Self-Assembling Peptides: From Design to Biomedical Applications
Abstract
:1. Introduction
2. Self-Assembly Process
2.1. Single Amino Acids
2.2. Dipeptides
2.3. D/L-Peptides
2.4. Cyclic Peptides
2.5. Stapled Peptides
2.6. Amphiphiles and Branched Amphiphilic Peptides
2.7. Bolaamphiphilic and Surfactant-Like Peptides
2.8. Multi-Domain Peptides
2.9. Lipidated Self-Assembly Peptides
3. Co-Assembly Process
4. Self-Assembly Peptides in Biomedical Applications
4.1. In Diagnostics
4.2. In Luminescence and Optoelectronics
4.3. For Bioprinting
4.4. As Antifouling, Antimicrobial, and Antiviral Agents
4.5. Self-Assembling Peptides as Drug Delivery Systems
4.6. Self-Assembly Peptides for Tissue Engineering and Regenerative Medicine
5. SAPs in the Pharmaceutical Market
5.1. RADA16: Hemostatic Agent for Surgery
5.2. PF11-4: Treatment of Dental Caries
5.3. Lanreotide: Treatment of Neuroendocrine Tumors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, S.; Sharma, A.K.; Kumar, P. Nanoscale Self-Assembly for Therapeutic Delivery. Front. Bioeng. Biotechnol. 2020, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-T.; Xia, Y.-Y.; Gao, J.-Q.; Xu, D.-H.; Han, M. Recent Progress in the Design and Medical Application of In Situ Self-Assembled Polypeptide Materials. Pharmaceutics 2021, 13, 753. [Google Scholar] [CrossRef] [PubMed]
- La Manna, S.; Roviello, V.; Scognamiglio, P.L.; Diaferia, C.; Giannini, C.; Sibillano, T.; Morelli, G.; Novellino, E.; Marasco, D. Amyloid fibers deriving from the aromatic core of C-terminal domain of nucleophosmin 1. Int. J. Biol. Macromol. 2019, 122, 517–525. [Google Scholar] [CrossRef]
- Inoue, S.; Sugiyama, S.; Travers, A.A.; Ohyama, T. Self-Assembly of Double-Stranded DNA Molecules at Nanomolar Concentrations. Biochemistry 2007, 46, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Kentsis, A.; Borden, K.L. Physical Mechanisms and Biological Significance of Supramolecular Protein Self-Assembly. Curr. Protein Pept. Sci. 2004, 5, 125–134. [Google Scholar] [CrossRef]
- Mendes, A.C.L.; Baran, E.T.; Reis, R.L.; Azevedo, H.S. Self-assembly in nature: Using the principles of nature to create complex nanobiomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 582–612. [Google Scholar] [CrossRef]
- Di Natale, C.; Monaco, A.; Pedone, C.; Tessitore, A.; De Mase, A.; Tedeschi, G.; Netti, P.A.; Abrescia, P. The level of 24-hydroxycholesteryl esters decreases in plasma of patients with Parkinson’s disease. Neurosci. Lett. 2018, 672, 108–112. [Google Scholar] [CrossRef]
- Gelain, F.; Luo, Z.; Rioult, M.; Zhang, S. Self-assembling peptide scaffolds in the clinic. npj Regen. Med. 2021, 6, 1–8. [Google Scholar] [CrossRef]
- Di Natale, C.; Florio, D.; Di Somma, S.; Di Matteo, A.; Federici, L.; Netti, P.A.; Morelli, G.; Malfitano, A.M.; Marasco, D. Proteostasis unbalance of nucleophosmin 1 in Acute Myeloid Leukemia: An aggregomic perspective. Int. J. Biol. Macromol. 2020, 164, 3501–3507. [Google Scholar] [CrossRef]
- Di Natale, C.; Natale, C.F.; Florio, D.; Netti, P.A.; Morelli, G.; Ventre, M.; Marasco, D. Effects of surface nanopatterning on internalization and amyloid aggregation of the fragment 264-277 of Nucleophosmin 1. Colloids Surf. B Biointerfaces 2021, 197, 111439. [Google Scholar] [CrossRef]
- Florio, D.; Di Natale, C.; Scognamiglio, P.L.; Leone, M.; La Manna, S.; Di Somma, S.; Netti, P.A.; Malfitano, A.M.; Marasco, D. Self-assembly of bio-inspired heterochiral peptides. Bioorgan. Chem. 2021, 114, 105047. [Google Scholar] [CrossRef]
- Di Natale, C.; La Manna, S.; De Benedictis, I.; Brandi, P.; Marasco, D. Perspectives in Peptide-Based Vaccination Strategies for Syndrome Coronavirus 2 Pandemic. Front. Pharmacol. 2020, 11, 578382. [Google Scholar] [CrossRef] [PubMed]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef] [PubMed]
- La Manna, S.; Di Natale, C.; Florio, D.; Marasco, D. Peptides as Therapeutic Agents for Inflammatory-Related Diseases. Int. J. Mol. Sci. 2018, 19, 2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.A.; Shi, S.; Wang, B.K.; Li, I.-C.; Jalan, A.A.; Sarkar, B.; Wickremasinghe, N.C.; Hartgerink, J.D. Drug-Triggered and Cross-Linked Self-Assembling Nanofibrous Hydrogels. J. Am. Chem. Soc. 2015, 137, 4823–4830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lock, L.L.; Li, Y.; Mao, X.; Chen, H.; Staedtke, V.; Bai, R.; Ma, W.; Lin, R.; Li, Y.; Liu, G.; et al. One-Component Supramolecular Filament Hydrogels as Theranostic Label-Free Magnetic Resonance Imaging Agents. ACS Nano 2017, 11, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Karavasili, C.; Fatouros, D.G. Self-assembling peptides as vectors for local drug delivery and tissue engineering applications. Adv. Drug Deliv. Rev. 2021, 174, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, M.; Nakamura, R.; Kishimoto, Y.; Yurie, H.; Hayashi, Y.; Kaba, S.; Ohnishi, H.; Yamashita, M.; Tateya, I.; Omori, K. Recurrent laryngeal nerve regeneration using a self-assembling peptide hydrogel. Laryngoscope 2020, 130, 2420–2427. [Google Scholar] [CrossRef]
- Lee, S.; Trinh, T.H.; Yoo, M.; Shin, J.; Lee, H.; Kim, J.; Hwang, E.; Lim, Y.-B.; Ryou, C. Self-Assembling Peptides and Their Application in the Treatment of Diseases. Int. J. Mol. Sci. 2019, 20, 5850. [Google Scholar] [CrossRef] [Green Version]
- Matson, J.; Zha, R.; Stupp, S.I. Peptide self-assembly for crafting functional biological materials. Curr. Opin. Solid State Mater. Sci. 2011, 15, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Marchini, A.; Favoino, C.; Gelain, F. Multi-Functionalized Self-Assembling Peptides as Reproducible 3D Cell Culture Systems Enabling Differentiation and Survival of Various Human Neural Stem Cell Lines. Front. Neurosci. 2020, 14, 413. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Chen, X.; Lu, Y.; Yang, W. Self-Assembled Peptide Hydrogel as a Smart Biointerface for Enzyme-Based Electrochemical Biosensing and Cell Monitoring. ACS Appl. Mater. Interfaces 2016, 8, 25036–25042. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, W.; Ye, C.; Sun, W.; Xie, H.; Yang, X.; Zhang, Q.; Ma, Y. Local mucosal immunization of self-assembled nanofibers elicits robust antitumor effects in an orthotopic model of mouse genital tumors. Nanoscale 2020, 12, 3076–3089. [Google Scholar] [CrossRef]
- Sankar, S.; O’Neill, K.; D’Arc, M.B.; Rebeca, F.; Buffier, M.; Aleksi, E.; Fan, M.; Matsuda, N.; Gil, E.S.; Spirio, L. Clinical Use of the Self-Assembling Peptide RADA16: A Review of Current and Future Trends in Biomedicine. Front. Bioeng. Biotechnol. 2021, 9, 679525. [Google Scholar] [CrossRef] [PubMed]
- Doberdoli, D.; Bommer, C.; Begzati, A.; Haliti, F.; Heinzel-Gutenbrunner, M.; Juric, H. Randomized Clinical Trial investigating Self-Assembling Peptide P11-4 for Treatment of Early Occlusal Caries. Sci. Rep. 2020, 10, 1–9. [Google Scholar]
- Wolin, E.M.; Manon, A.; Chassaing, C.; Lewis, A.; Bertocchi, L.; Richard, J.; Phan, A.T. Lanreotide Depot: An Antineoplastic Treatment of Carcinoid or Neuroendocrine Tumors. J. Gastrointest. Cancer 2016, 47, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, A.; Mondal, J.H.; Das, D. Peptide hydrogels. RSC Adv. 2013, 3, 9117–9149. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, H.; Fu, D.; Gao, P.; Lam, J.K.; Xu, B. Enzymatic Formation of Supramolecular Hydrogels. Adv. Mater. 2004, 16, 1440–1444. [Google Scholar] [CrossRef]
- Ryan, D.M.; Nilsson, B.L. Self-assembled amino acids and dipeptides as noncovalent hydrogels for tissue engineering. Polym. Chem. 2012, 3, 18–33. [Google Scholar] [CrossRef]
- Ryan, D.M.; Anderson, S.B.; Nilsson, B.L. The influence of side-chain halogenation on the self-assembly and hydrogelation of Fmoc-phenylalanine derivatives. Soft Matter 2010, 6, 3220–3231. [Google Scholar] [CrossRef]
- Ryan, D.M.; Doran, T.M.; Anderson, S.B.; Nilsson, B.L. Effect of C-Terminal Modification on the Self-Assembly and Hydrogelation of Fluorinated Fmoc-Phe Derivatives. Langmuir 2011, 27, 4029–4039. [Google Scholar] [CrossRef] [PubMed]
- Görbitz, C.H. The structure of nanotubes formed by diphenylalanine, the core recognition motif of Alzheimer’s β-amyloid polypeptide. Chem. Commun. 2006, 22, 2332–2334. [Google Scholar] [CrossRef] [PubMed]
- Lampel, A.; Ulijn, R.V.; Tuttle, T. Guiding principles for peptide nanotechnology through directed discovery. Chem. Soc. Rev. 2018, 47, 3737–3758. [Google Scholar] [CrossRef] [Green Version]
- Arakawa, H.; Takeda, K.; Higashi, S.L.; Shibata, A.; Kitamura, Y.; Ikeda, M. Self-assembly and hydrogel formation ability of Fmoc-dipeptides comprising α-methyl-L-phenylalanine. Polym. J. 2020, 52, 923–930. [Google Scholar] [CrossRef]
- Martin, A.D.; Thordarson, P. Beyond Fmoc: A review of aromatic peptide capping groups. J. Mater. Chem. B 2020, 8, 863–877. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Casting Metal Nanowires Within Discrete Self-Assembled Peptide Nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Revel, S.; Morris, K.; Serpell, L.; Adams, D. Effect of Molecular Structure on the Properties of Naphthalene−Dipeptide Hydrogelators. Langmuir 2010, 26, 13466–13471. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Self-assembly of peptide nanotubes and amyloid-like structures by charged-termini-capped diphenylalanine peptide analogues. Isr. J. Chem. 2005, 45, 363–371. [Google Scholar] [CrossRef]
- Smith, A.; Williams, R.; Tang, C.; Coppo, P.; Collins, R.F.; Turner, M.; Saiani, A.; Ulijn, R.V. Fmoc-Diphenylalanine Self Assembles to a Hydrogel via a Novel Architecture Based on π–π Interlocked β-Sheets. Adv. Mater. 2007, 20, 37–41. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Designed aromatic homo-dipeptides: Formation of ordered nanostructures and potential nanotechnological applications. Phys. Biol. 2006, 3, S10–S19. [Google Scholar] [CrossRef] [Green Version]
- Görbitz, C.H. Nanotubes from hydrophobic dipeptides: Pore size regulation through side chain substitution. New J. Chem. 2003, 27, 1789–1793. [Google Scholar] [CrossRef]
- Jeena, M.T.; Jeong, K.; Go, E.M.; Cho, Y.; Lee, S.; Jin, S.; Hwang, S.-W.; Jang, J.H.; Kang, C.S.; Bang, W.-Y.; et al. Heterochiral Assembly of Amphiphilic Peptides Inside the Mitochondria for Supramolecular Cancer Therapeutics. ACS Nano 2019, 13, 11022–11033. [Google Scholar] [CrossRef]
- Cringoli, M.C.; Romano, C.; Parisi, E.; Waddington, L.J.; Melchionna, M.; Semeraro, S.; De Zorzi, R.; Grönholm, M.; Marchesan, S. Bioadhesive supramolecular hydrogel from unprotected, short d,l-peptides with Phe-Phe and Leu-Asp-Val motifs. Chem. Commun. 2020, 56, 3015–3018. [Google Scholar] [CrossRef]
- Nagy, K.J.; Giano, M.C.; Jin, A.; Pochan, D.J.; Schneider, J.P. Enhanced Mechanical Rigidity of Hydrogels Formed from Enantiomeric Peptide Assemblies. J. Am. Chem. Soc. 2011, 133, 14975–14977. [Google Scholar] [CrossRef] [Green Version]
- Cringoli, M.C.; Bellotto, O.; DE Zorzi, R.; Vargiu, A.V.; Marchesan, S. Self-Assembling l-d-l-Tripeptides Dance the Twist. Synlett 2020, 31, 434–438. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Chu, L.; Zhang, Y.; Xu, H.; Kong, D.; Yang, Z.; Yang, C.; Ding, D. Self-Assembling Peptide of d-Amino Acids Boosts Selectivity and Antitumor Efficacy of 10-Hydroxycamptothecin. ACS Appl. Mater. Interfaces 2014, 6, 5558–5565. [Google Scholar] [CrossRef]
- Insua, I.; Montenegro, J. 1D to 2D Self Assembly of Cyclic Peptides. J. Am. Chem. Soc. 2019, 142, 300–307. [Google Scholar] [CrossRef]
- Ghadiri, M.R.; Granja, J.; Milligan, R.A.; McRee, D.E.; Khazanovich, N. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nat. Cell Biol. 1993, 366, 324–327. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, B.; Xia, F.; Xie, Y.; Jiang, S.; Su, R.; Lu, Y.; Wu, W. Transmembrane delivery of anticancer drugs through self-assembly of cyclic peptide nanotubes. Nanoscale 2016, 8, 7127–7136. [Google Scholar] [CrossRef]
- Argudo, P.G.; Giner-Casares, J.J. Folding and self-assembly of short intrinsically disordered peptides and protein regions. Nanoscale Adv. 2021, 3, 1789–1812. [Google Scholar] [CrossRef]
- Mondal, S.; Adler-Abramovich, L.; Lampel, A.; Bram, Y.; Lipstman, S.; Gazit, E. Formation of functional super-helical assemblies by constrained single heptad repeat. Nat. Commun. 2015, 6, 8615. [Google Scholar] [CrossRef]
- La Manna, S.; Scognamiglio, P.L.; Di Natale, C.; Leone, M.; Mercurio, F.A.; Malfitano, A.M.; Cianfarani, F.; Madonna, S.; Caravella, S.; Albanesi, C.; et al. Characterization of linear mimetic peptides of Interleukin-22 from dissection of protein interfaces. Biochimie 2017, 138, 106–115. [Google Scholar] [CrossRef]
- Mercurio, F.; Di Natale, C.; Pirone, L.; Marasco, D.; Calce, E.; Vincenzi, M.; Pedone, E.M.; De Luca, S.; Leone, M. Design and analysis of EphA2-SAM peptide ligands: A multi-disciplinary screening approach. Bioorgan. Chem. 2019, 84, 434–443. [Google Scholar] [CrossRef]
- Mercurio, F.A.; Di Natale, C.; Pirone, L.; Scognamiglio, P.L.; Marasco, D.; Pedone, E.M.; Saviano, M.; Leone, M. Peptide Fragments of Odin-Sam1: Conformational Analysis and Interaction Studies with EphA2-Sam. ChemBioChem 2015, 16, 1629–1636. [Google Scholar] [CrossRef]
- Mercurio, F.; Pirone, L.; Di Natale, C.; Marasco, D.; Pedone, E.M.; Leone, M. Sam domain-based stapled peptides: Structural analysis and interaction studies with the Sam domains from the EphA2 receptor and the lipid phosphatase Ship2. Bioorgan. Chem. 2018, 80, 602–610. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.; Yang, F.; Wan, C.; Cai, X.; Liu, J.; Zhang, Q.; Li, Z.; Han, W. Molecular design of stapled pentapeptides as building blocks of self-assembled coiled coil–like fibers. Sci. Adv. 2021, 7, eabd0492. [Google Scholar] [CrossRef]
- Gore, T.; Dori, Y.; Talmon, Y.; Tirrell, M.; Bianco-Peled, H. Self-Assembly of Model Collagen Peptide Amphiphiles. Langmuir 2001, 17, 5352–5360. [Google Scholar] [CrossRef]
- Qiu, F.; Chen, Y.; Tang, C.; Zhao, X. Amphiphilic peptides as novel nanomaterials: Design, self-assembly and application. Int. J. Nanomed. 2018, 13, 5003–5022. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Singh, I.; Sharma, A.K.; Kumar, P. Ultrashort Peptide Self-Assembly: Front-Runners to Transport Drug and Gene Cargos. Front. Bioeng. Biotechnol. 2020, 8, 504. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, X.; Feng, F.; Xu, J.; Wu, C.; Dai, G.; Yue, W.; Zhong, W.; Xu, K. Molecular design of peptide amphiphiles for controlled self-assembly and drug release. J. Mater. Chem. B 2021, 9, 3326–3334. [Google Scholar] [CrossRef]
- Adams, D.J.; Holtzmann, K.; Schneider, A.C.; Butler, M.F. Self-Assembly of Surfactant-like Peptides. Langmuir 2007, 23, 12729–12736. [Google Scholar] [CrossRef]
- Qiu, F.; Chen, Y.; Tang, C.; Zhang, J.; Gong, M.; Su, B. Self-assembling surfactant-like peptide A6K as potential delivery system for hydrophobic drugs. Int. J. Nanomed. 2015, 10, 847–858. [Google Scholar] [CrossRef] [Green Version]
- Fariya, M.; Jain, A.; Dhawan, V.; Shah, S.; Nagarsenker, M.S. Bolaamphiphiles: A Pharmaceutical Review. Adv. Pharm. Bull. 2014, 4, 483–491. [Google Scholar]
- Li, J.; Wang, J.; Zhao, Y.; Zhou, P.; Carter, J.; Li, Z.; Waigh, T.A.; Lu, J.R.; Xu, H. Surfactant-like peptides: From molecular design to controllable self-assembly with applications. Co-ord. Chem. Rev. 2020, 421, 213418. [Google Scholar] [CrossRef]
- Qiu, F.; Tang, C.; Chen, Y. Amyloid-like aggregation of designer bolaamphiphilic peptides: Effect of hydrophobic section and hydrophilic heads. J. Pept. Sci. 2017, 24, e3062. [Google Scholar] [CrossRef]
- Lopez-Silva, T.L.; Leach, D.G.; Li, I.-C.; Wang, X.; Hartgerink, J.D. Self-Assembling Multidomain Peptides: Design and Characterization of Neutral Peptide-Based Materials with pH and Ionic Strength Independent Self-Assembly. ACS Biomater. Sci. Eng. 2019, 5, 977–985. [Google Scholar] [CrossRef]
- Moore, A.N.; Hartgerink, J.D. Self-Assembling Multidomain Peptide Nanofibers for Delivery of Bioactive Molecules and Tissue Regeneration. Acc. Chem. Res. 2017, 50, 714–722. [Google Scholar] [CrossRef]
- Dehsorkhi, A.; Castelletto, V.; Hamley, I.W. Self-assembling amphiphilic peptides. J. Pept. Sci. 2014, 20, 453–467. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Hendricks, M.P.; Palmer, L.C.; Stupp, S.I. Peptide supramolecular materials for therapeutics. Chem. Soc. Rev. 2018, 47, 7539–7551. [Google Scholar] [CrossRef]
- Hamley, I.W.; Castelletto, V. Self-Assembly of Peptide Bioconjugates: Selected Recent Research Highlights. Bioconj. Chem. 2017, 28, 731–739. [Google Scholar] [CrossRef] [Green Version]
- Bech, E.M.; Pedersen, S.L.; Jensen, K.J. Chemical Strategies for Half-Life Extension of Biopharmaceuticals: Lipidation and Its Alternatives. ACS Med. Chem. Lett. 2018, 9, 577–580. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, J.A.; Hamley, I.W.; Torras, J.; Alemán, C.; Seitsonen, J.; Ruokolainen, J. Self-Assembly of Lipopeptides Containing Short Peptide Fragments Derived from the Gastrointestinal Hormone PYY3–36: From Micelles to Amyloid Fibrils. J. Phys. Chem. B 2019, 123, 614–621. [Google Scholar] [CrossRef]
- Hutchinson, J.A.; Burholt, S.; Hamley, I.W.; Lundback, A.-K.; Uddin, S.; dos Santos, A.L.G.; Reza, M.; Seitsonen, J.; Ruokolainen, J. The Effect of Lipidation on the Self-Assembly of the Gut-Derived Peptide Hormone PYY3–36. Bioconj. Chem. 2018, 29, 2296–2308. [Google Scholar] [CrossRef] [Green Version]
- Giraud, T.; Bouguet-Bonnet, S.; Stébé, M.-J.; Richaudeau, L.; Pickaert, G.; Averlant-Petit, M.-C.; Stefan, L. Co-assembly and multicomponent hydrogel formation upon mixing nucleobase-containing peptides. Nanoscale 2021, 13, 10566–10578. [Google Scholar] [CrossRef]
- Tajima, A.; Liu, W.; Pradhan, I.; Bertera, S.; Bagia, C.; Trucco, M.; Meng, W.S.; Fan, Y. Bioengineering mini functional thymic units with EAK16-II/EAKIIH6 self-assembling hydrogel. Clin. Immunol. 2015, 160, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Boothroyd, S.; Saiani, A.; Miller, A.F. Controlling network topology and mechanical properties of co-assembling peptide hydrogels. Biopolymers 2013, 101, 669–680. [Google Scholar] [CrossRef]
- Seroski, D.T.; Dong, X.; Wong, K.M.; Liu, R.; Shao, Q.; Paravastu, A.K.; Hall, C.K.; Hudalla, G.A. Charge guides pathway selection in β-sheet fibrillizing peptide co-assembly. Commun. Chem. 2020, 3, 1–11. [Google Scholar] [CrossRef]
- Szondy, Z.; Korponay-Szabó, I.; Király, R.; Sarang, Z.; Tsay, G.J. Transglutaminase 2 in human diseases. BioMedicine 2017, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Zhang, Y.; Wu, S.; Jalalah, M.; Alsareii, S.A.; Yin, Y.; Harraz, F.A.; Li, G. Co-assembly of Peptides and Carbon Nanodots: Sensitive Analysis of Transglutaminase 2. ACS Appl. Mater. Interfaces 2021, 13, 36919–36925. [Google Scholar] [CrossRef]
- Ajovalasit, A.; Redondo-Gómez, C.; Sabatino, M.A.; Okesola, B.O.; Braun, K.; Mata, A.; Dispenza, C. Carboxylated-xyloglucan and peptide amphiphile co-assembly in wound healing. Regen. Biomater. 2021, 8, 040. [Google Scholar] [CrossRef]
- Acar, H.; Srivastava, S.; Chung, E.J.; Schnorenberg, M.R.; Barrett, J.C.; LaBelle, J.L.; Tirrell, M. Self-assembling peptide-based building blocks in medical applications. Adv. Drug Deliv. Rev. 2017, 110-111, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Besenius, P.; Heynens, J.L.M.; Straathof, R.; Nieuwenhuizen, M.M.L.; Bomans, P.H.H.; Terreno, E.; Aime, S.; Strijkers, G.; Nicolay, K.; Meijer, E.W. Paramagnetic self-assembled nanoparticles as supramolecular MRI contrast agents. Contrast Media Mol. Imaging 2012, 7, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.-Y.; Shen, Y.-Y.; Wang, J.-D.; Li, L.; Liang, G.-L. Controlled intracellular self-assembly of gadolinium nanoparticles as smart molecular MR contrast agents. Sci. Rep. 2013, 3, srep01024. [Google Scholar] [CrossRef] [Green Version]
- Gallo, E.; Diaferia, C.; Di Gregorio, E.; Morelli, G.; Gianolio, E.; Accardo, A. Peptide-Based Soft Hydrogels Modified with Gadolinium Complexes as MRI Contrast Agents. Pharmaceuticals 2020, 13, 19. [Google Scholar] [CrossRef] [Green Version]
- Machnev, A.; Ofer, D.; Shishkin, I.; Kozlov, V.; Diaferia, C.; Accardo, A.; Morelli, G.; Apter, B.; Inberg, A.; Rosenman, G.; et al. Amplified spontaneous emission and gain in highly concentrated Rhodamine-doped peptide derivative. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Apter, B.; Fainberg, B.; Handelman, A.; Lapsker, I.; Accardo, A.; Diaferia, C.; Morelli, G.; Rosenman, G. Long-Range Fluorescence Propagation in Amyloidogenic β-Sheet Films and Fibers. Adv. Opt. Mater. 2020, 8, 2000056. [Google Scholar] [CrossRef]
- Li, C.; Iscen, A.; Sai, H.; Sato, K.; Sather, N.A.; Chin, S.M.; Álvarez, Z.; Palmer, L.C.; Schatz, G.C.; Stupp, S.I. Supramolecular–covalent hybrid polymers for light-activated mechanical actuation. Nat. Mater. 2020, 19, 900–909. [Google Scholar] [CrossRef]
- Loo, Y.; Hauser, C.A.E. Bioprinting synthetic self-assembling peptide hydrogels for biomedical applications. Biomed. Mater. 2015, 11, 014103. [Google Scholar] [CrossRef]
- Raphael, B.; Khalil, T.; Workman, V.; Smith, A.; Brown, C.; Streuli, C.; Saiani, A.; Domingos, M. 3D cell bioprinting of self-assembling peptide-based hydrogels. Mater. Lett. 2017, 190, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Rauf, S.; Susapto, H.H.; Kahin, K.; Alshehri, S.; Abdelrahman, S.; Lam, J.H.; Asad, S.; Jadhav, S.; Sundaramurthi, D.; Gao, X.; et al. Self-assembling tetrameric peptides allow in situ 3D bioprinting under physiological conditions. J. Mater. Chem. B 2021, 9, 1069–1081. [Google Scholar] [CrossRef]
- Loo, Y.; Lakshmanan, A.; Ni, M.; Toh, L.L.; Wang, S.; Hauser, C.A.E. Peptide Bioink: Self-Assembling Nanofibrous Scaffolds for Three-Dimensional Organotypic Cultures. Nano Lett. 2015, 15, 6919–6925. [Google Scholar] [CrossRef]
- Gelain, F.; Luo, Z.; Zhang, S. Self-Assembling Peptide EAK16 and RADA16 Nanofiber Scaffold Hydrogel. Chem. Rev. 2020, 120, 13434–13460. [Google Scholar] [CrossRef]
- Cofiño, C.; Perez-Amodio, S.; Semino, C.E.; Engel, E.; Mateos-Timoneda, M.A. Development of a Self-Assembled Peptide/Methylcellulose-Based Bioink for 3D Bioprinting. Macromol. Mater. Eng. 2019, 304, 1900353. [Google Scholar] [CrossRef]
- Jian, H.; Wang, M.; Dong, Q.; Li, J.; Wang, A.; Li, X.; Ren, P.; Bai, S. Dipeptide Self-Assembled Hydrogels with Tunable Mechanical Properties and Degradability for 3D Bioprinting. ACS Appl. Mater. Interfaces 2019, 11, 46419–46426. [Google Scholar] [CrossRef]
- Holm, E.R. Barnacles and Biofouling. Integr. Comp. Biol. 2012, 52, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Damodaran, V.B.; Murthy, N.S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20. [Google Scholar] [CrossRef] [Green Version]
- Sakala, G.P.; Reches, M. Peptide-Based Approaches to Fight Biofouling. Adv. Mater. Interfaces 2018, 5, 1800073. [Google Scholar] [CrossRef]
- Maity, S.; Nir, S.; Zada, T.; Reches, M. Self-assembly of a tripeptide into a functional coating that resists fouling. Chem. Commun. 2014, 50, 11154–11157. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Chang, H.-Y.; Lu, J.-K.; Huang, Y.-C.; Harroun, S.G.; Tseng, Y.-T.; Li, Y.-J.; Huang, C.-C.; Chang, H.-T. Self-Assembly of Antimicrobial Peptides on Gold Nanodots: Against Multidrug-Resistant Bacteria and Wound-Healing Application. Adv. Funct. Mater. 2015, 25, 7189–7199. [Google Scholar] [CrossRef]
- Janković, P.; Šantek, I.; Pina, A.S.; Kalafatovic, D. Exploiting Peptide Self-Assembly for the Development of Minimalistic Viral Mimetics. Front. Chem. 2021, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Mizuguchi, Y.; Kimizuka, N. Peptide nanospheres self-assembled from a modified β-annulus peptide of Sesbania mosaic virus. Biopolymers 2016, 106, 470–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, M.; Wang, Y.; Zhao, W.; Qi, R.; Han, Y.; Wu, R.; Wang, Y.; Xu, H. Peptide-Induced DNA Condensation into Virus-Mimicking Nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 24349–24360. [Google Scholar] [CrossRef]
- Di Natale, C.; De Rosa, D.; Profeta, M.; Jamaledin, R.; Attanasio, A.; Lagreca, E.; Scognamiglio, P.L.; Netti, P.A.; Vecchione, R. Design of biodegradable bi-compartmental microneedles for the stabilization and the controlled release of the labile molecule collagenase for skin healthcare. J. Mater. Chem. B 2021, 9, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Fotticchia, T.; Vecchione, R.; Scognamiglio, P.L.; Guarnieri, D.; Calcagno, V.; Di Natale, C.; Attanasio, C.; De Gregorio, M.; Di Cicco, C.; Quagliariello, V.; et al. Enhanced Drug Delivery into Cell Cytosol via Glycoprotein H-Derived Peptide Conjugated Nanoemulsions. ACS Nano 2017, 11, 9802–9813. [Google Scholar] [CrossRef] [PubMed]
- Jamaledin, R.; Di Natale, C.; Onesto, V.; Taraghdari, Z.B.; Zare, E.N.; Makvandi, P.; Vecchione, R.; Netti, P.A. Progress in Microneedle-Mediated Protein Delivery. J. Clin. Med. 2020, 9, 542. [Google Scholar] [CrossRef] [Green Version]
- Jamaledin, R.; Sartorius, R.; Di Natale, C.; Vecchione, R.; De Berardinis, P.; Netti, P.A. Recombinant Filamentous Bacteriophages Encapsulated in Biodegradable Polymeric Microparticles for Stimulation of Innate and Adaptive Immune Responses. Microorganisms 2020, 8, 650. [Google Scholar] [CrossRef]
- Lagreca, E.; Onesto, V.; Di Natale, C.; La Manna, S.; Netti, P.A.; Vecchione, R. Recent advances in the formulation of PLGA microparticles for controlled drug delivery. Prog. Biomater. 2020, 9, 153–174. [Google Scholar] [CrossRef]
- Yang, J.; An, H.-W.; Wang, H. Self-Assembled Peptide Drug Delivery Systems. ACS Appl. Bio Mater. 2021, 4, 24–46. [Google Scholar] [CrossRef]
- Makvandi, P.; Jamaledin, R.; Chen, G.; Baghbantaraghdari, Z.; Zare, E.N.; Di Natale, C.; Onesto, V.; Vecchione, R.; Lee, J.; Tay, F.R.; et al. Stimuli-responsive transdermal microneedle patches. Mater. Today 2021, 47, 206–222. [Google Scholar] [CrossRef]
- Lam, K.S.; Salmon, S.E.; Hersh, E.M.; Hruby, V.J.; Kazmierski, W.M.; Knapp, R.J. A new type of synthetic peptide library for identifying ligand-binding activity. Nat. Cell Biol. 1991, 354, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, C.; De Benedictis, I.; De Benedictis, A.; Marasco, D. Metal–Peptide Complexes as Promising Antibiotics to Fight Emerging Drug Resistance: New Perspectives in Tuberculosis. Antibiotics 2020, 9, 337. [Google Scholar] [CrossRef]
- Qi, G.-B.; Gao, Y.-J.; Wang, L.; Wang, H. Self-Assembled Peptide-Based Nanomaterials for Biomedical Imaging and Therapy. Adv. Mater. 2018, 30, e1703444. [Google Scholar] [CrossRef]
- Cao, M.; Lu, S.; Wang, N.; Xu, H.; Cox, H.; Li, R.; Waigh, T.A.; Han, Y.; Wang, Y.; Lu, J.R. Enzyme-Triggered Morphological Transition of Peptide Nanostructures for Tumor-Targeted Drug Delivery and Enhanced Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 16357–16366. [Google Scholar] [CrossRef]
- Qiao, Z.-Y.; Zhao, W.-J.; Gao, Y.-J.; Cong, Y.; Zhao, L.; Hu, Z.; Wang, H. Reconfigurable Peptide Nanotherapeutics at Tumor Microenvironmental pH. ACS Appl. Mater. Interfaces 2017, 9, 30426–30436. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhou, J.; Liang, C.; Liu, B.; Pan, X.; Zhang, Y.; Wang, Y.; Yan, B.; Xie, W.; Liu, F.; et al. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater. Sci. 2019, 7, 2920–2933. [Google Scholar] [CrossRef]
- Ren, C.; Chu, L.; Huang, F.; Yang, L.; Fan, H.; Liu, J.; Yang, C. A novel H2O2responsive supramolecular hydrogel for controllable drug release. RSC Adv. 2017, 7, 1313–1317. [Google Scholar] [CrossRef] [Green Version]
- Moyer, T.J.; Finbloom, J.A.; Chen, F.; Toft, D.J.; Cryns, V.L.; Stupp, S.I. pH and Amphiphilic Structure Direct Supramolecular Behavior in Biofunctional Assemblies. J. Am. Chem. Soc. 2014, 136, 14746–14752. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Ji, C.; Wu, P.; Jin, C.; Qian, H. Human umbilical cord mesenchymal stem cells and exosomes: Bioactive ways of tissue injury repair. Am. J. Transl. Res. 2019, 11, 1230–1240. [Google Scholar] [PubMed]
- Tian, R.; Wang, H.; Niu, R.; Ding, D. Drug delivery with nanospherical supramolecular cell penetrating peptide–taxol conjugates containing a high drug loading. J. Colloid Interface Sci. 2015, 453, 15–20. [Google Scholar] [CrossRef]
- Law, B.; Weissleder, R.; Tung, C.H. Peptide-based biomaterials for protease-enhanced drug delivery. Biomacromolecules 2006, 7, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Sangtani, A.; Petryayeva, E.; Wu, M.; Susumu, K.; Oh, E.; Huston, A.L.; Lasarte-Aragones, G.; Medintz, I.L.; Algar, W.R.; Delehanty, J.B. Intracellularly actuated quantum dot–peptide–doxorubicin nanobioconjugates for controlled drug delivery via the endocytic pathway. Bioconjug. Chem. 2018, 29, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Callmann, C.E.; Barback, C.V.; Thompson, M.P.; Hall, D.J.; Mattrey, R.F.; Gianneschi, N.C. Therapeutic Enzyme-Responsive Nanoparticles for Targeted Delivery and Accumulation in Tumors. Adv. Mater. 2015, 27, 4611–4615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, Y.; Dalhaimer, P.; Cai, S.; Tsai, R.; Tewari, M.; Minko, T.; Discher, D.E. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007, 2, 249–255. [Google Scholar] [CrossRef]
- Nguyen, M.M.; Carlini, A.S.; Chien, M.-P.; Sonnenberg, S.; Luo, C.; Braden, R.L.; Osborn, K.G.; Li, Y.; Gianneschi, N.C.; Christman, K.L. Enzyme-Responsive Nanoparticles for Targeted Accumulation and Prolonged Retention in Heart Tissue after Myocardial Infarction. Adv. Mater. 2015, 27, 5547–5552. [Google Scholar] [CrossRef]
- Cheng, D.-B.; Zhang, X.-H.; Gao, Y.-J.; Ji, L.; Hou, D.; Wang, Z.; Xu, W.; Qiao, Z.-Y.; Wang, H. Endogenous Reactive Oxygen Species-Triggered Morphology Transformation for Enhanced Cooperative Interaction with Mitochondria. J. Am. Chem. Soc. 2019, 141, 7235–7239. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.-H.; He, C.; Riviere, J.E.; Monteiro-Riviere, N.A.; Lin, Z. Meta-Analysis of Nanoparticle Delivery to Tumors Using a Physiologically Based Pharmacokinetic Modeling and Simulation Approach. ACS Nano 2020, 14, 3075–3095. [Google Scholar] [CrossRef] [Green Version]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Cong, Y.; Ji, L.; Gao, Y.; Liu, F.; Cheng, D.; Hu, Z.; Qiao, Z.; Wang, H. Microenvironment-Induced In Situ Self-Assembly of Polymer–Peptide Conjugates That Attack Solid Tumors Deeply. Angew. Chem. Int. Ed. 2019, 58, 4632–4637. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, H.; Zhou, R.; Li, J.; Xu, B. Enzyme-Instructed Assembly and Disassembly Processes for Targeting Downregulation in Cancer Cells. J. Am. Chem. Soc. 2017, 139, 3950–3953. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-H.; Cong, Y.; Qi, G.-B.; Ji, L.; Qiao, Z.-Y.; Wang, H. Near-Infrared Laser-Driven in Situ Self-Assembly as a General Strategy for Deep Tumor Therapy. Nano Lett. 2018, 18, 6577–6584. [Google Scholar] [CrossRef]
- Chen, J.; Zou, X. Self-assemble peptide biomaterials and their biomedical applications. Bioact. Mater. 2019, 4, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.; Boekhoven, J.; Dickerson, M.B.; Naik, R.R.; Stupp, S.I. Biopolymers and supramolecular polymers as biomaterials for biomedical applications. MRS Bull. 2015, 40, 1089–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eren, E.D.; Tansik, G.; Tekinay, A.B.; Guler, M.O.; Tansık, G. Mineralized Peptide Nanofiber Gels for Enhanced Osteogenic Differentiation. ChemNanoMat 2018, 4, 837–845. [Google Scholar] [CrossRef]
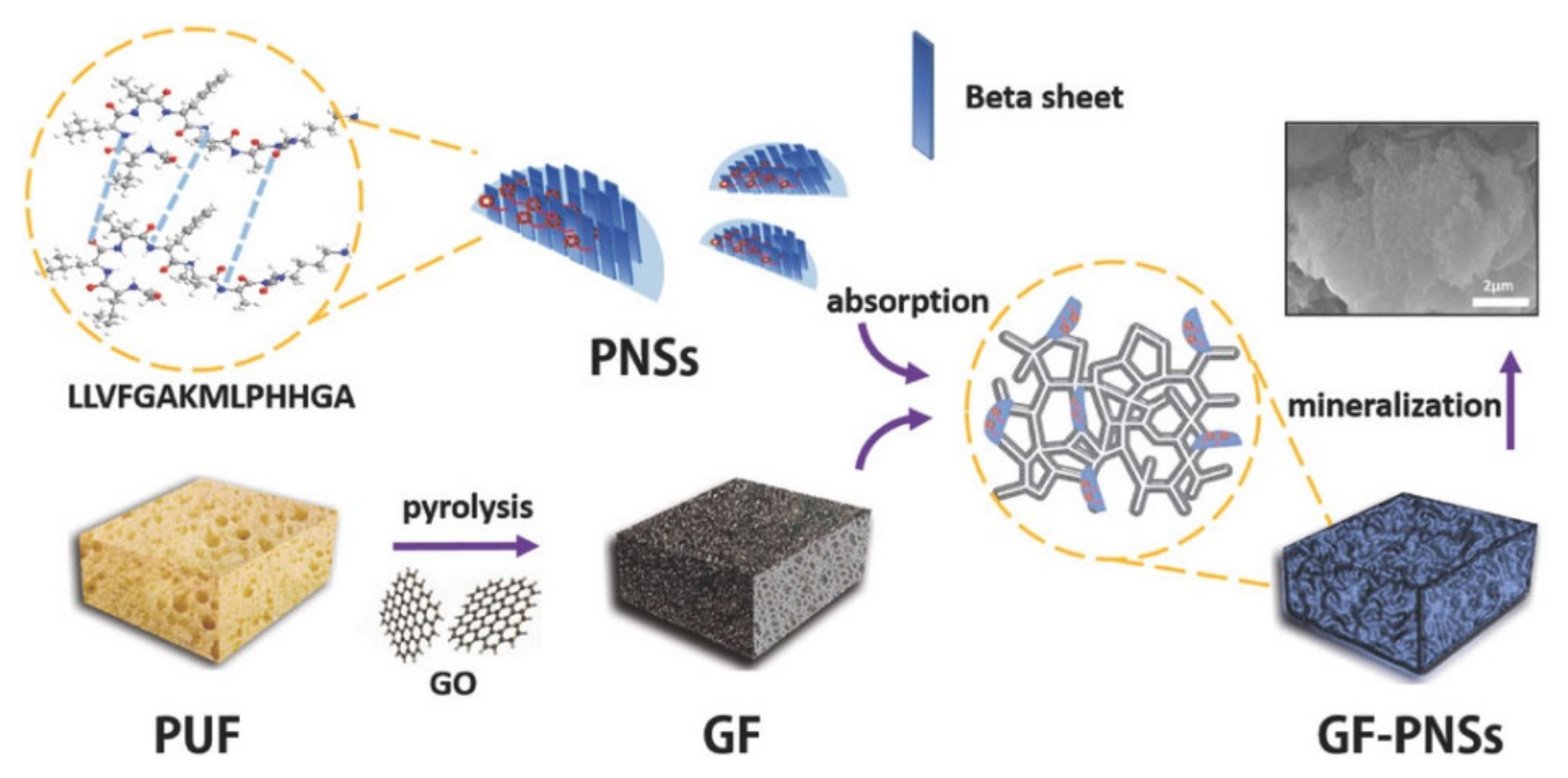
- Li, K.; Zhang, Z.; Li, D.; Zhang, W.; Yu, X.; Liu, W.; Gong, C.; Wei, G.; Su, Z. Biomimetic Ultralight, Highly Porous, Shape-Adjustable, and Biocompatible 3D Graphene Minerals via Incorporation of Self-Assembled Peptide Nanosheets. Adv. Funct. Mater. 2018, 28, 1801056. [Google Scholar] [CrossRef]
- Quan, C.; Zhang, Z.; Liang, P.; Zheng, J.; Wang, J.; Hou, Y.; Tang, Q. Bioactive gel self-assembled from phosphorylate biomimetic peptide: A potential scaffold for enhanced osteogenesis. Int. J. Biol. Macromol. 2018, 121, 1054–1060. [Google Scholar] [CrossRef]
- Motamed, S.; Del Borgo, M.P.; Kulkarni, K.; Habila, N.; Zhou, K.; Perlmutter, P.; Forsythe, J.S.; Aguilar, M.I. A self-assembling β-peptide hydrogel for neural tissue engineering. Soft Matter. 2016, 12, 2243–2246. [Google Scholar] [CrossRef]
- Wan, S.; Borland, S.; Richardson, S.M.; Merry, C.L.; Saiani, A.; Gough, J.E. Self-assembling peptide hydrogel for intervertebral disc tissue engineering. Acta Biomater. 2016, 46, 29–40. [Google Scholar] [CrossRef]
- Smith, D.J.; Brat, G.A.; Medina, S.H.; Tong, D.; Huang, Y.; Grahammer, J.; Furtmüller, G.J.; Oh, B.C.; Nagy-Smith, K.J.; Walczak, P.; et al. A multiphase transitioning peptide hydrogel for suturing ultrasmall vessels. Nat. Nanotechnol. 2016, 11, 95–102. [Google Scholar] [CrossRef]
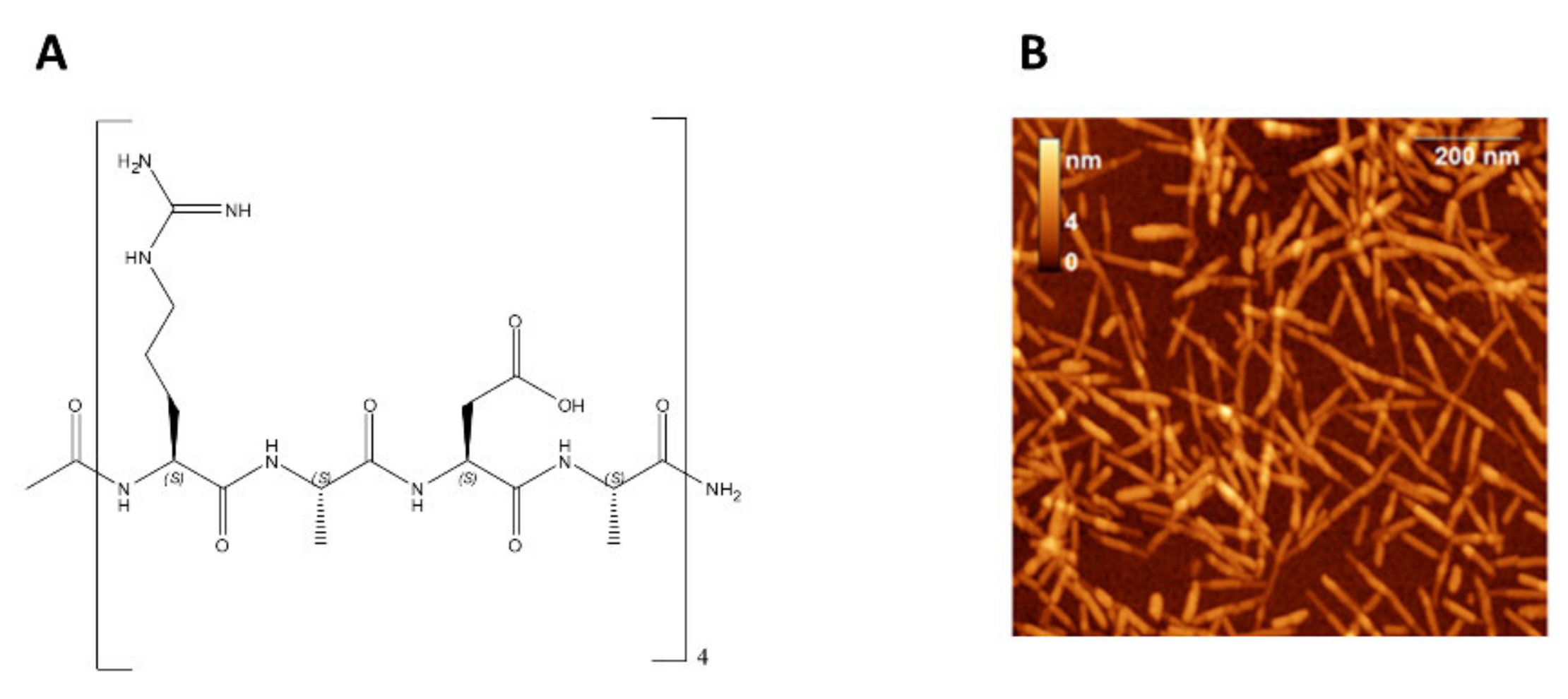
- Jun, S.; Hong, Y.; Imamura, H.; Ha, B.-Y.; Bechhoefer, J.; Chen, P. Self-Assembly of the Ionic Peptide EAK16: The Effect of Charge Distributions on Self-Assembly. Biophys. J. 2004, 87, 1249–1259. [Google Scholar] [CrossRef] [Green Version]
- Wierichs, R.; Carvalho, T.; Wolf, T. Efficacy of a self-assembling peptide to remineralize initial caries lesions - A systematic review and meta-analysis. J. Dent. 2021, 109, 103652. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Phan, A.T.; Ćwikła, J.B.; Sedláčková, E.; Thanh, X.-M.T.; Wolin, E.M.; Ruszniewski, P.; CLARINET Investigators. Lanreotide autogel/depot in advanced enteropancreatic neuroendocrine tumours: Final results of the CLARINET open-label extension study. Endocrinology 2021, 71, 502–513. [Google Scholar]
- Arosio, P.; Owczarz, M.; Wu, H.; Butté, A.; Morbidelli, M. End-to-End Self-Assembly of RADA 16-I Nanofibrils in Aqueous Solutions. Biophys. J. 2012, 102, 1617–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; Zhao, X.; Zhang, S. Structural Dynamic of a Self-Assembling Peptide d-EAK16 Made of Only D-Amino Acids. PLoS ONE 2008, 3, e2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Wang, Z.; Guo, Y.; Li, H.; Chen, Z. Design of a RADA16-based self-assembling peptide nanofiber scaffold for biomedical applications. J. Biomater. Sci. Polym. Ed. 2019, 30, 713–736. [Google Scholar] [CrossRef]
- Bagrov, D.; Gazizova, Y.; Podgorsky, V.; Udovichenko, I.; Danilkovich, A.; Prusakov, K.; Klinov, D. Morphology and aggregation of RADA-16-I peptide Studied by AFM, NMR and molecular dynamics simulations. Biopolymers 2016, 106, 72–81. [Google Scholar] [CrossRef]
- Burgess, K.A. Peptide Hydrogels for Advanced 3D Cell Culture; The University of Manchester: Manchester, UK, 2019. [Google Scholar]
- Kondelova, P.S.; Mannaa, A.; Bommer, C.; Abdelaziz, M.; Daeniker, L.; Di Bella, E.; Krejci, I. Efficacy of P11-4 for the treatment of initial buccal caries: A randomized clinical trial. Sci. Rep. 2020, 10, 20211. [Google Scholar] [CrossRef]
- Suda, S.; Takamizawa, T.; Takahashi, F.; Tsujimoto, A.; Akiba, S.; Nagura, Y.; Kurokawa, H.; Miyazaki, M. Application of the Self- Assembling Peptide P11-4 for Prevention of Acidic Erosion. Oper. Dent. 2018, 43, E166–E172. [Google Scholar] [CrossRef]
- Alkilzy, M.; Santamaria, R.; Schmoeckel, J.; Splieth, C. Treatment of Carious Lesions Using Self-Assembling Peptides. Adv. Dent. Res. 2018, 29, 42–47. [Google Scholar] [CrossRef]
- Gomes-Porras, M.; Cárdenas, J.J.; Álvarez-Escolá, C. Somatostatin Analogs in Clinical Practice: A Review. Int. J. Mol. Sci. 2020, 21, 1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
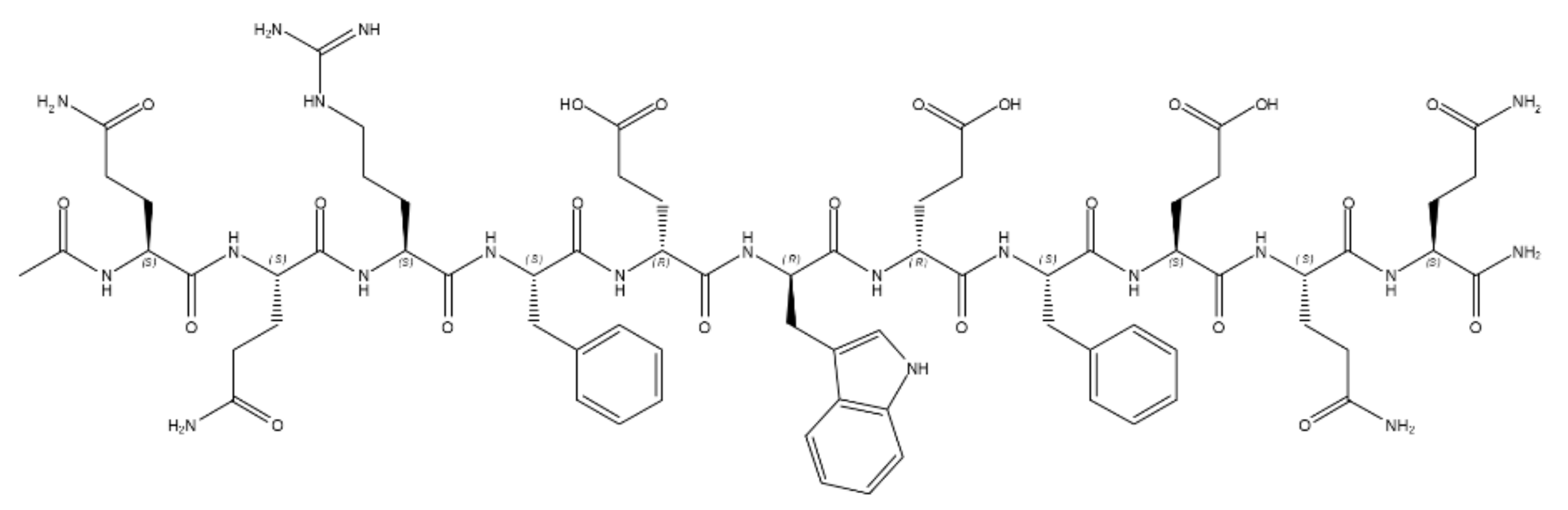
- Pandit, A.; Fay, N.; Bordes, L.; Valéry, C.; Cherif-Cheikh, R.; Robert, B.; Artzner, F.; Paternostre, M. Self-assembly of the octapeptide lanreotide and lanreotide-based derivatives: The role of the aromatic residues. J. Pept. Sci. 2007, 14, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Pouget, E.; Fay, N.; Dujardin, E.; Jamin, N.; Berthault, P.; Perrin, L.; Pandit, A.; Rose, T.; Valéry, C.; Thomas, D.; et al. Elucidation of the Self-Assembly Pathway of Lanreotide Octapeptide into β-Sheet Nanotubes: Role of Two Stable Intermediates. J. Am. Chem. Soc. 2010, 132, 4230–4241. [Google Scholar] [CrossRef] [PubMed]
- Valéry, C.; Paternostre, M.; Robert, B.; Gulik-Krzywicki, T.; Narayanan, T.; Dedieu, J.-C.; Keller, G.; Torres, M.-L.; Cherif-Cheikh, R.; Calvo, P.; et al. Biomimetic organization: Octapeptide self-assembly into nanotubes of viral capsid-like dimension. Proc. Natl. Acad. Sci. USA 2003, 100, 10258–10262. [Google Scholar] [CrossRef] [Green Version]
- Ronchi, C.L.; Boschetti, M.; Degli Uberti, E.C.; Mariotti, S.; Grottoli, S.; Loli, P.; Lombardi, G.; Tamburrano, G.; Arvigo, M.; Angeletti, G.; et al. Efficacy of a slow-release formulation of lanreotide (Autogel®120 mg) in patients with acromegaly previously treated with octreotide long acting release (LAR): An open, multicentre longitudinal study. Clin. Endocrinol. 2007, 67, 512–519. [Google Scholar] [CrossRef]
- Pavel, M.E.; Phan, A.T.; Wolin, E.M.; Mirakhur, B.; Liyanage, N.; Lowenthal, S.P.; Fisher, G.A.; Vinik, A.I.; CLARINET Study Investigators. Effect of Lanreotide Depot/Autogel on Urinary 5-Hydroxyindoleacetic Acid and Plasma Chromogranin A Biomarkers in Nonfunctional Metastatic Enteropancreatic Neuroendocrine Tumors. Oncologist 2019, 24, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Rinke, A.; Maintz, C.; Müller, L.; Weber, M.M.; Lahner, H.; Pavel, M.; Saeger, W.; Houchard, A.; Ungewiss, H.; Petersenn, S. Multicenter, Observational Study of Lanreotide Autogel for the Treatment of Patients with Neuroendocrine Tumors in Routine Clinical Practice in Germany and Austria. Exp. Clin. Endocrinol. Diabetes 2021, 129, 500–509. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Manna, S.; Di Natale, C.; Onesto, V.; Marasco, D. Self-Assembling Peptides: From Design to Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 12662. https://doi.org/10.3390/ijms222312662
La Manna S, Di Natale C, Onesto V, Marasco D. Self-Assembling Peptides: From Design to Biomedical Applications. International Journal of Molecular Sciences. 2021; 22(23):12662. https://doi.org/10.3390/ijms222312662
Chicago/Turabian StyleLa Manna, Sara, Concetta Di Natale, Valentina Onesto, and Daniela Marasco. 2021. "Self-Assembling Peptides: From Design to Biomedical Applications" International Journal of Molecular Sciences 22, no. 23: 12662. https://doi.org/10.3390/ijms222312662
APA StyleLa Manna, S., Di Natale, C., Onesto, V., & Marasco, D. (2021). Self-Assembling Peptides: From Design to Biomedical Applications. International Journal of Molecular Sciences, 22(23), 12662. https://doi.org/10.3390/ijms222312662








