The Role of Pectobacterium atrosepticum Exopolysaccharides in Plant–Pathogen Interactions
Abstract
1. Introduction
2. Results
2.1. The Viscosity of Pba EPS Solutions
2.2. Formation of Supramolecular Aggregates of Pba EPS
2.3. Antioxidant Properties of Pba EPS
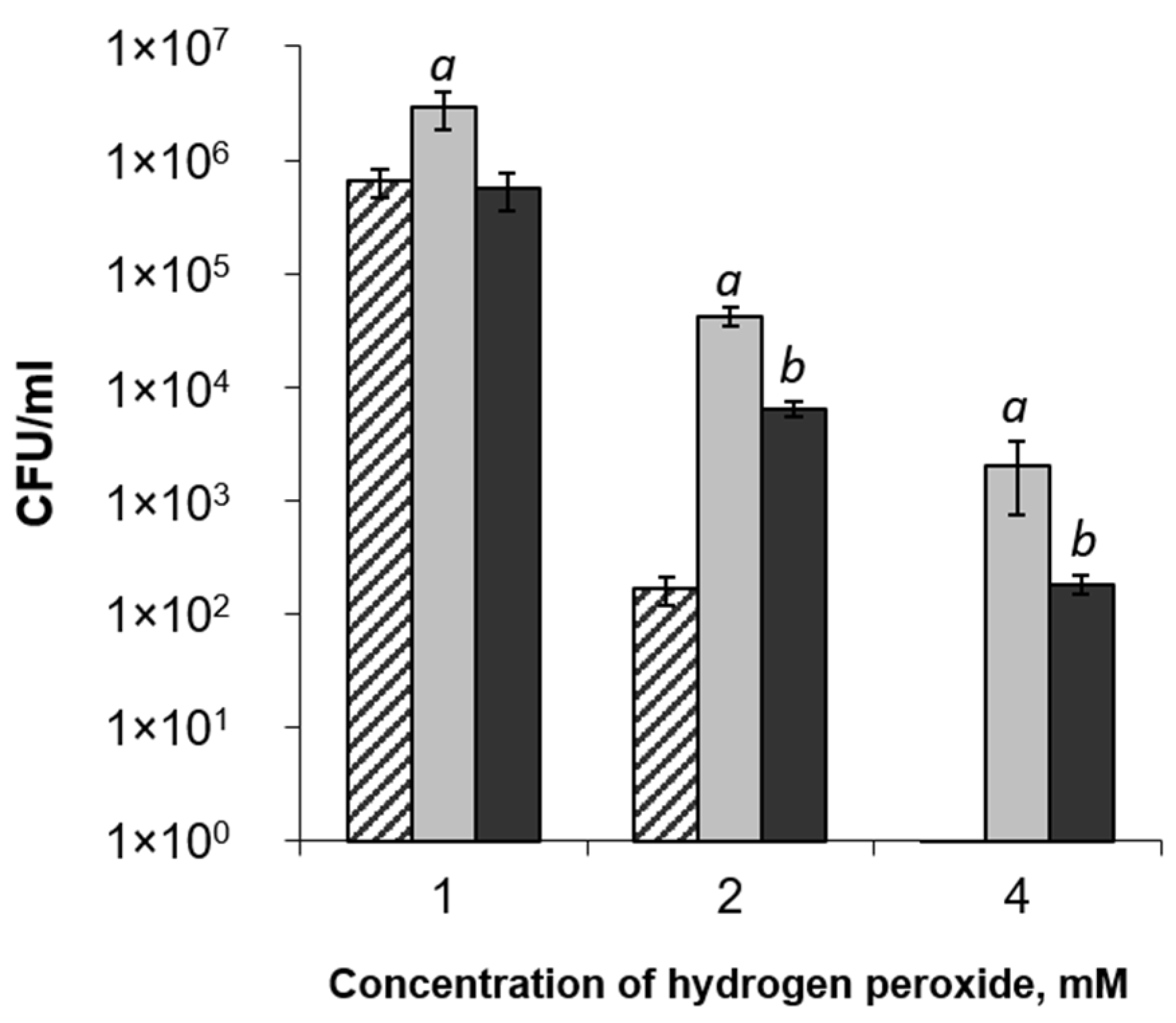
2.4. Phytoimmune Properties of Pba EPS
3. Discussion
4. Materials and Methods
4.1. Collection of the Pba EPS Samples
4.2. Rheological Measurements
4.3. Dynamic Light Scattering
4.4. Reactive Oxygen Species Scavenging Assays
4.5. Analysis of the Phytoimmune Properties
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Czajkowski, R.; De Boer, W.J.; Van Veen, J.A.; Van Der Wolf, J.M. Downward vascular translocation of a green fluorescent protein-tagged strain of Dickeya sp. (biovar 3) from stem and leaf inoculation sites on potato. Phytopathology 2010, 100, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, R.; De Boer, W.J.; Velvis, H.; Van Der Wolf, J.M. Systemic colonization of potato plants by a soilborne, green fluorescent protein-tagged strain of Dickeya sp. biovar 3. Phytopathology 2010, 100, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Kubheka, G.C.; Coutinho, T.A.; Moleleki, N.; Moleleki, L.N. Colonization patterns of an mCherry-tagged Pectobacterium carotovorum subsp. brasiliense strain in potato plants. Phytopathology 2013, 103, 1268–1279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gorshkov, V.; Daminova, A.; Ageeva, M.; Petrova, O.; Gogoleva, N.; Tarasova, N.; Gogolev, Y. Dissociation of a population of Pectobacterium atrosepticum SCRI1043 in tobacco plants: Formation of bacterial emboli and dormant cells. Protoplasma 2014, 251, 499–510. [Google Scholar] [CrossRef]
- Moleleki, L.N.; Pretorius, R.G.; Tanui, C.K.; Mosina, G.; Theron, J. A quorum sensing-defective mutant of Pectobacterium carotovorum ssp. brasiliense 1692 is attenuated in virulence and unable to occlude xylem tissue of susceptible potato plant stems. Mol. Plant Pathol. 2017, 18, 32–44. [Google Scholar] [CrossRef]
- Gorshkov, V.; Tsers, I.; Islamov, B.; Ageeva, M.; Gogoleva, N.; Mikshina, P.; Parfirova, O.; Petrova, O.; Gorshkov, T.; Gogolev, Y. The Modification of plant cell wall polysaccharides in potato plants during Pectobacterium atrosepticum-caused infection. Plants 2021, 10, 1407. [Google Scholar] [CrossRef]
- Gorshkov, V.Y.; Daminova, A.G.; Mikshina, P.V.; Petrova, O.E.; Ageeva, M.V.; Salnikov, V.V.; Gorshkov, T.; Gogolev, Y. Pathogen-induced conditioning of the primary xylem vessels–A prerequisite for the formation of bacterial emboli by Pectobacterium atrosepticum. Plant Biol. 2016, 18, 609–617. [Google Scholar] [CrossRef]
- Gorshkov, V.; Gubaev, R.; Petrova, O.; Daminova, A.; Gogoleva, N.; Ageeva, M.; Parfirova, O.; Prokchorchik, M.; Nikolaichi, Y.; Gogolev, Y. Transcriptome profiling helps to identify potential and true molecular switches of stealth to brute force behavior in Pectobacterium atrosepticum during systemic colonization of tobacco plants. Eur. J. Plant Pathol. 2018, 152, 957–976. [Google Scholar] [CrossRef]
- Gorshkov, V.; Islamov, B.; Mikshina, P.; Petrova, O.; Burygin, G.; Sigida, E.; Shashkov, A.; Daminova, A.; Ageeva, M.; Idiyatullin, B.; et al. Pectobacterium atrosepticum exopolysaccharides: Identification, molecular structure, formation under stress and in planta conditions. Glycobiology 2017, 27, 1016–1026. [Google Scholar] [CrossRef]
- Yang, B.Y.; Gray, J.S.; Montgomery, R. Extracellular polysaccharide of Erwinia chrysanthemi CU643. Carbohydr. Res. 1999, 316, 138–154. [Google Scholar] [CrossRef]
- Yang, B.Y.; Brand, J.M.; Gray, J.S.; Montgomery, R. Extracellular polysaccharides of modified strains of Erwinia spp. Carbohydr. Res. 2001, 333, 295–302. [Google Scholar] [CrossRef]
- Yang, B.Y.; Ding, Q.; Montgomery, R. Hydrodynamic properties of oxidized extracellular polysaccharides from Erwinia chrysanthemi spp. Carbohydr. Res. 2003, 338, 2763–2771. [Google Scholar] [CrossRef]
- Gray, J.S.; Brand, J.M.; Koerner, T.A.; Montgomery, R. Structure of an extracellular polysaccharide produced by Erwinia chrysanthemi. Carbohydr. Res. 1993, 245, 271–287. [Google Scholar] [CrossRef]
- Gray, J.S.; Yang, B.Y.; Montgomery, R. Extracellular polysaccharide of Erwinia chrysanthemi A350 and ribotyping of Erwinia chrysanthemi spp. Carbohydr. Res. 2000, 324, 255–267. [Google Scholar] [CrossRef]
- Azeredo, J.; Oliveira, R. The role of exopolymers produced by Sphingomonas paucimobilis in biofilm formation and composition. Biofouling 2000, 16, 17–27. [Google Scholar] [CrossRef]
- Sutherland, I.W. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef]
- Sutherland, I.W. The biofilm matrix—an immobilized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef]
- Ghafoor, A.; Hay, I.D.; Rehm, B.H. Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture. Appl. Environ. Microbiol. 2011, 77, 5238–5246. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial exopolysaccharides: Functionality and prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef]
- Maunders, E.; Welch, M. Matrix exopolysaccharides; the sticky side of biofilm formation. FEMS Microbiol. Lett. 2017, 364, fnx120. [Google Scholar] [CrossRef]
- Yu, J.; Peñaloza-Vázquez, A.; Chakrabarty, A.M.; Bender, C.L. Involvement of the exopolysaccharide alginate in the virulence and epiphytic fitness of Pseudomonas syringae pv. syringae. Mol. Microbiol. 1999, 33, 712–720. [Google Scholar] [CrossRef]
- Gilbert, P.; Das, J.; Foley, I. Biofilm susceptibility to antimicrobials. Adv. Dent. Res. 1997, 11, 160–167. [Google Scholar] [CrossRef]
- Wadström, T.; Eliasson, I.; Holder, I.; Ljungh, A. (Eds.) Pathogenesis of Wound and Biomaterial-Associated Infections; Springer Science & Business Media: London, UK, 2012; pp. 1–565. [Google Scholar]
- Andrew, M.; Jayaraman, G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2020, 487, 107881. [Google Scholar] [CrossRef] [PubMed]
- Lattner, D.; Flemming, H.C.; Mayer, C. 13C-NMR study of the interaction of bacterial alginate with bivalent cations. Int. J. Biol. Macromol. 2003, 33, 81–88. [Google Scholar] [CrossRef]
- Yun, M.H.; Torres, P.S.; El Oirdi, M.; Rigano, L.A.; Gonzalez-Lamothe, R.; Marano, M.R.; Castagnaro, A.P.; Dankert, M.A.; Bouarab, K.; Vojnov, A.A. Xanthan induces plant susceptibility by suppressing callose deposition. Plant Physiol. 2006, 141, 178–187. [Google Scholar] [CrossRef]
- Bradshaw-Rouse, J.J.; Whatley, M.H.; Coplin, D.L.; Woods, A.; Sequeira, L.; Kelman, A. Agglutination of Erwinia stewartii strains with a corn agglutinin: Correlation with extracellular polysaccharide production and pathogenicity. Appl. Environ. Microbiol. 1981, 42, 344–350. [Google Scholar] [CrossRef]
- Young, D.H.; Sequeira, L. Binding of Pseudomonas solanacearum fimbriae to tobacco leaf cell walls and its inhibition by bacterial extracellular polysaccharides. Physiol. Mol. Plant Path. 1986, 28, 393–402. [Google Scholar] [CrossRef]
- Menggad, M.; Laurent, J. Mutations in ams genes of Erwinia amylovora affect the interactions with host plants. Eur. J. Plant Pathol. 1998, 104, 313–322. [Google Scholar] [CrossRef]
- Leigh, J.A.; Coplin, D.L. Exopolysaccharides in plant-bacterial interactions. Annu. Rev. Microbiol. 1992, 46, 307–346. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, A.; Nizampatnam, N.R.; Kondreddy, A.; Pradhan, B.B.; Chatterjee, S. Xanthomonas campestris cell–cell signalling molecule DSF (diffusible signal factor) elicits innate immunity in plants and is suppressed by the exopolysaccharide xanthan. J. Exp. Bot. 2015, 66, 6697–6714. [Google Scholar] [CrossRef]
- Romeiro, R.S.; Kimura, O. Induced resistance in pepper leaves infiltrated with purified bacterial elicitors from Xanthomonas campestris pv. vesicatoria. J. Phytopath. 1997, 145, 495–498. [Google Scholar] [CrossRef]
- De Pinto, M.C.; Lavermicocca, P.; Evidente, A.; Corsaro, M.M.; Lazzaroni, S.; De Gara, L. Exopolysaccharides produced by plant pathogenic bacteria affect ascorbate metabolism in Nicotiana tabacum. Plant Cell Physiol. 2003, 44, 803–810. [Google Scholar] [CrossRef][Green Version]
- Xu, R.; Shang, N.; Li, P. In vitro and in vivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis RH. Anaerobe 2011, 17, 226–231. [Google Scholar] [CrossRef]
- Ding, Q.; LaBelle, M.; Yang, B.Y.; Montgomery, R. Physicochemical studies of extracellular polysaccharides of Erwinia chrysanthemi spp. Carbohydr. Polym. 2003, 51, 333–346. [Google Scholar] [CrossRef]
- Cross, M.M. Rheology of non-Newtonian fluids: A new flow equation for pseudoplastic systems. J. Colloid Interface Sci. 1965, 20, 417–437. [Google Scholar] [CrossRef]
- Shibayama, M.; Karino, T.; Okabe, S. Distribution analyses of multi-modal dynamic light scattering data. Polymer 2006, 47, 6446–6456. [Google Scholar] [CrossRef]
- Wyatt, N.B.; Liberatore, M.W. Rheology and viscosity scaling of the polyelectrolyte xanthan gum. J. Appl. Polym. Sci. 2009, 114, 4076–4084. [Google Scholar] [CrossRef]
- Niknezhad, S.V.; Najafpour-Darzi, G.; Morowvat, M.H.; Ghasemi, Y. Exopolysaccharide production of Pantoea sp. BCCS 001 GH: Physical characterizations, emulsification, and antioxidant activities. Int. J. Biol. Macromol. 2018, 118, 1103–1111. [Google Scholar] [CrossRef]
- Peng, J.; Xu, W.; Ni, D.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Preparation of a novel water-soluble gel from Erwinia amylovora levan. Int. J. Biol. Macromol. 2019, 122, 469–478. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Carvalheira, M.; Costa, N.; Oliveira, R.; Reis, M.A. Emulsifying behaviour and rheological properties of the extracellular polysaccharide produced by Pseudomonas oleovorans grown on glycerol byproduct. Carbohydr. Polym. 2009, 78, 549–556. [Google Scholar] [CrossRef]
- Andhare, P.; Delattre, C.; Pierre, G.; Michaud, P.; Pathak, H. Characterization and rheological behaviour analysis of the succinoglycan produced by Rhizobium radiobacter strain CAS from curd sample. Food Hydrocoll. 2017, 64, 1–8. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Q.; Bai, Y.; Yu, S.; Zhang, T.; Jiang, B.; Mu, W. Physicochemical properties of a high molecular weight levan from Brenneria sp. EniD312. Int. J. Biol. Macromol. 2018, 109, 810–818. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Taherian, A.R.; Bakhtiyari, M.; Farahnaky, A.; Askari, H. Structural and rheological properties of succinoglycan biogums made from low-quality date syrup or sucrose using Agrobacterium radiobacter inoculation. Food Bioproc. Tech. 2012, 5, 638–647. [Google Scholar] [CrossRef]
- Zhou, F.; Wu, Z.; Chen, C.; Han, J.; Ai, L.; Guo, B. Exopolysaccharides produced by Rhizobium radiobacter S10 in whey and their rheological properties. Food Hydrocoll. 2014, 36, 362–368. [Google Scholar] [CrossRef]
- Cai, S.; He, X.; Liu, K.; Rodrigues, A.M.; Zhang, R. Macromolecular interactions and synergy in xanthan/HPAM aqueous solutions. RSC Adv. 2017, 7, 41630–41639. [Google Scholar] [CrossRef]
- Kavitake, D.; Delattre, C.; Devi, P.B.; Pierre, G.; Michaud, P.; Shetty, P.H.; Andhare, P. Physical and functional characterization of succinoglycan exopolysaccharide produced by Rhizobium radiobacter CAS from curd sample. Int. J. Biol. Macromol. 2019, 134, 1013–1021. [Google Scholar] [CrossRef]
- Yildiz, H.; Karatas, N. Microbial exopolysaccharides: Resources and bioactive properties. Process Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Nguyen, T.T.; Bui, D.C.; Hong, P.T.; Hoang, Q.K.; Nguyen, H.T. Exopolysaccharide production by lactic acid bacteria: The manipulation of environmental stresses for industrial applications. AIMS Microbiol. 2020, 6, 451. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, X.; Mu, H.; Liang, X.; Guan, H. Structure and protective effect of exopolysaccharide from P. agglomerans strain KFS-9 against UV radiation. Microbiol. Res. 2007, 162, 124–129. [Google Scholar] [CrossRef]
- Sran, K.S.; Bisht, B.; Mayilraj, S.; Choudhury, A.R. Structural characterization and antioxidant potential of a novel anionic exopolysaccharide produced by marine Microbacterium aurantiacum FSW-25. Int. J. Biol. 2019, 131, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Milling, A.; Babujee, L.; Allen, C. Ralstonia solanacearum extracellular polysaccharide is a specific elicitor of defense responses in wilt-resistant tomato plants. PLoS ONE 2011, 6, e15853. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.I.; Toum, L.; Yaryura, P.M.; Mielnichuk, N.; Gudesblat, G.E.; Roeschlin, R.; Marano, M.R.; Ielpi, L.; Vojnov, A.A. Xanthan pyruvilation is essential for the virulence of Xanthomonas campestris pv. campestris. Mol. Plant Microbe Interact. 2016, 29, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.N.; Newman, M.A.; Erbs, G.; Morrissey, K.L.; Chinchilla, D.; Boller, T.; Jensen, T.T.; De Castro, C.; Ierano, T.; Molinaro, T.; et al. Bacterial polysaccharides suppress induced innate immunity by calcium chelation. Curr. Biol. 2008, 18, 1078–1083. [Google Scholar] [CrossRef]
- Bugert, P.; Geider, K. Molecular analysis of the ams operon required for exopolysaccharide synthesis of Erwinia amylovora. Mol. Microbiol. 1995, 15, 917–933. [Google Scholar] [CrossRef]
- Araud-Razou, I.; Vasse, J.; Montrozier, H.; Etchebar, C.; Trigalet, A. Detection and visualization of the major acidic exopolysaccharide of Ralstonia solanacearum and its role in tomato root infection and vascular colonization. Eur. J. Plant Pathol. 1998, 104, 795–809. [Google Scholar] [CrossRef]
- Kemp, B.P.; Horne, J.; Bryant, A.; Cooper, R.M. Xanthomonas axonopodis pv. manihotis gumD gene is essential for EPS production and pathogenicity and enhances epiphytic survival on cassava (Manihot esculenta). Physiol. Mol. Plant Pathol. 2004, 64, 209–218. [Google Scholar] [CrossRef]
- Keshavarzi, M.; Soylu, S.; Brown, I.; Bonas, U.; Nicole, M.; Rossiter, J.; Mansfield, J. Basal defenses induced in pepper by lipopolysaccharides are suppressed by Xanthomonas campestris pv. vesicatoria. Mol. Plant Microbe Interact. 2004, 17, 805–815. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Fry, S.C. The Growing Plant Cell Wall: Chemical and Metabolic Analysis; Longman Group Limited Publisher: London, UK, 1988; pp. 1–333. [Google Scholar]
- Bellincampi, D.; Dipierro, N.; Salvi, G.; Cervone, F.; De Lorenzo, G. Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol. 2000, 122, 1379–1386. [Google Scholar] [CrossRef]





| Pba EPS Concentration (%) | η0 [mPa∙s] | η∞ [mPa∙s] | λ [s] | n [−] |
|---|---|---|---|---|
| 5.0 | 16.973 | 15.839 | 0.2785 | 0.9557 |
| 2.5 | 11.093 | 10.546 | 0.0499 | 1.3592 |
| 1.25 | 4.893 | 4.688 | 0.0566 | 1.6465 |
| 0.625 | 2.362 | 2.362 | - | - |
| EPS Name, Bacterial Species | Viscosity at Different Concenrtations (C, %) at Different Shear Rates η0 | Reference | |||
|---|---|---|---|---|---|
| 0.4 ≤ C ≤ 0.6 | 1 ≤ C ≤ 1.25 | 2 ≤ C ≤ 3 | 4 ≤ C ≤ 10 | ||
| EPS, Pectobacterium atrosepticum | 2.4/2.4/2.4 (0.6%) | 4.9/4.7/4.8 (1.25%) | 11/11/11 (2.5%) | 17/16/16 (5%) | This study |
| EPS80, Erwinia chrysanthemi * | 32/32/23 (0.5%) | [36] | |||
| EPS9, Erwinia chrysanthemi * | 112/109/47 (0.5%) | [36] | |||
| Levan, Erwinia amylovora | 44/38/33 (2%) | 101,700/20,600/4387 (8%) | [41] | ||
| CAS EPS, Rhizobium radiobacter | 102/102/27 (2%) | 186/186/43 (6%) | [43] | ||
| Alginate, Pseudomonas oleovorans | - | 26/25/25 (10%) | [42] | ||
| Levan, Brenneria sp. | 0.6/0.6/0.6 (3%) | 643/26/12 (6%) | [44] | ||
| Succinoglycan, Agrobacterium radiobacter | 29/17/5 (0.5%) | 37/33/7.4 (1%) | 112/95/25 (2%) | [45] | |
| EPS, Pantoea sp. | 1250/1250/1250 (0.5%) | 34,300/17,180/2500 (1%) | 61,200/30,000/2600 (2%) | [40] | |
| Xanthan gum, Xanthamonas sp. | 39,000/343/62 (0.4%) | [39] | |||
| EPS S10, Rhizobium radiobacter | 4400/173/51 (0.5%) | 1.4 × 106/6373/576 (1%) | [46] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islamov, B.; Petrova, O.; Mikshina, P.; Kadyirov, A.; Vorob’ev, V.; Gogolev, Y.; Gorshkov, V. The Role of Pectobacterium atrosepticum Exopolysaccharides in Plant–Pathogen Interactions. Int. J. Mol. Sci. 2021, 22, 12781. https://doi.org/10.3390/ijms222312781
Islamov B, Petrova O, Mikshina P, Kadyirov A, Vorob’ev V, Gogolev Y, Gorshkov V. The Role of Pectobacterium atrosepticum Exopolysaccharides in Plant–Pathogen Interactions. International Journal of Molecular Sciences. 2021; 22(23):12781. https://doi.org/10.3390/ijms222312781
Chicago/Turabian StyleIslamov, Bakhtiyar, Olga Petrova, Polina Mikshina, Aidar Kadyirov, Vladimir Vorob’ev, Yuri Gogolev, and Vladimir Gorshkov. 2021. "The Role of Pectobacterium atrosepticum Exopolysaccharides in Plant–Pathogen Interactions" International Journal of Molecular Sciences 22, no. 23: 12781. https://doi.org/10.3390/ijms222312781
APA StyleIslamov, B., Petrova, O., Mikshina, P., Kadyirov, A., Vorob’ev, V., Gogolev, Y., & Gorshkov, V. (2021). The Role of Pectobacterium atrosepticum Exopolysaccharides in Plant–Pathogen Interactions. International Journal of Molecular Sciences, 22(23), 12781. https://doi.org/10.3390/ijms222312781






