The Significance of Biomechanics and Scaffold Structure for Bladder Tissue Engineering
Abstract
:1. Introduction
1.1. The Need for a Biomaterials-Based Approach to Bladder Reconstruction
1.2. Unmet Needs for Bladder Reconstruction
2. Methods
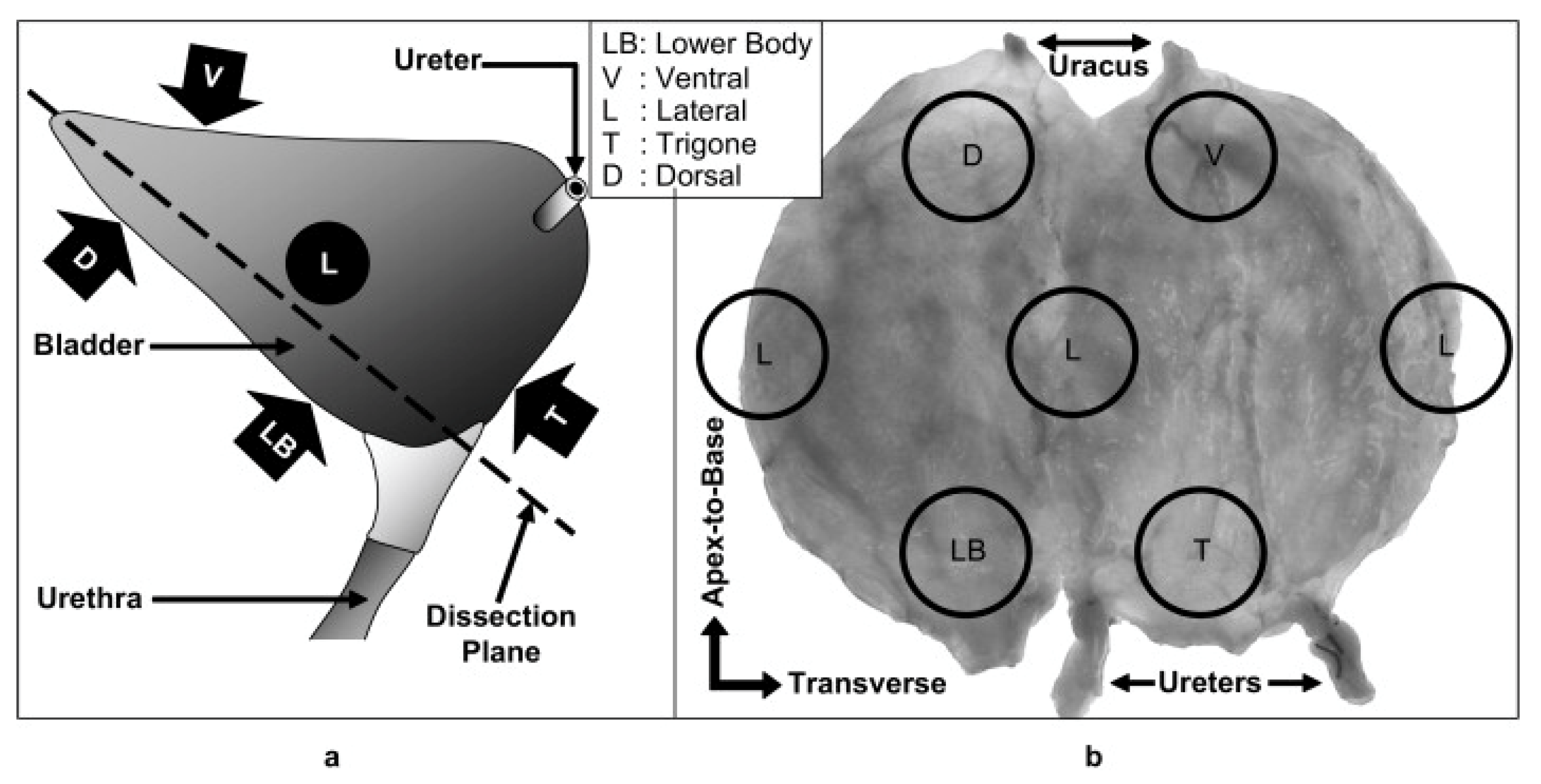
3. Bladder Structure
4. Biomechanical Properties of the Bladder
4.1. Strain
4.2. Stress
4.3. Stress-Strain Curve
4.4. Viscoelasticity
4.5. Preconditioning
4.6. Mechanical Testing
4.6.1. Uniaxial Tests
4.6.2. Biaxial and Ball Burst Tests
4.7. Urodynamic Testing
4.8. Summary
5. Biomaterials for Bladder Reconstruction
5.1. Pre-Seeding Cells into Scaffolds
5.2. Quantification of Biomechanical Properties
5.3. Urodynamic Studies on Biomaterials
5.4. The Importance of Scaffold Structure on Successful Outcomes
6. Conclusions and Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pattison, M.A.; Wurster, S.; Webster, T.J.; Haberstroh, K.M. Three-dimensional, nano-structured PLGA scaffolds for bladder tissue replacement applications. Biomaterials 2005, 26, 2491–2500. [Google Scholar] [CrossRef]
- Biers, S.M.; Venn, S.N.; Greenwell, T.J. The past, present and future of augmentation cystoplasty. BJU Int. 2012, 109, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, J.; Pokrywczynska, M.; Van Breda, S.V.; Kloskowski, T.; Drewa, T. Concise review: Tissue engineering of urinary bladder; we still have a long way to go? Stem Cells Transl. Med. 2017, 6, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Khan, S.A.; Hayn, M.H.; Agarwal, P.K.; Badani, K.K.; Balbay, M.D.; Castle, E.P.; Dasgupta, P.; Ghavamian, R.; Guru, K.A.; et al. Analysis of intracorporeal compared with extracorporeal urinary diversion after robot-assisted radical cystectomy: Results from the International Robotic Cystectomy Consortium. Eur. Urol. 2014, 65, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.H.; Hayn, M.; Murray, P. The use of bowel in urologic reconstructive surgery. Surg. Clin. N. Am. 2016, 96, 567–582. [Google Scholar] [CrossRef] [PubMed]
- van Hemelrijck, M.; Thorstenson, A.; Smith, P.; Adolfsson, J.; Akre, O. Risk of in-hospital complications after radical cystectomy for urinary bladder carcinoma: Population-based follow-up study of 7608 patients. BJU Int. 2013, 112, 1113–1120. [Google Scholar] [CrossRef]
- Çetinel, B.; Kocjancic, E.; Demirdağ, Ç. Augmentation cystoplasty in neurogenic bladder. Investig. Clin. Urol. 2016, 57, 316–323. [Google Scholar] [CrossRef]
- Buscarini, M.; Pasin, E.; Stein, J.P. Complications of radical cystectomy. Minerva Urol. Nefrol. 2007, 59, 67–87. [Google Scholar]
- Adamowicz, J.; Kuffel, B.; Van Breda, S.V.; Pokrwczynska, M.; Drewa, T. Reconstructive urology and tissue engineering: Converging developmental paths. J. Tissue Eng. Regen. Med. 2019, 13, 522–533. [Google Scholar] [CrossRef]
- Bohne, A.W.; Urwiller, K.L. Experience with urinary bladder regeneration. J. Urol. 1957, 77, 725–732. [Google Scholar] [CrossRef]
- Sanchez, R.P.; Blanco, F.L.; Santamarina, A.; Roa, J.C.; Mata, J.; Kaufman, A. Vesical regeneration in the human after total cystectomy and implantation of a plastic mould. Br. J. Urol. 1958, 30, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Tsulukidze, A.; Murvanidze, D.; Dvali, R.; Ivashchenko, G. Formation of a bladder by a plastic shell after total cystectomy. Br. J. Urol. 1964, 36, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Orikasa, S.; Tsuji, I. Enlargement of contracted bladder by use of gelatin sponge bladder. J. Urol. 1970, 104, 107–110. [Google Scholar] [CrossRef]
- Tsuji, I.; Kuroda, K.; Fujieda, J.; Shiraishi, Y.; Kunishima, K. Clinical experiences of bladder reconstruction using preserved bladder and gelatin sponge bladder in the case of bladder cancer. J. Urol. 1967, 98, 91–92. [Google Scholar] [CrossRef]
- Taguchi, H.; Ishizuka, E.; Saito, K. Cystoplasty by regeneration of the bladder. J. Urol. 1977, 118, 752–756. [Google Scholar] [CrossRef]
- Fujita, K. The use of resin-sprayed thin paper for urinary bladder regeneration. Investig. Urol. 1978, 15, 355–357. [Google Scholar]
- Kelâmi, A. Duraplasty of the urinary bladder—results after two to six years. Eur. Urol. 1975, 1, 178–181. [Google Scholar] [CrossRef]
- Arikan, N.; Ozdiler, E.; Yaman, O.; Gögüs, O. Augmentation duracystoplasty in neurogenic bladder dysfunction. Int. J. Urol. 1995, 2, 172–175. [Google Scholar] [CrossRef]
- Moon, S.J.; Kim, D.H.; Jo, J.K.; Chung, J.H.; Lee, J.Y.; Park, S.Y.; Kim, Y.T.; Park, H.K.; Choi, H.Y.; Moon, H.S. Bladder reconstruction using bovine pericardium in a case of enterovesical fistula. Korean J. Urol. 2011, 52, 150–153. [Google Scholar] [CrossRef] [Green Version]
- Caione, P.; Boldrini, R.; Salerno, A.; Nappo, S.G. Bladder augmentation using acellular collagen biomatrix: A pilot experience in exstrophic patients. Pediatr. Surg. Int. 2012, 28, 421–428. [Google Scholar] [CrossRef]
- Ajalloueian, F.; Lemon, G.; Hilborn, J.; Chronakis, I.S.; Fossum, M. Bladder biomechanics and the use of scaffolds for regenerative medicine in the urinary bladder. Nat. Rev. Urol. 2018, 15, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.E.; Farhat, W.A.; Ming, J.M.; McCammon, K.A. Review of clinical experience on biomaterials and tissue engineering of urinary bladder. World J. Urol. 2020, 38, 2081–2093. [Google Scholar] [CrossRef] [PubMed]
- Khademolqorani, S.; Tavanai, H.; Chronakis, I.S.; Boisen, A.; Ajalloueian, F. The determinant role of fabrication technique in final characteristics of scaffolds for tissue engineering applications: A focus on silk fibroin-based scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111867. [Google Scholar] [CrossRef]
- Khandelwal, P.; Abraham, S.N.; Apodaca, G. Cell biology and physiology of the uroepithelium. Am. J. Physiol. Renal Physiol. 2009, 297, F1477–F1501. [Google Scholar] [CrossRef] [Green Version]
- Davis, N.F.; Callanan, A.; McGuire, B.B.; Flood, H.D.; McGloughlin, T.M. Evaluation of viability and proliferative activity of human urothelial cells cultured onto xenogenic tissue-engineered extracellular matrices. Urology 2011, 77, 1007.e1–1007.e7. [Google Scholar] [CrossRef]
- Groen, J.; Pannek, J.; Diaz, D.C.; Del Popolo, G.; Gross, T.; Hamid, R.; Karsenty, G.; Kessler, T.M.; Schneider, M.; Blok, B.; et al. Summary of European Association of Urology (EAU) Guidelines on Neuro-Urology. Eur. Urol. 2016, 69, 324–333. [Google Scholar] [CrossRef]
- Zhang, Y.; Atala, A. Regenerative medicine of the bladder. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1263–1279. [Google Scholar]
- Abelson, B.; Sun, D.; Que, L.; Nebel, R.A.; Baker, D.; Popiel, P.; Amundsen, C.L.; Chai, T.; Close, C.; DiSanto, M.; et al. Sex differences in lower urinary tract biology and physiology. Biol. Sex Differ. 2018, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Birder, L.A.; Kullmann, F.A.; Hornsby, J.; Watton, P.N.; Watkins, S.; Thompson, M.; Robertson, A.M. Layer-dependent role of collagen recruitment during loading of the rat bladder wall. Biomech. Model. Mechanobiol. 2018, 17, 403–417. [Google Scholar] [CrossRef]
- Bolla, S.R.; Odeluga, N.; Jetti, R. Histology, Bladder; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Adamowicz, J.; Kloskowski, T.; Tworkiewicz, J.; Pokrywczyńska, M.; Drewa, T. Urine is a highly cytotoxic agent: Does it influence stem cell therapies in urology? Transplant. Proc. 2012, 44, 1439–1441. [Google Scholar] [CrossRef]
- Hu, P.; Deng, F.M.; Liang, F.X.; Hu, C.M.; Auerbach, A.B.; Shapiro, E.; Wu, X.R.; Kachar, B.; Sun, T.T. Ablation of uroplakin III gene results in small urothelial plaques, urothelial leakage, and vesicoureteral reflux. J. Cell Biol. 2000, 151, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Meyers, S.; Liang, F.-X.; Deng, F.-M.; Kachar, B.; Zeidel, M.L.; Sun, T.-T. Role of membrane proteins in permeability barrier function: Uroplakin ablation elevates urothelial permeability. Am. J. Physiol. Renal Physiol. 2002, 283, F1200–F1207. [Google Scholar] [CrossRef] [Green Version]
- Abrams, P. Urodynamics; Springer: London, UK, 2006. [Google Scholar]
- Andersson, K.-E.; McCloskey, K.D. Lamina propria: The functional center of the bladder? Neurourol. Urodyn. 2014, 33, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Miodoński, A.J.; Litwin, J.A. Microvascular architecture of the human urinary bladder wall: A corrosion casting study. Anat. Rec. 1999, 254, 375–381. [Google Scholar] [CrossRef]
- Boerckel, J.D.; Gemmiti, C.V.; Mason, D.E.; Kolambkar, Y.M.; Porter, B.D.; Guldberg, R.E. Physical stress as a factor in tissue growth and remodeling. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 417–436. [Google Scholar]
- Alexander, R.S. Mechanical properties of urinary bladder. Am. J. Physiol. 1971, 220, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Fung, Y.C.; Skalak, R. Biomechanics: Mechanical properties of living tissues. J. Biomech. Eng. 1981, 103, 231. [Google Scholar] [CrossRef]
- Nagatomi, J.; Gloeckner, D.C.; Chancellor, M.B.; DeGroat, W.C.; Sacks, M.S. Changes in the biaxial viscoelastic response of the urinary bladder following spinal cord injury. Ann. Biomed. Eng. 2004, 32, 1409–1419. [Google Scholar] [CrossRef]
- Nagatomi, J.; Toosi, K.K.; Chancellor, M.B.; Sacks, M.S. Contribution of the extracellular matrix to the viscoelastic behavior of the urinary bladder wall. Biomech. Model Mechanobiol. 2008, 7, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Robi, K.; Jakob, N.; Matevz, K.; Matjaz, V. The physiology of sports injuries and repair processes. In Current Issues in Sports and Exercise Medicine; Hamlin, M., Ed.; BoD—Books on Demand: Norderstedt, Germany, 2013. [Google Scholar]
- Zanetti, E.M.; Perrini, M.; Bignardi, C.; Audenino, A.L. Bladder tissue passive response to monotonic and cyclic loading. Biorheology 2012, 49, 49–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkelstein, B. Orthopaedic Biomechanics; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- García Páez, J.M.; Jorge Herrero, E.; Carrera Sanmartín, A.; Millán, I.; Cordon, A.; Martín Maestro, M.; Rocha, A.; Arenaz, B.; Castillo-Olivares, J.L. Comparison of the mechanical behaviors of biological tissues subjected to uniaxial tensile testing: Pig, calf and ostrich pericardium sutured with Gore-Tex. Biomaterials 2003, 24, 1671–1679. [Google Scholar] [CrossRef]
- Dahms, S.E.; Piechota, H.J.; Dahiya, R.; Lue, T.F.; Tanagho, E.A. Composition and biomechanical properties of the bladder acellular matrix graft: Comparative analysis in rat, pig and human. Br. J. Urol. 1998, 82, 411–419. [Google Scholar] [CrossRef]
- Korossis, S.; Bolland, F.; Southgate, J.; Ingham, E.; Fisher, J. Regional biomechanical and histological characterisation of the passive porcine urinary bladder: Implications for augmentation and tissue engineering strategies. Biomaterials 2009, 30, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Martins, P.A.L.S.; Filho, A.L.S.; Fonseca, A.M.R.M.; Santos, A.; Santos, L.; Mascarenhas, T.; Jorge, R.M.N.; Ferreira, A.J.M. Uniaxial mechanical behavior of the human female bladder. Int. Urogynecol. J. 2011, 22, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.D.; Chung, Y.G.; Gil, E.S.; Seth, A.; Franck, D.; Cristofaro, V.; Sullivan, M.P.; Di Vizio, D.; Gomez, P.; Adam, R.M.; et al. Bladder tissue regeneration using acellular bi-layer silk scaffolds in a large animal model of augmentation cystoplasty. Biomaterials 2013, 34, 8681–8689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jokandan, M.S.; Ajalloueian, F.; Edinger, M.; Stubbe, P.R.; Baldursdottir, S.; Chronakis, I.S. Bladder wall biomechanics: A comprehensive study on fresh porcine urinary bladder. J. Mech. Behav. Biomed. Mater. 2018, 79, 92–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwell, T.J.; Venn, S.N.; Mundy, A.R. Augmentation cystoplasty. BJU Int. 2001, 88, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.H.; Karsdal, M.A. Elastin. In Biochemistry of Collagens, Laminins and Elastin; Elsevier: Amsterdam, The Netherlands, 2016; pp. 197–201. [Google Scholar]
- Chen, J.; Drzewiecki, B.A.; Merryman, W.D.; Pope, J.C. Murine bladder wall biomechanics following partial bladder obstruction. J. Biomech. 2013, 46, 2752–2755. [Google Scholar] [CrossRef]
- Parekh, A.; Cigan, A.D.; Wognum, S.; Heise, R.L.; Chancellor, M.B.; Sacks, M.S. Ex vivo deformations of the urinary bladder wall during whole bladder filling: Contributions of extracellular matrix and smooth muscle. J. Biomech. 2010, 43, 1708–1716. [Google Scholar] [CrossRef]
- Natali, A.N.; Audenino, A.L.; Artibani, W.; Fontanella, C.G.; Carniel, E.L.; Zanetti, E.M. Bladder tissue biomechanical behavior: Experimental tests and constitutive formulation. J. Biomech. 2015, 48, 3088–3096. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, J.H. The significance of natural bladder filling by the production of urine during cystometry. Neurourol. Urodyn. 2008, 27, 772–774. [Google Scholar] [CrossRef]
- Gloeckner, D.C.; Sacks, M.S.; Fraser, M.O.; Somogyi, G.T.; de Groat, W.C.; Chancellor, M.B. Passive biaxial mechanical properties of the rat bladder wall after spinal cord injury. J. Urol. 2002, 167, 2247–2252. [Google Scholar] [CrossRef]
- Freytes, D.O.; Rundell, A.E.; Vande Geest, J.; Vorp, D.A.; Webster, T.J.; Badylak, S.F. Analytically derived material properties of multilaminated extracellular matrix devices using the ball-burst test. Biomaterials 2005, 26, 5518–5531. [Google Scholar] [CrossRef]
- Freytes, D.O.; Stoner, R.M.; Badylak, S.F. Uniaxial and biaxial properties of terminally sterilized porcine urinary bladder matrix scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Freytes, D.O.; Badylak, S.F.; Webster, T.J.; Geddes, L.A.; Rundell, A.E. Biaxial strength of multilaminated extracellular matrix scaffolds. Biomaterials 2004, 25, 2353–2361. [Google Scholar] [CrossRef] [PubMed]
- Whitson, B.A.; Cheng, B.C.; Kokini, K.; Badylak, S.F.; Patel, U.; Morff, R.; O’Keefe, C.R. Multilaminate resorbable biomedical device under biaxial loading. J. Biomed. Mater. Res. 1998, 43, 277–281. [Google Scholar] [CrossRef]
- Brookoff, D. Genitourinary pain syndromes: Interstitial cystitis, chronic prostatitis, pelvic floor dysfunction, and related disorders. In Current Therapy in Pain; Elsevier: Amsterdam, The Netherlands, 2009; pp. 205–215. [Google Scholar]
- Pfeiffer, R.F. Bladder and sexual function and dysfunction. In Neurology and Clinical Neuroscience; Elsevier: Amsterdam, The Netherlands, 2007; pp. 362–371. [Google Scholar]
- Harris, R.L.; Cundiff, G.W.; Theofrastous, J.P.; Bump, R.C. Bladder compliance in neurologically intact women. Neurourol. Urodyn. 1996, 15, 483–488. [Google Scholar] [CrossRef]
- Boron, W.F.; Boulpaep, E.L. (Eds.) Medical Physiology; Elsevier: Philadelphia, PA, USA, 2017. [Google Scholar]
- Zhu, Y.; Wagner, W.R. Design principles in biomaterials and scaffolds. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 505–522. [Google Scholar]
- Joseph, D.B.; Borer, J.G.; De Filippo, R.E.; Hodges, S.J.; McLorie, G.A. Autologous cell seeded biodegradable scaffold for augmentation cystoplasty: Phase II study in children and adolescents with spina bifida. J. Urol. 2014, 191, 1389–1395. [Google Scholar] [CrossRef]
- Scarritt, M.; Murdock, M.; Badylak, S.F. Biologic scaffolds composed of extracellular matrix for regenerative medicine. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 613–626. [Google Scholar]
- Qiu, Y.-L.; Chen, X.; Hou, Y.-L.; Hou, Y.-J.; Tian, S.-B.; Chen, Y.-H.; Yu, L.; Nie, M.-H.; Liu, X.-Q. Characterization of different biodegradable scaffolds in tissue engineering. Mol. Med. Rep. 2019, 19, 4043–4056. [Google Scholar] [CrossRef] [Green Version]
- Tottey, S.; Johnson, S.A.; Crapo, P.M.; Reing, J.E.; Zhang, L.; Jiang, H.; Medberry, C.J.; Reines, B.; Badylak, S.F. The effect of source animal age upon extracellular matrix scaffold properties. Biomaterials 2011, 32, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Stankus, J.J.; Freytes, D.O.; Badylak, S.F.; Wagner, W.R. Hybrid nanofibrous scaffolds from electrospinning of a synthetic biodegradable elastomer and urinary bladder matrix. J. Biomater. Sci. Polym. Ed. 2008, 19, 635–652. [Google Scholar] [CrossRef] [Green Version]
- Ajalloueian, F.; Zeiai, S.; Rojas, R.; Fossum, M.; Hilborn, J. One-stage tissue engineering of bladder wall patches for an easy-to-use approach at the surgical table. Tissue Eng. Part C Methods 2013, 19, 688–696. [Google Scholar] [CrossRef]
- Ajalloueian, F.; Zeiai, S.; Fossum, M.; Hilborn, J.G. Constructs of electrospun PLGA, compressed collagen and minced urothelium for minimally manipulated autologous bladder tissue expansion. Biomaterials 2014, 35, 5741–5748. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; He, Y.; Guo, J.-H.; Wu, J.-S.; Zhou, Z.; Zhang, M.; Li, W.; Zhou, J.; Xiao, D.-D.; Wang, Z.; et al. Time-dependent bladder tissue regeneration using bilayer bladder acellular matrix graft-silk fibroin scaffolds in a rat bladder augmentation model. Acta Biomater. 2015, 23, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, S.; Ostendorff, R.; Fleishman, B.; Nagatomi, J. Tetronic®-based composite hydrogel scaffolds seeded with rat bladder smooth muscle cells for urinary bladder tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2015, 26, 196–210. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Hedrick, M.; Pareek, G.; Renzulli, J.; Haleblian, G.; Webster, T.J. Nanostructured polyurethane-poly-lactic-co-glycolic acid scaffolds increase bladder tissue regeneration: An in vivo study. Int. J. Nanomed. 2013, 8, 3285–3296. [Google Scholar]
- Del Gaudio, C.; Vianello, A.; Bellezza, G.; Maulà, V.; Sidoni, A.; Zucchi, A.; Bianco, A.; Porena, M. Evaluation of electrospun bioresorbable scaffolds for tissue-engineered urinary bladder augmentation. Biomed. Mater. 2013, 8, 045013. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, S.; Amoroso, N.; Gu, X.; Purves, J.T.; Hughes, F.M.; Wagner, W.R.; Nagatomi, J. Evaluation of Poly (Carbonate-Urethane) Urea (PCUU) Scaffolds for Urinary Bladder Tissue Engineering. Ann. Biomed. Eng. 2019, 47, 891–901. [Google Scholar] [CrossRef]
- Horst, M.; Madduri, S.; Milleret, V.; Sulser, T.; Gobet, R.; Eberli, D. A bilayered hybrid microfibrous PLGA—Acellular matrix scaffold for hollow organ tissue engineering. Biomaterials 2013, 34, 1537–1545. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.-H.; Sacks, M.S.; Chung, S.Y.; Gloeckner, D.C.; Pruchnic, R.; Huard, J.; de Groat, W.C.; Chancellor, M.B. Biaxial mechanical properties of muscle-derived cell seeded small intestinal submucosa for bladder wall reconstitution. Biomaterials 2005, 26, 443–449. [Google Scholar] [CrossRef]
- Schaefer, M.; Kaiser, A.; Stehr, M.; Beyer, H.J. Bladder augmentation with small intestinal submucosa leads to unsatisfactory long-term results. J. Pediatr. Urol. 2013, 9, 878–883. [Google Scholar] [CrossRef]
- Seth, A.; Chung, Y.G.; Gil, E.S.; Tu, D.; Franck, D.; Di Vizio, D.; Adam, R.M.; Kaplan, D.L.; Estrada, C.R.; Mauney, J.R. The performance of silk scaffolds in a rat model of augmentation cystoplasty. Biomaterials 2013, 34, 4758–4765. [Google Scholar] [CrossRef] [Green Version]
- Bury, M.I.; Fuller, N.J.; Meisner, J.W.; Hofer, M.D.; Webber, M.J.; Chow, L.W.; Prasad, S.; Thaker, H.; Yue, X.; Menon, V.S.; et al. The promotion of functional urinary bladder regeneration using anti-inflammatory nanofibers. Biomaterials 2014, 35, 9311–9321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokrywczynska, M.; Jundzill, A.; Adamowicz, J.; Kowalczyk, T.; Warda, K.; Rasmus, M.; Buchholz, L.; Krzyzanowska, S.; Nakielski, P.; Chmielewski, T.; et al. Is the poly (L-lactide-co-caprolactone) nanofibrous membrane suitable for urinary bladder regeneration? PLoS ONE 2014, 9, e105295. [Google Scholar]
- Mauney, J.R.; Cannon, G.M.; Lovett, M.L.; Gong, E.M.; Di Vizio, D.; Gomez, P.; Kaplan, D.L.; Adam, R.M.; Estrada, C.R. Evaluation of gel spun silk-based biomaterials in a murine model of bladder augmentation. Biomaterials 2011, 32, 808–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kropp, B.P.; Rippy, M.K.; Badylak, S.F.; Adams, M.C.; Keating, M.A.; Rink, R.C.; Thor, K.B. Regenerative urinary bladder augmentation using small intestinal submucosa: Urodynamic and histopathologic assessment in long-term canine bladder augmentations. J. Urol. 1996, 155, 2098–2104. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, H.-K.; Frimberger, D.; Epstein, R.B.; Kropp, B.P. Growth of bone marrow stromal cells on small intestinal submucosa: An alternative cell source for tissue engineered bladder. BJU Int. 2005, 96, 1120–1125. [Google Scholar] [CrossRef]
- Zhang, Y.; Frimberger, D.; Cheng, E.Y.; Lin, H.-K.; Kropp, B.P. Challenges in a larger bladder replacement with cell-seeded and unseeded small intestinal submucosa grafts in a subtotal cystectomy model. BJU Int. 2006, 98, 1100–1105. [Google Scholar] [CrossRef]
- Caione, P.; Capozza, N.; Zavaglia, D.; Palombaro, G.; Boldrini, R. In vivo bladder regeneration using small intestinal submucosa: Experimental study. Pediatr. Surg. Int. 2006, 22, 593–599. [Google Scholar] [CrossRef]
- Ashley, R.A.; Roth, C.C.; Palmer, B.W.; Kibar, Y.; Routh, J.C.; Fung, K.-M.; Frimberger, D.; Lin, H.-K.; Kropp, B.P. Regional variations in small intestinal submucosa evoke differences in inflammation with subsequent impact on tissue regeneration in the rat bladder augmentation model. BJU Int. 2010, 105, 1462–1468. [Google Scholar] [CrossRef]
- Roth, C.C.; Mondalek, F.G.; Kibar, Y.; Ashley, R.A.; Bell, C.H.; Califano, J.A.; Madihally, S.V.; Frimberger, D.; Lin, H.-K.; Kropp, B.P. Bladder regeneration in a canine model using hyaluronic acid-poly(lactic-co-glycolic-acid) nanoparticle modified porcine small intestinal submucosa. BJU Int. 2011, 108, 148–155. [Google Scholar] [CrossRef]
- Sharma, A.K.; Bury, M.I.; Marks, A.J.; Fuller, N.J.; Meisner, J.W.; Tapaskar, N.; Halliday, L.C.; Matoka, D.J.; Cheng, E.Y. A nonhuman primate model for urinary bladder regeneration using autologous sources of bone marrow-derived mesenchymal stem cells. Stem Cells 2011, 29, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Eberli, D.; Susaeta, R.; Yoo, J.J.; Atala, A. Tunica repair with acellular bladder matrix maintains corporal tissue function. Int. J. Impot. Res. 2007, 19, 602–609. [Google Scholar] [CrossRef]
- Adamowicz, J.; Juszczak, K.; Poletajew, S.; Van Breda, S.V.; Pokrywczynska, M.; Drewa, T. Scented Candles as an Unrecognized Factor that Increases the Risk of Bladder Cancer; Is There Enough Evidence to Raise a Red Flag? Cancer Prev. Res. 2019, 12, 645–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.J.; Meng, J.; Oberpenning, F.; Atala, A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology 1998, 51, 221–225. [Google Scholar] [CrossRef]
- Coutu, D.L.; Mahfouz, W.; Loutochin, O.; Galipeau, J.; Corcos, J. Tissue engineering of rat bladder using marrow-derived mesenchymal stem cells and bladder acellular matrix. PLoS ONE 2014, 9, e111966. [Google Scholar] [CrossRef]
- Pokrywczynska, M.; Gubanska, I.; Drewa, G.; Drewa, T. Application of bladder acellular matrix in urinary bladder regeneration: The state of the art and future directions. BioMed Res. Int. 2015, 2015, 613439. [Google Scholar] [CrossRef]
- Sloff, M.; Simaioforidis, V.; de Vries, R.; Oosterwijk, E.; Feitz, W. Tissue engineering of the bladder—Reality or myth? A systematic review. J. Urol. 2014, 192, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Puranik, A.S.; Gauvin, R.; Edalat, F.; Carrillo-Conde, B.; Peppas, N.A.; Khademhosseini, A. Building vascular networks. Sci. Transl. Med. 2012, 4, 160ps23. [Google Scholar] [CrossRef] [Green Version]
- Adamowicz, J.; Pasternak, I.; Kloskowski, T.; Gniadek, M.; Van Breda, S.V.; Buhl, M.; Balcerczyk, D.; Gagat, M.; Grzanka, D.; Strupinski, W.; et al. Development of a conductive biocomposite combining graphene and amniotic membrane for replacement of the neuronal network of tissue-engineered urinary bladder. Sci. Rep. 2020, 10, 5824. [Google Scholar] [CrossRef] [PubMed]
- Smolar, J.; Horst, M.; Sulser, T.; Eberli, D. Bladder regeneration through stem cell therapy. Expert Opin. Biol. Ther. 2018, 18, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.F.; Cunnane, E.M.; Mulvihill, J.J.; Quinlan, M.R.; Bolton, D.M.; Walsh, M.T.; Jack, G.S. The role of stem cells for reconstructing the lower urinary tracts. Curr. Stem Cell Res. Ther. 2018, 13, 458–465. [Google Scholar] [CrossRef] [PubMed]













| Author | Samples | Ultimate Tensile Strain (%) | Ultimate Tensile Strength (MPa) | |
|---|---|---|---|---|
| Dahms et al., 1998 [47] | Different bladders | Human | 69 ± 17 | 0.27 ± 0.14 |
| Pig | 166 ± 31 | 0.32 ± 0.1 | ||
| Rat | 203 ± 44 | 0.72 ± 0.21 | ||
| Korossis et al., 2009 [48] | Porcine Bladder | Ventral | 350 ± 50 | 0.9 ± 0.2 |
| Dorsal | 290 ± 30 | 1.0 ± 0.2 | ||
| Lateral | 290 ± 30 | 1.1 ± 0.1 | ||
| Martins et al., 2011 [49] | Human female bladder | Bladder dome (no direction indicated) | - | 0.9 ± 0.1 |
| Tu et al., 2013 [50] | Fresh Porcine Bladder | No site indicated | 700 ± 10 | 0.21 ± 0.04 |
| Jokandan et al., 2018 [51] | Fresh Porcine Bladder | Circumferential | 435 ± 69 | 0.28 ± 0.03 |
| Longitudinal | 358 ± 21 | 0.34 ± 0.014 | ||
| Ball burst | 389.9 ± 58.9 | 1.45 ± 0.39 | ||
| Author | Scaffold Type | Regeneration Site | Cells Seeded | Results | Sample Type | Ultimate Tensile Stress (Mpa) | Ultimate Tensile Strain (%) |
|---|---|---|---|---|---|---|---|
| Qiu et al., 2019 [70] | SIS | none | none | The actual mechanical properties of sis can differ depending on animal age [71]. | SIS | 0.05 ± 0.0017 | 470.3 ± 1.93 |
| Stankus et al., 2008 [72] | urinary bladder matrix and PEUU electrospun mats | subcutaneous injection | vascular smooth muscle cells | With higher PEUU content higher tensile strength and strain were observed, however more PEUU was associated with higher inflammation. | PEUU content 25% | 2 ± 0.1 | 40 ± 0.6 |
| PEUU content 50% | 4.9 ± 1.6 | 83 ± 31 | |||||
| PEUU content 75% | 11.8 ± 0.7 | 143 ± 10 | |||||
| PEUU content 100% | 12.9 ± 1.7 | 220 ± 77 | |||||
| Ajalloueian et al., 2013 [73] | hybrid of plastic-compressed collagen with PLGA electrospun fibres | Petri dish | the minced pig bladder mucosa | Increase in tensile strength for the hybrid scaffold, the cells from mince infiltrated the construct and after 4 weeks formed urothelium typical to urothelial histology. | Plastic-compressed collagen | 0.6 ± 0.12 | 5 |
| Hybrid of plastic-compressed collagen and PLGA nanofibers | 3.57 ± 1.1 | 81 | |||||
| Ajalloueian et al., 2014 [74] | hybrid of plastic-compressed collagen with PCL-knitted material | petri dish | the minced pig bladder mucosa | Both scaffolds support the growth of the urothelium. The hybrid showed higher tensile strength and remained stable while the collagen significantly contracted. | Plastic-compressed collagen | 0.6 ± 0.12 | 5 |
| Hybrid of plastic-compressed collagen and PCL knitted mesh | 17.9 ± 2.6 | 67 | |||||
| Zhao et al., 2015 [75] | hybrid of porous silk fibroin and BAM graft | rat bladder | none | No significant loss of tissue response or systemic toxicity. The material supports regeneration. The procedure increases bladder capacity compared to the control group. | BAM-silk fibroin hybrid scaffold | 0.39 ± 0.09 | 88.17 ± 18.16 |
| Sivaraman et al., 2015 [76] | composite hydrogel Tetronic (BASF) 1107-acrylate (T1107A) blend with collagen and hyaluronic acid | petri dish | bladder smooth muscle cells | The constructs were showing higher stiffness the more time the cells were cultured on the scaffold. The study hypothesized that prolonging the culture might lead to matching properties to the bladder. | acellular | 0.0041 ± 0.0012 | 121 ± 123 |
| cellular 7 days | 0.0052 ± 0.0006 | 123 ± 4 | |||||
| cellular 14 days | 0.0116 ± 0.0022 | 139 ± 12 | |||||
| Yao et al., 2013 [77] | PLGA and polyurethane nanoscaffolds composite | minipig model | none | Composite scaffold supported regeneration in a minipig model compared to ileal segments. The engineered scaffold layer was easily separable after tissue regeneration. | PLGA and polyurethane nanoscaffolds composite | 0.71 ± 0.15 | NA |
| Tu et al., 2013 [50] | silk fibroin scaffold created using solvent-casting or solvent-casting and silk film casting | Yorkshire swine | none | Animals showed high rates of survival. An increase in bladder capacity and compliance was seen. The scaffold also supported the tissue regeneration with innervation and vascularisation. | solvent-casting pre-operatively | 0.05 ± 0.03 | 30 ± 10 |
| solvent-casting and silk film casting pre-operatively | 0.14 ± 0.06 | 45 ± 12.5 | |||||
| solvent-casting post-operatively | 0.26 ± 0.02 | 550 ± 10 | |||||
| solvent-casting and silk film casting post-operatively | 0.25 ± 0.03 | 300 ± 50 | |||||
| Khademolqorani et al., 2021 [24] | weft knit, cast film and electrospun scaffolds from silk fibroin material | petri dish | mouse fibroblasts | The knit scaffold showed superiority when considering properties important for bladder regeneration, such as lowest stiffness and highest strength and high cell infiltration. | weft knit course direction | 7.8 ± 0.9 | 377.7 ± 15.4 |
| weft knit wale direction | 9.4 ± 1.3 | 138.7 ± 14.1 | |||||
| cast film | 0.2 ± 0.1 | 77.7 ± 9.1 | |||||
| electrospun fibres | 1.0 ± 0.2 | 19.8 ± 3.4 | |||||
| Del Daudio et al., 2013 [78] | electrospun PCL and PHBV blend 50:50 | rat model | none | Scaffold showed some regenerative capabilities with urothelium coverage and muscle cell infiltration. | 50:50 PCL and PHBV blend electrospun mats | 1.4 ± 0.3 | 270 ± 80 |
| Sivaraman et al., 2013 [79] | PCUU, PGS-PCL and PEUU electrospun scaffolds | rat model | none | The scaffolds were tested using cytocompatibility studies and based on the results the PCUU scaffold was selected for bladder augmentation. Bladder augmentation increased animal survival but was associated with stone formation. | PGS-PCL | 0.072 ± 0.005 (N/mm) | 215 ± 28 |
| PEUU | 1.75 ± 0.68 (N/mm) | 247 ± 52 | |||||
| PCUU | 0.43 ± 0.029 (N/mm) | 243 ± 26 | |||||
| Dahms et al., 1998 [47] | rat, pig and human BAM | none | none | The scaffolds show different levels of collagen that comprise the matrix. The differences between the mechanical properties were not found to be statistically significantly different. | rat BAM | 0.68 ± 0.21 | 0.73 ± 0.23 |
| pig BAM | 0.29 ± 0.05 | 1.86 ± 0.51 | |||||
| human BAM | 0.13 ± 0.05 | 0.91 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanczar, M.; Moazen, M.; Day, R. The Significance of Biomechanics and Scaffold Structure for Bladder Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 12657. https://doi.org/10.3390/ijms222312657
Hanczar M, Moazen M, Day R. The Significance of Biomechanics and Scaffold Structure for Bladder Tissue Engineering. International Journal of Molecular Sciences. 2021; 22(23):12657. https://doi.org/10.3390/ijms222312657
Chicago/Turabian StyleHanczar, Marta, Mehran Moazen, and Richard Day. 2021. "The Significance of Biomechanics and Scaffold Structure for Bladder Tissue Engineering" International Journal of Molecular Sciences 22, no. 23: 12657. https://doi.org/10.3390/ijms222312657
APA StyleHanczar, M., Moazen, M., & Day, R. (2021). The Significance of Biomechanics and Scaffold Structure for Bladder Tissue Engineering. International Journal of Molecular Sciences, 22(23), 12657. https://doi.org/10.3390/ijms222312657






