Novel Bioactive Adhesive Monomer CMET Promotes Odontogenic Differentiation and Dentin Regeneration
Abstract
:1. Introduction
2. Results
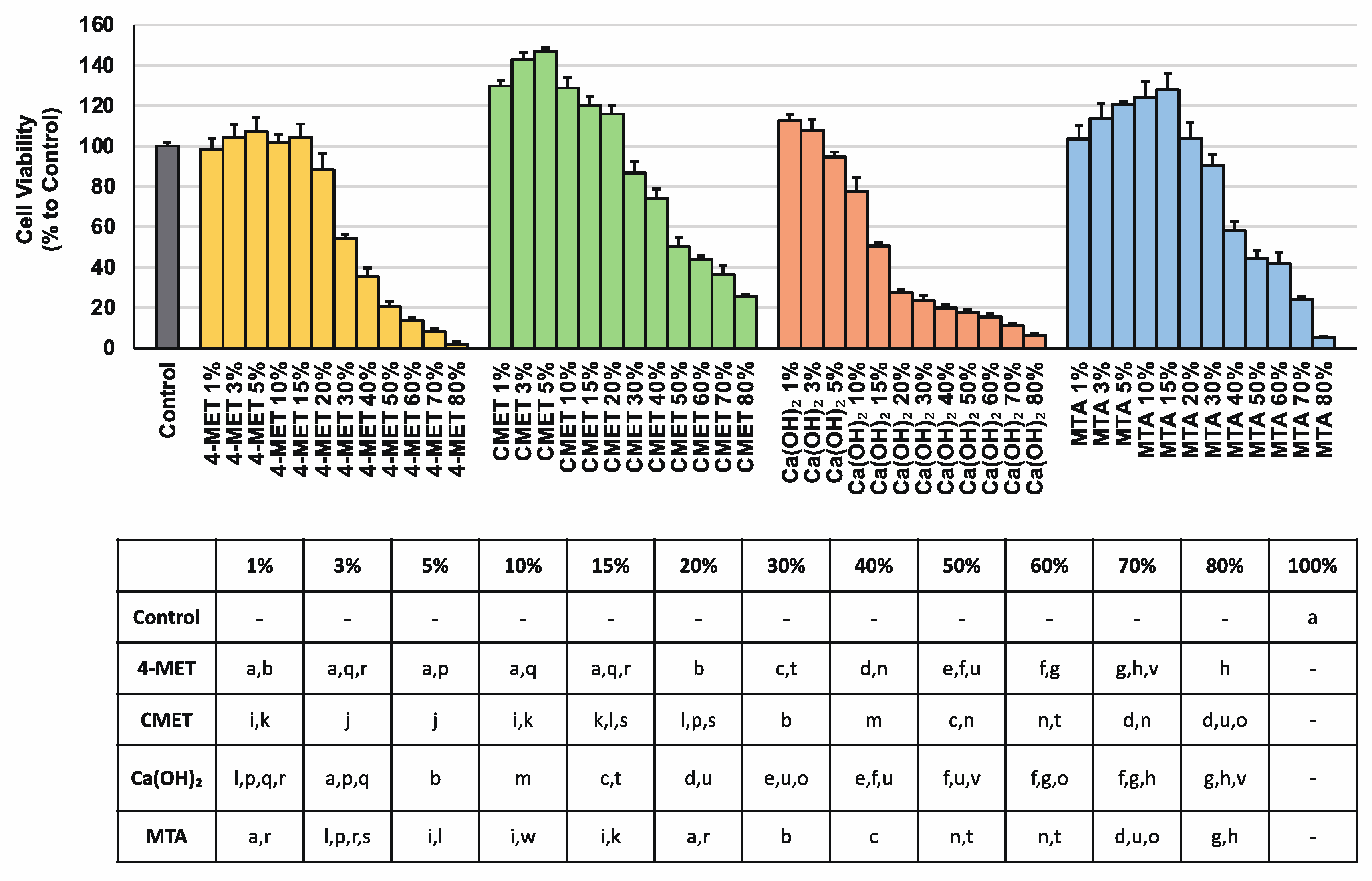
2.1. Cell Viability
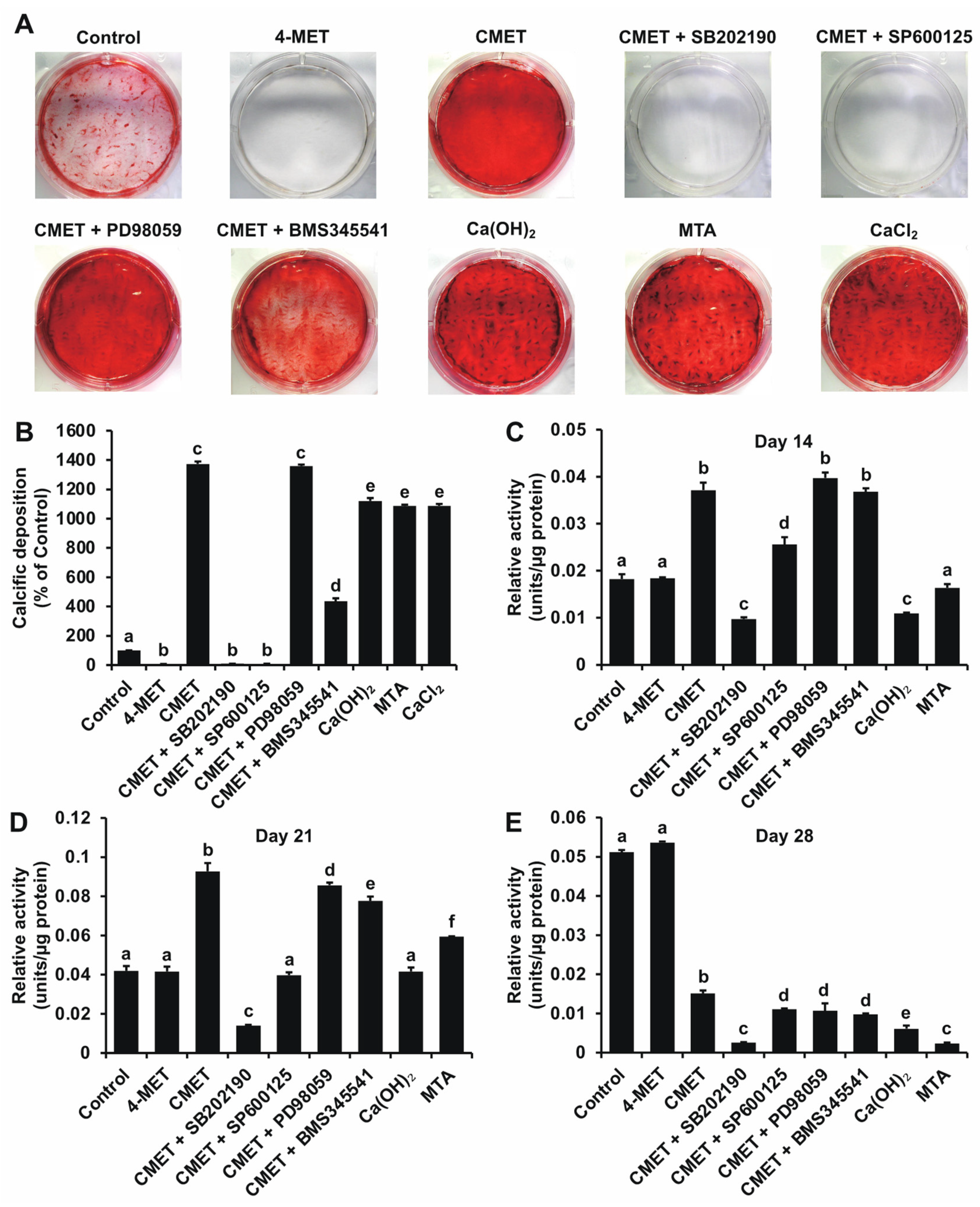
2.2. Alizarin Red S (ARS) Staining
2.3. Relative Alkaline Phosphatase (ALPase) Activity
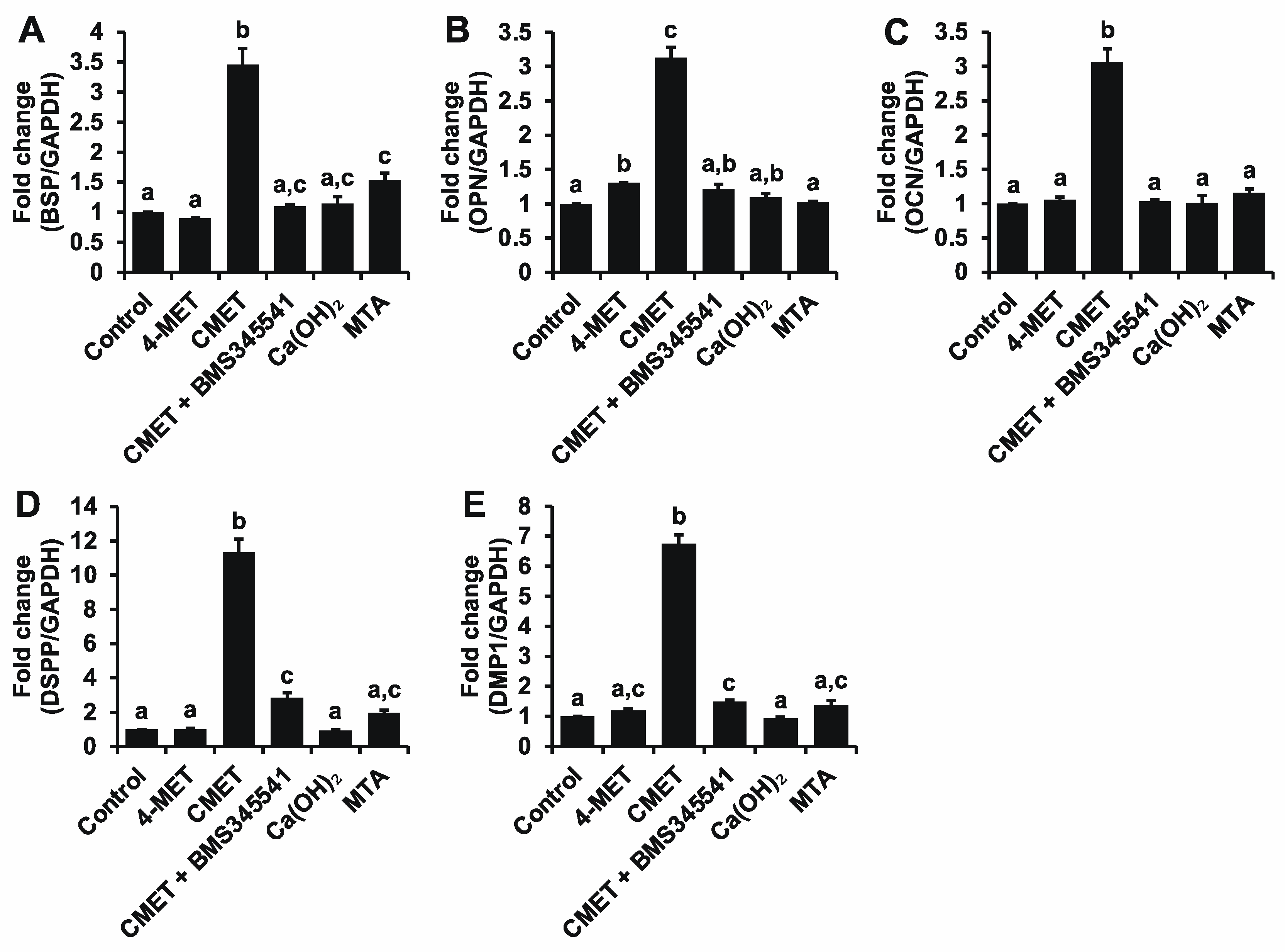
2.4. Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction (Real-Time RT-PCR)
2.5. Histomorphological Features
3. Discussion
4. Materials and Methods
4.1. Preparation of Aqueous Solutions of Different Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. ARS Staining
4.5. ALPase Activity Assay
4.6. Real-Time RT-PCR
4.7. Selective Blockade of NF-κB and MAPK
4.8. Direct Pulp Capping and Histological Observation
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kunert, M.; Lukomska-Szymanska, M. Bio-Inductive Materials in Direct and Indirect Pulp Capping—A Review Article. Materials 2020, 13, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mente, J.; Hufnagel, S.; Leo, M.; Michel, A.; Gehrig, H.; Panagidis, D.; Saure, D.; Pfefferle, T. Treatment Outcome of Mineral Trioxide Aggregate or Calcium Hydroxide Direct Pulp Capping: Long-Term Results. J. Endod. 2014, 40, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.R.J.; Berdal, A.; Cooper, P.R.; Lumley, P.J.; Tomson, P.L.; Smith, A.J. Dentin-Pulp Complex Regeneration. Adv. Dent. Res. 2011, 23, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi, T.; Zhu, Q.; Eberhart, R.; Imai, Y. Current Status of Direct Pulp-Capping Materials for Permanent Teeth. Dent. Mater. J. 2016, 35, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Dammaschke, T.; Stratmann, U.; Wolff, P.; Sagheri, D.; Schäfer, E. Direct Pulp Capping with Mineral Trioxide Aggregate: An Immunohistologic Comparison with Calcium Hydroxide in Rodents. J. Endod. 2010, 36, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, N.; Takigawa, T.; Horie, T.; Maeda, H.; Yamamoto, Y.; Momoi, Y.; Yamamoto, K.; Okiji, T. A Review of the Literature on the Efficacy of Mineral Trioxide Aggregate in Conservative Dentistry. Dent. Mater. J. 2019, 38, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral Trioxide Aggregate and Other Bioactive Endodontic Cements: An Updated Overview—Part I: Vital Pulp Therapy. Int. Endod. J. 2018, 51, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Marconyak, L.J.; Kirkpatrick, T.C.; Roberts, H.W.; Roberts, M.D.; Aparicio, A.; Himel, V.T.; Sabey, K.A. A Comparison of Coronal Tooth Discoloration Elicited by Various Endodontic Reparative Materials. J. Endod. 2016, 42, 470–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedi-Amin, A.; Luzi, A.; Giovarruscio, M.; Paolone, G.; Darvizeh, A.; Agulló, V.V.; Sauro, S. Innovative Root-End Filling Materials Based on Calcium-Silicates and Calcium-Phosphates. J. Mater. Sci. 2017, 28, 31. [Google Scholar] [CrossRef]
- da Rosa, W.L.O.; Cocco, A.R.; da Silva, T.M.; Mesquita, L.C.; Galarça, A.D.; da Silva, A.F.; Piva, E. Current Trends and Future Perspectives of Dental Pulp Capping Materials: A Systematic Review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1358–1368. [Google Scholar] [CrossRef]
- Giraud, T.; Jeanneau, C.; Rombouts, C.; Bakhtiar, H.; Laurent, P.; About, I. Pulp Capping Materials Modulate the Balance between Inflammation and Regeneration. Dent. Mater. 2019, 35, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, L.; Ribeiro, A.P.D.; de Oliveira Carrilho, M.R.; Pashley, D.H.; de Souza Costa, C.A.; Hebling, J. Transdentinal Cytotoxicity of Experimental Adhesive Systems of Different Hydrophilicity Applied to Ethanol-Saturated Dentin. Dent. Mater. 2013, 29, 980–990. [Google Scholar] [CrossRef] [Green Version]
- da Fonseca Roberti Garcia, L.; Pontes, E.C.V.; Basso, F.G.; Hebling, J.; de Souza Costa, C.A.; Soares, D.G. Transdentinal Cytotoxicity of Resin-Based Luting Cements to Pulp Cells. Clin. Oral Investig. 2016, 20, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Toz, T.; Kiremitçi, A.; Sera Çakmak, A.; Ünsal Tan, O.; Palaska, E.; Gümüşderelioğlu, M.; Özcan, M. A Comparative Study on Monomer Elution and Cytotoxicity of Different Adhesive Restoration Materials. J. Adhes. Sci. Technol. 2017, 31, 414–429. [Google Scholar] [CrossRef] [Green Version]
- Nishida, M.; Imazato, S.; Takahashi, Y.; Ebisu, S.; Ishimoto, T.; Nakano, T.; Yasuda, Y.; Saito, T. The Influence of the Antibacterial Monomer 12-Methacryloyloxydodecylpyridinium Bromide on the Proliferation, Differentiation and Mineralization of Odontoblast-like Cells. Biomaterials 2010, 31, 1518–1532. [Google Scholar] [CrossRef] [PubMed]
- Marigo, L.; Nocca, G.; Fiorenzano, G.; Callà, C.; Castagnola, R.; Cordaro, M.; Paolone, G.; Sauro, S. Influences of Different Air-Inhibition Coatings on Monomer Release, Microhardness, and Color Stability of Two Composite Materials. BioMed Res. Int. 2019, 2019, 4240264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.; Iijima, M.; Motai, F.; Mizoguchi, I.; Saito, T. Effects of Calcium Salts of Acidic Monomers on Mineral Induction of Phosphoprotein Immobilized to Agarose Beads. J. Biomed. Mater. Res. Part A 2012, 100A, 2760–2765. [Google Scholar] [CrossRef]
- Motai, F.; Ito, S.; Al Nomann, N.; Saito, T. Dentine Bond Strength and Remineralization Ability of Sealing Coat Material Containing a New Developed Adhesive Monomer, CMET. Jpn. J. Conserv. Dent. 2015, 58, 143–156. [Google Scholar]
- Thaweboon, S.; Saito, T.; Nagano, K.; Thaweboon, B. Evaluation of an Adhesive Containing Calcium Salt of Acidic Monomers on Inhibition of Biofilm Formation of Bacteria Related to Root Caries. Key Eng. Mater. 2020, 853, 41–45. [Google Scholar] [CrossRef]
- Qiu, Y.J.; Tang, J.; Saito, T. A Novel Bio-active Adhesive Monomer Induces Odontoblast Differentiation: A Comparative Study. Int. Endod. J. 2020, 53, 1413–1429. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal Human Dental Pulp Stem Cells (DPSCs) in Vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [Green Version]
- Bjørndal, L.; Simon, S.; Tomson, P.L.; Duncan, H.F. Management of Deep Caries and the Exposed Pulp. Int. Endod. J. 2019, 52, 949–973. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N. Characterisation of Dental Pulp Stem Cells: A New Horizon for Tissue Regeneration? Arch. Oral Biol. 2012, 57, 1439–1458. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem Cell Properties of Human Dental Pulp Stem Cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Moreau, J.L.; Xu, H.H.K. Mesenchymal Stem Cell Proliferation and Differentiation on an Injectable Calcium Phosphate—Chitosan Composite Scaffold. Biomaterials 2009, 30, 2675–2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takita, T.; Hayashi, M.; Takeichi, O.; Ogiso, B.; Suzuki, N.; Otsuka, K.; Ito, K. Effect of Mineral Trioxide Aggregate on Proliferation of Cultured Human Dental Pulp Cells. Int. Endod. J. 2006, 39, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, W.; Song, Z.; Tong, Z.; Li, S.; Ni, L. Mineral Trioxide Aggregate Promotes Odontoblastic Differentiation via Mitogen-Activated Protein Kinase Pathway in Human Dental Pulp Stem Cells. Mol. Biol. Rep. 2012, 39, 215–220. [Google Scholar] [CrossRef]
- An, S.; Gao, Y.; Huang, Y.; Jiang, X.; Ma, K.; Ling, J. Short-Term Effects of Calcium Ions on the Apoptosis and Onset of Mineralization of Human Dental Pulp Cells in Vitro and in Vivo. Int. J. Mol. Med. 2015, 36, 215–221. [Google Scholar] [CrossRef] [Green Version]
- An, S.; Gao, Y.; Ling, J.; Wei, X.; Xiao, Y. Calcium Ions Promote Osteogenic Differentiation and Mineralization of Human Dental Pulp Cells: Implications for Pulp Capping Materials. J. Mater. Sci. Mater. Med. 2012, 23, 789–795. [Google Scholar] [CrossRef]
- Simon, S.; Smith, A.J.; Berdal, A.; Lumley, P.J.; Cooper, P.R. The MAP Kinase Pathway Is Involved in Odontoblast Stimulation via P38 Phosphorylation. J. Endod. 2010, 36, 256–259. [Google Scholar] [CrossRef]
- Woo, S.M.; Seong, K.J.; Oh, S.J.; Park, H.J.; Kim, S.H.; Kim, W.J.; Jung, J.Y. 17β-Estradiol Induces Odontoblastic Differentiation via Activation of the c-Src/MAPK Pathway in Human Dental Pulp Cells. Biochem. Cell Biol. 2015, 93, 587–595. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.A.C.; Junior, C.R.; Garlet, G.P.; Nogueira, A.V.B.; Cirelli, J.A. Modulation of Host Cell Signaling Pathways as a Therapeutic Approach in Periodontal Disease. J. Appl. Oral Sci. 2012, 20, 128–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, S.P.; Reddy, H.; Caivano, M.; Cohen, P. Specificity and Mechanism of Action of Some Commonly Used Protein Kinase Inhibitors. Biochem. J. 2000, 351, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Boyle, D.L.; Chang, L.; Bennett, B.; Karin, M.; Yang, L.; Manning, A.M.; Firestein, G.S. C-Jun N-Terminal Kinase Is Required for Metalloproteinase Expression and Joint Destruction in Inflammatory Arthritis. J. Clin. Investig. 2001, 108, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Paula-Silva, F.W.G.; Ghosh, A.; Silva, L.A.B.; Kapila, Y.L. TNF-Alpha Promotes an Odontoblastic Phenotype in Dental Pulp Cells. J. Dent. Res. 2009, 88, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Zhang, C.; Tani-Ishii, N.; Shi, S.; Wang, C.-Y. NF-ΚB Activation in Human Dental Pulp Stem Cells by TNF and LPS. J. Dent. Res. 2005, 84, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.R.; Pattoli, M.A.; Gregor, K.R.; Brassil, P.J.; MacMaster, J.F.; McIntyre, K.W.; Yang, X.; Iotzova, V.S.; Clarke, W.; Strnad, J.; et al. BMS-345541 Is a Highly Selective Inhibitor of IκB Kinase That Binds at an Allosteric Site of the Enzyme and Blocks NF-ΚB-Dependent Transcription in Mice. J. Biol. Chem. 2003, 278, 1450–1456. [Google Scholar] [CrossRef] [Green Version]
- Jones, W.K.; Brown, M.; Ren, X.; He, S.; McGuinness, M. NF-ΚB as an Integrator of Diverse Signaling Pathways: The Heart of Myocardial Signaling? Cardiovasc. Toxicol. 2003, 3, 229–254. [Google Scholar] [CrossRef]
- Duarte, M.A.H.; Martins, C.S.; de Oliveira Cardoso Demarchi, A.C.; de Godoy, L.F.; Kuga, M.C.; Yamashita, J.C. Calcium and Hydroxide Release from Different Pulp-Capping Materials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 104, e66–e69. [Google Scholar] [CrossRef]
- Rashid, F.; Shiba, H.; Mizuno, N.; Mouri, Y.; Fujita, T.; Shinohara, H.; Ogawa, T.; Kawaguchi, H.; Kurihara, H. The Effect of Extracellular Calcium Ion on Gene Expression of Bone-Related Proteins in Human Pulp Cells. J. Endod. 2003, 29, 104–107. [Google Scholar] [CrossRef]
- de Mendonça PETTA, T.; Pedroni, A.C.F.; Saavedra, D.F.; do Carmo Freitas FAIAL, K.; Marques, M.M.; Couto, R.S.D. The Effect of Three Different Pulp Capping Cements on Mineralization of Dental Pulp Stem Cells. Dent. Mater. J. 2020, 39, 222–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandolfi, M.G.; Siboni, F.; Primus, C.M.; Prati, C. Ion Release, Porosity, Solubility, and Bioactivity of MTA Plus Tricalcium Silicate. J. Endod. 2014, 40, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Tran, X.V.; Gorin, C.; Willig, C.; Baroukh, B.; Pellat, B.; Decup, F.; Opsahl Vital, S.; Chaussain, C.; Boukpessi, T. Effect of a Calcium-Silicate-Based Restorative Cement on Pulp Repair. J. Dent. Res. 2012, 91, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Dammaschke, T.; Wolff, P.; Sagheri, D.; Stratmann, U.; Schäfer, E. Mineral Trioxide Aggregate for Direct Pulp Capping: A Histologic Comparison with Calcium Hydroxide in Rat Molars. Quintessence Int. 2010, 41, e20–e30. [Google Scholar] [PubMed]
- Yaemkleebbua, K.; Osathanon, T.; Nowwarote, N.; Limjeerajarus, C.N.; Sukarawan, W. Analysis of Hard Tissue Regeneration and Wnt Signalling in Dental Pulp Tissues after Direct Pulp Capping with Different Materials. Int. Endod. J. 2019, 52, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Ricucci, D.; Loghin, S.; Lin, L.M.; Spångberg, L.S.W.; Tay, F.R. Is Hard Tissue Formation in the Dental Pulp after the Death of the Primary Odontoblasts a Regenerative or a Reparative Process? J. Dent. 2014, 42, 1156–1170. [Google Scholar] [CrossRef]
- Krifka, S.; Petzel, C.; Bolay, C.; Hiller, K.-A.; Spagnuolo, G.; Schmalz, G.; Schweikl, H. Activation of Stress-Regulated Transcription Factors by Triethylene Glycol Dimethacrylate Monomer. Biomaterials 2011, 32, 1787–1795. [Google Scholar] [CrossRef]
- Eckhardt, A.; Müller, P.; Hiller, K.-A.; Krifka, S.; Bolay, C.; Spagnuolo, G.; Schmalz, G.; Schweikl, H. Influence of TEGDMA on the Mammalian Cell Cycle in Comparison with Chemotherapeutic Agents. Dent. Mater. 2010, 26, 232–241. [Google Scholar] [CrossRef]
- Spagnuolo, G.; Desiderio, C.; Rivieccio, V.; Amato, M.; Rossetti, D.V.; D’Antò, V.; Schweikl, H.; Lupi, A.; Rengo, S.; Nocca, G. In Vitro Cellular Detoxification of Triethylene Glycol Dimethacrylate by Adduct Formation with N-Acetylcysteine. Dent. Mater. 2013, 29, e153–e160. [Google Scholar] [CrossRef] [PubMed]
- Nocca, G.; Callà, C.; Martorana, G.E.; Cicillini, L.; Rengo, S.; Lupi, A.; Cordaro, M.; Luisa Gozzo, M.; Spagnuolo, G. Effects of Dental Methacrylates on Oxygen Consumption and Redox Status of Human Pulp Cells. BioMed Res. Int. 2014, 2014, 956579. [Google Scholar] [CrossRef] [Green Version]
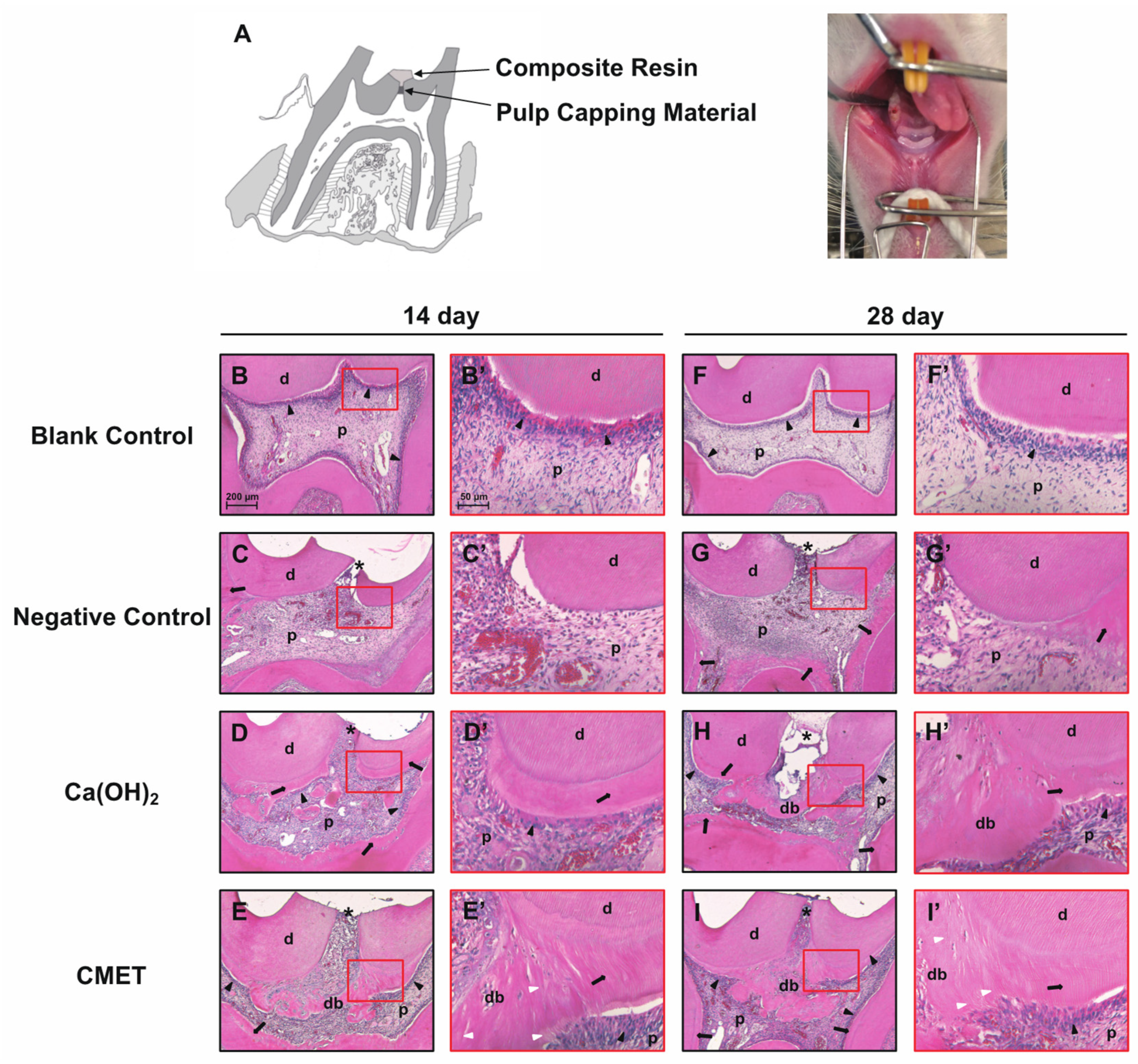
| Continuity | Morphology | Thickness | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| 14 days | ||||||||||||
| Negative Control | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 |
| Ca(OH)2 | 0 | 0 | 6 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 6 | 0 |
| CMET | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 5 | 1 | 0 |
| 28 days | ||||||||||||
| Negative Control | 0 | 0 | 0 | 6 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 6 |
| Ca(OH)2 | 6 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 |
| CMET | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 5 | 1 | 0 | 0 |
| Gene | Primer Sequences | Product Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| BSP | Forward: AAGGGCACCTCGAAGACAAC | 119 | 62.8 |
| Reverse: CCCTCGTATTCAACGGTGGT | |||
| OPN | Forward: TCCCTGTGTTGGTGGAGGAT | 158 | 59.9 |
| Reverse: GAGTTTTCCTTGGTCGGCGT | |||
| OCN | Forward: CGCAGCTCCCAACCACAATA | 238 | 62.8 |
| Reverse: GTGTGAGGGCTCTCATGGTG | |||
| DSPP | Forward: TGCTGGCCTGGATAATTCCG | 136 | 66 |
| Reverse: CTCCTGGCCCTTGCTGTTAT | |||
| DMP1 | Forward: ACAGCAGCTCAGCAGAGAGT | 235 | 62.8 |
| Reverse: TAATAGCCGTCTTGGCAGTC | |||
| GAPDH | Forward: CACTAGGCGCTCACTGTTCTCT | 250 | 66 |
| Reverse: CGTTCTCAGCCTTGACGGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Saito, T. Novel Bioactive Adhesive Monomer CMET Promotes Odontogenic Differentiation and Dentin Regeneration. Int. J. Mol. Sci. 2021, 22, 12728. https://doi.org/10.3390/ijms222312728
Qiu Y, Saito T. Novel Bioactive Adhesive Monomer CMET Promotes Odontogenic Differentiation and Dentin Regeneration. International Journal of Molecular Sciences. 2021; 22(23):12728. https://doi.org/10.3390/ijms222312728
Chicago/Turabian StyleQiu, Youjing, and Takashi Saito. 2021. "Novel Bioactive Adhesive Monomer CMET Promotes Odontogenic Differentiation and Dentin Regeneration" International Journal of Molecular Sciences 22, no. 23: 12728. https://doi.org/10.3390/ijms222312728
APA StyleQiu, Y., & Saito, T. (2021). Novel Bioactive Adhesive Monomer CMET Promotes Odontogenic Differentiation and Dentin Regeneration. International Journal of Molecular Sciences, 22(23), 12728. https://doi.org/10.3390/ijms222312728







