Mesenchymal Stromal Cells Adapt to Chronic Tendon Disease Environment with an Initial Reduction in Matrix Remodeling
Abstract
:1. Introduction
2. Results
2.1. MSC Culture as Aggregates and on Healthy Tendon Scaffolds
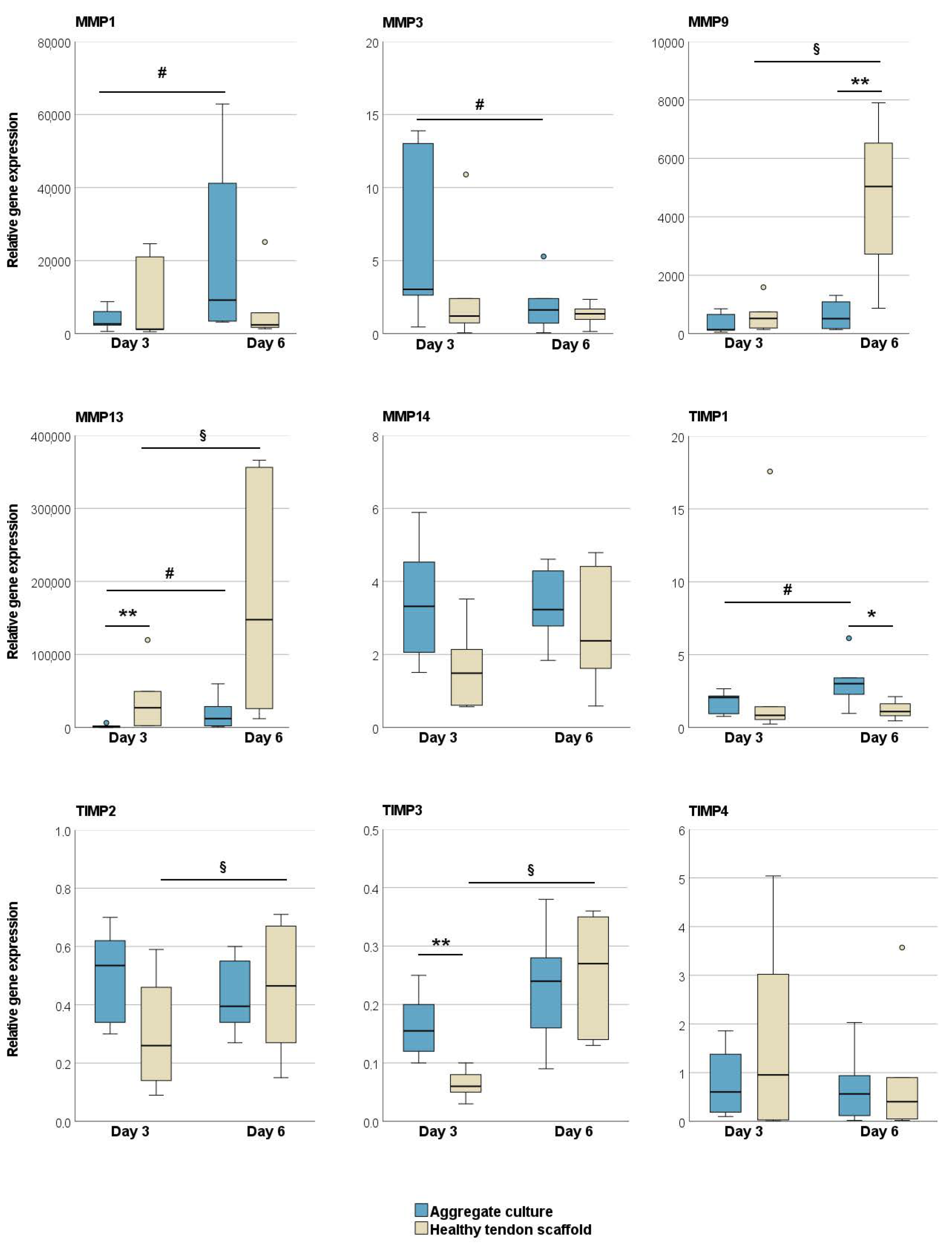
2.1.1. Tendon Marker, Growth Factor, MMP, and TIMP Gene Expression
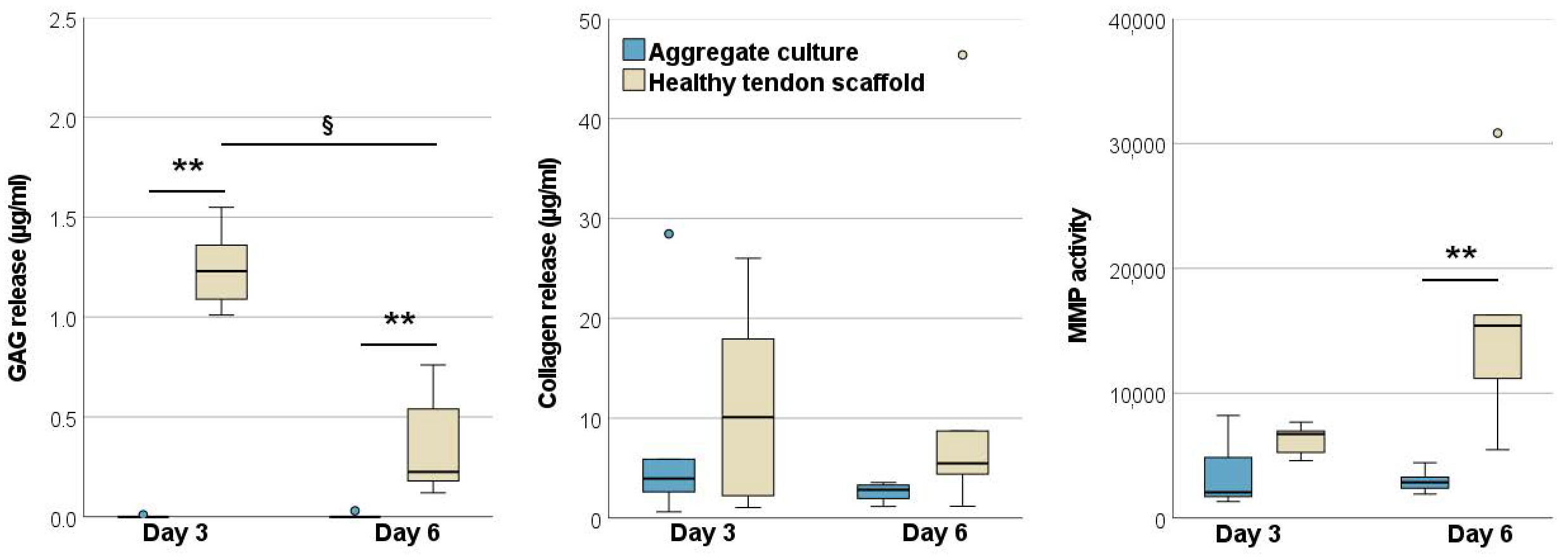
2.1.2. Glycosaminoglycan and Total Collagen Release and MMP Activity
2.2. MSC Culture on Healthy and Chronic Tendon Disease Scaffolds
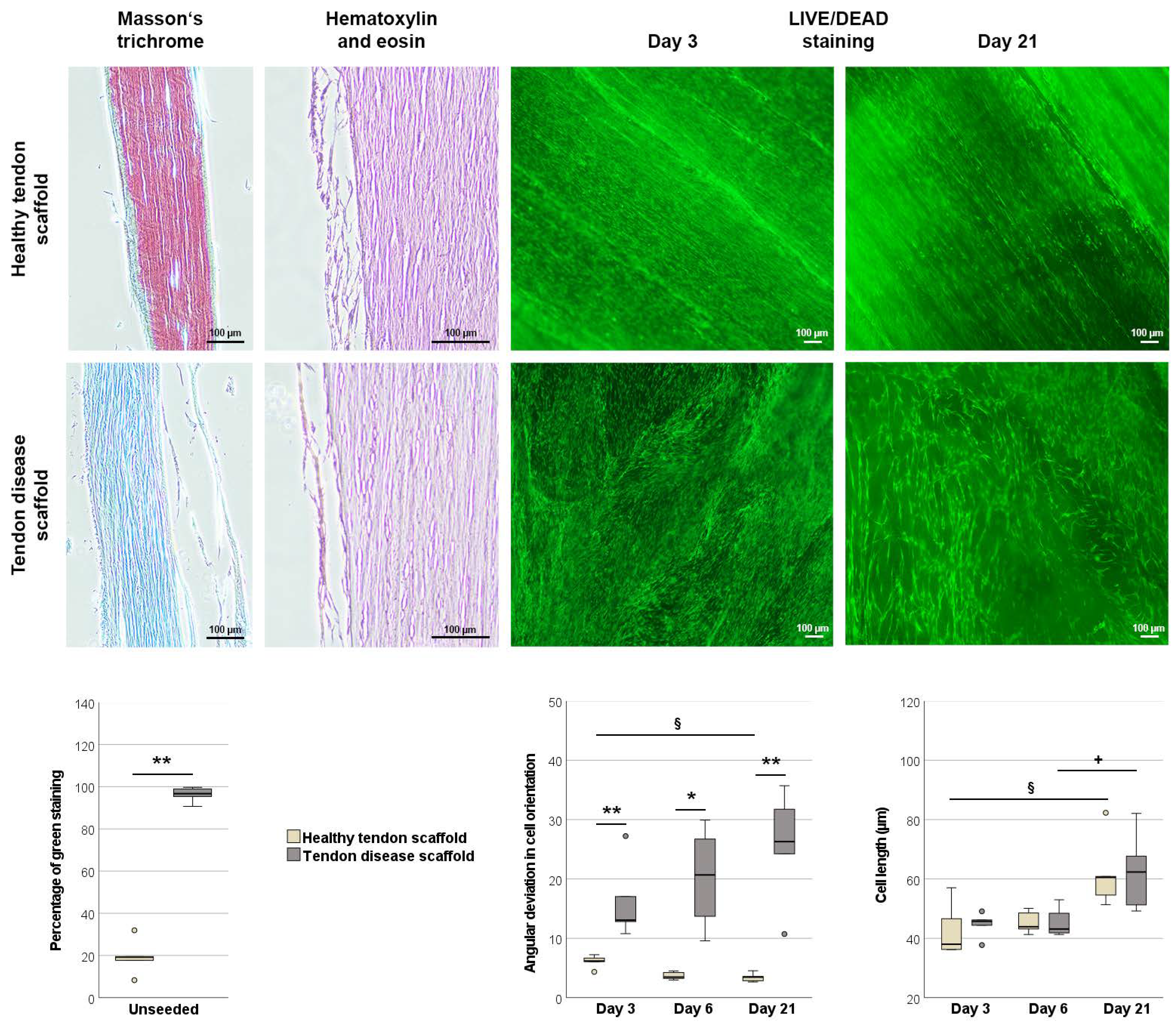
2.2.1. Morphology of Scaffolds and Scaffold Cultures
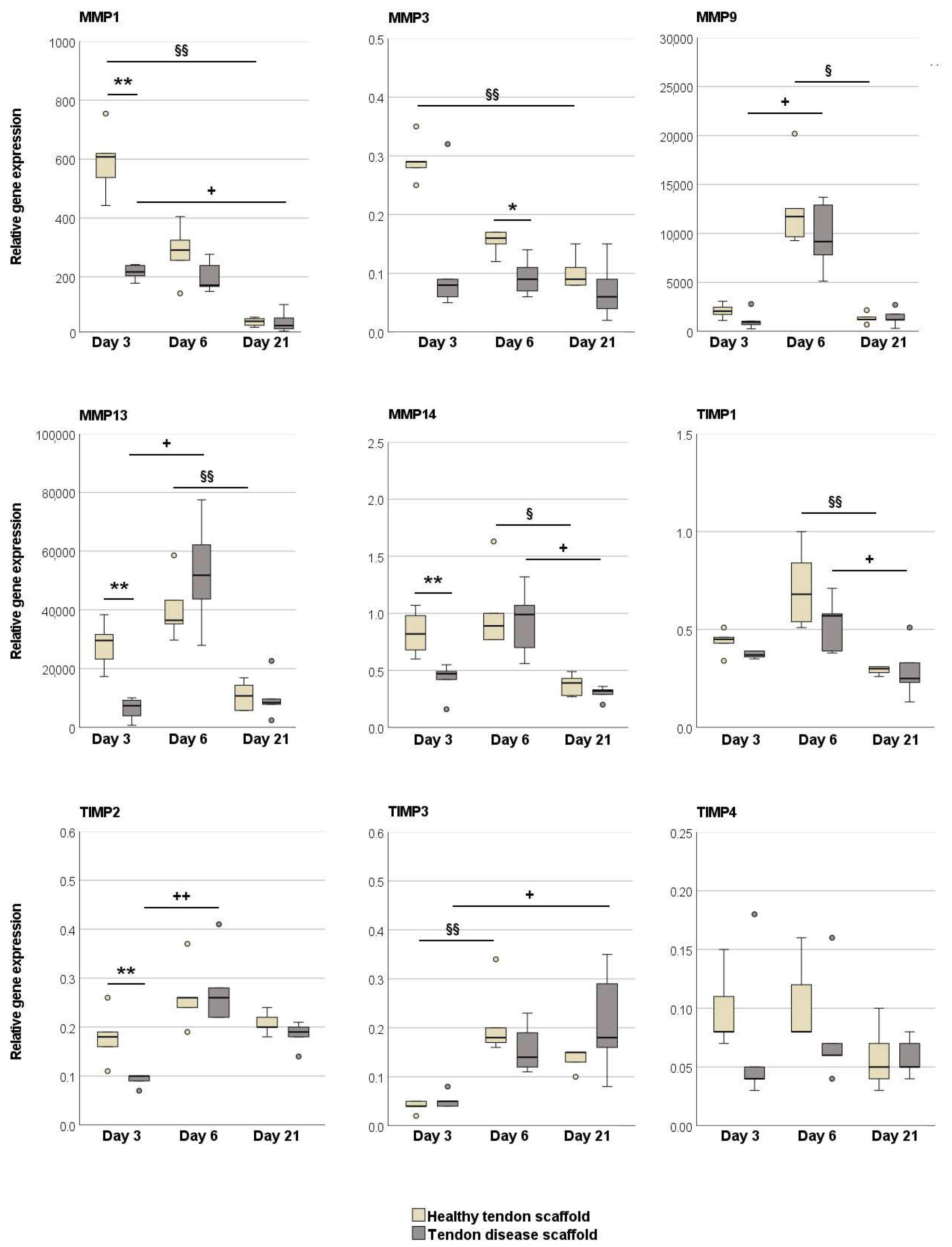
2.2.2. Tendon Marker, Growth Factor, MMP, and TIMP Gene Expression
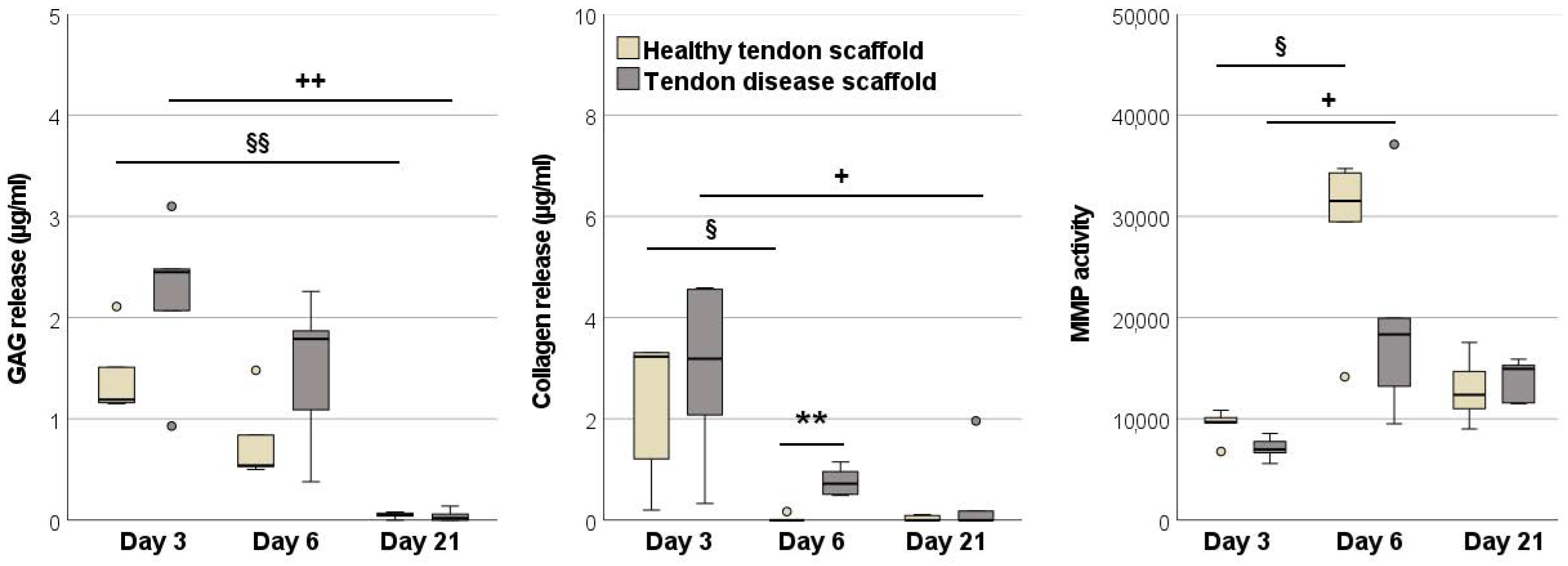
2.2.3. Glycosaminoglycan and Total Collagen Release and MMP Activity
3. Discussion
4. Materials and Methods
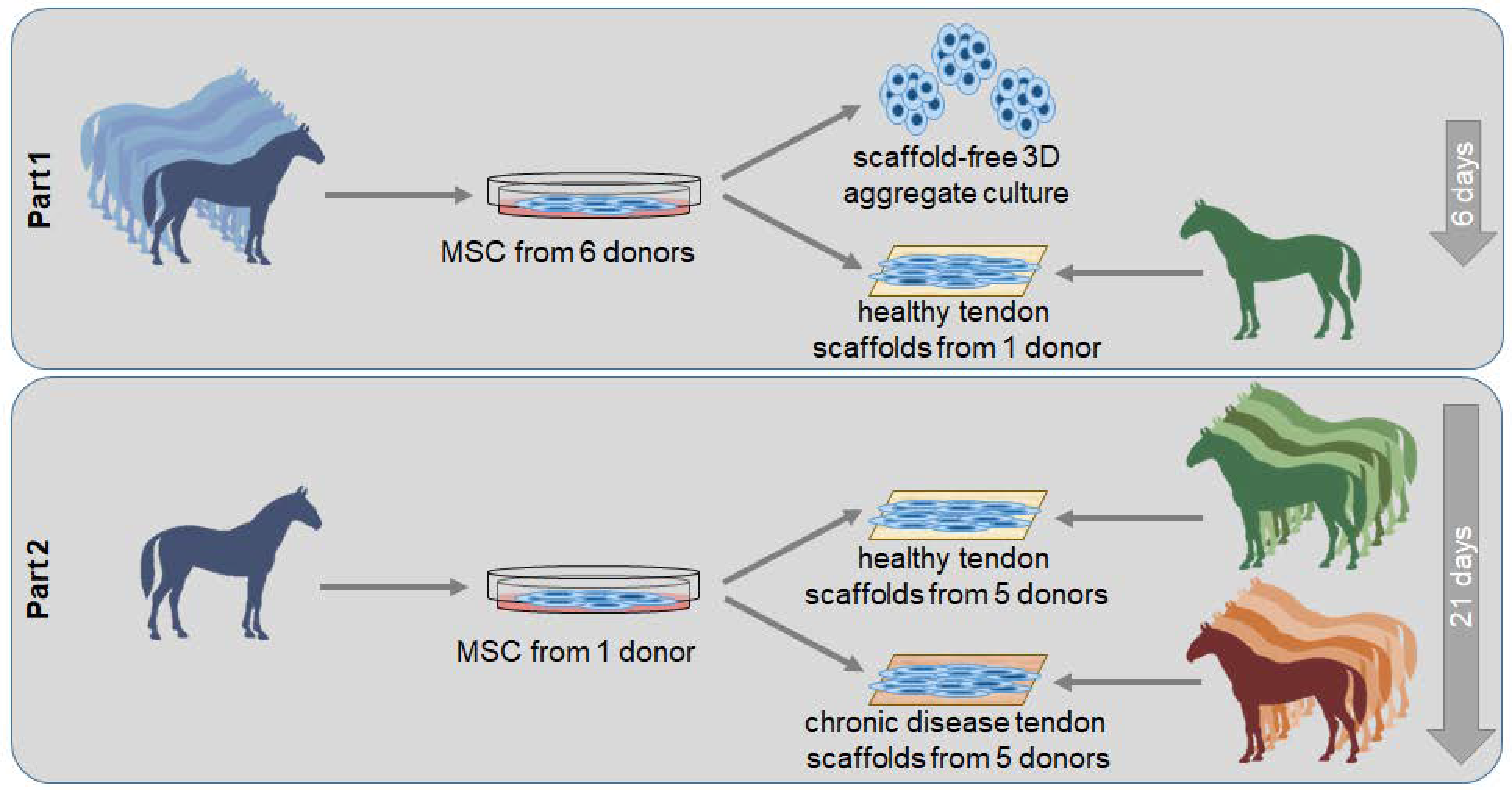
4.1. Study Design
4.2. Tendon Scaffold Preparation
4.3. Mesenchymal Stromal Cell Isolation
4.4. Cell Culture Experiments
4.5. Histology
4.6. Live/Dead Staining
4.7. Quantitative RT-PCR
4.8. Supernatant Analyses
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, R.B.; Harkins, L.S.; Hammond, C.J.; Wood, J.L. Racehorse injuries, clinical problems and fatalities recorded on British racecourses from flat racing and National Hunt racing during 1996, 1997 and 1998. Equine Vet. J. 2001, 33, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Snedeker, J.G.; Foolen, J. Tendon injury and repair-A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017, 63, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, L.; Mohammed, H.; Nixon, A. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J. Orthop. Res. 2005, 23, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.C.; Corps, A.N.; Pennington, C.J.; Clark, I.M.; Edwards, D.R.; Bradley, M.M.; Hazleman, B.L.; Riley, G.P. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human achilles tendon. Arthritis Rheum. 2006, 54, 832–842. [Google Scholar] [CrossRef] [Green Version]
- Patterson-Kane, J.C.; Becker, D.L.; Rich, T. The pathogenesis of tendon microdamage in athletes: The horse as a natural model for basic cellular research. J. Comp. Pathol. 2012, 147, 227–247. [Google Scholar] [CrossRef] [Green Version]
- Ely, E.; Avella, C.; Price, J.; Smith, R.; Wood, J.; Verheyen, K. Descriptive epidemiology of fracture, tendon and suspensory ligament injuries in National Hunt racehorses in training. Equine Vet. J. 2009, 41, 372–378. [Google Scholar] [CrossRef]
- Spaas, J.; Guest, D.; Van de Walle, G. Tendon Regeneration in Human and Equine Athletes Ubi Sumus-Quo Vadimus (Where are We and Where are We Going to)? Sports Med. 2012, 42, 871–890. [Google Scholar] [CrossRef]
- Godwin, E.; Young, N.; Dudhia, J.; Beamish, I.; Smith, R. Implantation of bone marrow-derived mesenchymal stemcells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet. J. 2011, 44, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Burk, J.; Brehm, W. Stammzellentherapie von Sehnenverletzungen–klinische Ergebnisse von 98 Fällen [Stem cell therapy for tendon injuries-clinical results of 98 cases]. Pferdeheilkunde 2011, 27, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Del Bue, M.; Riccò, S.; Ramoni, R.; Conti, V.; Gnudi, G.; Grolli, S. Equine adipose-tissue derived mesenchymal stem cells and platelet concentrates: Their association in vitro and in vivo. Vet. Res. Commun. 2008, 32, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.K.; Webbon, P.M. Harnessing the stem cell for the treatment of tendon injuries: Heralding a new dawn? Br. J. Sports Med. 2005, 39, 582–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burk, J. Mechanisms of Action of Multipotent Mesenchymal Stromal Cells in Tendon Disease. In Tendons; IntechOpen: Leipzig, Germany, 2019. [Google Scholar]
- Wu, Y.; Peng, Y.; Gao, D.; Feng, C.; Yuan, X.; Li, H.; Wang, Y.; Yang, L.; Huang, S.; Fu, X. Mesenchymal stem cells suppress fibroblast proliferation and reduce skin fibrosis through a TGF-β3-dependent activation. Int J. Low. Extrem. Wounds 2015, 14, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Guan, C.Y.; Tian, S.; Lv, X.D.; Li, J.H.; Ma, X.; Xia, H.F. Therapeutic effect of human umbilical cord-derived mesenchymal stem cells on injured rat endometrium during its chronic phase. Stem Cell Res. Ther. 2018, 9, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiwatashi, N.; Bing, R.; Kraja, I.; Branski, R. Mesenchymal stem cells have antifibrotic effects on transforming growth factor-β1-stimulated vocal fold fibroblasts. Laryngoscope 2017, 127, E35–E41. [Google Scholar] [CrossRef] [PubMed]
- Lozito, T.; Jackson, W.; Nesti, L.; Tuan, R. Human mesenchymal stem cells generate a distinct pericellular zone of MMP activities via binding of MMPs and secretion of high levels of TIMPs. Matrix Biol. 2014, 34, 132–143. [Google Scholar] [CrossRef]
- Lozito, T.; Tuan, R. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J. Cell Physiol. 2011, 226, 385–396. [Google Scholar] [CrossRef]
- Roth, S.P.; Schubert, S.; Scheibe, P.; Groß, C.; Brehm, W.; Burk, J. Growth Factor-Mediated Tenogenic Induction of Multipotent Mesenchymal Stromal Cells Is Altered by the Microenvironment of Tendon Matrix. Cell Transplant. 2018, 27, 1434–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youngstrom, D.W.; Barrett, J.G. Engineering Tendon: Scaffolds, Bioreactors, and Models of Regeneration. Stem Cells Int. 2016, 2016, 3919030. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, Y.; Song, L.; Chen, J.; Ma, Y.; Chen, Y.; Fan, S.; Su, M.; Lin, X. Decellularized tendon as a prospective scaffold for tendon repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Samiric, T.; Parkinson, J.; Ilic, M.Z.; Cook, J.; Feller, J.A.; Handley, C.J. Changes in the composition of the extracellular matrix in patellar tendinopathy. Matrix Biol. 2009, 28, 230–236. [Google Scholar] [CrossRef]
- Berner, D.; Brehm, W.; Gerlach, K.; Offhaus, J.; Scharner, D.; Burk, J. Variation in the MRI signal intensity of naturally occurring equine superficial digital flexor tendinopathies over a 12-month period. Vet. Rec. 2020, 187, e53. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, P.R.; McDevitt, T.C. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell Tissue Res. 2012, 347, 701–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beachley, V.; Kasyanov, V.; Nagy-Mehesz, A.; Norris, R.; Ozolanta, I.; Kalejs, M.; Stradins, P.; Baptista, L.; da Silva, K.; Grainjero, J.; et al. The fusion of tissue spheroids attached to pre-stretched electrospun polyurethane scaffolds. J. Tissue Eng. 2014, 5, 2041731414556561. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Gögele, C.; Ondruschka, B.; Hammer, N.; Kohl, B.; Schulze-Tanzil, G. Migrating Myofibroblastic Iliotibial Band-Derived Fibroblasts Represent a Promising Cell Source for Ligament Reconstruction. Int. J. Mol. Sci. 2019, 20, 1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, I.; Baumgartner, W.; Groninger, O.; Stark, W.J.; Marsmann, S.; Calcagni, M.; Cinelli, P.; Wolint, P.; Buschmann, J. 3D microtissue-derived human stem cells seeded on electrospun nanocomposites under shear stress: Modulation of gene expression. J. Mech. Behav. Biomed. Mater. 2020, 102, 103481. [Google Scholar] [CrossRef]
- Frith, J.E.; Thomson, B.; Genever, P.G. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng. Part. C Methods 2010, 16, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G.; Mobasheri, A.; Clegg, P.D.; Sendzik, J.; John, T.; Shakibaei, M. Cultivation of human tenocytes in high-density culture. Histochem. Cell Biol. 2004, 122, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Jauković, A.; Abadjieva, D.; Trivanović, D.; Stoyanova, E.; Kostadinova, M.; Pashova, S.; Kestendjieva, S.; Kukolj, T.; Jeseta, M.; Kistanova, E.; et al. Specificity of 3D MSC Spheroids Microenvironment: Impact on MSC Behavior and Properties. Stem Cell Rev. Rep. 2020, 16, 853–875. [Google Scholar] [CrossRef]
- Cheng, N.C.; Chen, S.Y.; Li, J.R.; Young, T.H. Short-term spheroid formation enhances the regenerative capacity of adipose-derived stem cells by promoting stemness, angiogenesis, and chemotaxis. Stem Cells Transl. Med. 2013, 2, 584–594. [Google Scholar] [CrossRef]
- Hsu, S.H.; Hsieh, P.S. Self-assembled adult adipose-derived stem cell spheroids combined with biomaterials promote wound healing in a rat skin repair model. Wound Repair. Regen. 2015, 23, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Camões, S.P.; Filipe, E.; Cipriano, M.; Barcia, R.N.; Filipe, M.; Teixeira, M.; Simões, S.; Gaspar, M.; Mosqueira, D.; et al. Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing. Stem Cell Res. Ther. 2015, 6, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redondo-Castro, E.; Cunningham, C.J.; Miller, J.; Brown, H.; Allan, S.M.; Pinteaux, E. Changes in the secretome of tri-dimensional spheroid-cultured human mesenchymal stem cells in vitro by interleukin-1 priming. Stem Cell Res. Ther. 2018, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Joshi, J.; Abnavi, M.D.; Kothapalli, C.R. Synthesis and secretome release by human bone marrow mesenchymal stem cell spheroids within three-dimensional collagen hydrogels: Integrating experiments and modelling. J. Tissue Eng. Regen Med. 2019, 13, 1923–1937. [Google Scholar] [CrossRef]
- Yao, L.; Bestwick, C.S.; Bestwick, L.A.; Maffulli, N.; Aspden, R.M. Phenotypic drift in human tenocyte culture. Tissue Eng. 2006, 12, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Stoll, C.; John, T.; Endres, M.; Rosen, C.; Kaps, C.; Kohl, B.; Sittinger, M.; Ertel, W.; Schulze-Tanzil, G. Extracellular matrix expression of human tenocytes in three-dimensional air-liquid and PLGA cultures compared with tendon tissue: Implications for tendon tissue engineering. J. Orthop. Res. 2010, 28, 1170–1177. [Google Scholar] [CrossRef]
- Schneider, P.R.; Buhrmann, C.; Mobasheri, A.; Matis, U.; Shakibaei, M. Three-dimensional high-density co-culture with primary tenocytes induces tenogenic differentiation in mesenchymal stem cells. J. Orthop. Res. 2011, 29, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Hahner, J.; Hoyer, M.; Hillig, S.; Schulze-Tanzil, G.; Meyer, M.; Schroepfer, M.; Lohan, A.; Garbe, L.A.; Heinrich, G.; Breier, A. Diffusion chamber system for testing of collagen-based cell migration barriers for separation of ligament enthesis zones in tissue-engineered ACL constructs. J. Biomater. Sci. Polym. Ed. 2015, 26, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, M.; Meier, C.; Breier, A.; Hahner, J.; Heinrich, G.; Drechsel, N.; Meyer, M.; Rentsch, C.; Garbe, L.A.; Ertel, W.; et al. In vitro characterization of self-assembled anterior cruciate ligament cell spheroids for ligament tissue engineering. Histochem. Cell Biol. 2015, 143, 289–300. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Life is 3D: Boosting Spheroid Function for Tissue Engineering. Trends Biotechnol. 2017, 35, 133–144. [Google Scholar] [CrossRef]
- Kuo, C.K.; Marturano, J.E.; Tuan, R.S. Novel strategies in tendon and ligament tissue engineering: Advanced biomaterials and regeneration motifs. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2010, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Longo, U.G.; Lamberti, A.; Petrillo, S.; Maffulli, N.; Denaro, V. Scaffolds in tendon tissue engineering. Stem Cells Int. 2012, 2012, 517165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Pongkitwitoon, S.; Lu, H.; Lee, C.; Gelberman, R.; Thomopoulos, S. CTGF induces tenogenic differentiation and proliferation of adipose-derived stromal cells. J. Orthop. Res. 2019, 37, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Jayaram, R.; Yoneda, S.; Linderman, S.W.; Sakiyama-Elbert, S.E.; Xia, Y.; Gelberman, R.H.; Thom Poulos, S. The effect of adipose-derived stem cell sheets and CTGF on early flexor tendon healing in a canine model. Sci Rep. 2018, 8, 11078. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, Y.; Knops, N.; Elmonem, M.A.; Nguyen, T.Q.; Arcolino, F.O.; van den Heuvel, L.; Levtchenko, E.; Kuypers, D.; Goldschmeding, R. Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol. 2018, 68–69, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Riley, G.P.; Harrall, R.L.; Constant, C.R.; Chard, M.D.; Cawston, T.E.; Hazleman, B.L. Tendon degeneration and chronic shoulder pain: Changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann. Rheum. Dis. 1994, 53, 359–366. [Google Scholar] [CrossRef]
- Jacobson, E.; Dart, A.J.; Mondori, T.; Horadogoda, N.; Jeffcott, L.B.; Little, C.B.; Smith, M.M. Focal experimental injury leads to widespread gene expression and histologic changes in equine flexor tendons. PLoS ONE 2015, 10, e0122220. [Google Scholar] [CrossRef]
- Kolb, M.; Margetts, P.J.; Sime, P.J.; Gauldie, J. Proteoglycans decorin and biglycan differentially modulate TGF-beta-mediated fibrotic responses in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 280, L1327–L1334. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, Y.; Kirisawa, R.; Mafune, N.; Takehana, K. Downregulation of decorin and transforming growth factor-beta1 by decorin gene suppression in tendinocytes. Connect. Tissue Res. 2005, 46, 18–26. [Google Scholar] [CrossRef]
- Delgado Caceres, M.; Pfeifer, C.G.; Docheva, D. Understanding Tendons: Lessons from Transgenic Mouse Models. Stem Cells Dev. 2018, 27, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.R.; Jones, G.C.; Legerlotz, K.; Riley, G.P. Cyclical strain modulates metalloprotease and matrix gene expression in human tenocytes via activation of TGFβ. Biochim. Biophys. Acta 2013, 1833, 2596–2607. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Rothrauff, B.B.; Lin, H.; Gottardi, R.; Alexander, P.G.; Tuan, R.S. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials 2013, 34, 9295–9306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, E.; Kuroyanagi, K.; Ando, Y.; Matsumoto, T. Effects of Substrate Stiffness on Morphology and MMP-1 Gene Expression in Tenocytes Stimulated With Interleukin-1β. J. Orthop. Res. 2020, 38, 150–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Sassmann, A.; Lauermann, A.; Kasper, C.; Schubert, S.; Burk, J. Context-sensitive extracellular matrix remodelling by human multipotent mesenchymal stromal cells. Acta Physiol. 2019, 227 (Suppl. 719), 70. [Google Scholar] [CrossRef] [Green Version]
- Minkwitz, S.; Schmock, A.; Kurtoglu, A.; Tsitsilonis, S.; Manegold, S.; Wildemann, B.; Klatte-Schulz, F. Time-Dependent Alterations of MMPs, TIMPs and Tendon Structure in Human Achilles Tendons after Acute Rupture. Int. J. Mol. Sci. 2017, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Hosaka, Y.; Kasashima, Y.; Ueda, H.; Takehana, K.; Kuwano, A.; Arai, K. Active expression of matrix metalloproteinase-13 mRNA in the granulation tissue of equine superficial digital flexor tendinitis. J. Vet. Med. Sci. 2007, 69, 637–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, G.P.; Curry, V.; DeGroot, J.; van El, B.; Verzijl, N.; Hazleman, B.L.; Bank, R.A. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002, 21, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Kessler, M.W.; Barr, J.; Greenwald, R.; Lane, L.B.; Dines, J.S.; Dines, D.M.; Drakos, M.C.; Grande, D.A.; Chahine, N.O. Enhancement of Achilles tendon repair mediated by matrix metalloproteinase inhibition via systemic administration of doxycycline. J. Orthop. Res. 2014, 32, 500–506. [Google Scholar] [CrossRef]
- Davis, M.E.; Gumucio, J.P.; Sugg, K.B.; Bedi, A.; Mendias, C.L. MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix. J. Appl. Physiol. 2013, 115, 884–891. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, S.J.; Kreplak, L.; Lee, J.M. MMP-9 selectively cleaves non-D-banded material on collagen fibrils with discrete plasticity damage in mechanically-overloaded tendon. J. Mech. Behav. Biomed. Mater. 2019, 95, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Karousou, E.; Ronga, M.; Vigetti, D.; Passi, A.; Maffulli, N. Collagens, proteoglycans, MMP-2, MMP-9 and TIMPs in human achilles tendon rupture. Clin. Orthop. Relat. Res. 2008, 466, 1577–1582. [Google Scholar] [CrossRef] [Green Version]
- Sahin, H.; Tholema, N.; Petersen, W.; Raschke, M.J.; Stange, R. Impaired biomechanical properties correlate with neoangiogenesis as well as VEGF and MMP-3 expression during rat patellar tendon healing. J. Orthop. Res. 2012, 30, 1952–1957. [Google Scholar] [CrossRef]
- Smith, R.; Werling, N.; Dakin, S.; Alam, R.; Goodship, A.; Dudhia, J. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS ONE 2013, 8, e5697. [Google Scholar] [CrossRef]
- Romero, A.; Barrachina, L.; Ranera, B.; Remacha, A.; Moreno, B.; Blas, I. Comparison of autologous bone marrow and adipose tissue derived mesenchymal stem cells, and platelet rich plasma, for treating surgically induced lesions of the equine superficial digital flexor tendon. Vet. J. 2017, 224, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Burk, J.; Erbe, I.; Berner, D.; Kacza, J.; Kasper, C.; Pfeiffer, B.; Winter, K.; Brehm, W. Freeze-thaw cycles enhance decellularization of large tendons. Tissue Eng. Part. C Methods 2014, 20, 276–284. [Google Scholar] [CrossRef]
- Paebst, F.; Piehler, D.; Brehm, W.; Heller, S.; Schroeck, C.; Tárnok, A.; Burk, J. Comparative immunophenotyping of equine multipotent mesenchymal stromal cells: An approach toward a standardized definition. Cytom. A 2014, 85, 678–687. [Google Scholar] [CrossRef]
- Hagen, A.; Lehmann, H.; Aurich, S.; Bauer, N.; Melzer, M.; Moellerberndt, J.; Patané, V.; Schnabel, C.L.; Burk, J. Scalable Production of Equine Platelet Lysate for Multipotent Mesenchymal Stromal Cell Culture. Front. Bioeng. Biotechnol. 2021, 8, 613621. [Google Scholar] [CrossRef]


| Gene | Primer Sequence | Accession No. | PCR Product (bp) |
|---|---|---|---|
| GAPDH | For: TGGAGAAAGCTGCCAAATACG Rev: GGCCTTTCTCCTTCTCTTGC | NM_001163856.1 | 309 |
| COL1A2 | For: CAACCGGAGATAGAGGACCA Rev: CAGGTCCTTGGAAACCTTGA | XM_001492939.3 | 243 |
| COL3A1 | For: AGGGGACCTGGTTACTGCTT Rev: TCTCTGGGTTGGGACAGTCT | XM_001917620.3 | 216 |
| SCX | For: TACCTGGGTTTTCTTCTGGTCACT Rev: TATCAAAGACACAAGATGCCAGC | NM_001105150.1 | 51 |
| TNC | For: TGAATATGGAATTGGAGTGTCTGC Rev: GGAGTTCTCCACACCAGAGTC | XM_023628743.1 | 147 |
| DCN | For: GGCTGGCGGATCATAAGTACA Rev: CCAGGTGGGCAGAAGTCATT | NM_001081925.2 | 88 |
| MKX | For: AAGATACTCTTGGCGCTCGG Rev: ACACTAAGCCGCTCAGCATT | XM_014737017.1 | 170 |
| CTGF | For: CGACTGGAAGACACGTTTGG Rev: GTCTTGGAACAGGCACTCCA | XM_023651101.1 | 89 |
| TGFB1 | For: AATTCCTGGCGCTACCTCAG Rev: GAACTGAACCCGTTGATGCC | NM_001081849.1 | 194 |
| TGFB3 | For: TGCCCCAAAGGAATCACCTC Rev: GCGTTGTTTGGCGATATGCT | XM_001492687.6 | 186 |
| MMP1 | For: TGCCAAATGGACTTCAAGCTG Rev: GCCGAAGGATCTGTGGATGT | NM_001081847.2 | 137 |
| MMP3 | For: CACTAGCCATGTGCGATCCT Rev: GTCCTGAAGGTTTTGCGCC | NM_001082495.2 | 106 |
| MMP9 | For: GGCCAGTTCCAGACCTTTGA Rev: GGCAAGTCTCCCGAGTAGTTT | NM_001111302.1 | 86 |
| MMP13 | For: CACCTACACTGGCAAAAGCC Rev: TGGGATGTTTAGGGTTCGGG | NM_001081804.1 | 104 |
| MMP14 | For: GGCATCCAGCAACTTTACGG Rev: GCTTATCAGGGACAGAGGGC | XM_023621963.1 | 94 |
| TIMP1 | For: GTCTCCGGCATTCTGTTGTTG Rev: GCCCTGATGACGAACTCAGA | NM_001082515.1 | 110 |
| TIMP2 | For: AGTTCATCTACACGGCTCCC Rev: GCCTTTCCTGCGATGAGGTA | XM_023651899.1 | 88 |
| TIMP3 | For: AGGACTCTGCAACTTCGTGG Rev: GTCACGTAGCAAGGCAGGTA | NM_001081870.2 | 129 |
| TIMP4 | For: TTGAGTGCTTGGTGCAAAGTG Rev: TCTCCTTTCCCCAACTAAAGCA | XM_001492577.4 | 91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doll, C.U.; Niebert, S.; Burk, J. Mesenchymal Stromal Cells Adapt to Chronic Tendon Disease Environment with an Initial Reduction in Matrix Remodeling. Int. J. Mol. Sci. 2021, 22, 12798. https://doi.org/10.3390/ijms222312798
Doll CU, Niebert S, Burk J. Mesenchymal Stromal Cells Adapt to Chronic Tendon Disease Environment with an Initial Reduction in Matrix Remodeling. International Journal of Molecular Sciences. 2021; 22(23):12798. https://doi.org/10.3390/ijms222312798
Chicago/Turabian StyleDoll, Carla U., Sabine Niebert, and Janina Burk. 2021. "Mesenchymal Stromal Cells Adapt to Chronic Tendon Disease Environment with an Initial Reduction in Matrix Remodeling" International Journal of Molecular Sciences 22, no. 23: 12798. https://doi.org/10.3390/ijms222312798
APA StyleDoll, C. U., Niebert, S., & Burk, J. (2021). Mesenchymal Stromal Cells Adapt to Chronic Tendon Disease Environment with an Initial Reduction in Matrix Remodeling. International Journal of Molecular Sciences, 22(23), 12798. https://doi.org/10.3390/ijms222312798






