Adamantane-Substituted Purines and Their β-Cyclodextrin Complexes: Synthesis and Biological Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Inclusion Complexes with β-CD
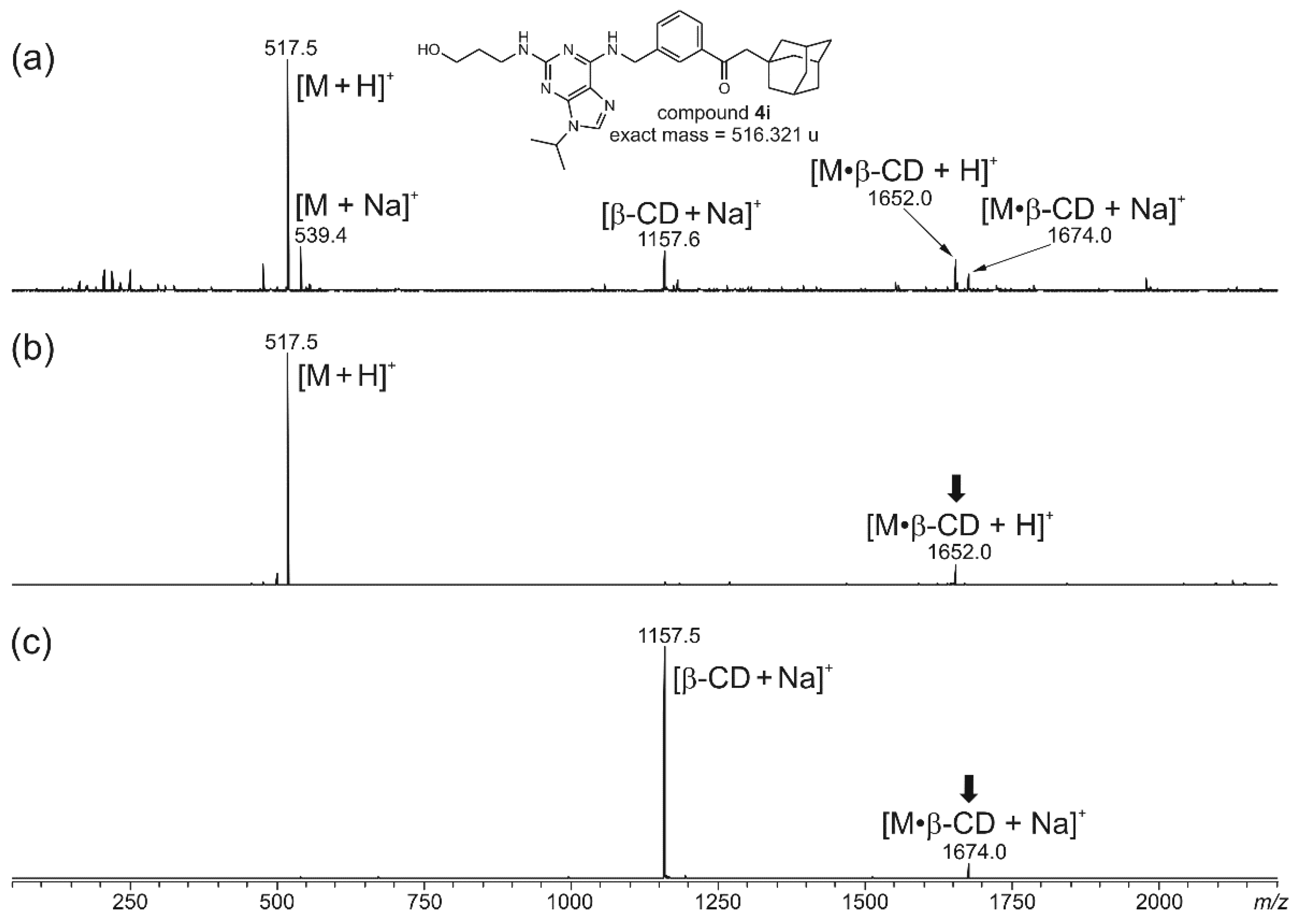
2.2.1. ESI-MS Analyses
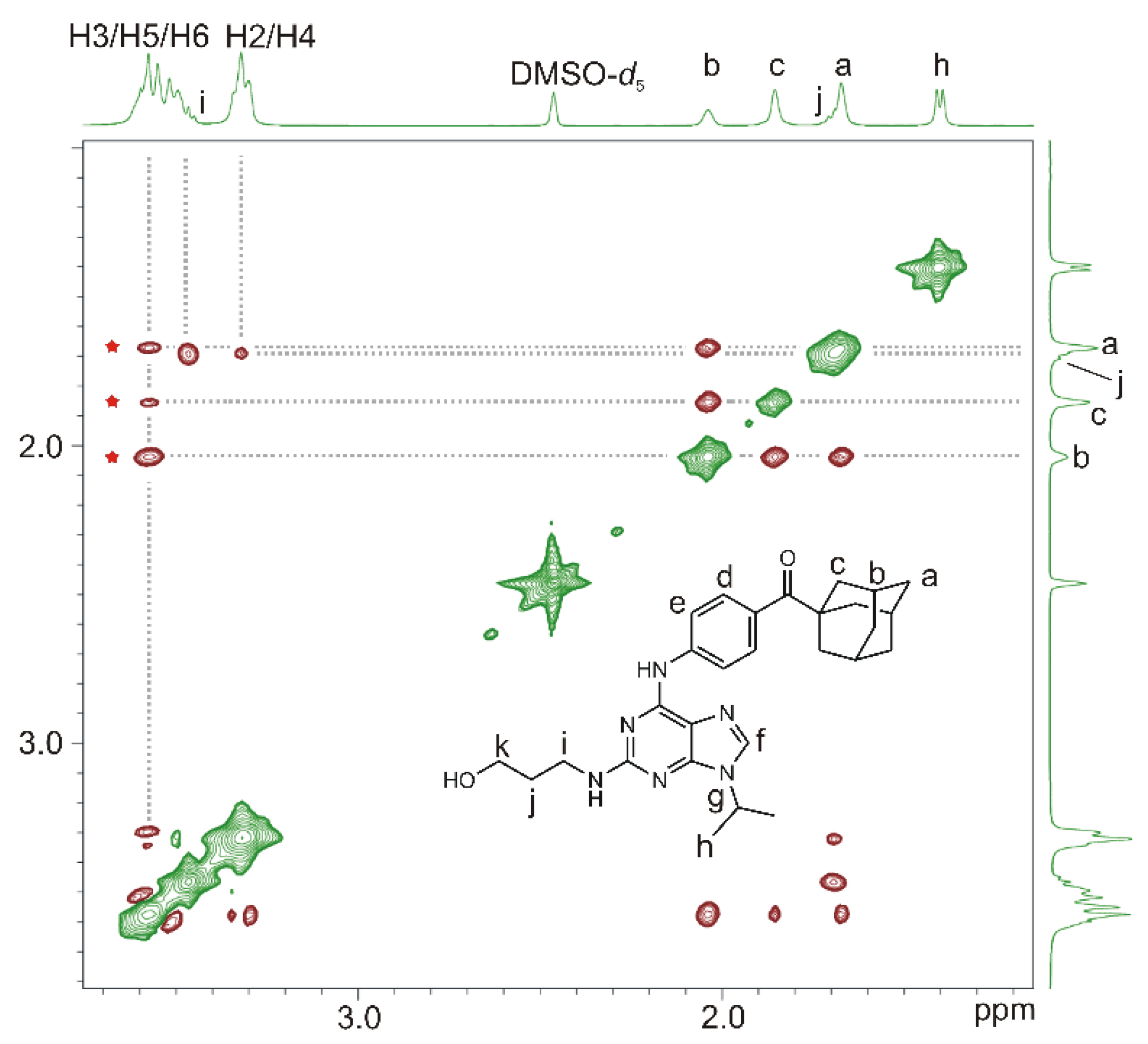
2.2.2. 1H-1H Rotating Frame Nuclear Overhauser Effect Spectroscopy (ROESY) NMR Experiments
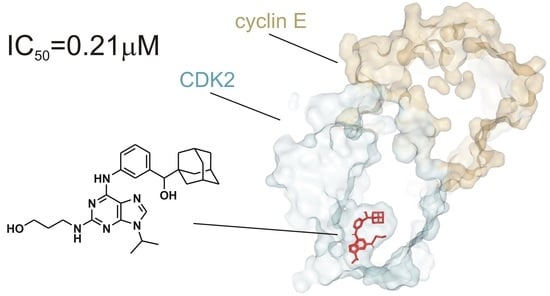
2.3. CDK Inhibition Activity
2.4. In Vitro Cytotoxicity
2.5. Docking Study
3. Materials and Methods
3.1. General Data
3.2. Molecular Docking Study
3.3. Alkylation of 2,6-Dichloro-9H-purine (1)
- 2,6-Dichloro-9-isopropyl-9H-purine (2a)
- 2,6-Dichloro-7-isopropyl-7H-purine (2b)
3.4. General Procedure for Preparation of 6-“Amino”-2-chloro-9-isopropyl-9H-purines (3a–k)
- (1-Adamantyl){3-[(2-chloro-9-isopropyl-9H-purin-6-yl)amino]phenyl}methanone (3a)
- (1-Adamantyl){3-[(2-chloro-9-isopropyl-9H-purin-6-yl)amino]phenyl}methanol (3b)
- N-[3-(1-Adamantylmethyl)phenyl]-2-chloro-9-isopropyl-9H-purin-6-amine (3c)
- (1-Adamantyl){4-[(2-chloro-9-isopropyl-9H-purin-6-yl)amino]phenyl}methanone (3d)
- (1-Adamantyl){4-[(2-chloro-9-isopropyl-9H-purin-6-yl)amino]phenyl}methanol (3e)
- N-[4-(1-Adamantylmethyl)phenyl]-2-chloro-9-isopropyl-9H-purin-6-amine (3f)
- 2-(1-Adamantyl)-1-{3-[(2-chloro-9-isopropyl-9H-purin-6-yl)amino]phenyl}ethan-1-ol (3g)
- N-{3-[2-(1-Adamantyl)ethyl]phenyl}-2-chloro-9-isopropyl-9H-purin-6-amine (3h)
- 2-(1-Adamantyl)-1-{3-[({2-chloro-9-isopropyl-9H-purin-6-yl}amino)methyl]phenyl}ethan-1-one (3i)
- 2-(1-Adamantyl)-1-{4-[({2-chloro-9-isopropyl-9H-purin-6-yl}amino)methyl]phenyl}ethan-1-one (3j)
- N-Benzyl-2-chloro-9-isopropyl-9H-purin-6-amine (3k)
3.5. General Procedure for Preparation of 2,6-“Diamino”-9-isopropyl-9H-purines (4a–k)
- (1-Adamantyl)[3-({2-[(3-hydroxypropyl)amino]-9-isopropyl-9H-purin-6-yl}amino)phenyl]methanone (4a)
- 3-{[6-({3-[1-Adamantyl(hydroxy)methyl]phenyl}amino)-9-isopropyl-9H-purin-2-yl]amino}propan-1-ol (4b)
- 3-{[6-{[3-(1-Adamantylmethyl)phenyl]amino}-9-isopropyl-9H-purin-2-yl]amino}propan-1-ol (4c)
- (1-Adamantyl)[4-({2-[(3-hydroxypropyl)amino]-9-isopropyl-9H-purin-6-yl}amino)phenyl]methanone (4d)
- 3-{[6-({4-[1-Adamantyl(hydroxy)methyl]phenyl}amino)-9-isopropyl-9H-purin-2-yl]amino}propan-1-ol (4e)
- 3-{[6-{[4-(1-Adamantylmethyl)phenyl]amino}-9-isopropyl-9H-purin-2-yl]amino}propan-1-ol (4f)
- 3-{[6-({3-[2-(1-Adamantyl)-1-hydroxyethyl]phenyl}amino)-9-isopropyl-9H-purin-2-yl]amino}propan-1-ol (4g)
- 3-{[6-({3-[2-(1-Adamantyl)ethyl]phenyl}amino)-9-isopropyl-9H-purin-2-yl]amino}propan-1-ol (4h)
- 2-(1-Adamantyl)-1-{3-[({2-(3-hydroxypropyl)amino]-9-isopropyl-9H-purin-6-yl}amino)methyl]phenyl}ethan-1-one (4i)
- 2-(1-Adamantyl)-1-{4-[({2-(3-hydroxypropyl)amino]-9-isopropyl-9H-purin-6-yl}amino)methyl]phenyl}ethan-1-one (4j)
- 3-{[6-(Benzylamino)-9-isopropyl-9H-purin-2-yl]amino}propan-1-ol (4k)
3.6. CDK Inhibition Assay
3.7. In Vitro Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Malumbres, M.; Barbacid, M. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 2005, 30, 630–641. [Google Scholar] [CrossRef]
- Shapiro, G.I. Cyclin-dependent kinases pathways as targets for cancer treatment. J. Clin. Oncol. 2006, 24, 1770–1783. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, S.; Giordano, A. Cell cycle kinases as therapeutic targets for cancer. Nat. Rev. Drug Discov. 2009, 8, 547–566. [Google Scholar] [CrossRef]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef] [Green Version]
- Hermanová, D.; Jorda, R.; Kryštof, V. How selective are clinical CDK4/6 inhibitors? Med. Res. Rev. 2021, 41, 1578–1598. [Google Scholar] [CrossRef]
- Legraverend, M.; Grierson, D.S. The purines: Potent and versatile small molecule inhibitors and modulators of key biological targets. Bioorg. Med. Chem. 2006, 14, 3987–4006. [Google Scholar] [CrossRef]
- Veselý, J.; Havlíček, L.; Strnad, M.; Blow, J.J.; Donella-Deana, A.; Pinna, L.; Letham, D.S.; Kato, J.-Y.; Detivaud, L.; Leclerc, S.; et al. Inhibition of cyclin-dependent kinases by purine analogues. Eur. J. Biochem. 1994, 224, 771–786. [Google Scholar] [CrossRef]
- Meijer, L.; Borgne, A.; Mulner, O.; Chong, J.P.J.; Blow, J.J.; Inagaki, N.; Inagaki, M.; Delcros, J.-G.; Moulinoux, J.-P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997, 243, 527–536. [Google Scholar] [CrossRef]
- Gray, N.S.; Wodicka, L.; Thunnissen, A.-M.W.H.; Norman, T.C.; Kwon, S.; Espinoza, F.H.; Morgan, D.O.; Barnes, G.; LeClerc, S.; Meijer, L.; et al. Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science 1998, 281, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Kryštof, R.V.; Lenobel, L.; Havlíček, M.; Kuzma, M.; Strnad, M. Synthesis and bilogical activity of olomoucine II. Bioorg. Med. Chem. Lett. 2002, 12, 3283–3286. [Google Scholar] [CrossRef]
- Gucký, T.; Jorda, R.; Zatloukal, M.; Bazgier, V.; Berka, K.; Řezníčková, E.; Béres, T.; Strnad, M.; Kryštof, V. A novel series of highly potent 2,6,9-trisubstituted purine cyclin-dependent kinase inhibitors. J. Med. Chem. 2013, 56, 6234–6247. [Google Scholar] [CrossRef] [PubMed]
- Jorda, R.; Paruch, K.; Kryštof, V. Cyclin-dependent kinase inhibitors inspired by roscovitine: Purine bioisosteres. Curr. Pharm. Design 2012, 18, 2974–2980. [Google Scholar] [CrossRef]
- Tadesse, S.; Caldon, E.C.; Tilley, W.; Wang, S. Cyclin-Dependent Kinase 2 Inhibitors in Cancer Therapy: An Update. J. Med. Chem. 2019, 62, 4233–4251. [Google Scholar] [CrossRef] [PubMed]
- Otyepka, M.; Kryštof, V.; Havlíček, L.; Siglerová, V.; Strnad, M.; Koča, J. Docking-based developtment of purine-like inhibitor of cyclin-dependent kinase-2. J. Med. Chem. 2000, 43, 2506–2513. [Google Scholar] [CrossRef] [PubMed]
- Jorda, R.; Havlíček, L.; McNae, I.W.; Walkinshaw, M.D.; Voller, J.; Šturc, A.; Navrátilová, J.; Kuzma, M.; Mistrík, M.; Bártek, J.; et al. Pyrazolo[4,3-d] bioisostere of roscovitine: Evaluation of a novel selective inhibitor of cyclin-dependent kinases with antiproliferative activity. J. Med. Chem. 2011, 54, 2980–2993. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, H.; Strnad, M.; Kryštof, V.; Havlíček, L.; van der Aa, A.; Lenjou, M.; Nijs, G.; Rodrigus, J.; Stockman, B.; van Onckelen, H.; et al. Antiproliferative effect of plant cytokinin analogues with an inhibitory activity on cyclin-dependent kinases. Leukemia 2002, 16, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Ren, T.; Côté, M.; Gholamreza, B.; Misasi, J.; Bruchez, A.; Cunningham, J. Inhibition of Ebola virus infection: Identification of Niemann-Pick C1 as the target by optimization of a chemical probe. ACS Med. Chem. Lett. 2013, 4, 239–243. [Google Scholar] [CrossRef]
- Torres, E.; Fernández, R.; Miquet, S.; Font-Bardia, M.; Vanderlinden, E.; Naesens, L.; Vázquez, S. Synthesis and anti-influenza A virus activity of 2,2-dialkylamantadines and related compounds. ACS Med. Chem. Lett. 2012, 3, 1065–1069. [Google Scholar] [CrossRef] [Green Version]
- Göktaş, F.; Vanderlinden, E.; Naesens, L.; Cesur, N.; Cesur, Z. Microwave assisted synthesis and anti-influenza virus activity of 1-adamantyl substituted N-(1-thia-4-azaspiro[4.5]decan-4-yl)carboxamide derivatives. Bioorg. Med. Chem. 2012, 20, 7155–7159. [Google Scholar] [CrossRef]
- Kwon, S.W.; Kang, S.K.; Lee, J.H.; Bok, J.H.; Kim, C.H.; Rhee, S.D.; Jung, W.H.; Kim, H.Y.; Bae, M.A.; Song, J.S.; et al. Synthesis and 11β hydroxysteroid dehydrogenase 1 inhibition of thiazolidine derivatives with an adamantyl group. Bioorg. Med. Chem. Lett. 2011, 21, 435–439. [Google Scholar] [CrossRef]
- Žák, F.; Turánek, J.; Kroutil, A.; Sova, P.; Mistr, A.; Poulová, A.; Mikolin, P.; Žák, Z.; Kašná, A.; Záluská, D.; et al. Platinum(IV) complex with adamantylamine as nonleaving amine group: Synthesis, characterization and in vitro antitumor activity against panel of cisplatin-resistant cancer cell lines. J. Med. Chem. 2004, 47, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Andring, J.T.; Fouch, M.A.; Akocak, S.; Angeli, A.; Supurnan, C.T.; Ilies, M.A.; McKenna, R. Structural Basis of Nanomolar Inhibition of Tumor-Associated Carbonic Anhydrase IX: X-Ray Crystallographic and Inhibition Study of Lipophilic Inhibitors with Acetazolamide Backbone. J. Med. Chem. 2020, 63, 13064–13075. [Google Scholar] [CrossRef] [PubMed]
- Marson, C.M. New and unusual scaffolds in medicinal chemistry. Chem. Soc. Rev. 2011, 40, 5514–5533. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Tsai, H.-J.; Liu, J.-Y.; Morisseau, C.; Hammock, B.D. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J. Med. Chem. 2007, 50, 3825–3840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Fujimoto, T.; Ogawa, Y.; Sugita, C.; Miyazaki, S.; Tamaki, K.; Takahashi, M.; Matsui, Y.; Nagayama, T.; Manabe, K.; et al. Discovery of DS-8108b, a novel orally bioavailable renin inhibitor. ACS Med. Chem. Lett. 2012, 3, 754–758. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Tomaszewski, J.E.; Hanrahan, C.; Coward, L.; Noker, P.; Gorman, G.; Nikonenko, B.; Protopopova, M. Pharmacodynamics and pharmacokinetics of SQ109, a new diamine-based antitubercular drug. Br. J. Pharmacol. 2005, 144, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Baraldi, P.G.; Saponaro, G.; Moorman, A.R.; Romagnoli, R.; Preti, D.; Baraldi, S.; Ruggiero, E.; Varani, K.; Targa, M.; Vincenzi, F.; et al. 7-Oxo-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxamides as CB2 cannabinoid receptor ligands: Structural investigation around a novel class of full agonists. J. Med. Chem. 2012, 55, 6608–6623. [Google Scholar] [CrossRef]
- Liu, J.; Obando, D.; Liao, V.; Lifa, T.; Codd, R. The many faces of the adamantyl group in drug design. Eur. J. Med. Chem. 2011, 46, 1949–1963. [Google Scholar] [CrossRef]
- Wanka, L.; Iqbal, K.; Schreiner, P.R. The lipophilic bullet hits the targets: Medicinal chemistry of adamantane derivatives. Chem. Rev. 2013, 113, 3516–3604. [Google Scholar] [CrossRef] [Green Version]
- Spilovská, K.; Zemek, F.; Korabečný, J.; Nepovimová, E.; Soukup, O.; Windish, M.; Kuča, K. Adamantane—A Lead Structure for Drugs in Clinical Practise. Curr. Med. Chem. 2016, 23, 3245–3266. [Google Scholar] [CrossRef]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, E.A.M.; Lira, M.C.B.; Rabello, M.M.; Cavalcanti, I.M.F.; Galdino, S.L.; Pitta, I.R.; Lima, M.C.A.; Pitta, M.G.R.; Hernandes, M.Z.; Santos-Magalhães, N.S. Enhanced antiproliferative activity of the new anticancer candidate LPSF/AC04 in cyclodextrin inclusion complexes encapsulated into liposomes. AAPS PharmSciTech 2012, 13, 1355–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreassi, E.; Zizzari, A.T.; Mori, M.; Filipi, I.; Belfiore, A.; Naldini, A.; Carraro, F.; Santucci, A.; Schenone, S.; Botta, M. 2-Hydroxypropyl-β-cyclodextrin strongly improves water solubility and anti-proliferative activity of pyrazolo[3,4-d]pyrimidines Src-Abl dual inhibitors. Eur. J. Med. Chem. 2010, 45, 5958–5964. [Google Scholar] [CrossRef]
- Azuma, H.; Aizawa, Y.; Higashitani, N.; Tsumori, T.; Kojima-Yuasa, A.; Matsui-Yuasa, I.; Nagasaki, T. Biological activity of water-soluble inclusion complexes of 1’-acetoxychavicol acetate with cyclodextrins. Bioorg. Med. Chem. 2011, 19, 3855–3863. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Jiang, K.; Li, K.; Cong, Y.; Pu, S.; Jin, Y.; Lin, J. Preparation, characterisation and antitumour activity of β-, γ- and HP-β-cyclodextrin inclusion complexes with oxalilplatin. Spectroc. Acta Pt. A Molec. Biomolec. Spectr. 2016, 152, 501–508. [Google Scholar] [CrossRef]
- Vaidya, B.; Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Damon, J.K.; Sarode, A.; Kanabar, D.; Garcia, J.V.; Mitragotri, S.; Muth, A.; et al. Cyclodextrin modified loaded PLGA nanoparticles for improved therapeutic efficacy against non-small cell lung cancer. Int. J. Biol. Macromol. 2019, 122, 338–347. [Google Scholar] [CrossRef]
- Thiabaud, G.; Harden-Bull, L.; Ghang, Y.-J.; Sen, S.; Chi, X.; Bachman, J.L.; Lych, V.M.; Siddik, Z.H.; Sessler, J.L. Platinum(IV)-Ferrocene Conjugates and Their Cyclodextrin Host–Guest Complexes. Inorg. Chem. 2019, 58, 7886–7894. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Oumata, M.; Bettayeb, K.; Ferandin, Y.; Demange, L.; Lopez-Giral, A.; Goddard, M.-L.; Myrianthopoulos, V.; Mikros, E.; Flajolet, M.; Greengard, P.; et al. Roscovitine-derived, dual-specifity inhibitors of cyclin-dependent kinases and casein kinases 1. J. Med. Chem. 2008, 51, 5229–5242. [Google Scholar] [CrossRef]
- Vícha, R.; Rouchal, M.; Kozubková, Z.; Kuřitka, I.; Marek, R.; Branná, P.; Čmelík, R. Novel adamantane-bearing anilines and properties of their supramolecular complexes with β-cyclodextrin. Supramol. Chem. 2011, 23, 663–677. [Google Scholar] [CrossRef]
- Rouchal, M.; Matelová, A.; Pires de Carvalho, F.; Bernat, R.; Grbić, D.; Kuřitka, I.; Babinský, M.; Marek, R.; Čmelík, R.; Vícha, R. Adamantane-bearing benzylamines and benzylamides: Novel building blocks for supramolecular systems with finely tuned binding properties toward β-cyclodextrin. Supramol. Chem. 2013, 25, 349–361. [Google Scholar] [CrossRef]
- Fioriny, M.T.; Abell, C. Solution-phase synthesis of 2,6,9-trisubstituted purines. Tetrahedron Lett. 1998, 39, 1827–1830. [Google Scholar] [CrossRef]
- Berry, D.J.; DiGiovanna, C.V.; Metrick, S.S.; Murugan, R. Catalysis by 4-dialkylaminopyridines. Arkivoc 2001, 2, 944–964. [Google Scholar] [CrossRef] [Green Version]
- Schow, S.R.; Mackman, R.L.; Blum, C.L.; Brooks, E.; Horsma, A.G.; Joly, A.; Kerwar, S.S.; Lee, G.; Shiffman, D.; Nelson, M.G.; et al. Synthesis and activity of 2,6,9-trisubstituted purines. Bioorg. Med. Chem. Lett. 1997, 7, 2697–2702. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Gray, N.S.; Rosania, G.R.; Sutherlin, D.P.; Kwon, S.; Norman, T.C.; Sarohia, R.; Leost, M.; Meijer, L.; Schultz, P.G. Synthesis and application of functionally diverse 2,6,9-trisubstituted purines libraries as CDK inhibitors. Chem. Biol. 1999, 6, 361–375. [Google Scholar] [CrossRef] [Green Version]
- Oumata, N.; Ferandin, Y.; Meijer, L.; Galons, H. Practical synthesis of roscovitine and CR8. Org. Process Res. Dev. 2009, 13, 641–644. [Google Scholar] [CrossRef]
- Hloušková, N.; Rouchal, M.; Nečas, M.; Vícha, R. 2,6-Dichloro-7-isopropyl-7H-purine. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o1585. [Google Scholar] [CrossRef]
- Rouchal, M.; Nečas, M.; Vícha, R. 4-(1-Adamantylmethyl)-N-(2-chloro-9-isopropyl-9H-purin-6-yl)aniline. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, o1676. [Google Scholar] [CrossRef] [Green Version]
- Rouchal, M.; Nečas, M.; de Carvalho, F.P.; Vícha, R. 2-(1-Adamantyl)-1-{4-[(2-chloro-9-isopropyl-9H-purin-6-yl)aminomethyl]phenyl}ethanol. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, o298–o299. [Google Scholar] [CrossRef]
- Popowycz, F.; Fornet, G.; Schneider, C.; Bettayeb, K.; Ferandin, Y.; Lamigeon, C.; Tirado, O.M.; Mateo-Lozano, S.; Notario, V.; Colas, P.; et al. Pyrazolo[1,5-a]triazine as a purine bioisostere: Access to potent cyclin-dependent kinase inhibitor (R)-roscovitine analogue. J. Med. Chem. 2009, 52, 655–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Lee, N.V.; Hu, W.; Xu, M.; Ferre, R.A.; Lam, H.; Bergqist, S.; Solowiej, J.; Diehl, W.; He, Z.-A.; et al. Spectrum and Degree of CDK Drug Interactions Predicts Clinical Performance. Mol. Cancer Ther. 2016, 15, 2273–2281. [Google Scholar] [CrossRef] [Green Version]
- Vícha, R.; Potáček, M. Influence of Catalytic System Composition on Formation of Adamantane Containing Ketones. Tetrahedron 2005, 61, 83–88. [Google Scholar] [CrossRef]
- Halgren, T.H. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- De Azevedo, W.F.; Leclerc, S.; Meijer, L.; Havlíček, L.; Strnad, M.; Kim, S.-H. Inhibition of Cyclin-Dependent Kinases by Purine Analogues. Eur. J. Biochem. 1997, 243, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Comp. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. In Chemical Biology. Methods in Molecular Biology; Hempel, J., Williams, C., Hong, C., Eds.; Humana Press: New York, NY, USA, 2015; Volume 1263. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucl. Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]




| Compound | CDK2/Cyclin E IC50 (μm) 1 | ||
|---|---|---|---|
| Purine | Purine·β-CD (1:1) | Purine·β-CD (1:10) | |
| 4a | 2.55 ± 0.08 | 5.5 ± 1.3 | >40 |
| 4b | 0.21 ± 0.02 | 0.16 | 0.3 ± 0.2 |
| 4c | 12 ± 8 | 1.85 | >40 |
| 4d | 0.6 ± 0.2 | 1.4 ± 0.5 | 7.57 |
| 4e | 1.6 ± 0.7 | 1.53 | 26.1 |
| 4f | >12.5 | >40 | >40 |
| 4g | 0.92 ± 0.18 | 1.24 | 7.15 |
| 4h | >12.5 | >40 | >40 |
| 4i | 14 ± 5 | >40 | 30.7 |
| 4j | 3.26 ± 0.01 | 15.7 | 20.9 |
| 4k (bohemine) | 0.7 ± 0.5 | 1.1 ± 0.6 | 1.11 ± 0.12 |
| 4l (roscovitine) | 0.22 2 | n.a. 3 | n.a. 3 |
| Compound | K-562 GI50 (μm) 1 | MCF-7 GI50 (μm) 1 | ||||
|---|---|---|---|---|---|---|
| Purine | Purine·β-CD (1:1) | Purine·β-CD (1:10) | Purine | Purine·β-CD (1:1) | Purine·β-CD (1:10) | |
| 4a | 6.16 | 15.04 ± 0.16 | >40 | >6.25 | 15.7 ± 0.9 | >40 |
| 4b | >6.25 | 34.69 ± 0.14 | >40 | >6.25 | 32 ± 3 | >40 |
| 4c | >6.25 | 28.7 ± 10.2 | >40 | >6.25 | >40 | >40 |
| 4d | 5.45 | 16.5 ± 0.7 | >40 | 5.72 | 13.8 ± 1.4 | >40 |
| 4e | 10.1 ± 0.8 | 18.7 ± 0.6 | >40 | 8.1 ± 0.7 | 17 ± 2 | >40 |
| 4f | 10.4 ± 0.7 | 24 ± 3 | >40 | > 12.5 | 32.9 ± 0.1 | >40 |
| 4g | >6.25 | 33.6 ± 1.2 | >40 | >6.25 | 17 ± 2 | >40 |
| 4h | >12.5 | >40 | >40 | >12.5 | >40 | >40 |
| 4i | >6.25 | 19.8 ± 0.5 | >40 | >6.25 | 30 ± 5 | >40 |
| 4j | >6.25 | 17.7 ± 0.6 | >40 | >6.25 | 21.51 ± 0.04 | >40 |
| 4k (bohemine) | 96.55 | >40 | >40 | 26.73 ± 6.36 | 36 ± 4 | 35 ± 4 |
| 4l (roscovitine) | 50.1 2 | n.a. 3 | n.a. 3 | 12.6 2 | n.a. 3 | n.a. 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouchal, M.; Rudolfová, J.; Kryštof, V.; Vojáčková, V.; Čmelík, R.; Vícha, R. Adamantane-Substituted Purines and Their β-Cyclodextrin Complexes: Synthesis and Biological Activity. Int. J. Mol. Sci. 2021, 22, 12675. https://doi.org/10.3390/ijms222312675
Rouchal M, Rudolfová J, Kryštof V, Vojáčková V, Čmelík R, Vícha R. Adamantane-Substituted Purines and Their β-Cyclodextrin Complexes: Synthesis and Biological Activity. International Journal of Molecular Sciences. 2021; 22(23):12675. https://doi.org/10.3390/ijms222312675
Chicago/Turabian StyleRouchal, Michal, Jana Rudolfová, Vladimír Kryštof, Veronika Vojáčková, Richard Čmelík, and Robert Vícha. 2021. "Adamantane-Substituted Purines and Their β-Cyclodextrin Complexes: Synthesis and Biological Activity" International Journal of Molecular Sciences 22, no. 23: 12675. https://doi.org/10.3390/ijms222312675
APA StyleRouchal, M., Rudolfová, J., Kryštof, V., Vojáčková, V., Čmelík, R., & Vícha, R. (2021). Adamantane-Substituted Purines and Their β-Cyclodextrin Complexes: Synthesis and Biological Activity. International Journal of Molecular Sciences, 22(23), 12675. https://doi.org/10.3390/ijms222312675