, W.; Kuhlmann, T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology 2009, 72, 1914–1921. [Google Scholar] [CrossRef] [PubMed]
- Seewann, A. Diffusely abnormal white matter in chronic multiple sclerosis: Imaging and histopathologic analysis. Arch. Neurol. 2009, 66, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.R.W. Dirty-appearing white matter in multiple sclerosis: Preliminary observations of myelin phospholipid and axonal loss. J. Neurol. 2008, 255, 1802–1811. [Google Scholar] [CrossRef]
- Luchicchi, A. Axon-Myelin Unit Blistering as Early Event in MS Normal Appearing White Matter. Ann. Neurol. 2021, 89, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Ranganathan, P.; Pramesh, C.; Aggarwal, R. Common pitfalls in statistical analysis: Measures of agreement. Perspect. Clin. Res. 2017, 8, 187–191. [Google Scholar] [CrossRef]
- Filippi, M.; Rocca, M.A. Dirty-Appearing White Matter: A Disregarded Entity in Multiple Sclerosis. Am. J. Neuroradiol. 2010, 31, 389–390. [Google Scholar] [CrossRef] [Green Version]
- Seewann, A.; Kooi, E.J.; Roosendaal, S.D.; Barkhof, F.; van der Valk, P.; Geurts, J.J.G. Translating pathology in multiple sclerosis: The combination of postmortem imaging, histopathology and clinical findings. Acta Neurol. Scand. 2009, 119, 349–355. [Google Scholar] [CrossRef]
- Ge, Y.; Grossman, R.I.; Babb, J.S.; He, J.; Mannon, L.J. Dirty-Appearing White Matter in Multiple Sclerosis: Volumetric MR Imaging and Magnetization Transfer Ratio Histogram Analysis. Am. J. Neuroradiol. 2003, 24, 1935–1940. [Google Scholar]
- Ropele, S. A Comparison of Magnetization Transfer Ratio, Magnetization Transfer Rate, and the Native Relaxation Time of Water Protons Related to Relapsing-remitting Multiple Sclerosis. Am. J. Neuroradiol. 2000, 21, 1885–1891. [Google Scholar] [PubMed]
- Love, S. Demyelinating diseases. J. Clin. Pathol. 2006, 59, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, K.; Rangasami, R.; Chandrasekharan, A. Magnetic resonance imaging findings in viral encephalitis: A pictorial essay. J. Neurosci. Rural. Pract. 2018, 9, 556–560. [Google Scholar] [CrossRef]
- Preziosa, P. Axonal degeneration as substrate of fractional anisotropy abnormalities in multiple sclerosis cortex. Brain 2019, 142, 1921–1937. [Google Scholar] [CrossRef]
- Gomez-Nicola, D.; Boche, D. Post-mortem analysis of neuroinflammatory changes in human Alzheimer’s disease. Alzheimer’s Res. Ther. 2015, 7, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fard, M.K. BCAS1 expression defines a population of early myelinating oligodendrocytes in multiple sclerosis lesions. Sci. Transl. Med. 2017, 9, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teo, W. Nile Red fluorescence spectroscopy reports early physicochemical changes in myelin with high sensitivity. Proc. Natl. Acad. Sci. USA 2021, 118, e2016897118. [Google Scholar] [CrossRef]
- Luchicchi, A. Disturbed Axon-Myelin Interaction in MS Normal Appearing White Matter. 2019. Available online: https://onlinelibrary.ectrims-congress.eu/ectrims/2019/stockholm/278402/antonio.luchicchi.disturbed.axon-myelin.interaction.in.ms.normal.appearing.html?f=listing%3D3%2Abrowseby%3D8%2Asortby%3D1%2Amedia%3D1 (accessed on 17 January 2020).
- Motavaf, M.; Sadeghizadeh, M.; Javan, M. Attempts to Overcome Remyelination Failure: Toward Opening New Therapeutic Avenues for Multiple Sclerosis. Cell. Mol. Neurobiol. 2017, 37, 1335–1348. [Google Scholar] [CrossRef]
- Jonkman, L.E.; Kenkhuis, B.; Geurts, J.J.G.; van de Berg, W.D.J. Post-Mortem MRI and Histopathology in Neurologic Disease: A Translational Approach. Neurosci. Bull. 2019, 35, 229–243. [Google Scholar] [CrossRef]
- Klaver, R.; de Vries, H.E.; Schenk, G.J.; Geurts, J.J.G. Grey matter damage in multiple sclerosis A pathology perspective. Prion 2013, 7, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- De Vet, H.C.W.; Terwee, C.B.; Knol, D.L.; Bouter, L.M. When to use agreement versus reliability measures. J. Clin. Epidemiol. 2006, 59, 1033–1039. [Google Scholar] [CrossRef] [Green Version]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keene, O.N. The log transformation is special. Stat. Med. 1995, 14, 811–819. [Google Scholar] [CrossRef] [PubMed]

| Lesion with Partial Remyelination (Score 2) | Black Pixel Percentages ((PB/PT) × 100%) | Threshold Value |
|---|---|---|
| Analysis A. small magnification 1 Lesion: 0.6x | (475,272/2,158,826) × 100% = 21.24% black pixels within ROI | 208 |
| Analysis B. bigger magnification 2 Lesion: 1.9x | (44,856/211,200) × 100% = 22.02% black pixels within ROI | 208 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huitema, M.J.D.; Strijbis, E.M.M.; Luchicchi, A.; Bol, J.G.J.M.; Plemel, J.R.; Geurts, J.J.G.; Schenk, G.J. Myelin Quantification in White Matter Pathology of Progressive Multiple Sclerosis Post-Mortem Brain Samples: A New Approach for Quantifying Remyelination. Int. J. Mol. Sci. 2021, 22, 12634. https://doi.org/10.3390/ijms222312634
Huitema MJD, Strijbis EMM, Luchicchi A, Bol JGJM, Plemel JR, Geurts JJG, Schenk GJ. Myelin Quantification in White Matter Pathology of Progressive Multiple Sclerosis Post-Mortem Brain Samples: A New Approach for Quantifying Remyelination. International Journal of Molecular Sciences. 2021; 22(23):12634. https://doi.org/10.3390/ijms222312634
Chicago/Turabian StyleHuitema, Marije J. D., Eva M. M. Strijbis, Antonio Luchicchi, John G. J. M. Bol, Jason R. Plemel, Jeroen J. G. Geurts, and Geert J. Schenk. 2021. "Myelin Quantification in White Matter Pathology of Progressive Multiple Sclerosis Post-Mortem Brain Samples: A New Approach for Quantifying Remyelination" International Journal of Molecular Sciences 22, no. 23: 12634. https://doi.org/10.3390/ijms222312634
APA StyleHuitema, M. J. D., Strijbis, E. M. M., Luchicchi, A., Bol, J. G. J. M., Plemel, J. R., Geurts, J. J. G., & Schenk, G. J. (2021). Myelin Quantification in White Matter Pathology of Progressive Multiple Sclerosis Post-Mortem Brain Samples: A New Approach for Quantifying Remyelination. International Journal of Molecular Sciences, 22(23), 12634. https://doi.org/10.3390/ijms222312634