Stability of Erythrocyte-Derived Nanovesicles Assessed by Light Scattering and Electron Microscopy
Abstract
:1. Introduction
2. Results
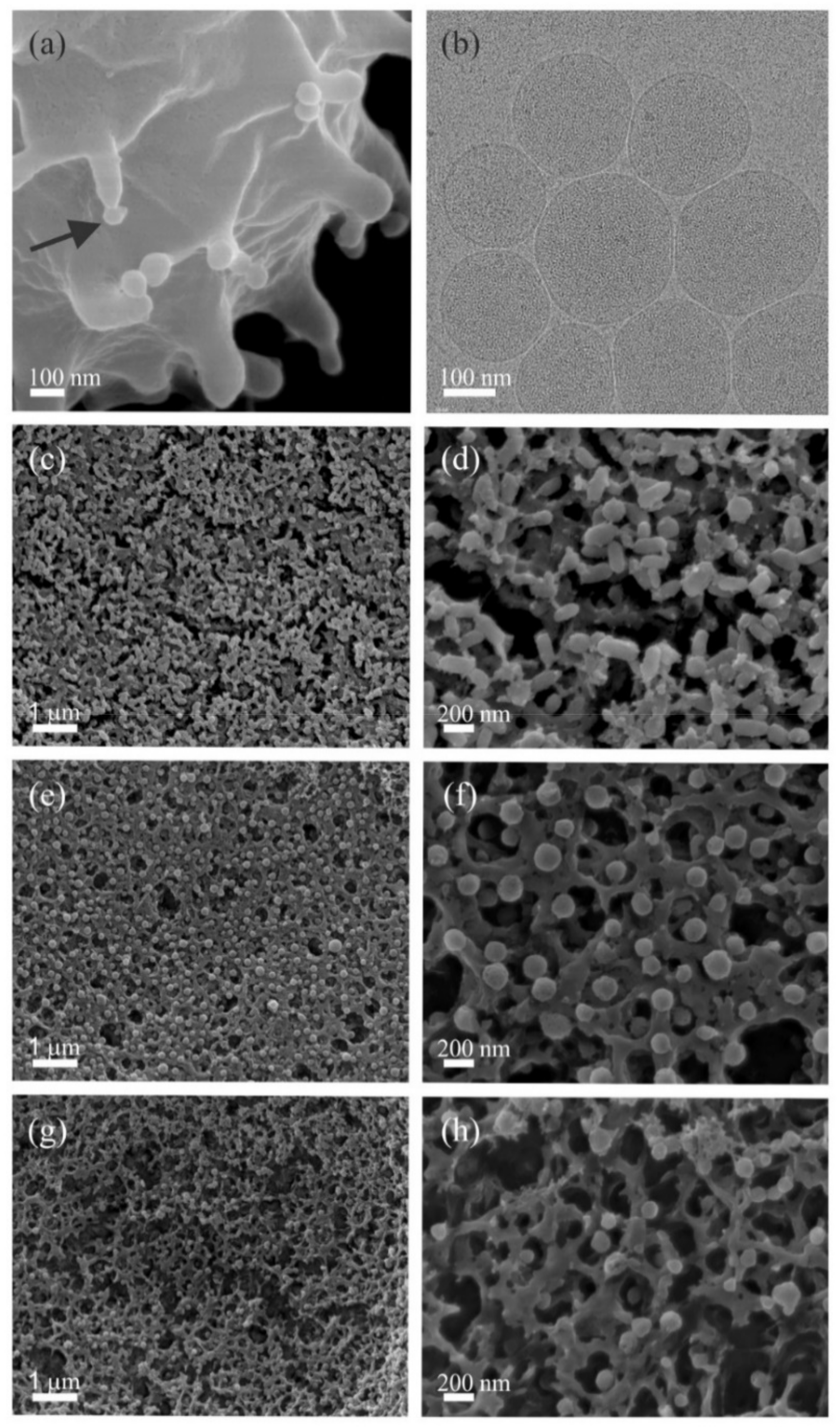
2.1. Mechanism of hbEVs Formation and Their Morphology
2.2. Stability of HbEVs with Respect to Osmolarity of the Suspension, pH, Temperature and Addition of Surfactant Triton X-100, Determined by LS
3. Discussion
3.1. Mechanism of hbEV Formation and Their Morphology
3.2. Stability of hbEVs
3.3. The Power and Limitations of LS Analysis for Evaluation of EV Integrity
4. Materials and Methods
4.1. Preparation of Vesicles
4.2. Preparation of Samples for Characterization and Evaluation of HbEV Stability
4.3. Scanning Electron Microscopy (SEM)
4.4. Cryo Electron Microscopy (Cryo-TEM)
4.5. Flow Cytometry (FCM)
4.6. Static (SLS) and Dynamic (DLS) Light Scattering (LS)
4.7. Estimation of Protein Content
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Analysis of Efficiency of hbEVs Isolation by Differential Centrifugation
- 300 g (10 min, 4 °C); centrifuge Centric 400 R (Domel, Slovenia) in 4 mL polypropylene culture tubes (ref. T405-1A, Simport scientific, Kanada);
- 2000 g (10 min, 4 °C); centrifuge Centric 400 R (Domel, Slovenia) in 4 mL polypropylene culture tubes (ref. T405-1A, Simport scientific, Kanada);
- 4000 g (10 min, 4 °C); centrifuge Centric 400 R (Domel, Slovenia) in 4 mL polypropylene culture tubes (ref. T405-1A, Simport scientific, Kanada);
- 10,000 g (10 min, 4 °C); centrifuge Centric 200 R with a swinging rotor “Lilliput” (Domel, Slovenia) in 1.5 mL conic polypropylene centrifuge tubes);
- 50,000 g (70 min at 4 °C); ultracentrifuge Beckman L9-70M, with rotor Type SW55Ti (Beckman Coulter Inc., USA) in 5 mL round-bottom polypropylene centrifuge tubes (ref. 326819, Beckman Coulter Inc., USA);
- 100,000 g (70 min at 4 °C); ultracentrifuge Beckman L9-70M with rotor Type SW55Ti (Beckman Coulter Inc., USA) in 5 mL round-bottom polypropylene centrifuge tubes (ref. 326819, Beckman Coulter Inc., USA).

Appendix B. Additional Cryo-TEM Images


Appendix C. Protein Analysis

Appendix D. Direct Analysis of hbEVs Samples by DLS, FCM and UV-Vis Spectrometry

Appendix E. Correlation Functions Obtained in Experiments of hbEVs Stability

Appendix F. Resolution of DLS to Detect Vesicles and Solubilised Components
| HBP Addition (Protein c, mg/mL) | Peak Rh,90 > 100 nm (hbEVs) | Peak Rh,90 < 30 nm (HBP) | |
|---|---|---|---|
| Rh,90,hbEVs | I90,hbEVs/I90,hbEVs,0,add | I90,Rh < 30 nm/I90,Rh < 30 nm,HBP,add | |
| - | 111.02 | 100% | nd |
| 0.16 | 110.76 | 100% | nd |
| 0.31 | 108.16 | 100% | 21% |
| 1.50 | 112.56 | 100% | 112% |
| 2.86 | 113.72 | 100% | 91% |
Appendix G

References
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Woith, E.; Fuhrmann, G.; Melzig, M.F. Extracellular Vesicles-Connecting Kingdoms. Int. J. Mol. Sci. 2019, 20, 5695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Cruz, L.; Romero, J.A.A.; Iglesia, R.P.; Lopes, M.H. Extracellular Vesicles: Decoding a New Language for Cellular Communication in Early Embryonic Development. Front. Cell Dev. Biol. 2018, 6, 94. [Google Scholar] [CrossRef]
- Capra, E.; Lange-Consiglio, A. The Biological Function of Extracellular Vesicles during Fertilization, Early Embryo-Maternal Crosstalk and Their Involvement in Reproduction: Review and Overview. Biomolecules 2020, 10, 1510. [Google Scholar] [CrossRef]
- Domingues, S.; Nielsen, K.M. Membrane vesicles and horizontal gene transfer in prokaryotes. Curr. Opin. Microbiol. 2017, 38, 16–21. [Google Scholar] [CrossRef]
- Emamalipour, M.; Seidi, K.; Zununi Vahed, S.; Jahanban-Esfahlan, A.; Jaymand, M.; Majdi, H.; Amoozgar, Z.; Chitkushev, L.T.; Javaheri, T.; Jahanban-Esfahlan, R.; et al. Horizontal Gene Transfer: From Evolutionary Flexibility to Disease Progression. Front. Cell Dev. Biol. 2020, 8, 229. [Google Scholar] [CrossRef]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Schnatz, A.; Müller, C.; Brahmer, A.; Krämer-Albers, E.-M. Extracellular Vesicles in neural cell interaction and CNS homeostasis. FASEB BioAdv. 2021, 3, 577–592. [Google Scholar] [CrossRef]
- Alberro, A.; Iparraguirre, L.; Fernandes, A.; Otaegui, D. Extracellular Vesicles in Blood: Sources, Effects, and Applications. Int. J. Mol. Sci. 2021, 22, 8163. [Google Scholar] [CrossRef]
- Mallia, A.; Gianazza, E.; Zoanni, B.; Brioschi, M.; Barbieri, S.S.; Banfi, C. Proteomics of Extracellular Vesicles: Update on Their Composition, Biological Roles and Potential Use as Diagnostic Tools in Atherosclerotic Cardiovascular Diseases. Diagnostics 2020, 10, 843. [Google Scholar] [CrossRef]
- Buzas, E.I.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [CrossRef]
- Van Hezel, M.E.; Nieuwland, R.; Van Bruggen, R.; Juffermans, N.P. The ability of extracellular vesicles to induce a pro-inflammatory host response. Int. J. Mol. Sci. 2017, 18, 1285. [Google Scholar] [CrossRef] [Green Version]
- Lo Cicero, A.; Stahl, P.D.; Raposo, G. Extracellular vesicles shuffling intercellular messages: For good or for bad. Curr. Opin. Cell Biol. 2015, 35, 69–77. [Google Scholar] [CrossRef]
- Bergsmedh, A.; Szeles, A.; Henriksson, M.; Bratt, A.; Folkman, M.J.; Spetz, A.L.; Holmgren, L. Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc. Natl. Acad. Sci. USA 2001, 98, 6407–6411. [Google Scholar] [CrossRef] [Green Version]
- Moloudizargari, M.; Asghari, M.H.; Goel, A. The therapeutic triad of extracellular vesicles: As drug targets, as drugs, and as drug carriers. Biochem. Pharmacol. 2021, 192, 114714. [Google Scholar] [CrossRef]
- De Jong, O.G.; Kooijmans, S.A.A.; Murphy, D.E.; Jiang, L.; Evers, M.J.W.; Sluijter, J.P.G.; Vader, P.; Schiffelers, R.M. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef] [Green Version]
- Nieuwland, R.; Falcón-Pérez, J.M.; Théry, C.; Witwer, K.W. Rigor and standardization of extracellular vesicle research: Paving the road towards robustness. J. Extracell. Vesicles 2020, 10, e12037. [Google Scholar] [CrossRef]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, T.L.; Russell, A.J.; Riley, P. Experimental limitations of extracellular vesicle-based therapies for the treatment of myocardial infarction. Trends Cardiovasc. Med. 2021, 31, 405–415. [Google Scholar] [CrossRef]
- Torres Crigna, A.; Fricke, F.; Nitschke, K.; Worst, T.; Erb, U.; Karremann, M.; Buschmann, D.; Elvers-Hornung, S.; Tucher, C.; Schiller, M.; et al. Inter-Laboratory Comparison of Extracellular Vesicle Isolation Based on Ultracentrifugation. Transfus. Med. Hemother. 2021, 48, 48–59. [Google Scholar] [CrossRef]
- Maas, S.L.; de Vrij, J.; van der Vlist, E.J.; Geragousian, B.; van Bloois, L.; Mastrobattista, E.; Schiffelers, R.M.; Wauben, M.H.; Broekman, M.L.; Nolte-’t Hoen, E.N. Possibilities and limitations of current technologies for quantification of biological extracellular vesicles and synthetic mimics. J. Control. Release 2015, 200, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Vogel, R.; Savage, J.; Muzard, J.; Camera, G.D.; Vella, G.; Law, A.; Marchioni, M.; Mehn, D.; Geiss, O.; Peacock, B.; et al. Measuring particle concentration of multimodal synthetic reference materials and extracellular vesicles with orthogonal techniques: Who is up to the challenge? J. Extracell. Vesicles 2021, 10, e12052. [Google Scholar] [CrossRef]
- Taylor, D.D.; Shah, S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods 2015, 87, 3–10. [Google Scholar] [CrossRef]
- Allelein, S.; Medina-Perez, P.; Lopes, A.L.H.; Rau, S.; Hause, G.; Kölsch, A.; Kuhlmeier, D. Potential and challenges of specifically isolating extracellular vesicles from heterogeneous populations. Sci. Rep. 2021, 11, 11585. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef] [Green Version]
- Veerman, R.E.; Teeuwen, L.; Czarnewski, P.; Güclüler Akpinar, G.; Sandberg, A.; Cao, X.; Pernemalm, M.; Orre, L.M.; Gabrielsson, S.; Eldh, M. Molecular evaluation of five different isolation methods for extracellular vesicles reveals different clinical applicability and subcellular origin. J. Extracell. Vesicles 2021, 10, e12128. [Google Scholar] [CrossRef]
- Tiruvayipati, S.; Wolfgeher, D.; Yue, M.; Duan, F.; Andrade, J.; Jiang, H.; Schuger, L. Variability in protein cargo detection in technical and biological replicates of exosome-enriched extracellular vesicles. PLoS ONE 2020, 15, e0228871. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Poncelet, P.; Robert, S.; Bouriche, T.; Bez, J.; Lacroix, R.; Dignat-George, F. Standardized counting of circulating platelet microparticles using currently available flow cytometers and scatter-based triggering: Forward or side scatter? Cytom. A 2016, 89, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Moore, J. Extracellular vesicles: Great potential, many challenges. Cytom. B Clin. Cytom. 2016, 90, 324–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valkonen, S.; van der Pol, E.; Böing, A.; Yuana, Y.; Yliperttula, M.; Nieuwland, R.; Laitinen, S.; Siljander, P.R. Biological reference materials for extracellular vesicle studies. Eur. J. Pharm. Sci. 2017, 98, 4–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geeurickx, E.; Lippens, L.; Rappu, P.; De Geest, B.G.; De Wever, O.; Hendrix, A. Recombinant extracellular vesicles as biological reference material for method development, data normalization and assessment of (pre-)analytical variables. Nat. Protoc. 2021, 16, 603–633. [Google Scholar] [CrossRef]
- Yuan, F.; Li, Y.M.; Wang, Z. Preserving extracellular vesicles for biomedical applications: Consideration of storage stability before and after isolation. Drug Deliv. 2021, 28, 1501–1509. [Google Scholar] [CrossRef]
- Jacobsohn, M.K.; Bazilian, L.S.; Hardiman, J.; Jacobsohn, G.M. Effect of pH on the affinity of phospholipids for cholesterol. Lipids 1989, 24, 375–382. [Google Scholar] [CrossRef]
- Tegmo-Larsson, I.M.; Hofmann, K.P.; Kreutz, W.; Yatvin, M.B. The effect of pH on vesicles composed of phosphatidylcholines and N-acylamino acids: A calcein release fluorescence study. J. Control. Release 1985, 1, 191–196. [Google Scholar] [CrossRef]
- Leung, C.-Y.; Palmer, L.C.; Kewalramani, S.; Qiao, B.; Stupp, S.I.; Olvera de la Cruz, M.; Bedzyk, M.J. Crystalline polymorphism induced by charge regulation in ionic membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 16309–16314. [Google Scholar] [CrossRef] [Green Version]
- Schlieper, P.; Steiner, R. Effect of pH and different substrates on the electrokinetic properties of (Na+, K+)-ATPase vesicles. Biophys. Struct. Mech. 1983, 9, 193–206. [Google Scholar] [CrossRef]
- Zheng, L.-Q.; Shui, L.-L.; Shen, Q.; Li, G.-Z.; Baba, T.; Minamikawa, H.; Hato, M. pH and salt-induced reversible aggregation of nonionic synthetic glycolipid vesicles. Colloids Surf. A Physicochem. Eng. 2002, 207, 215–221. [Google Scholar] [CrossRef]
- Mondal Roy, S.; Sarkar, M. Membrane fusion induced by small molecules and ions. J. Lipids 2011, 2011, 528784. [Google Scholar] [CrossRef] [Green Version]
- Thureson-Klein, A.; Klein, R.L.; Yen, S.H.C. Morphological effects of osmolarity on purified noradrenergic vesicles. J. Neurocytol. 1975, 4, 609–627. [Google Scholar] [CrossRef]
- De Michelis, M.I.; Pugliarello, M.C.; Rasi-Caldogno, F.; De Vecchi, L. Osmotic Behaviour and Permeability Properties of Vesicles in Microsomal Preparations from Pea Internodes. J. Exp. Bot. 1981, 32, 293–302. [Google Scholar] [CrossRef]
- Ohki, S. Effects of divalent cations, temperature, osmotic pressure gradient, and vesicle curvature on phosphatidylserine vesicle fusion. J. Membr. Biol. 1984, 77, 265–275. [Google Scholar] [CrossRef]
- Ibarguren, M.; Bomans, P.H.; Ruiz-Mirazo, K.; Frederik, P.M.; Alonso, A.; Goñi, F.M. Thermally-induced aggregation and fusion of protein-free lipid vesicles. Colloids Surf. B Biointerfaces 2015, 136, 545–552. [Google Scholar] [CrossRef]
- Eum, K.M.; Riedy, G.; Langley, K.H.; Roberts, M.F. Temperature-induced fusion of small unilamellar vesicles formed from saturated long-chain lecithins and diheptanoylphosphatidylcholine. Biochemistry 1989, 28, 8206–8213. [Google Scholar] [CrossRef]
- Howard, F.B.; Levin, I.W. Lipid vesicle aggregation induced by cooling. Int. J. Mol. Sci. 2010, 11, 754–761. [Google Scholar] [CrossRef]
- Cheng, Y.; Zeng, Q.; Han, Q.; Xia, W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell 2019, 10, 295–299. [Google Scholar] [CrossRef] [Green Version]
- Canham, P.B. The minimum energy of bending as a possible explanation of the biconcave shape of the human red blood cell. J. Theor. Biol. 1970, 26, 61–81. [Google Scholar] [CrossRef]
- Deuling, H.J.; Helfrich, W. Red blood cell shapes as explained on the basis of curvature elasticity. Biophys. J. 1976, 16, 861–868. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Balbuena, L.; Arteaga-Jiménez, A.; Hernández-Zapata, E.; Urrutia-Buñuelos, E. Application of the Helfrich elasticity theory to the morphology of red blood cells. Am. J. Phys. 2021, 89, 465–476. [Google Scholar] [CrossRef]
- Iglic, A. A possible mechanism determining the stability of spiculated red blood cells. J. Biomech. 1997, 30, 35–40. [Google Scholar] [CrossRef]
- Lim, H.W.G.; Wortis, M.; Mukhopadhyay, R. Stomatocyte-discocyte-echinocyte sequence of the human red blood cell: Evidence for the bilayer- couple hypothesis from membrane mechanics. Proc. Natl. Acad. Sci. USA 2002, 99, 16766–16769. [Google Scholar] [CrossRef] [Green Version]
- Yartsev, A. The Gibbs-Donnan Effect | Deranged Physiology. Available online: https://derangedphysiology.com/main/cicm-primary-exam/required-reading/cellular-physiology/Chapter%20121/gibbs-donnan-effect (accessed on 11 November 2021).
- Mesarec, L.; Góźdź, W.; Iglič, A.; Kralj-Iglič, V.; Virga, E.G.; Kralj, S. Normal red blood cells’ shape stabilized by membrane’s in-plane ordering. Sci. Rep. 2019, 9, 19742. [Google Scholar] [CrossRef] [Green Version]
- Mesarec, L.; Drab, M.; Penič, S.; Kralj-Iglič, V.; Iglič, A. On the Role of Curved Membrane Nanodomains, and Passive and Active Skeleton Forces in the Determination of Cell Shape and Membrane Budding. Int. J. Mol. Sci. 2021, 22, 2348. [Google Scholar] [CrossRef]
- Geekiyanage, N.; Sauret, E.; Saha, S.; Flower, R.; Gu, Y. Modelling of Red Blood Cell Morphological and Deformability Changes during In-Vitro Storage. Appl. Sci. 2020, 10, 3209. [Google Scholar] [CrossRef]
- Melzak, K.A.; Spouge, J.L.; Boecker, C.; Kirschhöfer, F.; Brenner-Weiss, G.; Bieback, K. Hemolysis Pathways during Storage of Erythrocytes and Inter-Donor Variability in Erythrocyte Morphology. Transfus. Med. Hemother. 2021, 48, 39–47. [Google Scholar] [CrossRef]
- Hägerstrand, H.; Kralj-Iglic, V.; Bobrowska-Hägerstrand, M.; Iglic, A. Membrane skeleton detachment in spherical and cylindrical microexovesicles. Bull. Math. Biol. 1999, 61, 1019–1030. [Google Scholar] [CrossRef]
- Iglic, A.; Svetina, S.; Zeks, B. Depletion of membrane skeleton in red blood cell vesicles. Biophys. J. 1995, 69, 274–279. [Google Scholar] [CrossRef]
- Penič, S.; Mesarec, L.; Fošnarič, M.; Mrówczyńska, L.; Hägerstrand, H.; Kralj-Iglič, V.; Iglič, A. Budding and Fission of Membrane Vesicles: A Mini Review. Front. Phys. 2020, 8, 342. [Google Scholar] [CrossRef]
- Greenwalt, T.J. The how and why of exocytic vesicles. Transfusion 2006, 46, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Kriebardis, A.G.; Antonelou, M.H.; Stamoulis, K.E.; Economou-Petersen, E.; Margaritis, L.H.; Papassideri, I.S. RBC-derived vesicles during storage: Ultrastructure, protein composition, oxidation, and signaling components. Transfusion 2008, 48, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, K.; Neerukonda, S.N.; Katneni, U.; Buehler, P.W. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int. J. Mol. Sci. 2020, 22, 153. [Google Scholar] [CrossRef] [PubMed]
- Ciana, A.; Achilli, C.; Gaur, A.; Minetti, G. Membrane Remodelling and Vesicle Formation During Ageing of Human Red Blood Cells. Cell Physiol. Biochem. 2017, 42, 1127–1138. [Google Scholar] [CrossRef]
- Rubin, O.; Delobel, J.; Prudent, M.; Lion, N.; Kohl, K.; Tucker, E.I.; Tissot, J.D.; Angelillo-Scherrer, A. Red blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion 2013, 53, 1744–1754. [Google Scholar] [CrossRef]
- Hashemi Tayer, A.; Amirizadeh, N.; Ahmadinejad, M.; Nikougoftar, M.; Deyhim, M.R.; Zolfaghari, S. Procoagulant Activity of Red Blood Cell-Derived Microvesicles during Red Cell Storage. Transfus. Med. Hemother. 2019, 46, 224–230. [Google Scholar] [CrossRef]
- Bosman, G.J.; Lasonder, E.; Groenen-Döpp, Y.A.; Willekens, F.L.; Werre, J.M.; Novotný, V.M. Comparative proteomics of erythrocyte aging in vivo and in vitro. J. Proteom. 2010, 73, 396–402. [Google Scholar] [CrossRef]
- Föller, M.; Huber, S.M.; Lang, F. Erythrocyte programmed cell death. IUBMB Life 2008, 60, 661–668. [Google Scholar] [CrossRef]
- Pompeo, G.; Girasole, M.; Cricenti, A.; Boumis, G.; Bellelli, A.; Amiconi, S. Erythrocyte death in vitro induced by starvation in the absence of Ca(2+). Biochim. Biophys. Acta 2010, 1798, 1047–1055. [Google Scholar] [CrossRef] [Green Version]
- Mannu, F.; Arese, P.; Cappellini, M.D.; Fiorelli, G.; Cappadoro, M.; Giribaldi, G.; Turrini, F. Role of hemichrome binding to erythrocyte membrane in the generation of band-3 alterations in beta-thalassemia intermedia erythrocytes. Blood 1995, 86, 2014–2020. [Google Scholar] [CrossRef] [Green Version]
- Hägerstrand, H.; Bobrowska-Hägerstrand, M.; Lillsunde, I.; Isomaa, B. Vesiculation induced by amphiphiles and ionophore A23187 in porcine platelets: A transmission electron microscopic study. Chem. Biol. Interact. 1996, 101, 115–126. [Google Scholar] [CrossRef]
- Hägerstrand, H.; Isomaa, B. Morphological characterization of exovesicles and endovesicles released from human erythrocytes following treatment with amphiphiles. Biochim. Biophys. Acta 1992, 1109, 117–126. [Google Scholar] [CrossRef]
- Schärtl, W. Light Scattering from Polymer Solutions and Nanoparticle Dispersions; Springer Laboratory: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 17319. [Google Scholar] [CrossRef]
- Laboratory of Molecular Human Genetics, Research Institute of Physical-Chemical Medicine, Moscow, Russia. Centrifugation Parameters Calculator. Available online: http://vesicles.niifhm.ru/index.php?do=1 (accessed on 10 October 2020).
- Kralj-Iglič, V.; Pocsfalvi, G.; Mesarec, L.; Šuštar, V.; Hägerstrand, H.; Iglič, A. Minimizing isotropic and deviatoric membrane energy—An unifying formation mechanism of different cellular membrane nanovesicle types. PLoS ONE 2020, 15, e0244796. [Google Scholar] [CrossRef]
- Pretorius, E.; du Plooy, J.N.; Bester, J. A Comprehensive Review on Eryptosis. Cell Physiol. Biochem. 2016, 39, 1977–2000. [Google Scholar] [CrossRef]
- Lang, K.S.; Duranton, C.; Poehlmann, H.; Myssina, S.; Bauer, C.; Lang, F.; Wieder, T.; Huber, S.M. Cation channels trigger apoptotic death of erythrocytes. Cell Death Differ. 2003, 10, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Foller, M.; Kasinathan, R.S.; Koka, S.; Lang, C.; Shumilina, E.; Birnbaumer, L.; Lang, F.; Huber, S.M. TRPC6 contributes to the Ca(2+) leak of human erythrocytes. Cell Physiol. Biochem. 2008, 21, 183–192. [Google Scholar] [CrossRef]
- Duranton, C.; Huber, S.M.; Lang, F. Oxidation induces a Cl(-)-dependent cation conductance in human red blood cells. J. Physiol. 2002, 539, 847–855. [Google Scholar] [CrossRef]
- Ghashghaeinia, M.; Cluitmans, J.C.; Akel, A.; Dreischer, P.; Toulany, M.; Köberle, M.; Skabytska, Y.; Saki, M.; Biedermann, T.; Duszenko, M.; et al. The impact of erythrocyte age on eryptosis. Br. J. Haematol. 2012, 157, 606–614. [Google Scholar] [CrossRef]
- Prudent, M.; Crettaz, D.; Delobel, J.; Seghatchian, J.; Tissot, J.D.; Lion, N. Differences between calcium-stimulated and storage-induced erythrocyte-derived microvesicles. Transfus. Apher. Sci. 2015, 53, 153–158. [Google Scholar] [CrossRef]
- Hägerstrand, H.; Isomaa, B. Vesiculation induced by amphiphiles in erythrocytes. Biochim. Biophys. Acta 1989, 982, 179–186. [Google Scholar] [CrossRef]
- Hallett, F.R.; Marsh, J.; Nickel, B.G.; Wood, J.M. Mechanical properties of vesicles. II. A model for osmotic swelling and lysis. Biophys. J. 1993, 64, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, K.; Kitano, T.; Kuwahara, H.; Tedani, M.; Aburai, K.; Futaki, S.; Abe, M.; Sakai, H.; Ohtaka, H.; Yamashita, Y. Effect of Vesicle Size on the Cytolysis of Cell-Penetrating Peptides (CPPs). Int. J. Mol. Sci. 2020, 21, 7405. [Google Scholar] [CrossRef]
- Nolan, J.P. Flow Cytometry of Extracellular Vesicles: Potential, Pitfalls, and Prospects. Curr. Protoc. Cytom. 2015, 73, 13.14.1–13.14.16. [Google Scholar] [CrossRef]
- Van der Pol, E.; de Rond, L.; Coumans, F.A.W.; Gool, E.L.; Böing, A.N.; Sturk, A.; Nieuwland, R.; van Leeuwen, T.G. Absolute sizing and label-free identification of extracellular vesicles by flow cytometry. Nanomedicine 2018, 14, 801–810. [Google Scholar] [CrossRef]
- Marcoux, G.; Duchez, A.-C.; Cloutier, N.; Provost, P.; Nigrovic, P.A.; Boilard, E. Revealing the diversity of extracellular vesicles using high-dimensional flow cytometry analyses. Sci. Rep. 2016, 6, 35928. [Google Scholar] [CrossRef]
- Šuštar, V.; Bedina-Zavec, A.; Štukelj, R.; Frank, M.; Bobojević, G.; Janša, R.; Ogorevc, E.; Kruljc, P.; Mam, K.; Šimunič, B.; et al. Nanoparticles isolated from blood: A reflection of vesiculability of blood cells during the isolation process. Int. J. Nanomed. 2011, 6, 2737–2748. [Google Scholar] [CrossRef] [Green Version]
- Božič, D.; Hočevar, M.; Kononenko, V.; Jeran, M.; Štibler, U.; Fiume, I.; Pajnič, M.; Pađen, L.; Kogej, K.; Drobne, D.; et al. Chapter Five—Pursuing mechanisms of extracellular vesicle formation. Effects of sample processing. In Advances in Biomembranes and Lipid Self-Assembly; Bongiovanni, A., Pocsfalvi, G., Manno, M., Kralj-Iglič, V., Eds.; Elsevier/Academic Press: London, UK, 2020; Volume 32, pp. 113–155. [Google Scholar] [CrossRef]
- Larson, M.C.; Hogg, N.; Hillery, C.A. Centrifugation Removes a Population of Large Vesicles, or “Macroparticles,” Intermediate in Size to RBCs and Microvesicles. Int. J. Mol. Sci. 2021, 22, 1243. [Google Scholar] [CrossRef]
- Glassman, P.M.; Hood, E.D.; Ferguson, L.T.; Zhao, Z.; Siegel, D.L.; Mitragotri, S.; Brenner, J.S.; Muzykantov, V.R. Red blood cells: The metamorphosis of a neglected carrier into the natural mothership for artificial nanocarriers. Adv. Drug Deliv. Rev. 2021, 178, 113992. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Controll. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Magnani, M.; Rossi, L.; D’Ascenzo, M.; Panzani, I.; Bigi, L.; Zanella, A. Erythrocyte engineering for drug delivery and targeting. Biotechnol. Appl. Biochem. 1998, 28, 1–6. [Google Scholar] [PubMed]
- Brown, W. Dynamic Light Scattering: The Method and Some Applications; Brown, W., Ed.; Clarendon Press: Oxford, UK, 1993. [Google Scholar]
- Deville, S.; Berckmans, P.; Van Hoof, R.; Lambrichts, I.; Salvati, A.; Nelissen, I. Comparison of extracellular vesicle isolation and storage methods using high-sensitivity flow cytometry. PLoS ONE 2021, 16, e0245835. [Google Scholar] [CrossRef] [PubMed]
- Jeyaram, A.; Jay, S.M. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2017, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Mattei, B.; Lira, R.B.; Perez, K.R.; Riske, K.A. Membrane permeabilization induced by Triton X-100: The role of membrane phase state and edge tension. Chem. Phys. Lipids 2017, 202, 28–37. [Google Scholar] [CrossRef]
- Drab, M.; Pandur, Ž.; Penič, S.; Iglič, A.; Kralj-Iglič, V.; Stopar, D. A Monte Carlo study of giant vesicle morphologies in nonequilibrium environments. Biophys. J. 2021, 120, 4418–4428. [Google Scholar] [CrossRef]
- Dalgarno, P.A.; Juan-Colás, J.; Hedley, G.J.; Piñeiro, L.; Novo, M.; Perez-Gonzalez, C.; Samuel, I.D.W.; Leake, M.C.; Johnson, S.; Al-Soufi, W.; et al. Unveiling the multi-step solubilization mechanism of sub-micron size vesicles by detergents. Sci. Rep. 2019, 9, 12897. [Google Scholar] [CrossRef] [Green Version]
- Božič, D.; Sitar, S.; Junkar, I.; Štukelj, R.; Pajnič, M.; Žagar, E.; Kralj-Iglič, V.; Kogej, K. Viscosity of Plasma as a Key Factor in Assessment of Extracellular Vesicles by Light Scattering. Cells 2019, 8, 1046. [Google Scholar] [CrossRef] [Green Version]
- Provencher, S.W. CONTIN: A General Purpose Constrained Regularization Program for Inverting Noisy Linear Algebraic and Integral Equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]



| DLS/SLS | ||
|---|---|---|
| Sample | I90/I90,hbEVs/300 mOsm/L PBS–citrate | ρ |
| hbEVs/300 mOsm/L PBS–citrate | 100% | 0.75 |
| hbEVs/50 mOsm/L PBS–citrate | 94% | 0.76 |
| hbEVs/100 μmol/L Triton X-100 | 76% | 0.77 |
| hbEVs/150 μmol/L Triton X-100 | 47% | 0.81 |
| Sample Designation | PBS–citrate (μL) | dH2O (μL) | 4 M NaCl (μL) | HbEVs Isolate (μL) | Methods | Assessment Focus |
|---|---|---|---|---|---|---|
| hbEVs/50 mOsm/L (PBS–citrate) | - | 833.0 | 167.0 | maLS, SEM | Effect of medium osmolarity, storage | |
| hbEVs/150 mOsm/L | - | 820.5 | 12.5 | 167.0 | saLS | Effect of medium osmolarity |
| hbEVs/150 mOsm/L PBS–citrate | 333.0 | 500.0 | - | 167.0 | maLS | storage |
| hbEVs/300 mOsm/L | - | 802.0 | 31.0 | 167.0 | saLS | Effect of medium osmolarity |
| hbEVs/300 mOsm/L PBS–citrate | 833.0 | - | - | 167.0 | maLS, SEM | Thermal and pH resistance, storage, solubilisation by Triton X-100 |
| hbEVs/450 mOsm/L | - | 783.0 | 50.0 | 167.0 | saLS | Effect of medium osmolarity |
| hbEVs/600 mOsm/L | - | 764.5 | 68.5 | 167.0 | saLS | Effect of medium osmolarity |
| hbEVs/750 mOsm/L | - | 745.5 | 87.5 | 167.0 | saLS | Effect of medium osmolarity |
| hbEVs/1000 mOsm/L | - | 718.5 | 114.5 | 167.0 | saLS | Effect of medium osmolarity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Božič, D.; Hočevar, M.; Kisovec, M.; Pajnič, M.; Pađen, L.; Jeran, M.; Bedina Zavec, A.; Podobnik, M.; Kogej, K.; Iglič, A.; et al. Stability of Erythrocyte-Derived Nanovesicles Assessed by Light Scattering and Electron Microscopy. Int. J. Mol. Sci. 2021, 22, 12772. https://doi.org/10.3390/ijms222312772
Božič D, Hočevar M, Kisovec M, Pajnič M, Pađen L, Jeran M, Bedina Zavec A, Podobnik M, Kogej K, Iglič A, et al. Stability of Erythrocyte-Derived Nanovesicles Assessed by Light Scattering and Electron Microscopy. International Journal of Molecular Sciences. 2021; 22(23):12772. https://doi.org/10.3390/ijms222312772
Chicago/Turabian StyleBožič, Darja, Matej Hočevar, Matic Kisovec, Manca Pajnič, Ljubiša Pađen, Marko Jeran, Apolonija Bedina Zavec, Marjetka Podobnik, Ksenija Kogej, Aleš Iglič, and et al. 2021. "Stability of Erythrocyte-Derived Nanovesicles Assessed by Light Scattering and Electron Microscopy" International Journal of Molecular Sciences 22, no. 23: 12772. https://doi.org/10.3390/ijms222312772
APA StyleBožič, D., Hočevar, M., Kisovec, M., Pajnič, M., Pađen, L., Jeran, M., Bedina Zavec, A., Podobnik, M., Kogej, K., Iglič, A., & Kralj-Iglič, V. (2021). Stability of Erythrocyte-Derived Nanovesicles Assessed by Light Scattering and Electron Microscopy. International Journal of Molecular Sciences, 22(23), 12772. https://doi.org/10.3390/ijms222312772









