Splice-Variant Knock-Out of TGFβ Receptors Perturbates the Proteome of Ovarian Carcinoma Cells
Abstract
1. Introduction
2. Results
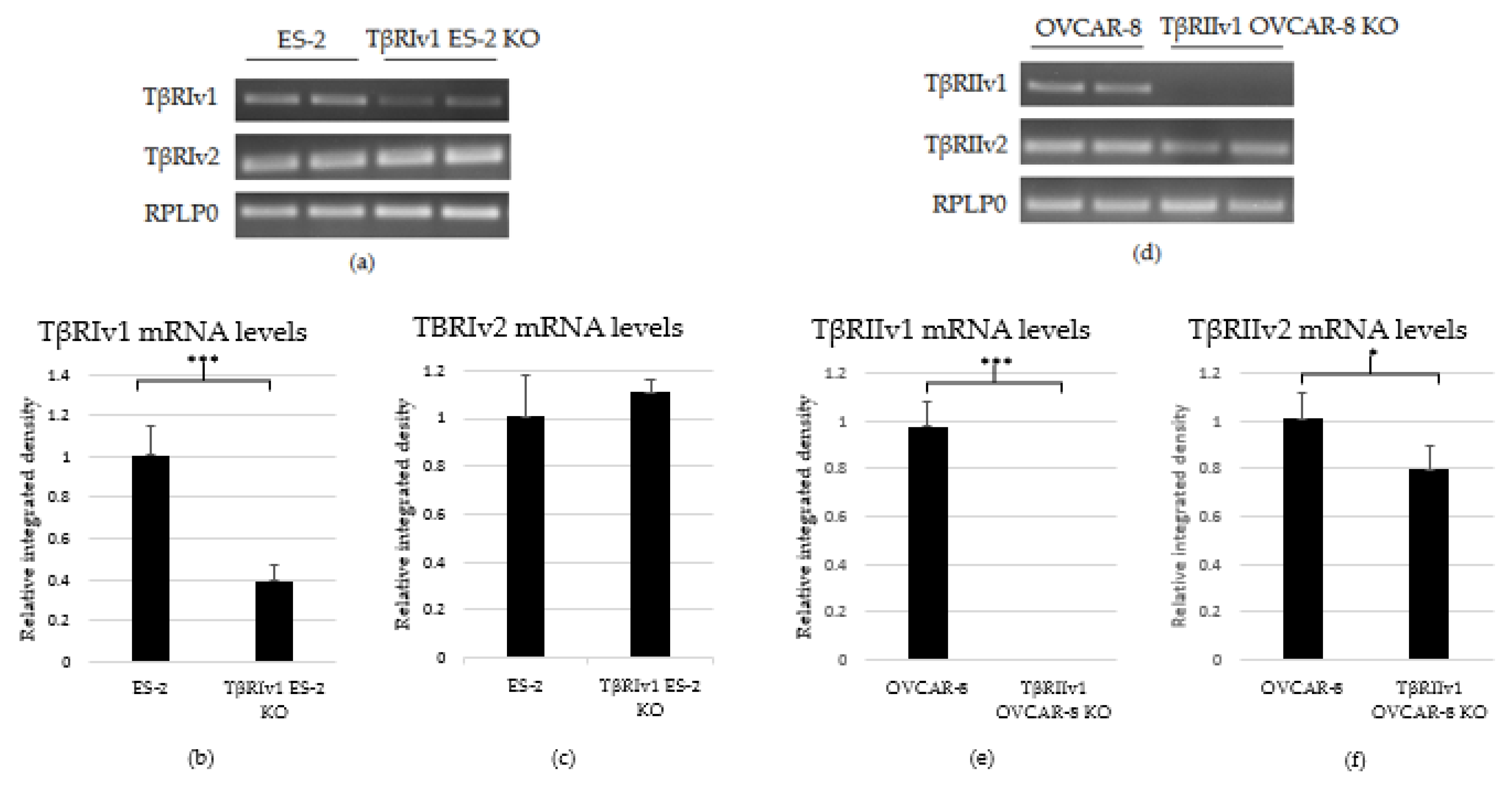
2.1. CRISPR/Cas9 KO in OC Cell Lines
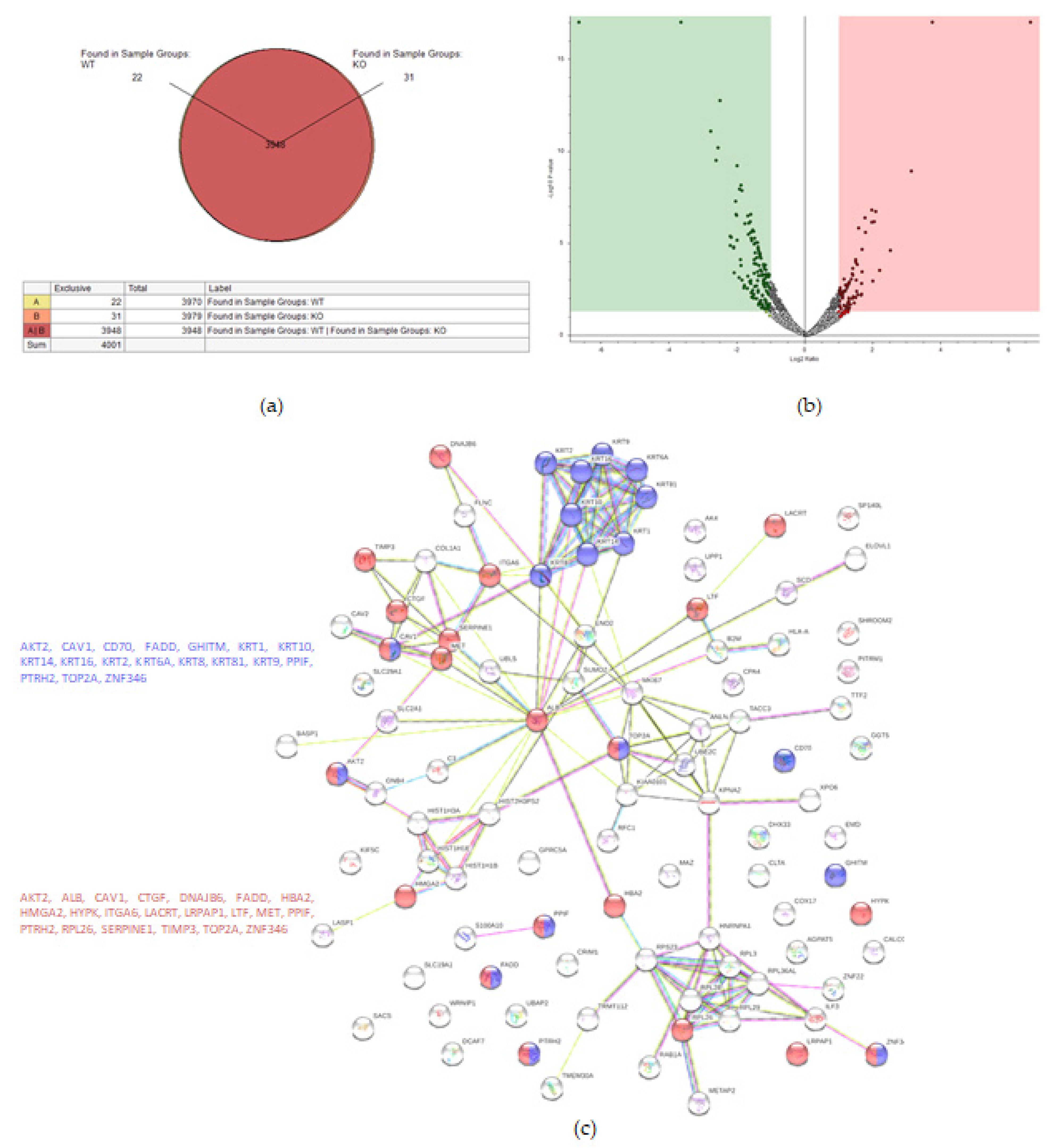
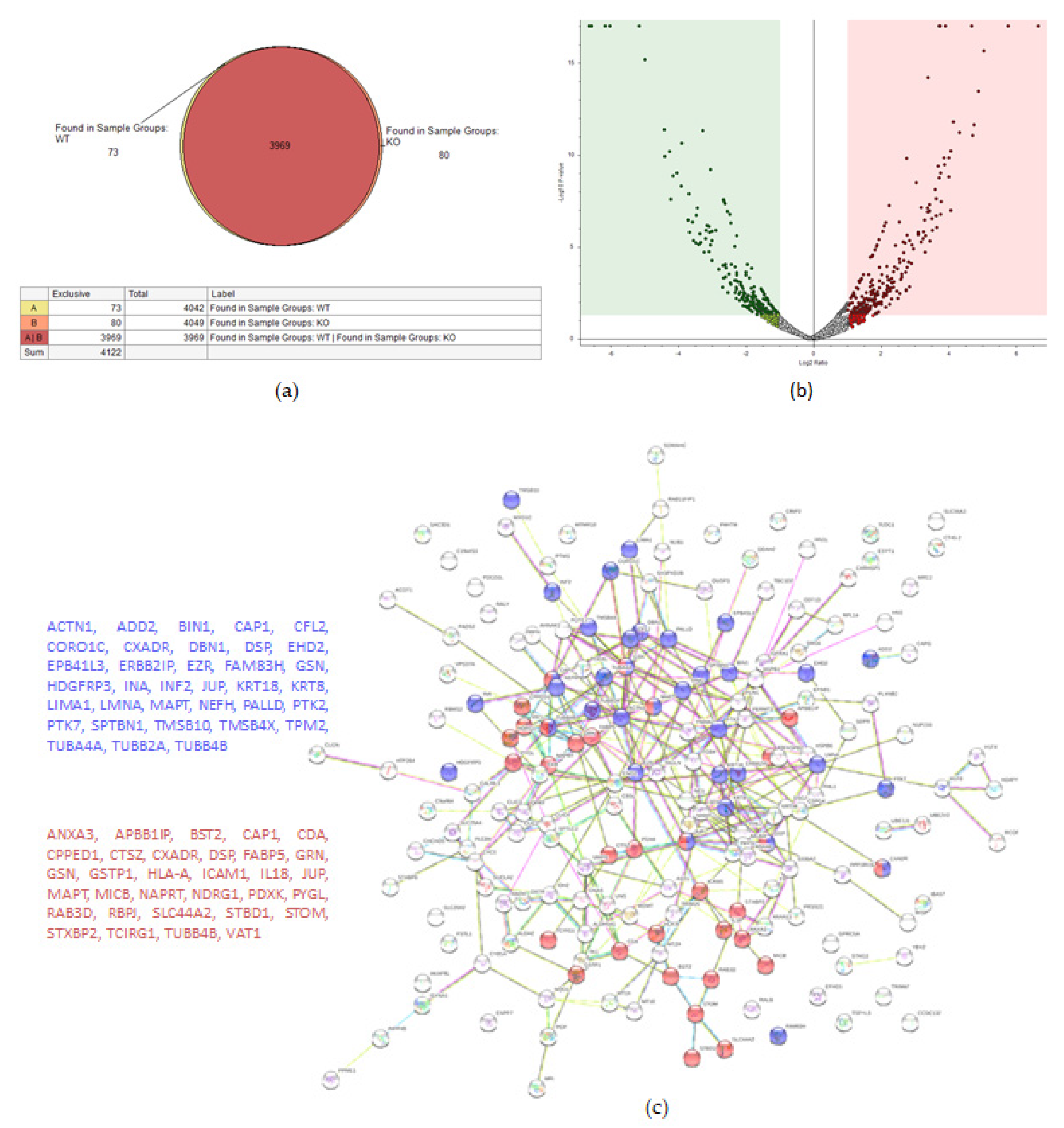
2.2. KO Formation Results in Significant Changes in the Cellular Proteome
2.2.1. ES-2 vs. TβRIv1-ES-2 KO
2.2.2. OVCAR-8 v.s. TβRIIv1 OVCAR-8 KO
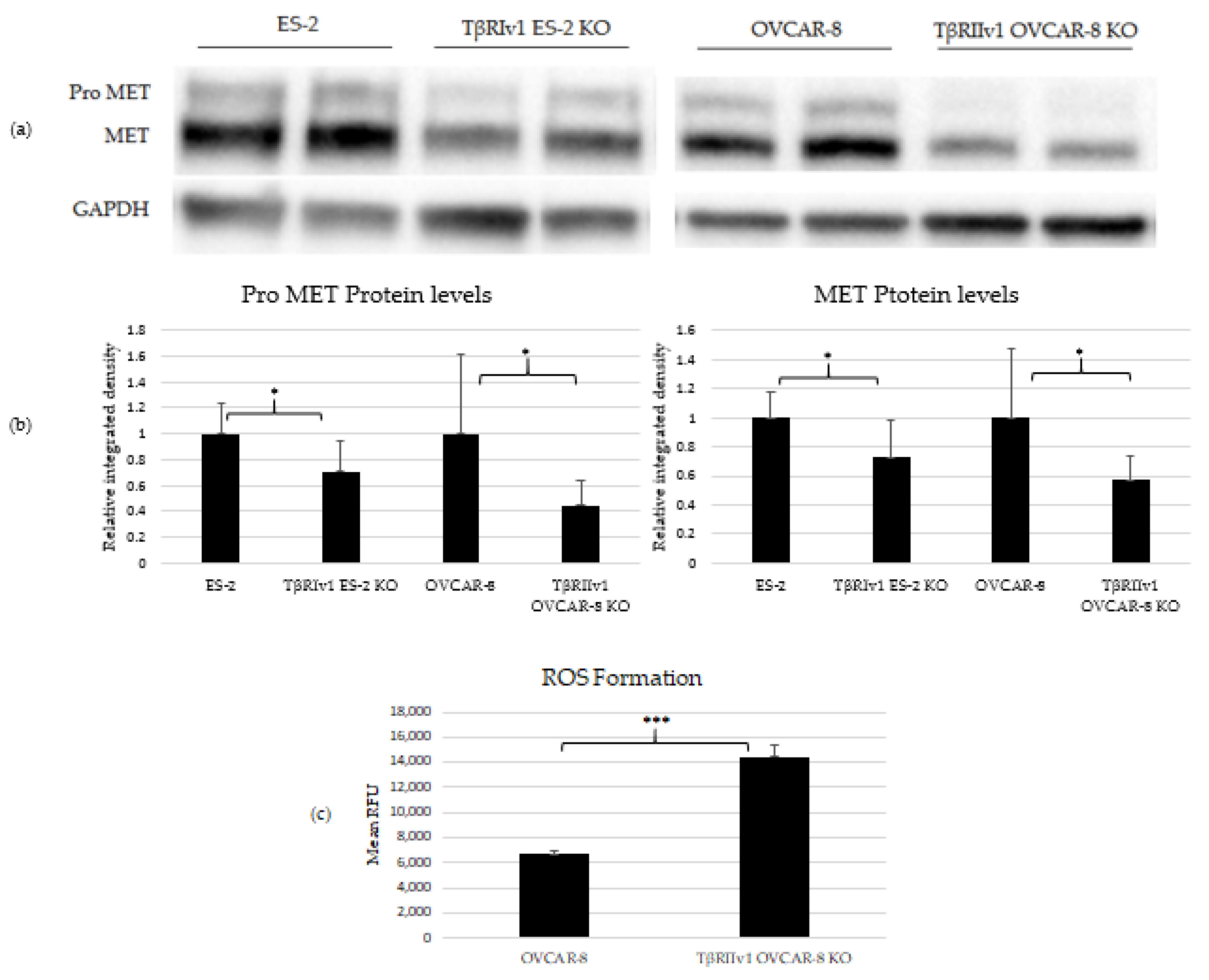
2.3. Pro-MET and MET Proteins Are Downregulated in the KOs’, and ROS Formation Is Upregulated in the TβRIIv1 OVCAR-8 KO
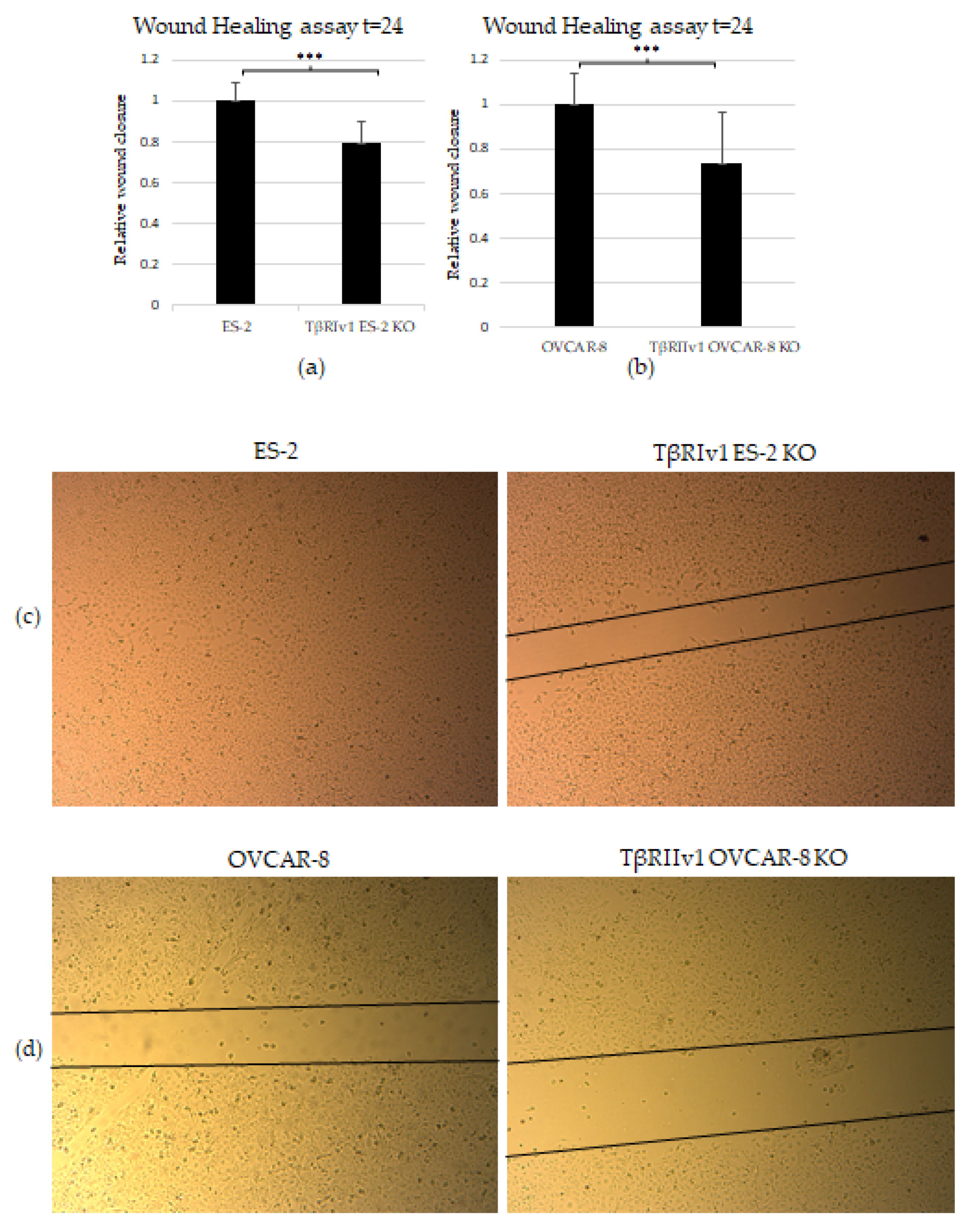
2.4. KO Formation Causes a Decrease in the Migratory and Invasive Capabilities of the Cells
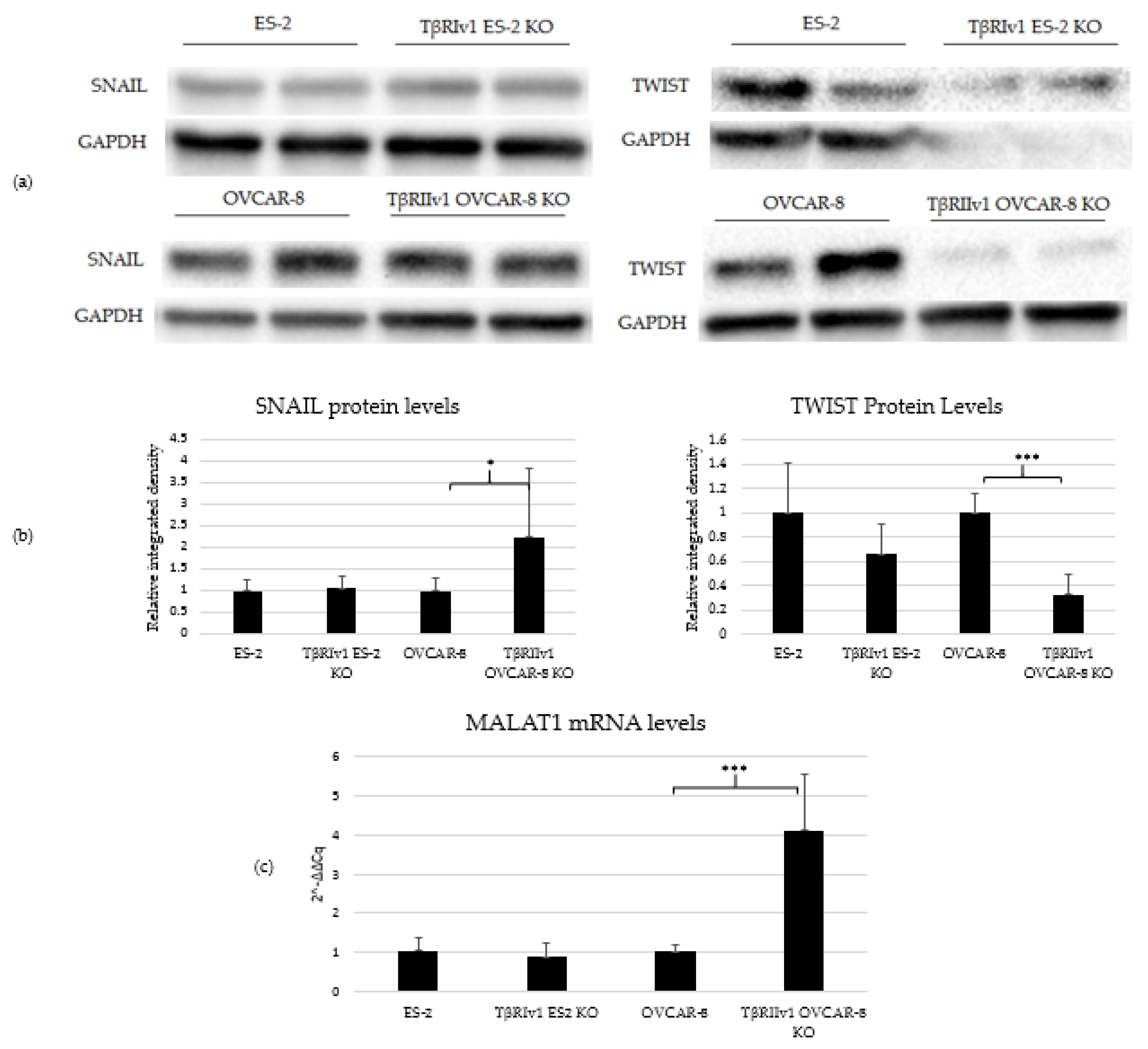
2.5. EMT Induction Is Splice-Variant Dependent
2.6. KO Formation Affects Downstream Signaling
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. CRISPR/Cas9
4.3. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
4.4. Quantitative Polymerase Chain Reaction (qPCR)
4.5. Proteomics
4.5.1. Sample Preparation
4.5.2. Proteomic Data Analysis
4.5.3. Proteomic Bioinformatics Analysis
4.6. Western Blot Analysis
4.7. ROS Quantification
4.8. Scratch Wound-Healing Assay
4.9. Matrigel Invasion Assay
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rafehi, S.; Bertrand, M.; McGee, J.; Préfontaine, M.; Sugimoto, A.; DiMattia, G.E.; Shepherd, T.G. TGFB signaling regulates Epithelial-mesenchymal plasticity in ovarian cancer ascites-derived spheroids. Endocr. Relat. Cancer 2016, 23, 147–159. [Google Scholar] [CrossRef]
- Massagué, J. TGFb in cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.; Bhattacharya, R.; Ray, U.; Mukhopadhyay, S.; Chatterjee, U.; Roy, S.S. Invasion of ovarian cancer cells is induced byPITX2-mediated activation of TGF-β and Activin-A. Mol. Cancer 2015, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gulei, D.; Mehterov, N.; Ling, H.; Stanta, G.; Braicu, C.; Berindan-Neagoe, I. The ‘good-cop bad-cop’ TGF-beta role in breast cancer modulated by non- coding RNAs. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 1661–1675. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-C.; Auersperg, N.; Leung, P.C.K.; Srivastava, R.K. TGF-Beta Induces Serous Borderline Ovarian Tumor Cell Invasion by Activating EMT but Triggers Apoptosis in Low-Grade Serous Ovarian Carcinoma Cells. PLoS ONE 2012, 7, e42436. [Google Scholar] [CrossRef][Green Version]
- Parvani, J.G.; Taylor, M.A.; Schiemann, W.P. Noncanonical TGF-β signaling during mammary tumorigenesis. J. Mammary Gland Biol. Neoplasia 2011, 16, 127–146. [Google Scholar] [CrossRef]
- Heldin, C.-H.; Moustakas, A. Signaling Receptors for TGF-β Family Members. Cold Spring Harb. Perspect. Biol. 2016, 8, a022053. [Google Scholar] [CrossRef]
- Konrad, L.; Scheiber, J.A.; Völck-Badouin, E.; Keilani, M.M.; Laible, L.; Brandt, H.; Schmidt, A.; Aumüller, G.; Hofmann, R. Alternative splicing of TGF-betas and their high-affinity receptors T beta RI, T beta RII and T beta RIII (betaglycan) reveal new variants in human prostatic cells. BMC Genom. 2007, 8, 318. [Google Scholar] [CrossRef]
- Parker, W.L.; Finnson, K.W.; Soe-Lin, H.; Knaus, P.; Philip, A. Expression and function of TβRII-B, a variant of the type II TGF-β receptor, in human chondrocytes. Osteoarthr. Cartil. 2007, 15, 442–453. [Google Scholar] [CrossRef][Green Version]
- Human chr9:99105089-99154192—UCSC Genome Browser v347. Available online: https://genome.ucsc.edu/cgi-bin/hgTracks?db=hg38&lastVirtModeType=default&lastVirtModeExtraState=&virtModeType=default&virtMode=0&nonVirtPosition=&position=chr9%3A99105089-99154192&hgsid=590221263_pzUq4zkoa3J6Zds6cii1XTjRX9pT (accessed on 30 April 2017).
- RCSB PDB—Protein Feature View—TGF-Beta Receptor Type-1-P36897 (TGFR1_HUMAN). Available online: http://www.rcsb.org/pdb/protein/P36897?evtc=Suggest&evta=ProteinFeatureView&evtl=autosearch_SearchBar_querySuggest (accessed on 30 April 2017).
- RCSB PDB—Protein Feature View—TGF-Beta Receptor Type-2-P37173 (TGFR2_HUMAN). Available online: http://www.rcsb.org/pdb/protein/P37173?evtc=Suggest&evta=ProteinFeatureView&evtl=autosearch_SearchBar_querySuggest (accessed on 30 April 2017).
- Gutgold, N.; Davidson, B.; Catane, L.J.; Holth, A.; Hellesylt, E.; Tropé, C.G.; Dørum, A.; Reich, R. TGFβ splicing and canonical pathway activation in high-grade serous carcinoma. Virchows Arch. 2017, 470, 665–678. [Google Scholar] [CrossRef]
- Davidson, B.; Tropé, C.G. Ovarian cancer: Diagnostic, biological and prognostic aspects. Womens Health 2014, 10, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Yang, Y.; Xu, S.Y.; Peng, J.; Jiang, J.H.; Li, C.Y. The CRISPR/Cas system inhibited the pro-oncogenic effects of alternatively spliced fibronectin extra domain A via editing the genome in salivary adenoid cystic carcinoma cells. Oral Dis. 2015, 21, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, C.; Song, K.; Chen, W.; Ungerleider, N.; Yao, L.; Ma, W.; Wu, T. The long-noncoding RNA MALAT1 regulates TGF-β/Smad signaling through formation of a lncRNA-protein complex with Smads, SETD2 and PPM1A in hepatic cells. PLoS ONE 2020, 15, e0228160. [Google Scholar] [CrossRef]
- Dong, N. Long Noncoding RNA MALAT1 Acts as a Competing Endogenous RNA to Regulate TGF- β 2 Induced Epithelial-Mesenchymal Transition of Lens Epithelial Cells by a MicroRNA-26a-Dependent Mechanism. Biomed. Res. Int. 2019, 2019, 1569638. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhang, Y.; Yong, T.; Qianhui, L. MALAT1 modulates TGF-β1-induced endothelial-to-mesenchymal transition through downregulation of miR-145. Cell. Physiol. Biochem. 2017, 43, 1309–1310. [Google Scholar] [CrossRef] [PubMed]
- Onallah, H.; Catane, L.J.; Tropé, C.G.; Hetland Falkenthal, T.E.; Reich, R.; Davidson, B. Activity and clinical relevance of autotaxin and lysophosphatidic acid pathways in high-grade serous carcinoma. Virchows Arch. 2018, 473, 463–470. [Google Scholar] [CrossRef]
- Hurteau, J.A.; Allison, B.; Sutton, G.P.; Moore, D.H.; Look, K.Y.; Hurd, W.; Bigsby, R.M. Transforming growth factor-β differentially inhibits epithelial ovarian carcinoma cells from primary and metastatic isolates without up-regulation of p21(WAF1). Cancer 1999, 85, 1810–1815. [Google Scholar] [CrossRef]
- Ho, C.M.; bi Shih, D.T.; Hsiao, C.C.; Huang, S.H.; Chang, S.F.; Cheng, W.F. Gene methylation of human ovarian carcinoma stromal progenitor cells promotes tumorigenesis. J. Transl. Med. 2015, 13, 367. [Google Scholar] [CrossRef]
- Liao, S.; Liu, J.; Lin, P.; Shi, T.; Jain, R.K.; Xu, L. TGF-β blockade controls ascites by preventing abnormalization of lymphatic vessels in orthotopic human ovarian carcinoma models. Clin. Cancer Res. 2011, 17, 1415–1424. [Google Scholar] [CrossRef]
- Rotzer, D.; Roth, M.; Lutz, M.; Lindemann, D.; Sebald, W.; Knaus, P. Type III TGF-β receptor-independent signalling of TGFβ2 via TβRII-B, an alternatively spliced TGF-β type II receptor. EMBO J. 2001, 20, 480–490. [Google Scholar] [CrossRef]
- Czyz, M. HGF/c-MET signaling in melanocytes and melanoma. Int. J. Mol. Sci. 2018, 19, 3844. [Google Scholar] [CrossRef]
- Kumari, A.; Shonibare, Z.; Monavarian, M.; Arend, R.C.; Lee, N.Y.; Inman, G.J.; Mythreye, K. TGFβ signaling networks in ovarian cancer progression and plasticity. Clin. Exp. Metastasis 2021, 38, 139–161. [Google Scholar] [CrossRef]
- Luan, W.; Zhang, X.; Ruan, H.; Wang, J.; Bu, X. Long noncoding RNA OIP5-AS1 acts as a competing endogenous RNA to promote glutamine catabolism and malignant melanoma growth by sponging miR-217. J. Cell. Physiol. 2019, 234, 16609–16618. [Google Scholar] [CrossRef]
- Liu, J.; Lin, A. Role of JNK activation in apoptosis: A double-edged sword. Cell Res. 2005, 15, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Optimized CRISPR Design. Available online: http://crispr.mit.edu:8079/? (accessed on 6 June 2017).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. US National Institutes of Health. Available online: http://imagej.nih.gov/ij/ (accessed on 6 June 2017).
- Thermo Fisher Scientific. Proteome Discoverer, Version 2.3; Thermo Fisher Scientific: Waltham, MA, USA, 2017.
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef]
- Gebäck, T.; Schulz, M.M.P.; Koumoutsakos, P.; Detmar, M. TScratch: A novel and simple software tool for automated analysis of monolayer wound healing assays: Short Technical Reports. Biotechniques 2009, 46, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Geback, T.; Schulz, M. TScratch, Version 1.0; CSE lab, ETH: Zurich, Switzerland, 2010.




| Gene | Flanking Region | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|---|
| TβRI | Upstream of Exon 3 | GTTGATGTTTATtTCACTCG | CGAGTGAAATAAACATCAAC |
| Downstream of Exon 3 | GCTTGATGTATATATGACTG | CAGTCATATATACATCAAGC | |
| TβRII | Upstream1 of Exon 2 | GAAAAAGAGAAACTAGTACC | GGTACTAGTTTCTCTTTTTC |
| Upstream2 of Exon 2 | TATGGGTGAAAGATCACCAG | CTGGTGATCTTTCACCCATA | |
| Upstream3 of Exon 2 | TGTCTAGTTATAATAATCTT | AAGATTATTATAACTAGACA | |
| Downstream1 of Exon 2 | ATTTAAGACTGGAGAATTTC | GAAATTCTCCAGTCTTAAAT | |
| Downstream2 of Exon2 | GCGATGGCAGTGTACTCATC | GATGAGTACACTGCCATCGC |
| Gene | Strand | Primer Sequence 5′→3′ |
|---|---|---|
| TβRI variant 1 | Sense | TGGACCAGTGTGCTTCGTCTG |
| Antisense | CGACCTTTGCCAATGCTTTCT | |
| TβRI variant 2 | Sense | TCCAACTACTGGTTTACCATTGCTT |
| Antisense | CGACCTTTGCCAATGCTTTCT | |
| TβRII variant 1 | Sense | ACTGCCCATCCACTGAGACAT |
| Antisense | TGCACTTTGGAGAAGCAGCAT | |
| TβRII variant 2 | Sense | CGTTCAGAAGTCGGTTAATAACGA |
| Antisense | CACAGGTGGAAAATCTCACATCA | |
| Malat1 | Sense | GATCCTAGACCAGCATGCC |
| Antisense | AAAGGTTACCATAAGTAAGTTCCAGAAAA | |
| RPLP0 | Sense | CCAACTACTTCCTTAAGATCATCCAACTA |
| Antisense | ACATGCGGATCTGCTGCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobs Catane, L.; Moshel, O.; Smith, Y.; Davidson, B.; Reich, R. Splice-Variant Knock-Out of TGFβ Receptors Perturbates the Proteome of Ovarian Carcinoma Cells. Int. J. Mol. Sci. 2021, 22, 12647. https://doi.org/10.3390/ijms222312647
Jacobs Catane L, Moshel O, Smith Y, Davidson B, Reich R. Splice-Variant Knock-Out of TGFβ Receptors Perturbates the Proteome of Ovarian Carcinoma Cells. International Journal of Molecular Sciences. 2021; 22(23):12647. https://doi.org/10.3390/ijms222312647
Chicago/Turabian StyleJacobs Catane, Liora, Ofra Moshel, Yoav Smith, Ben Davidson, and Reuven Reich. 2021. "Splice-Variant Knock-Out of TGFβ Receptors Perturbates the Proteome of Ovarian Carcinoma Cells" International Journal of Molecular Sciences 22, no. 23: 12647. https://doi.org/10.3390/ijms222312647
APA StyleJacobs Catane, L., Moshel, O., Smith, Y., Davidson, B., & Reich, R. (2021). Splice-Variant Knock-Out of TGFβ Receptors Perturbates the Proteome of Ovarian Carcinoma Cells. International Journal of Molecular Sciences, 22(23), 12647. https://doi.org/10.3390/ijms222312647






