Sulfur-Induced Resistance against Pseudomonas syringae pv. actinidiae via Triggering Salicylic Acid Signaling Pathway in Kiwifruit
Abstract
:1. Introduction
2. Results
2.1. Soil Treatment of Sulfur Enhances Kiwifruit against Pseudomonas syringae pv. actinidiae
2.2. Sulfur Improved Photosynthetic Characteristics of Kiwifruit
2.3. Effect of Sulfur on Chlorophyll Content in Kiwifruit Leaves
2.4. Sulfur Activates the SA Signaling Pathways and Inhibits the JA Signaling Pathway
2.5. Expression of Resistance-Genes in Leaf and Stem of Kiwifruit under Sulfur Treatment
2.6. Induction of Higher Lignin Accumulation and Deposition
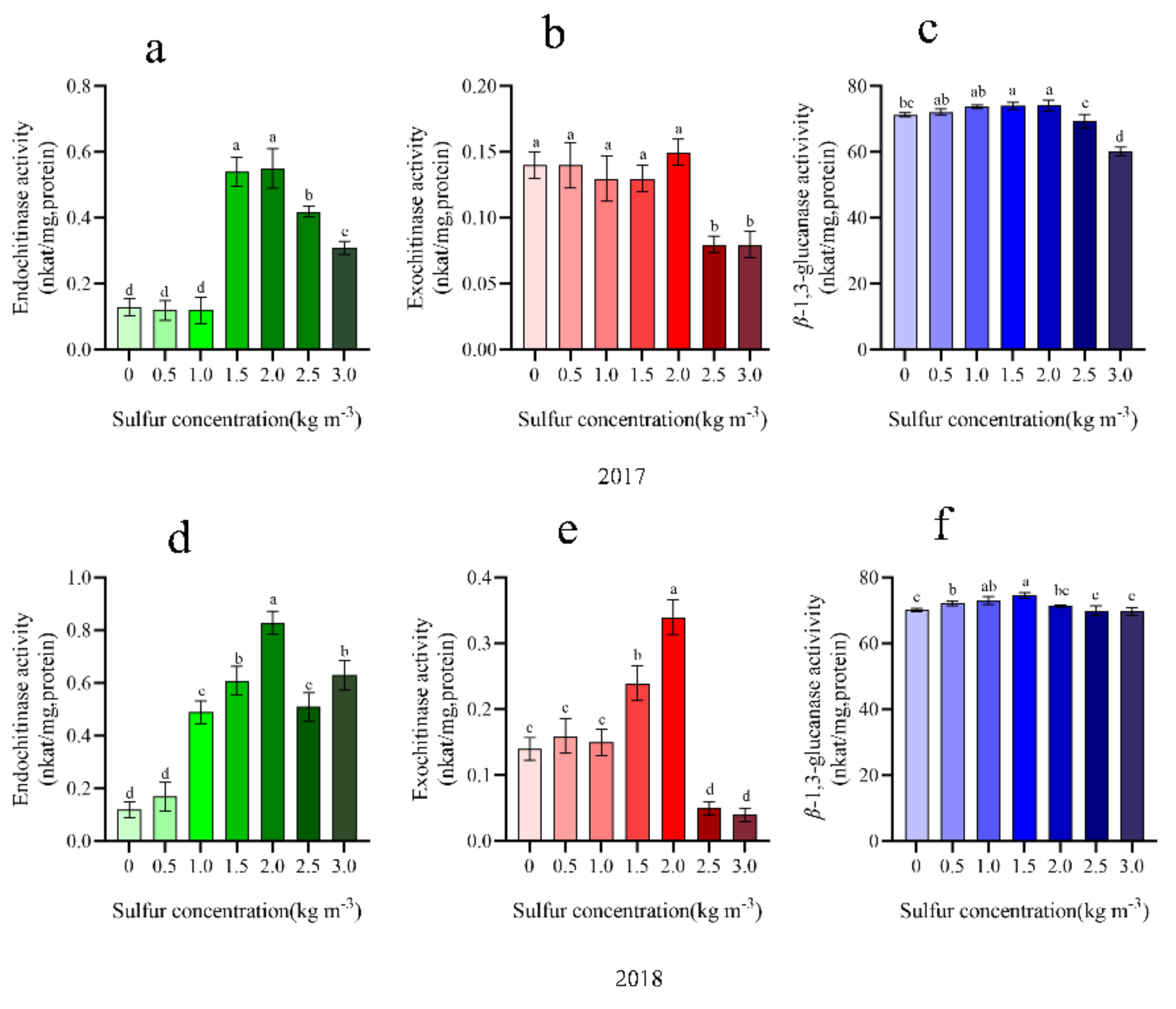
2.7. Chitinase and ß-1,3-Glucanase Activity
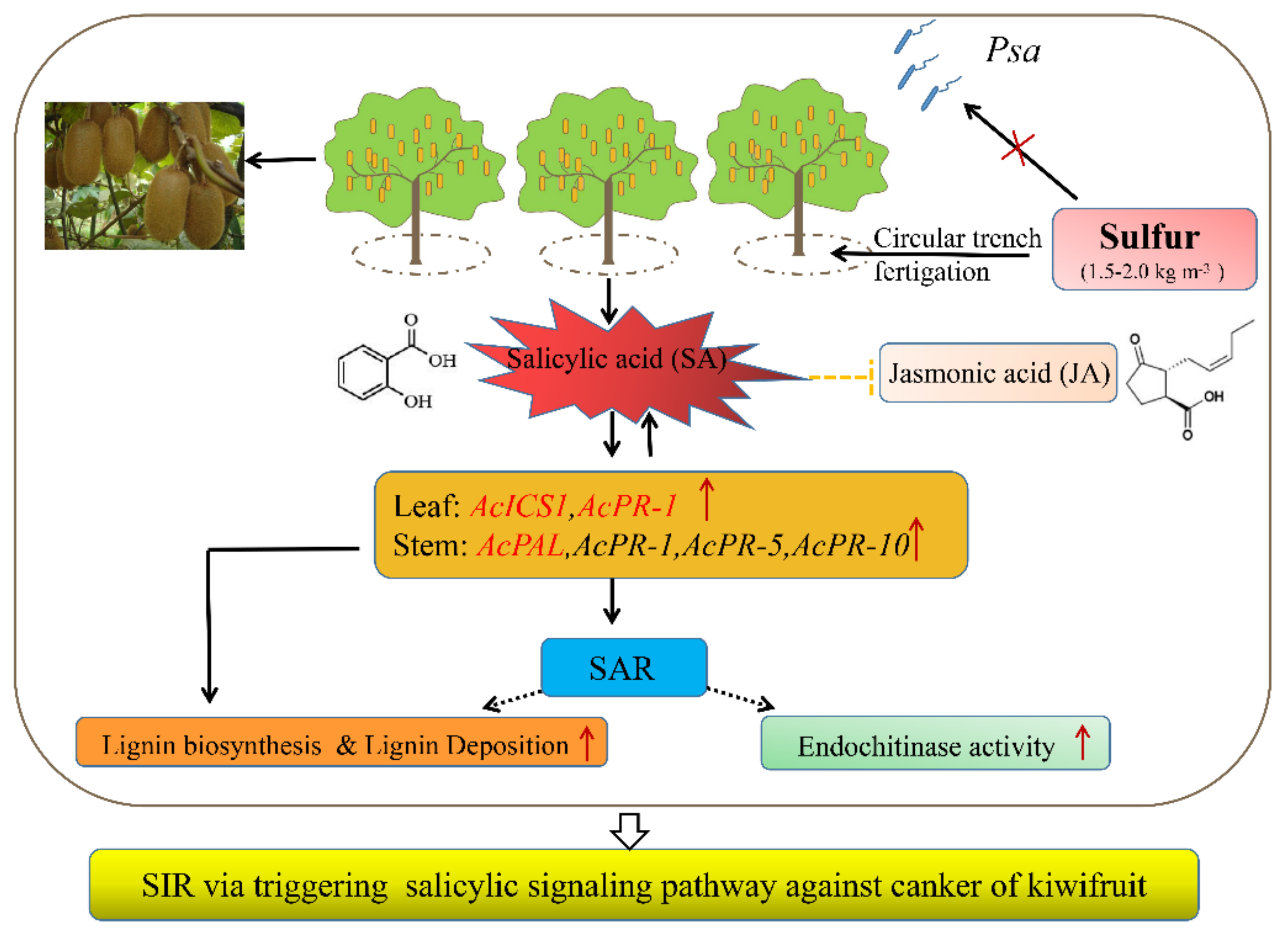
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Field Experiments
4.3. Assessment of Disease Incidence: Disease Severity Index and Induction Effect
4.4. Photosynthesis Parameters and Fluorescence Parameters
4.5. Leaf Chlorophyll Content
4.6. Salicylic Acid (SA) and Jasmonic Acid (JA) Contents Measurements
4.7. RNA Extraction and Quantitative RT-PCR
4.8. Measurement of Lignin Content and Histochemical Analysis
4.9. Extraction and Assay of β-1,3-Glucanase and Chitinase
4.9.1. Assay for Chitinase Activity
4.9.2. Assay for β-1,3-Glucanase Activity
5. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Psa | Pseudomonas syringae pv. actinidiae: |
| SA | Salicylic Acid |
| JA | Jasmonic Acid |
| SIR | Sulfur-induced resistance |
| SAR | Activates systemic acquired resistance |
References
- Scortichini, M.; Marcelletti, S.; Ferrante, P.; Petriccione, M.; Firrao, G. Pseudomonas syringae pv. actinidiae: A re-emerging, multi-faceted, pandemic pathogen. Mol. Plant. Pathol. 2012, 13, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, S.; Gullino, M.L.; Spadaro, D. Pseudomonas syringae pv. actinidiae isolated from Actinidia chinensis Var. deliciosa in Northern Italy: Genetic diversity and virulence. Eur. J. Plant Pathol. 2018, 150, 191–204. [Google Scholar] [CrossRef]
- Cunty, A.; Cesbron, S.; Poliakoff, F.; Jacques, M.A.; Manceau, C. Origin of the Outbreak in France of Pseudomonas syringae pv. actinidiae Biovar 3, the Causal Agent of Bacterial Canker of Kiwifruit, Revealed by a Multilocus Variable-Number Tandem-Repeat Analysis. Appl. Environ. Microbiol. 2015, 81, 6773–6789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulter, R.T.M.; Ho, J.; Handley, T.; Taiaroa, G.; Butler, M.I. Comparison between complete genomes of an isolate of Pseudomonas syringae pv. actinidiae from Japan and a New Zealand isolate of the pandemic lineage. Sci. Rep. 2018, 8, 10915. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.F.; Xiao, X.Z.; Dao, Y.W. Preliminary study on kiwifruit diseases in Hunan. Sichuan Fruit Tree Sci. Technol. 1996, 18, 19–28. [Google Scholar]
- Jez, J.M. Structural biology of plant sulfur metabolism: From sulfate to glutathione. J. Exp. Bot. 2019, 70, 4089–4103. [Google Scholar] [CrossRef]
- Kopriva, S.; Talukdar, D.; Takahashi, H.; Hell, R.; Sirko, A.; D’Souza, S.F.; Talukdar, T. Editorial: Frontiers of Sulfur Metabolism in Plant Growth, Development, and Stress Response. Front. Plant Sci. 2016, 6, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Rennenberg, H.; Brunold, C.; Kok, L.J.D.; Stulen, I. Sulfur Nutrition and Sulfur Assimilation in Higher Plants: Molecular, Biochemical and Physiological Aspects; SPB Academic Publishing: Hague, The Netherlands, 2000; pp. 56–68. [Google Scholar]
- Yin, X.H.; Wang, M.; Long, Y.H.; Tian, X.L.; Zhu, L.H.; Li, X.Q.; Xu, C.Y.; Wang, Y. Effects of sulfur treatment on chloroplast ultrastructure and fruit quality. J. Fruit Sci. 2017, 34, 454–463. [Google Scholar]
- Wang, M. Prevention and Control Effect of Sulfur on Kiwi Canker Safety; Gui Zhou University: Guiyang, China, 2016. [Google Scholar]
- Bloem, E.; Haneklaus, S.; Schung, E. Milestones in plant sulfur research on sulfur-induced-resistance (SIR) in Europe. Front. Plant Sci. 2015, 5, 779. [Google Scholar] [CrossRef]
- Kiraly, L.; Kuenstler, A.; Hoeller, K.; Fattinger, M.; Juhasz, C.; Mueller, M.; Gullner, G.; Zechmann, B. Sulfate supply influences compartment specific glutathione metabolism and confers enhanced resistance to Tobacco mosaic virus during a hypersensitive response. Plant Physiol. Biochem. 2012, 59, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Kruse, C.; Jost, R.; Lipschis, M.; Kopp, B.; Hartmann, M.; Hell, R. Sulfur-enhanced defence: Effects of sulfur metabolism, nitrogen supply, and pathogen lifestyle. Plant Biol. 2007, 9, 608–619. [Google Scholar] [CrossRef]
- Lima, R.d.C.M.d.; Stamford, N.P.; Santos, C.E.d.R.e.S.; Lira Júnior, M.d.A.; Dias, S.H.L. Eficiência e efeito residual de biofertilizantes de rochas com PK e enxofre com Acidithiobacillus em alface. Hortic. Bras. 2007, 25, 402–407. [Google Scholar] [CrossRef]
- Silveira Rabelo, F.H.; de Alcantara da Silva, B.K.; Borgo, L.; Keunen, E.; Rossi, M.L.; Lima Reis Borges, K.; dos Santos, E.F.; dos Reise, A.R.; Martinelli, A.P.; Azevedo, R.A.; et al. Enzymatic antioxidants-Relevant or not to protect the photosynthetic system against cadmium-induced stress in Massai grass supplied with sulfur? Environ. Exp. Bot. 2018, 155, 702–717. [Google Scholar] [CrossRef]
- Kopriva, S.; Malagoli, M.; Takahashi, H. Sulfur nutrition: Impacts on plant development, metabolism, and stress responses. J. Exp. Bot. 2019, 70, 4069–4073. [Google Scholar] [CrossRef]
- Hussain, S.J.; Masood, A.; Anjum, N.A.; Khan, N.A. Sulfur-mediated control of salinity impact on photosynthesis and growth in mungbean cultivars screened for salt tolerance involves glutathione and proline metabolism, and glucose sensitivity. Acta Physiol. Plant 2019, 41, 1–13. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Garcia-Angulo, P.; Goulao, L.F.; Acebes, J.L.; Amancio, S. Mineral stress affects the cell wall composition of grapevine (Vitis vinifera L.). Plant Sci. 2013, 205, 111–120. [Google Scholar] [CrossRef]
- Saito, K. Sulfur assimilatory metabolism. The long and smelling road. Plant Physiol. 2004, 136, 2443–2450. [Google Scholar] [CrossRef] [Green Version]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Zhang, B.B.; Guo, L.; Song, Z.Z.; Yu, M.L.; Ma, R.J. Effect of salicylic acid on freezing injury in peach floral organs and the expressions of CBF genes. Biol. Plant 2017, 61, 622–630. [Google Scholar] [CrossRef]
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic acid to decrease plant stress. Environ. Chem. Lett. 2017, 15, 101–123. [Google Scholar] [CrossRef]
- Moosa, A.; Farzand, A.; Sahi, S.T.; Khan, S.A.; Aslam, M.N.; Zubair, M. Salicylic acid and Cinnamomum verum confer resistance against Penicillium rot by modulating the expression of defense linked genes in Citrus reticulata Blanco. Postharvest Biol. Technol. 2021, 181, 111649. [Google Scholar] [CrossRef]
- Lemarie, S.; Robert-Seilaniantz, A.; Lariagon, C.; Lemoine, J.; Marnet, N.; Jubault, M.; Manzanares-Dauleux, M.J.; Gravot, A. Both the Jasmonic Acid and the Salicylic Acid Pathways Contribute to Resistance to the Biotrophic Clubroot Agent Plasmodiophora brassicae in Arabidopsis. Plant Cell Physiol. 2015, 56, 2158–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Sonbol, F.-M.; Huot, B.; Gu, Y.; Withers, J.; Mwimba, M.; Yao, J.; He, S.Y.; Dong, X. Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat. Commun. 2016, 7, 13099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, W.; Li, X.; Wang, X.; Cao, J.; Jiang, W. Chlorogenic acid induces resistance against Penicillium expansum in peach fruit by activating the salicylic acid signaling pathway. Food Chem. 2018, 260, 274–282. [Google Scholar] [CrossRef]
- Kasprzewska, A. Plant chitinases—Regulation and function. Cell. Mol. Biol. Lett. 2003, 8, 809–824. [Google Scholar]
- Grover, A. Plant Chitinases: Genetic Diversity and Physiological Roles. Crit. Rev. Plant Sci. 2012, 31, 57–73. [Google Scholar] [CrossRef]
- Khan, M.F.; Umar, U.U.D. Application of a robust microplate assay to determine induced β-1,3-glucanase and chitinase activity in the cotton plant. BioTechniques 2021, 70, 202–208. [Google Scholar] [CrossRef]
- Jia, X.; Meng, Q.; Zeng, H.; Wang, W.; Yin, H. Chitosan oligosaccharide induces resistance to Tobacco mosaic virus in Arabidopsis via the salicylic acid-mediated signalling pathway. Sci. Rep. 2016, 6, 26144. [Google Scholar] [CrossRef]
- Nechaeva, T.L.; Nikolaeva, T.N.; Zagoskina, N.V. Salicylic and Hydroxybenzoic Acids Affect the Accumulation of Phenolic Compounds in Tea-Plant Cultures in vitro. Biol. Bull. 2020, 47, 374–380. [Google Scholar] [CrossRef]
- Cesarino, I. Structural features and regulation of lignin deposited upon biotic and abiotic stresses. Curr. Opin. Biotechnol. 2019, 56, 209–214. [Google Scholar] [CrossRef]
- Lee, M.-H.; Jeon, H.S.; Kim, S.H.; Chung, J.H.; Roppolo, D.; Lee, H.-J.; Cho, H.J.; Tobimatsu, Y.; Ralph, J.; Park, O.K. Lignin-based barrier restricts pathogens to the infection site and confers resistance in plants. EMBO J. 2019, 38, 1–17. [Google Scholar] [CrossRef]
- Long, Y.; Yin, X.; Wang, M.; Wu, X.; Li, R.; Tian, X.; Li, M. Effects of Sulfur on Kiwifruit Canker Caused by Pseudomonas syringae pv. actinidae. Bangladesh J. Bot. 2017, 46, 1183–1192. [Google Scholar]
- Vanneste, J. The Scientific, economic, and social impacts of the New Zealand outbreak of bacterial canker of kiwifruit (Pseudomonas syringae pv. actinidiae). Annu. Rev. Phytopathol. 2017, 55, 377–399. [Google Scholar] [CrossRef]
- Froud, K.J.; Beresford, R.M.; Cogger, N.C. Impact of kiwifruit bacterial canker on productivity of cv. Hayward kiwifruit using observational data and multivariable analysis. Plant Pathol. 2018, 67, 671–681. [Google Scholar] [CrossRef]
- Beatrice, C.; Linthorst, J.M.; Cinzia, F.; Luca, R. Enhancement of PR1 and PR5 gene expressions by chitosan treatment in kiwifruit plants inoculated with Pseudomonas syringae pv. actinidiae. Eur. J. Plant Pathol. 2017, 148, 163–179. [Google Scholar] [CrossRef]
- Garcia-Mina, J.M. Plant nutrition and defense mechanism: Frontier knowledge. In Advances in Citrus Nutrition; Universidad de Navarra: Navarra, Spain, 2012; pp. 1–12. [Google Scholar]
- Zhang, Y.; Suzuki, K.; Liu, H.; Nukaya, A.; Kiriiwa, Y. Fruit yellow-shoulder disorder as related to mineral element uptake of tomatoes grown in high temperature. Sci. Hortic. 2018, 242, 25–29. [Google Scholar] [CrossRef]
- Romera, F.J.; Garcia, M.J.; Lucena, C.; Martinez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcantara, E.; Angulo, M.; Perez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 17. [Google Scholar] [CrossRef]
- McKenna, P.; Cannon, N.; Conway, J. Soil mineral nitrogen availability predicted by herbage yield and disease resistance in red clover (Trifolium pratense) cropping. Nutr. Cycl. Agroecosyst. 2018, 112, 303–315. [Google Scholar] [CrossRef]
- Lopez-Zaplana, A.; Barzana, G.; Agudelo, A.; Carvajal, M. Foliar Mineral Treatments for The Reduction of Melon (Cucumis melo L.) Fruit Cracking. Agronomy 2020, 10, 1815. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, R.; Kaushik, M.; Hasanuzzaman, M.; Pereira, E.; Ahmad, I.; Tuteja, N.; Gill, S.S. ATP-sulfurylase, sulfur-compounds, and plant stress tolerance. Front. Plant Sci. 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, C.; Angeles Bermudez, M.; Romero, L.C.; Gotor, C.; Garcia, I. Cysteine homeostasis plays an essential role in plant immunity. New Phytol. 2012, 193, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Datta, R.; Chattopadhyay, S. Glutathione as a crucial modulator of phytohormonesignalling during pathogen defence in plants. Proc. Indian Natl. Sci. Acad. 2018, 84, 581–597. [Google Scholar] [CrossRef]
- Gullner, G.; Juhasz, C.; Nemeth, A.; Barna, B. Reactions of tobacco genotypes with different antioxidant capacities to powdery mildew and Tobacco mosaic virus infections. Plant Physiol. Biochem. 2017, 119, 232–239. [Google Scholar] [CrossRef]
- Hoeller, K.; Kiraly, L.; Kuenstler, A.; Mueller, M.; Gullner, G.; Fattinger, M.; Zechmann, B. Enhanced Glutathione Metabolism Is Correlated with Sulfur-Induced Resistance in Tobacco mosaic virus-Infected Genetically Susceptible Nicotiana tabacum Plants. Mol. Plant-Microbe Interact. 2010, 23, 1448–1459. [Google Scholar] [CrossRef] [Green Version]
- Moniuszko, G.; Skoneczny, M.; Zientara-Rytter, K.; Wawrzynska, A.; Glow, D.; Cristescu, S.M.; Harren, F.J.M.; Sirko, A. Tobacco LSU-like protein couples sulphur-deficiency response with ethylene signalling pathway. J. Exp. Bot. 2013, 64, 5173–5182. [Google Scholar] [CrossRef] [Green Version]
- Rajab, H.; Khan, M.S.; Malagoli, M.; Hell, R.; Wirtz, M. Sulfate-Induced Stomata Closure Requires the Canonical ABA Signal Transduction Machinery. Plants 2019, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Kruse, C.; Haas, F.H.; Jost, R.; Reiser, B.; Reichelt, M.; Wirtz, M.; Gershenzon, J.; Schnug, E.; Hell, R. Improved sulfur nutrition provides the basis for enhanced production of sulfur-containing defense compounds in Arabidopsis thaliana upon inoculation with Alternaria brassicicola. J. Plant Physiol. 2012, 169, 740–743. [Google Scholar] [CrossRef]
- Han, Y.; Chaouch, S.; Mhamdi, A.; Queval, G.; Zechmann, B.; Noctor, G. Functional Analysis of Arabidopsis Mutants Points to Novel Roles for Glutathione in Coupling H2O2 to Activation of Salicylic Acid Accumulation and Signaling. Antioxid. Redox Signal. 2013, 18, 2106–2121. [Google Scholar] [CrossRef] [Green Version]
- Appa, B.; Hfa, C.; Et, A. Nitrogen, phosphorous and potassium levels affected growth indices, leaf gas exchange parameters and biomass production of henna (Lawsonia inermis L.) ecotypes. Ind. Crop. Prod. 2021, 163, 113297. [Google Scholar] [CrossRef]
- Broekaert, W.F.; Terras, F.; Cammue, B. Induced and Preformed Antimicrobial Proteins. In Mechanisms of Resistance to Plant Diseases; RWTH: Aachen, Germany, 2000; pp. 371–477. [Google Scholar]
- Pettongkhao, S.; Bilanglod, A.; Khompatara, K.; Churngchow, N. Sulphated Polysaccharide from Acanthophora spicifera Induced Hevea brasiliensis Defense Responses Against Phytophthora palmivora Infection. Plants 2019, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Wang, Y. Research progress of plant pathogenesis related protein PR-10. Zhiwu Shengli Xuebao/Plant Physiol. J. 2017, 53, 2057–2068. [Google Scholar]
- Li, X.; Zhu, L.; Tu, L.; Liu, L.; Yuan, D.; Li, J.; Lu, L.; Zhang, X. Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-Seq-dependent transcriptional analysis and histochemistry. J. Exp. Bot. 2011, 62, 5607–5621. [Google Scholar] [CrossRef] [Green Version]
- Onohata, T.; Gomi, K. Overexpression of jasmonate-responsive OsbHLH034 in rice results in the induction of bacterial blight resistance via an increase in lignin biosynthesis. Plant Cell Rep. 2020, 39, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Huang, C.; Luo, L.; Zheng, S.; Zhong, Y.; Sun, J.; Gui, J.; Li, L. Cell-Specific Suppression of 4-Coumarate-CoA Ligase Gene Reveals Differential Effect of Lignin on Cell Physiological Function in Populus. Front. Plant Sci. 2020, 17. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, F.; Xing, H.; Mao, K.; Chen, J. Correlation Analysis of Lignin Accumulation and Expression of Key Genes Involved in Lignin Biosynthesis of Ramie (Boehmeria nivea). Genes 2019, 10, 389. [Google Scholar] [CrossRef] [Green Version]
- Chatelet, D.S.; Wistrom, C.M.; Purcell, A.H.; Rost, T.L.; Matthews, M.A. Xylem structure of four grape varieties and 12 alternative hosts to the xylem-limited bacterium Xylella fastidious. Ann. Bot. 2011, 108, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.; Li, Z.; Zhang, X.; Wang, R.; Yang, S. Expression Analysis of Lignin-Associated Genes in Hard End Pear (Pyrus pyrifolia Whangkeumbae) and Its Response to Calcium Chloride Treatment Conditions. J. Plant Growth Regul. 2015, 34, 251–262. [Google Scholar] [CrossRef]
- Schurt, D.A.; Rodrigues, F.A.; Colodette, J.L.; Carre-Missio, V. Effect of silicon on lignin and sugar concentrations of leaf sheaths in rice plants infected by Rhizoctonia solani. Bragantia 2013, 72, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, E.R.; Oliveira, J.A.; Reis, L.V.; Ferreira, E.T.F. Mn foliar sobre a qualidade sanitária e lignina de sementes de soja convencional e resistente ao glifosato1. Rev. Ciênc. Agronmica 2014, 46, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Gorshkov, V.Y.; Daminova, A.G.; Mikshina, P.V.; Petrova, O.E.; Ageeva, M.V.; Salnikov, V.V.; Gorshkova, T.A.; Gogolev, Y.V. Pathogen-induced conditioning of the primary xylem vessels—A prerequisite for the formation of bacterial emboli by Pectobacterium atrosepticum. Plant Biol. 2016, 18, 609–617. [Google Scholar] [CrossRef]
- Zhang, D.; Hrmova, M.; Wan, C.-H.; Wu, C.; Balzen, J.; Cai, W.; Wang, J.; Densmore, L.D.; Fincher, G.B.; Zhang, H.; et al. Members of a new group of chitinase-like genes are expressed preferentially in cotton cells with secondary walls. Plant Mol. Biol. 2004, 54, 353–372. [Google Scholar] [CrossRef]
- Gupta, P.; Ravi, I.; Sharma, V. Induction of β-1,3-glucanase and chitinase activity in the defense response of Eruca sativa plants against the fungal pathogen Alternaria brassicicola. J. Plant Interact. 2013, 8, 155–161. [Google Scholar] [CrossRef]
- Yoshida, K.; Ogino, A.; Yamada, K.; Sonod, R. Induction of Disease Resistance in Tea (Camellia sinensis L.) by Plant Activators. Japan Agricultural Research Quarterly 2010, 44, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Chemical and Microbiological Properties; American Society of Agronomy: Washington, DC, USA, 1982; pp. 86–110. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Analysis 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- De Sá, M.; Ferreira, J.P.; Queiroz, V.T.; Vilas-Boas, L.; Silva, M.C.; Almeida, M.H.; Guerra-Guimarães, L.; Bronze, M.R. A liquid chromatography/electrospray ionisation tandem mass spectrometry method for the simultaneous quantification of salicylic, jasmonic and abscisic acids in Coffea arabica leaves. J. Sci. Food Agric. 2014, 94, 529–536. [Google Scholar] [CrossRef]
- Liang, M.; Davis, E.; Gardner, D.; Cai, X.; Wu, Y. Involvement of AtLAC15 in lignin synthesis in seeds and in root elongation of Arabidopsis. Planta 2006, 224, 1185–1196. [Google Scholar] [CrossRef]
- Pomar, F.; Novo, M.; Bernal, M.A.; Merino, F.; Barchelo, A.R. Changes in stem lignins (monomer composition and crosslinking) and peroxidase are related with the maintenance of leaf photosynthetic integrity during Verticillium wilt in Capsicum annuum. New Phytol. 2004, 163, 111–123. [Google Scholar] [CrossRef]
- Boller, T.; Gehri, A.; Mauch, F.; Vogeli, U. Chitinase in bean leaves: Induction by ethylene, purification, properties, and possible function. Planta 1983, 157, 22–31. [Google Scholar] [CrossRef]
- Pan, S.Q.; Ye, X.S.; Kuć, J. Association of β-1,3-glucanase activity and isoform pattern with systemic resistance to blue mould in tobacco induced by stem injection with Peronospora tabacina or leaf inoculation with tobacco mosaic virus. Physiol. Mol. Plant Pathol. 1991, 39, 25–39. [Google Scholar] [CrossRef]
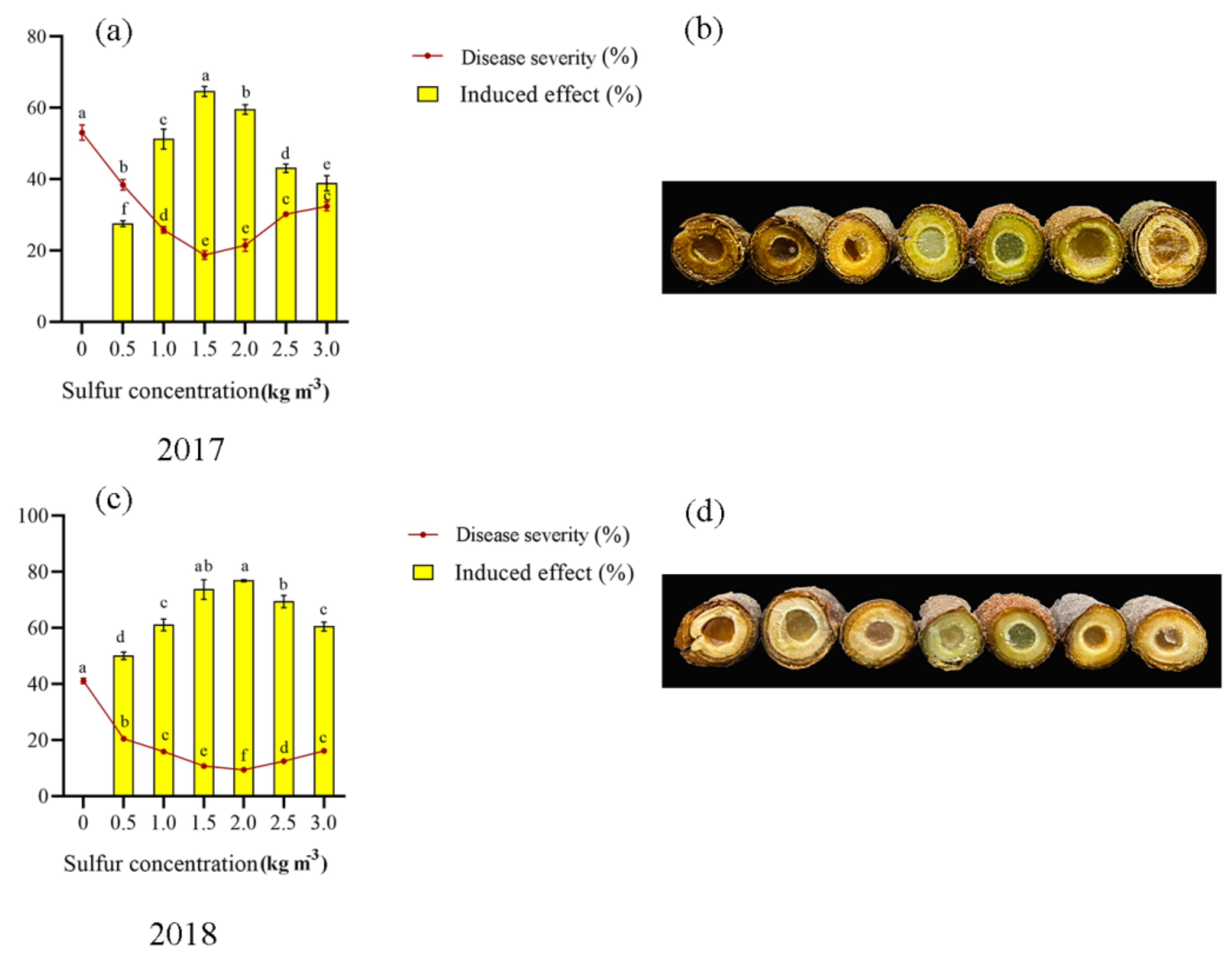
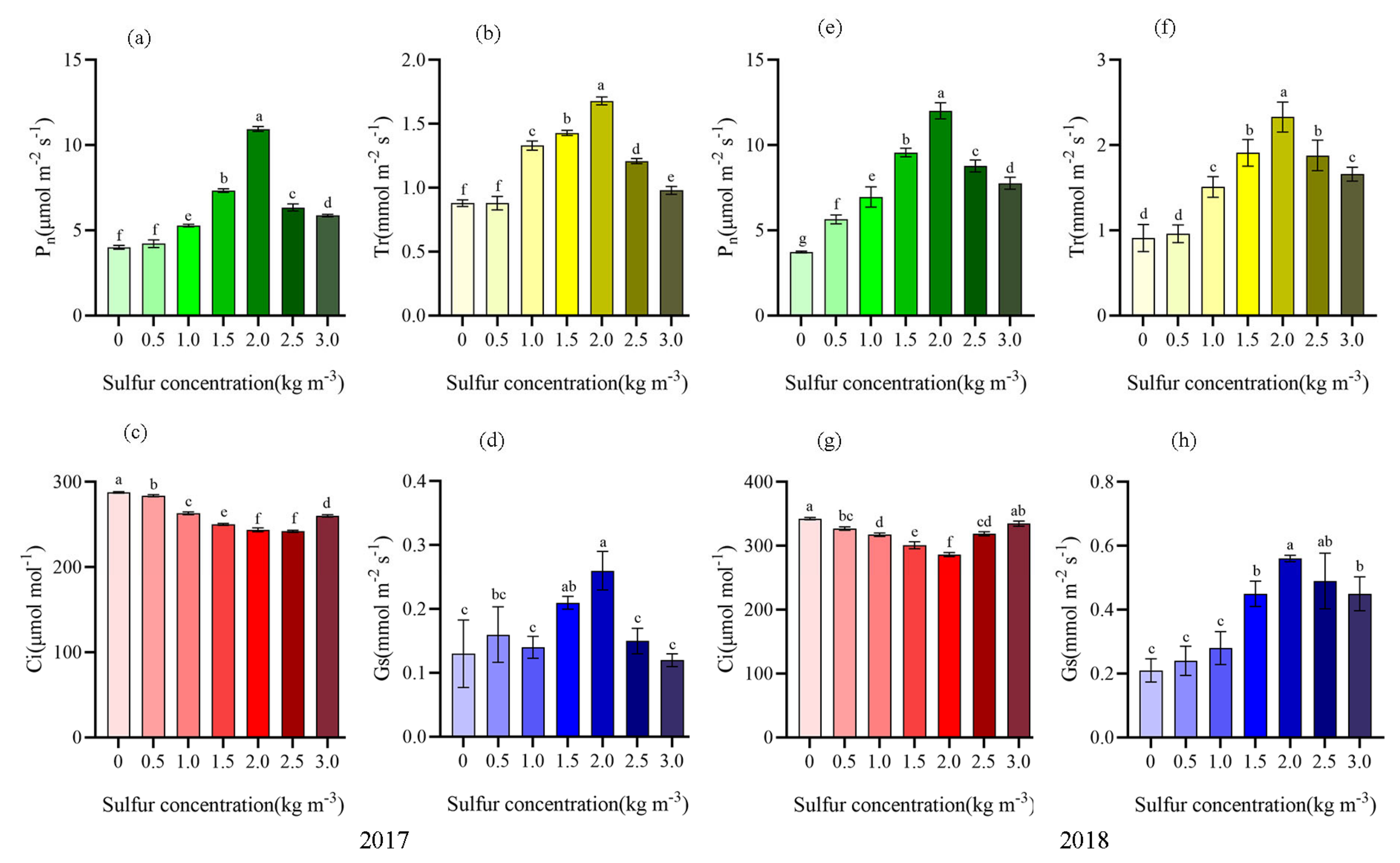
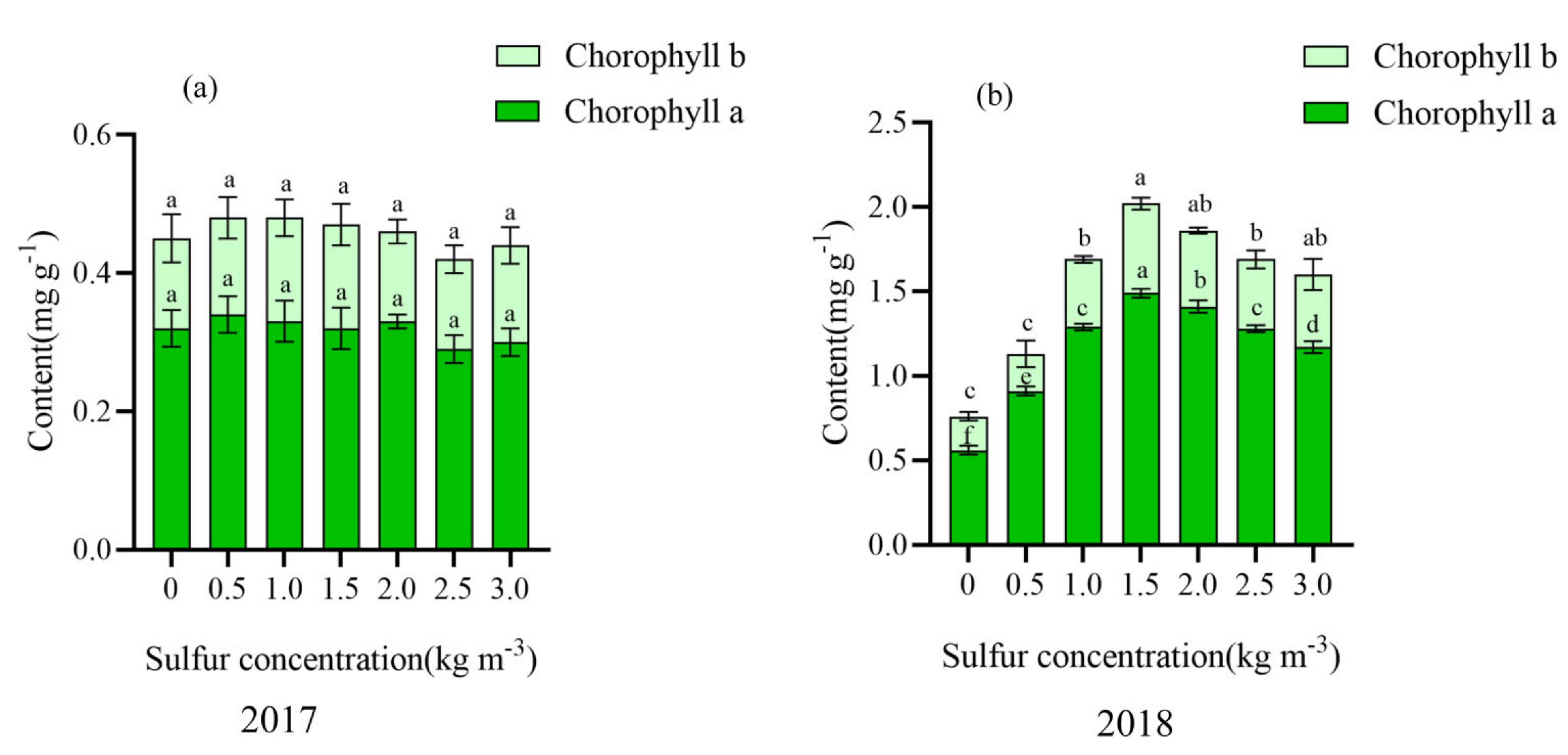

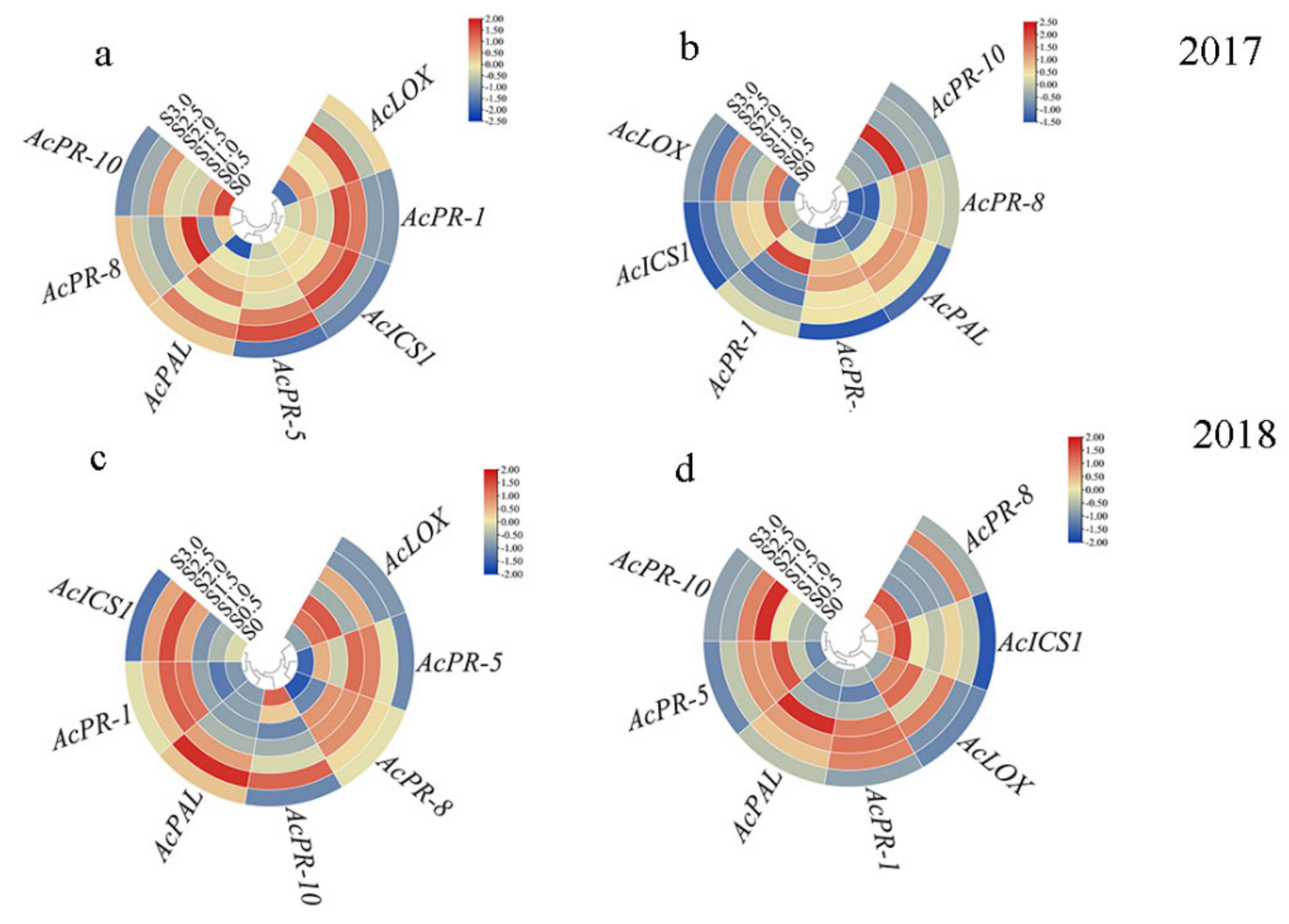


| Number | Treatment |
|---|---|
| S0 | Sulfur-deficiency+10 kg m−3 organic fertilizer (as a control) |
| S1.0 | 1.0 kg m−3 Sulfur powder +10 kg organic fertilizer |
| S1.5 | 1.5 kg m−3 Sulfur powder +10 kg organic fertilizer |
| S2.0 | 2.0 kg m−3 Sulfur powder +10 kg organic fertilizer |
| S2.5 | 2.5 kg m−3 Sulfur powder +10 kg organic fertilizer |
| S3.0 | 3.0 kg m−3 Sulfur powder +10 kg organic fertilizer |
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| AcActin | CACCCTGTGCTGCTTACAGA | GAGAGAGAACGGCCTGAATG |
| AcPR-1 | GCCCCCGGTAAGGTTTGT | CGAACCAAGACCCACTATTGC |
| AcPR-5 | TTCACCAACCTCAGTTCT | ATCGTAAGCGTAACTATAAGC |
| AcPR-8 | TTTGGATGGAATTGACTTTGACA | TTCTTGCCACGACTGCTATA |
| AcPR-10 | TGCTACACTTTAATTGAAGGC | TTGCTTGTCATCTTAGTAATCG |
| AcPAL | CGGAGCAACACAACCAAGA | CCTGACATAGTGCGACTACATAG |
| AcICS1 | AGGCGAGGCTTCTAATTG | ACAGCAAACTCACTCTCTC |
| AcLOX | GGAGAAGCCATTGCCAAT | GGACGGTAATAAGTTGTGAAGTA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Long, Y.; Yin, X.; Yang, S. Sulfur-Induced Resistance against Pseudomonas syringae pv. actinidiae via Triggering Salicylic Acid Signaling Pathway in Kiwifruit. Int. J. Mol. Sci. 2021, 22, 12710. https://doi.org/10.3390/ijms222312710
Zhang Z, Long Y, Yin X, Yang S. Sulfur-Induced Resistance against Pseudomonas syringae pv. actinidiae via Triggering Salicylic Acid Signaling Pathway in Kiwifruit. International Journal of Molecular Sciences. 2021; 22(23):12710. https://doi.org/10.3390/ijms222312710
Chicago/Turabian StyleZhang, Zhuzhu, Youhua Long, Xianhui Yin, and Sen Yang. 2021. "Sulfur-Induced Resistance against Pseudomonas syringae pv. actinidiae via Triggering Salicylic Acid Signaling Pathway in Kiwifruit" International Journal of Molecular Sciences 22, no. 23: 12710. https://doi.org/10.3390/ijms222312710






