Recent Advances in Biological Activity, New Formulations and Prodrugs of Ferulic Acid
Abstract
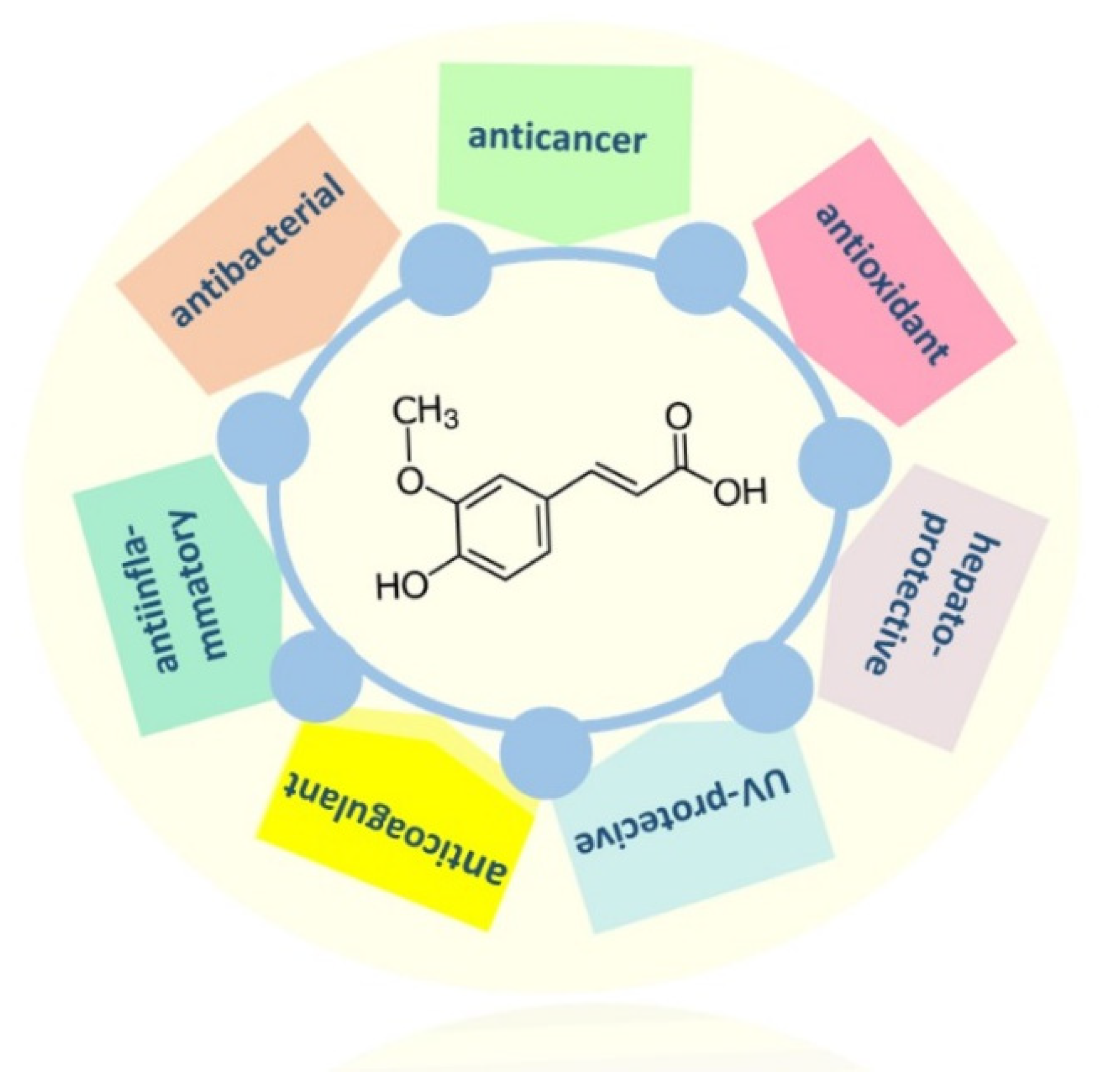
:1. Introduction
2. Main Pharmacological Properties of FA
2.1. Detoxification and Hepatoprotective Effects
2.2. Anticancer Activity
2.3. Other Properties of Ferulic Acid
3. Novel Strategies for Ferulic Acid Drug Delivery
4. Prodrugs of FA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stompor, M.; Żarowska, B. Antimicrobial activity of xanthohumol and its selected structural analogues. Molecules 2016, 21, 608. [Google Scholar] [CrossRef]
- Panek-Krzyśko, A.; Stompor-Gorący, M. The pro-health benefits of morusin administration—An update review. Nutrients 2021, 13, 3043. [Google Scholar] [CrossRef]
- Stompor-Gorący, M.; Bajek-Bil, A.; Machaczka, M. Chrysin: Perspectives on contemporary status and future possibilities as pro-health agent. Nutrients 2021, 13, 2038. [Google Scholar] [CrossRef]
- Stompor, M.; Świtalska, M.; Wietrzyk, J. The influence of a single and double biotinylation of xanthohumol on its anticancer activity. Acta Bioch. Pol. 2019, 66, 2876. [Google Scholar] [CrossRef]
- Stompor, M. 6-Acetamidoflavone obtained by microbial and chemical methods and its antioxidant activity. J. Biotechnol. 2016, 237, 25–34. [Google Scholar] [CrossRef]
- Lyashenko, S.; Fabrikov, D.; González-Fernández, J.M.; Gómez-Mercado, F.; Ruiz, R.L.; Fedorov, A.; de Bélair, G.; Urrestarazu, M.; Rodríguez-García, I.; Alvarez-Corral, M.; et al. Phenolic composition and in vitro antiproliferative activity of Borago ssp. seed extracts on HT-29 cancer cells. Food Biosci. 2021, 42, 101043. [Google Scholar] [CrossRef]
- Yadav, M.P.; Kaur, A.; Singh, B.; Simon, S.; Kaur, N.; Powell, M.; Sarker, M. Extraction and characterization of lipids and phenolic compounds from the brans of different wheat varieties. Food Hydrocoll. 2021, 117, 106734. [Google Scholar] [CrossRef]
- Turghun, C.; Bakri, M.; Liu, G.Y.; Bobakulov, K.; Aisa, H.A. Phenolic glycosides from Nitraria sibirica leaves and their in vitro biological activities. Nat. Prod. Res. 2021, 35, 1388–1392. [Google Scholar] [CrossRef]
- Drawbridge, P.C.; Apea-Bah, F.; Silveira Hornung, P.; Beta, T. Bioaccessibility of phenolic acid in Canadian hulless barley varieties. Food Chem. 2021, 358, 129905. [Google Scholar] [CrossRef]
- Wu, S.; Shen, D.; Wang, R.; Li, Q.; Mo, R.; Zheng, Y.; Zhou, Y.; Liu, Y. Phenolic profiles and antioxidant activities of free, esterified and bound phenolic compounds in walnut kernel. Food Chem. 2021, 350, 129217. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Xiang, J.; Zheng, B.; Yuan, Y.; Luo, D.; Fan, J. Comparative evaluation on phenolic profiles, antioxidant properties and α-glucosidase inhibitory effects of different milling fractions of foxtail millet. J. Cereal Sci. 2021, 99, 103217. [Google Scholar] [CrossRef]
- Naveen, J.; Baskaran, R.; Baskaran, V. Profiling of bioactives and in vitro evaluation of antioxidant and antidiabetic property of polyphenols of marine algae Padina tetrastromatica. Algal Res. 2021, 55, 102250. [Google Scholar] [CrossRef]
- Joshi, P.; Joshi, S.; Semwal, D.K.; Bisht, A.; Sharma, S.; Dwivedi, J. Chemical composition, antioxidative and antimicrobial activities of turmeric spent oleoresin. Ind. Crop. Prod. 2021, 162, 113278. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Jafarzadeh-Moghaddam, M.; Pateiro, M.; Lorenzo, J.M.; Domínguez, R. Physicochemical, thermal and rheological properties of pectin extracted from sugar beet pulp using subcritical water extraction process. Molecules 2021, 26, 1413. [Google Scholar] [CrossRef]
- Ozkok, A.; Keskin, M.; Tanugur Samanci, A.E.; Yorulmaz Onder, E.; Takma, C. Determination of antioxidant activity and phenolic compounds for basic standardization of Turkish propolis. Appl. Biol. Chem. 2021, 64, 37. [Google Scholar] [CrossRef]
- Zhu, S.; Bai, X.; Zhu, J.; Li, W.; Wang, B. Multi-spectral techniques and molecular docking to investigation of the interaction between ferulic acid and pepsin. Spectrochim. Acta A Mol. Biomol. Spectr. 2021, 251, 119442. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Hao, Y.; Liu, Y. Identification of the DPPH radical scavenging reaction adducts of ferulic acid and sinapic acid and their structure-antioxidant activity relationship. LWT 2021, 146, 111411. [Google Scholar] [CrossRef]
- Grzelczyk, J.; Budryn, G.; Peña-García, J.; Szwajgier, D.; Gałązka-Czarnecka, I.; Oracz, J.; Pérez-Sácnchez, H. Evaluation of the inhibition of monoamine oxidase A by bioactive coffee compounds protecting serotonin degradation. Food Chem. 2021, 348, 129108. [Google Scholar] [CrossRef]
- Chen, J.; Lin, D.; Zhang, C.; Li, G.; Zhang, N.; Ruan, L.; Yan, Q.; Li, J.; Yu, X.; Xie, X.; et al. Antidepressant-like effects of ferulic acid: Involvement of serotonergic and norepinergic systems. Metab. Brain Dis. 2014, 30, 129–136. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, J.K.; Kim, K.M.; Lee, H.J.; Kim, S. In vitro and in vivo antithrombotic and cytotoxicity effects of ferulic acid. J. Biochem. Mol. Toxicol. 2018, 32, e22004. [Google Scholar] [CrossRef]
- Sun, X.; Sun, P.; Liu, L.; Jiang, P.; Li, Y. Ferulic acid attenuates microglia-mediated neuroinflammation in retinal degeneration. BMC Ophthalmol. 2021, 21, 13. [Google Scholar] [CrossRef]
- Wu, X.; Lin, L.; Wu, H. Ferulic acid alleviates lipopolysaccharide-induced acute lung injury through inhibiting TLR4/NF-κB signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22664. [Google Scholar] [CrossRef]
- Yasmin, S.; Cercjia, C.; Badavath, V.N.; Laghezza, A.; Dal Piaz, F.; Mondal, S.K.; Atli, O.; Baysal, M.; Vadielan, S.; Shankar, S.; et al. A series of ferulic acid amides reveals unexpected peroxiredoxin 1 inhibitory activity with in vivo antidiabetic and hypolipidemic effects. ChemMedChem 2021, 16, 484–498. [Google Scholar] [CrossRef]
- Zhang, L.W.; Al-Suwayeh, S.A.; Hsieh, P.W.; Fang, J.Y. A comparison of skin delivery of ferulic acid and its derivatives: Evaluation of their efficacy and safety. Int. J. Pharm. 2010, 399, 44–51. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Lureiro, J.A.; Pereira, M.C. The biophysical interaction of ferulic acid with liposomes as biological membrane model: The effect of the lipid bilayer composition. J. Mol. Liq. 2021, 324, 114689. [Google Scholar] [CrossRef]
- Wei, Z.; Xue, Y.; Xue, Y.; Cheng, J.; Lv, G.; Chu, L.; Ma, Z.; Guan, S. Ferulic acid at-tenuates non-alcoholic steatohepatitis by reducing oxidative stress and inflammation through inhibition of the ROCK/NF-ĸB signaling pathways. J. Pharmacol. Sci. 2021, 147, 72–80. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, X.; Qiang, S.; Su, J.; Li, J. Anti-oxidation and anti-inflammatory po-tency evaluation of ferulic acid derivatives obtained through virtual screening. Int. J. Mol. Sci. 2021, 22, 11305. [Google Scholar] [CrossRef]
- Perumal, E.; Eswaran, S.; Parvin, R.; Balasubramanian, S. Mitigation of arsenic induced developmental cardiotoxicity by ferulic acid in zebrafish. Comperativ. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 244, 109021. [Google Scholar] [CrossRef]
- Yu, C.; Pan, S.; Zhang, J.; Li, X.; Niu, Y. Ferulic acid exerts Nrf2-dependent protection against prenatal lead exposure-induced cognitive impairment in offspring mice. J. Nutr. Biochem. 2021, 91, 108603. [Google Scholar] [CrossRef]
- Kassab, R.B.; Lokman, M.S.; Daabo, H.M.A.; Gaber, D.A.; Habotta, O.A.; Hafez, M.M.; Zhery, A.S.; Moneim, A.E.A.; Fouda, M.S. Ferulic acid influences Nrf2 activation to restore testicular tissue from cadmium-induced oxidative challenge, inflammation, and apoptosis in rats. J. Food Biochem. 2020, 44, e13505. [Google Scholar] [CrossRef]
- Guvvala, P.R.; Ravindra, J.P.; Selvaraju, S.; Arangasamy, A.; Venkata, K.M. Ellagic and ferulic acids protect arsenic-induced male reproductive toxicity via regulating Nfe2l2, Ppargc1a and StAR expressions in testis. Toxicology 2019, 413, 1–12. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, J.; Lee, K.H.; Lee, D.U.; Kwak, J.H.; Kim, Y.S.; Lee, S.M. Ferulic acid protects against carbon tetrachloride-induced liver injury in mice. Toxicology 2011, 282, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Aswar, U.; Mahajan, U.; Kandhare, A.; Aswar, M. Ferulic acid ameliorates doxorubicin-induced cardiac toxicity in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Bami, E.; Ozakpinar, O.B.; Ozdemir-Kumral, Z.N.; Köroglu, K.; Ercan, F.; Cirakli, Z.; Sekerler, T.; Izzettin, F.V.; Sancar, M.; Okuyan, B. Protective effect of ferulic acid on cisplatin induced nephrotoxicity in rats. Environ. Toxicol. Pharmacol. 2017, 54, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.G.; Mahajan, U.B.; Shinde, S.D.; Surana, S.J. Cardioprotective role of FA against isoproterenol induced cardic toxicity. Mol. Biol. Rep. 2018, 45, 1357–1365. [Google Scholar] [CrossRef]
- Kelainy, E.G.; Laila, I.M.I.; Ibrahim, S.R. The effect of ferulic acid against lead-induced oxidative stress and DNA damage in kidney and testes of rats. Environ. Sci. Poll. Res. 2019, 26, 31675–31684. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, K.; Lv, L.; Wu, S.; Guo, Z. Ferulic acid ameliorates nonalcoholic fatty liver disease and modulates the gut microbiota composition in high-fat diet fed ApoE−/− mice. Biomed. Pharmacother. 2019, 113, 108753. [Google Scholar] [CrossRef]
- Xu, T.; Song, Q.; Zhou, L.; Yang, W.; Wu, X.; Qian, Q.; Chai, H.; Han, Q.; Pan, H.; Dou, X.; et al. Ferulic acid alleviates lipotoxicity-induced hepatocellular death through the SIRT1-regulated autophagy pathway and independently of AMPK and Akt in AML-12 hepatocytes. Nutr. Metabol. 2021, 18, 13. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, Z.; Zhang, H.; Summah, B.S.; Liu, H.; An, D.; Zhan, Q.; Lai, W.; Zeng, Q.; Ren, H.; et al. Ferulic acid increases intestinal Lactobacillus and improves cardiac function in TCA mice. Biomed. Pharmacother. 2019, 120, 109482. [Google Scholar] [CrossRef]
- Salau, V.; Erukainure, O.L.; Koorbanally, N.A.; Islam, S. Ferulic acid promotes muscle glucose uptake and modulate dysregulated redox balance and metabolic pathway in ferric-induced pancreatic oxidative injury. J. Food Biochem. 2021, e13641. [Google Scholar] [CrossRef]
- Cao, L.; Li, Z.; Yang, Z.; Wang, M.; Zhang, W.; Ren, Y.; Li, L.; Hu, J.; Sun, Z.; Nie, S. Ferulic acid positively modulates the inflammatory response to septic liver injury through the GSK-3β/NF-κB/CREB pathway. Life Sci. 2021, 277, 119584. [Google Scholar] [CrossRef] [PubMed]
- Sevgi, K.; Tepe, B.; Sarikurkcu, C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem. Toxicol. 2015, 77, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Nakahara, M.; Matsugi, E.; Soda, M.; Hattori, T.; Hara, K.; Usami, A.; Kusumoto, C.; Higashiyama, S.; Kitaichi, K. Protective Effect of Ferulic Acid against Hydrogen Peroxide Induced Apoptosis in PC12 Cells. Molecules 2021, 26, 90. [Google Scholar] [CrossRef]
- Ani, G.; Tanya, T.Y.; Reneta, T. Antitumor and Protective effect of ferulic acid against hydrogen peroxide induced apoptosis in PC12 cells apoptogenic effects of ferulic acid on cervical carcinoma cells. Res. J. Biotechnol. 2021, 16, 6–11. [Google Scholar]
- Zhang, X.; Lin, D.; Jiang, R.; Li, H.; Wan, J.; Li, H. Ferulic acid antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 2016, 36, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Helmy, S.A.; El-Mofty, S.; El Gayar, A.M.; El-Sherbiny, I.M.; El-Far, Y.M. Novel doxorubicin/folate-targeted trans-ferulic acid–loaded doxorubicin/folate-targeted trans-ferulic acid–loaded PLGA nanoparticles combination: In vivo superiority over the standard chemotherapeutic regimen for breast cancer treatment. Biomed. Pharmacother. 2022, 145, 112376. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Baby, A.R.; de Almeida, T.S.; Costa, J.G. In vitro cytotoxicity assessment of ferulic, caffeic and p-coumaric acids on human renal cancer cells. Biomed. Biopharm. Res. 2020, 17. [Google Scholar] [CrossRef]
- Karimvand, M.N.; Kalantar, H.; Khodayar, M.J. Cytotoxic and apoptotic effects of ferulic acid on renal carcinoma cell line (ACHN). Jundishapur J. Nat. Pharm. Prod. 2021, 15, e81969. [Google Scholar] [CrossRef]
- Peng, C.C.; Chyau, C.C.; Wang, H.E.; Chang, C.H.; Chen, K.C.; Chou, K.Y.; Peng, R.Y. Cytotoxicity of ferulic acid on T24 cell line differentiated by different microenvironments. BioMed Res. Int. 2013, 2013, 579859. [Google Scholar] [CrossRef]
- Wang, T.; Gong, X.; Jiang, R.; Li, H.; Du, W.; Kuang, G. Ferulic acid inhibits proliferation and promotes apoptosis via blockage of PI3K/Akt pathway in osteosarcoma cell. Am. J. Transl. Res. 2016, 8, 968–980. [Google Scholar]
- ElKhazendar, M.; Chalak, J.; El-Huneidi, W.; Vinod, A.; Abdel-Rahman, W.M.; Abu-Gharbieh, E. Antiproliferative and proapoptotic activities of ferulic acid in breast and liver cancer cell lines. Trop. J. Pharm. Res. 2019, 18, 2571–2576. [Google Scholar]
- Gao, J.; Yu, H.; Guo, W.; Kong, Y.; Gu, L.; Li, Q.; Yang, S.; Zhang, Y.; Wang, Y. The anticancer effects of ferulic acid is associated with induction of cell cycle arrest and autophagy in cervical cancer cells. Cancer Cell 2018, 18, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouzaiene, N.N.; Kilani Jaziri, S.; Kovacic, H.; Chekir-Ghedira, L.; Ghedira, K.; Luis, J. The effects of caffeic, coumaric and ferulic acids on proliferation, superoxide production, adhesion and migration of human tumor cells in vitro. Eur. J. Pharmacol. 2015, 766, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.J.; Zhang, P.P.; Luo, Q.Q.; Deng, S.M.; Jia, A.Q. The chemosensitizer ferulic acid enhances epirubicin-induced apoptosis in MDA-MB-231 cells. J. Funct. Foods 2020, 73, 104130. [Google Scholar] [CrossRef]
- Das, U.; Manna, K.; Adhikary, A.; Mishra, S.; Saha, K.D.; Sharma, R.D.; Majumder, B.; Dey, S. Ferulic acid enhances the radiation sensitivity of lung and liver carcinoma cells by collapsing redox homeostasis: Mechanistic involvement of Akt/p38 MAPK signalling pathway. Free Rad. Res. 2019, 53, 944–967. [Google Scholar] [CrossRef]
- Wang, F.; Lu, W.; Zhang, T.; Dong, J.; Gao, H.; Li, P.; Wang, S.; Zhang, J. Development of novel ferulic acid derivatives as potent histone deacetylase inhibitors. Bioorg. Med. Chem. 2013, 21, 6973–6980. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simỵões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Zhou, Z.; Shi, T.; Hou, J.; Li, M. Ferulic acid alleviates atopic dermatitis-like symptoms in mice via its potent anti-inflammatory effect. Immunopharmacol. Immunotoxicol. 2020, 42, 1–10. [Google Scholar] [CrossRef]
- Peres, D.D.; Sarruf, F.D.; De Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic acid photoprotective properties in association with UV filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B 2018, 185, 46–49. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, R.; Li, Y.; Li, Y.; Yang, Z.; Yang, H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion–induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 2017, 40, 1444–1456. [Google Scholar] [CrossRef] [Green Version]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid exerts its antidiabetic effect by modulating insulin-signalling molecules in the liver of high-fat diet and fructose-induced type-2 diabetic adult male rat. Appl. Physiol. Nutr. Metab. 2015, 40, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.K.; Prasad, R.; Ali, S.; Doble, M. Synergistic interaction of ferulic acid with commercial hypoglycemic drugs in streptozotocin induced diabetic rats. Phytomedicine 2013, 20, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Ijabadeniyi, O.A.; Govender, A.; Olagunju, O.F.; Oyedeji, A.B. The antimicrobial activity of two phenolic acids against foodborne Escherichia coli and Listeria monocytogenes and their effectiveness in a meat system. Ital. J. Food Sci. 2021, 33, 39–45. [Google Scholar] [CrossRef]
- Shen, R.; Wang, H.; Wu, K.; Gao, J.; Li, J. Characterization and antimicrobial properties of ferulic acid grafted self-assembled bacterial cellulose-chitosan membranes. J. Appl. Polym. Sci. 2021, 138, 50824. [Google Scholar] [CrossRef]
- Liu, W.; Xie, J.; Li, L.; Xue, B.; Li, X.; Gan, J.; Shao, Z.; Sun, T. Properties of phenolic acid-chitosan composite films and preservative effect on Penaeus vannamei. J. Mol. Sci. 2021, 1239, 130531. [Google Scholar] [CrossRef]
- Bacanli, M.; Aydin, S.; Taner, G.; Göktaş, H.G.; Sahin, T.; Başaran, A.A.; Başaran, N. The protective role of ferulic acid on sepsis-induced oxidative damage in Wistar albino rats. Environ. Toxicol. Pharmacol. 2014, 38, 774–782. [Google Scholar] [CrossRef]
- Yu, Q.; Fan, L. Understanding the combined effect and inhibition mechanism of 4-hydroxycinnamic acid and ferulic acid as tyrosinase inhibitors. Food Chem. 2021, 352, 129369. [Google Scholar] [CrossRef]
- Qi, D.; Li, Q.; Chen, C.; Wang, X. Ferulic acid modification enhances the anti-oxidation activity of natural Hb in vitro. Artif. Cell Nanomed. Biotechnol. 2018, 46, 1191–1199. [Google Scholar] [CrossRef] [Green Version]
- Yao, N.; Sun, S. Hydrophilic glyceryl ferulates preparation catalyzed by free lipase b from Candida antartica. J. Oleo Sci. 2020, 69, 43–53. [Google Scholar] [CrossRef]
- Oehlke, K.; Behsnilian, D.; Mayer-Miebach, E.; Weidler, P.G.; Greiner, R. Edible solid lipid nanoparticles (SLN) as carriers system for antioxidants of different lipophilicity. PLoS ONE 2017, 12, e0171662. [Google Scholar] [CrossRef]
- Waibel, J.S.; Rudnick, A. Laser assisted delivery to treat facial scars. Facinal Plast. Surgey Clin. N. Am. 2017, 25, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Waibel, J.S.; Mi, Q.S.; Ozog, D.; Qu, L.; Zhou, L.; Rudnick, A.; Al-Niaimi, F.; Woodward, J.; Campos, V.; Mordon, S. Laser-assisted delivery of vitamin C, vitamin E, and ferulic acid formula serum decreases fractional laser postoperative recovery by increased beta fibroblast growth factor expression. Laser Surg. Med. 2016, 48, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Du, S.; Bai, J.; Shang, K.; Lu, Y.; Li, P. Studies on transdermal delivery of ferulic acid through rat skin treated by microneedle arrays. China J. Chin. Mater. Med. 2014, 39, 4773–4777. [Google Scholar]
- Bai, J.; Lu, Y.; Li, P.Y.; Liu, C.M.; Wu, H.C.; Wen, R.; Du, S.Y. Development and in vitro evaluation of a transdermal hydrogel patch for ferulic acid. Pak. J. Pharm. Sci. 2014, 27, 369–375. [Google Scholar] [PubMed]
- Tao, L.; He, L.F.; Guan, Y.M.; Chen, L.H.; Zhu, W.F.; Jin, C.; Wu, L. Preparation and transdermal permeation of triptolide and ferulic acid ethosomes gel in vitro. Zhongguo Zhongyao Zazhi 2018, 43, 1139–1144. [Google Scholar]
- Hassanzadeh, P.; Arbabi, E.; Rostami, F.; Atyabi, F.; Dinarvand, R. Aerosol delivery of ferulic acid-loaded nanostructured lipid carriers: A promising treatment approach against the respiratory disorders. Physiol. Pharmacol. 2017, 21, 331–342. [Google Scholar]
- Del Olmo, N.S.; González, C.E.P.; Rojas, J.D.; Gómez, R.; Ortega, P.; Escarpa, A.; de la Mata, F.J. Antioxidant and antibacterial properties of carbosilane dendrimers functionalized with polyphenolic moieties. Pharmaceutics 2021, 12, 698. [Google Scholar] [CrossRef]
- Anbazhagan, R.; Muthusamy, G.; Krishnamoorthi, R.; Kumaresan, S.; Prasad, N.R.; Lai, J.Y.; Yang, J.M.; Tsai, H.C. PAMAM G 4.5 dendrimers for targeted delivery of ferulic acid and paclitaxel to overcome P-glycoprotein-mediated multidrug resistance. Biotechnol. Bioeng. 2021, 118, 1213–1223. [Google Scholar] [CrossRef]
- Vashisth, P.; Sharma, M.; Nikhil, K.; Singh, H.; Panwar, R.; Pruthi, P.A.; Pruthi, V. Antiproliferative activity of ferulic acid-encapsulated electrospun PLGA/PEO nanofibers against MCF-7 human breast carcinoma cells. 3 Biotech 2015, 5, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, I.; Ponrasu, T.; Rajaram, R.; Suguna, L. The apoptotic effect of ferulic acid-synthesized gold nanoparticles against human epidermoid carcinoma (A431) cells via activation of caspase-3 pathway. J. Drug Deliv. Sci. Technol. 2021, 63, 102478. [Google Scholar] [CrossRef]
- Johnson, E.M.; Lee, H.; Jayabalan, R.; Suh, J.W. Ferulic acid grafted self-assembled fructo-oligosaccharide micro particle for targeted delivery to colon. Carbohyd. Polym. 2020, 247, 116550. [Google Scholar] [CrossRef]
- Casadey, R.; Broglia, M.; Barbero, C.; Criado, S.; Rivarola, C. Controlled release systems of natural phenolic antioxidants encapsulated inside biocompatible hydrogels. React. Funct. Polym. 2020, 156, 104729. [Google Scholar] [CrossRef]
- Rezaeiroshan, A.; Saeedi, M.; Morteza-Semnani, K.; Akbari, J.; Gahsemi, M.; Nokhodchi, A. development of trans-ferulic acid niosome: An optimization and an in-vivo study. J. Drug Deliv. Sci. Technol. 2020, 59, 101854. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Arbabi, E.; Rostami, F. Coating of ferulic acid-loaded silk fibroin nanoparticles with neutrophil membranes: A promising strategy against the acute pancreatitis. Life Sci. 2021, 270, 119128. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Hsiao, C.Y.; Tsai, K.L.; Cheng, Y.H. Injectable thermosensitive chitosan-based hydrogel containing ferulic acid for treating peripheral arterial disease. J. Tissue Eng. Regen. Med. 2020, 14, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Telange, D.R.; Jain, S.P.; Pethe, A.M.; Kharkar, P.S.; Rarokar, N.R. Use of combined nanocarrier system based on chitosan nanoparticles and phospholipids complex for improved delivery of ferulic acid. Int. J. Biol. Macromol. 2021, 171, 288–307. [Google Scholar] [CrossRef]
- Dhayanandamoorthy, Y.; Antoniraj, M.G.; Kandregula, C.A.B.; Kandasamy, R. Aerosolized hyaluronic acid decorated, ferulic acid loaded chitosan nanoparticle: A promising asthma control strategy. Int. J. Pharm. 2020, 591, 119958. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Fang, K.; He, W.; Li, K.; Jiang, Y.; Li, J. Evaluation of chitosan-ferulic acid microcapsules for sustained drug delivery: Synthesis, characterizations, and release kinetics in vitro. J. Mol. Sci. 2021, 1227, 129353. [Google Scholar] [CrossRef]
- Mancuso, A.; Cristiano, M.C.; Pandolfo, R.; Greco, M.; Fresta, M.; Paolino, D. Improvement of ferulic acid antioxidant activity by multiple emulsions: In vitro and in vivo evaluation. Nanomaterials 2021, 11, 425. [Google Scholar] [CrossRef] [PubMed]
- Praphakar, R.A.; Munusamy, M.A.; Rajan, M. Development of extended-voyaging anti-oxidant linked amphiphilic polymeric nanomicells for anti-tuberculosis drug delivery. Int. J. Pharm. 2017, 524, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Poornima, B.; Korrapati, P.S. Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr. Polym. 2017, 157, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Granata, G.; Consoli, G.M.L.; Lo Nigro, R.; Geraci, C. Hydroxycinnamic acids loaded in lipid-core nanocapsules. Food Chem. 2018, 245, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Panwar, R.; Sharma, A.K.; Kaloti, M.; Dutt, D.; Pruthi, V. Characterization and anticancer potential of ferulic acid-loaded chitosan nanoparticles against ME-180 human cervical cancer cell lines. Appl. Nanosci. 2016, 6, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Li, J.B. Preparation of chitosan-ferulic acid conjugate: Structure characterization and in the application of pharmaceuticals. Int. J. Biol. Macromol. 2017, 105, 1539–1543. [Google Scholar] [CrossRef]
- Thakkar, A.; Chenreddy, S.; Wang, J.; Prabhu, S. Ferulic acid combined with aspirin demonstrates chemopreventive potential towards pancreatic cancer when delivered using chitosan-coated solid-lipid nanoparticles. Cell Biosci. 2015, 5, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, F.I.C.; Costa, A.B.S.M.; Filho, J.F.V.; Freite, V.L.P.; Freire, J.V.A.; Converti, A.; Ferrari, M.; Gomes, A.P.B.; Ostrosky, E.A.; Lima, Á.A.N. In vitro release studies of ferulic acid in semi-solid formulations with optimized synthetic membrane. J. Drug Deliv. Sci. Technol. 2021, 61, 102106. [Google Scholar] [CrossRef]
- Hou, J.; Yang, J.; Zheng, X.; Wang, M.; Liu, Y.; Yu, D.G. A nanofiber-based drug depot with high drug loading for sustained release. Int. J. Pharm. 2020, 583, 119397. [Google Scholar] [CrossRef]
- Martins, G.N.; Spínola, V.; Castilho, P.C. Release of adsorbed ferulic acid in simulated gastrointestinal conditions. Eur. Food Res. Technol. 2020, 246, 1297–1306. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Concheiro, A.; Alvarez-Lorenzo, C. Crosslinked hyaluronan electrospun nanofibers for ferulic acid ocular delivery. Pharmaceutics 2020, 12, 274. [Google Scholar] [CrossRef] [Green Version]
- Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Cytosine-functionalized bioinspired hydrogels for ocular delivery of antioxidant transferulic acid. Biomater. Sci. 2020, 8, 1171–1180. [Google Scholar] [CrossRef]
- Romeo, A.; Muscumeci, T.; Carbone, C.; Bonaccorso, A.; Corvo, S.; Lupo, G.; Anfuso, C.D.; Puglisi, G.; Pignatello, R. Ferulic acid-loaded polymeric nanoparticles for potential ocular delivery. Pharmaceutics 2021, 13, 687. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Chen, L.; Hu, Y.N.; Dai, J.L.; Ma, B.; Tang, Q.F.; Tan, X.M. Self-microemulsifying drug delivery system for improved oral delivery and hypnotic efficacy of ferulic acid. Int. J. Nanomed. 2020, 15, 2059–2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zyaitdinov, D.R.; Ewteew, A.V.; Bannikova, A.V. Immobilization of oat bran polyphenols in complex coacervates of whey protein and malthodextrin. Food Proc. Techniq. Technol. 2020, 50, 460–469. [Google Scholar] [CrossRef]
- Heep, G.; Almeida, A.; Marcano, R.; Vieira, D.; Mainardes, R.M.; Khalil, N.M.; Sarmento, B. Zein-casein-lysine multicomposite nanoparticles are effective in modulate the intestinal permeability of ferulic acid. Int. J. Biol. Macromol. 2020, 138, 244–251. [Google Scholar] [CrossRef]
- Awadalla, A.; Hussein, A.M.; El-Far, Y.M.; Barakat, N.; Hamam, E.T.; El-Sherbiny, M.; El-Shafey, M.; Shokeir, A.A. Effect of zinc oxide nanoparticles and ferulic acid on renal ischemia/reperfusion injury: Possible underlying mechanisms. Biomed. Pharmacol. 2021, 140, 111686. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; You, X.; Chen, L.; Huang, J.; Wang, L.; Wu, J.; Guan, S. Biotherapeutic nanoparticles of poly(ferulic acid) delivering doxorubicin for cancer therapy. J. Biomed. Nanotechnol. 2019, 15, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Zhang, Z.H.; Qiao, Z.R.; Cai, W.D.; Yan, J.K. Construction and characterization of antioxidative ferulic acid-grafted carboxylic curdlan conjugates and their contributions on β-carotene storage stability. Food Chem. 2021, 349, 129166. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.B.; Cai, W.D.; Wang, Z.W.; Yan, J.K. Emulsifying properties of a ferulic acid-grafted curdlan conjugate and its contribution to the chemical stability of β-carotene. Food Chem. 2021, 339, 128053. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, G. Synbiotic encapsulation of probiotic Lactobacillus plantarum by alginate-arabinoxylan composite microspheres. LWT 2018, 93, 135–141. [Google Scholar] [CrossRef]
- Puris, E.; Gynther, M.; Huttunen, J.; Auriola, S.; Huttunen, K.M. L-type amino acid transporter 1 utilizing prodrugs of ferulic acid revealed structural features supporting the design of prodrugs for brain delivery. Eur. J. Pharm. Sci. 2019, 129, 99–109. [Google Scholar] [CrossRef]
- Trombino, S.; Ferrarelli, T.; Cassano, R. A New pro-prodrug aminoacid-based for trans-ferulic acid and silybin intestinal release. J. Funct. Biomater. 2014, 5, 99–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamato, S.; Epifano, F.; Curini, M.; Genovese, S.; Kim, M.; Ishigamori-Suzuki, R.; Yasui, Y.; Shigeyuki, S. A novel prodrug of 4′-gelanyloxy-ferulic acid suppresses colitis-related colon carcinogenesis in mice. Nutr. Cancer 2008, 60, 675–684. [Google Scholar] [CrossRef]
- Tan, M.X.; Wang, Z.F.; Qin, Q.P.; Zou, B.Q.; Liang, H. Complexes of oxoplatin with rhein and ferulic acid ligands as platinum(IV) prodrugs with high anti-tumor activity. Dalton Trans. 2020, 49, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Kaki, S.S.; Kunduru, K.R.; Kanjilal, S.; Pradad, R.B.N. Synthesis and characterization of a novel phenolic lipid for use as potential lipophilic antioxidant and as a prodrug of butyric acid. J. Oleo Sci. 2015, 64, 845–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Formulation | Physicochemical Characteristic | Activity | Reference |
|---|---|---|---|
| FA-SLN Solid-lipid nanoparticles loaded with ferulic acid | Zeta potential: −25 to −43 mV Final FA contents in the SLN: 0.56 and 2.80 mg g−1 of dispersion | Stable antioxidant activity | [70] |
| Laser-assisted method of delivery of ferulic acid together with vitamins C | No data | ↑ Wound healing and prevents scarring | [71] |
| Transdermal hydrogel patches with ferulic acid, on the basis of glycerin, dihydroxyaluminum aminoacetate and tartaric acid | No data | ↑ Release of FA from the paste; difficult to permeate through the skin barrier | [74] |
| FA-loaded NLCs Aerosol delivery of ferulic acid-loaded nanostructured lipid carriers | Particle size: 54.9–148.6 nm Polydispersity index: 0.15–0.37 Zeta potential: (−19.8)–(−25.3) mV Entrapment efficiency: 44.3–94.3% | ↑ Period of cytotoxicity time against lung cancer cells (A549); ↑ Pharmacokinetic profile of FA | [76] |
| Gn-[Si(CH2)3NHC(O)FA]2 (G1) Gn-[Si(CH2)3NHC(O)FA]8 (G2) First-generation carbosilane dendrimers functionalized with ferulic acid | NMR characterized | No improving the antioxidant activity (DPPH) Antibacterial activity: MIC (ppm) > 16 (S. aureus and E. coli) % Viability of HFF cells: 95.4 (G1) 92.9 (G2) | [77] |
| RGD-PAMAM-FP Ferulic acid (FA) and paclitaxel (PTX) co-loaded polyamidoamine (PAMAM) dendrimers G 4.5 conjugated with arginyl-glycyl-aspartic acid (RGD) | Zeta potential: −31.3 mV Size: 144.6 nm | ↑ Release of FA; ↑ effectiveness of drug therapy, especially in the treatment of MDR cancers; ↓ P-glycoprotein expression | [78] |
| FA-encapsulated PLGA/PEO nanofibers | Fiber diameter: 150 ± 47.4 to 200 ± 79 nm | Morphological changes in MCF- 7 cells signs for antiapoptotic effect; ↓ viability of HEK- 293 cells | [79] |
| FA-AuNPs | Size: 34.2 nm Polydispersity index (PDI) = 0.137 | Antiangiogenic properties; encouraged programmed cell death in A431 cells. Proapoptotic: ↓ Mitochondrial membrane potential, Improved the ROS; ↑activation of caspase-3 leading to apoptosis | [80] |
| Biocompatible hydrogels based on poly-(N-isopropylacrylamide) (PNIPAM) and copolymers crosslinked with N,N-methylenebisacrylamide (BIS) | No data | ↑ Antioxidant properties; ↑ time release | [82] |
| Niosomal biogel of TFA (trans-ferulic acid) | EE = 21.64% Particle size: 158.7 nm | Anti-inflammatory effect; inhibited the oedema about 21.37% | [83] |
| FA-SF-NPs nanoformulation based on the silk fibroin | Size: 186.3 nm PDI: 0.17 Zeta potential: −36.4 mV | ↓ Levels of enzymes; prevented the significant enhancement of the inflammatory cytokine levels IL-1β, TNF-α and IL-6; and selective accumulation of FA in the inflammatory lesions of the pancreas | [84] |
| FA-gel chitosan/gelatin-based hydrogel containing encapsulated ferulic acid | Gelation time: 64.75 ± 3.31 s at 37 °C | Antioxidant effect; decreasing endogenous reactive oxygen species production, inflammation-related gene expression and apoptosis level; improves blood flow and muscle regeneration; and decreases inflammation in veins | [85] |
| Chitosan nanoparticles loaded with phospholipid complex (FA-FAPLC CNP) | Particle size ~123.27 nm, PDI value ~0.31 Zeta potential: ~32 mV Spherical-shaped morphology | ↑ Aqueous solubility of FA around ~(12-fold), ↑ antioxidant activity and ↑ oral bioavailability | [86] |
| Aerosolized hyaluronic acid decorated, ferulic acid–loaded chitosan nanoparticles | Size: 164.2 ± 9.7 nm Zeta potential: (24.0 ± 0.5 mV) Entrapment efficiency: (EE%) (65.0 ± 1.5) Loading capacity: (LC%) (18.5 ± 0.4) Mass median aerodynamic diameter (MMAD) of 1.81 ± 0.15 µm | ↑ Interaction and transportation across mucus barrier | [87] |
| Ferulic acid delivered in the form of stable w/o/w emulsions | ↑ Percutaneous permeation; possible topical application in photo-induced erythema | [89] | |
| CS-g-PCL/FA chitosan with ɛ-caprolactone and covalently bonded FA | Average size: 100–210 nm | Potential for delivery of hydrophobic antitubercular drugs | [90] |
| FA-chitosan-polycaprolactone nanofibers | Size: 200–240 nm | Antioxidant activity Cytocompatible and able to provide sustained Release of bioactive to support keratinocytes growth in vitro non-hemolytic activity Improve keratinocytes migration in vitro | [91] |
| FA-NC nanocapsules based on poly(ε caprolactone) polymer, loaded with FA | Nanoparticles loaded with hydroxycinnamic acids (HA-NCs) have diameter of 224–253 nm, encapsulation efficiency of 53–78%, and are stable over time (30 days). Zeta potential: −7 mV EE: 62% pH: 4.2 PDI: 0.08 FA loaded amount: 0.62 mg/mL | Protect the HAs in simulated gastric fluid (SGF) and release them in simulated intestinal fluid (SIF) | [92] |
| FA/CS–TPP NPs chitosan–tripolyphosphate pentasodium (CS–TPP) nanoparticles (NPs) with ferulic acid | No data | Antiproliferative activity against ME-180 cells | [93] |
| Microencapsulates of BSA with ferulic acid–grafted chitosan | Primary absorption peak at 350 nm | ↑ Sustained-release effection | [94] |
| Chitosan-coated solid-lipid nanoparticles | Particle sizes: 183 ± 46 and 229 ± 67 nm Encapsulation efficiency of 80 and 78% Zeta potential of 39.1 and 50.3 mV | Chemopreventive effects on 40-fold decreases in dose of FA against human pancreatic cancer cells MIA PaCa-2 and Panc-1 suppressed the growth of the tumor by 45%; decrease expression of proliferation proteins PCNA and MKI67; and also increased expression of apoptotic proteins p-RB, p21 and p-ERK1/2 | [95] |
| FA-cellulose acetate nanostructures | Average diameter of 760 ± 130 nm | Drug loading: 71.5% | [97] |
| FA-Lewatit® Immobilize FA in the solid | Changes in the FTIR-ATR peaks 1685/cm (FA) 1267/cm (C=O) and 1184/cm (O–C) | Average release of 32 mg FA/g of dry loaded resin (recovery of 22%) | [98] |
| FA-NA-ε-PL-PVP Hyaluronan nanofibers and ε-polylysine | mean thickness of 270 ± 21 µm and 273 ± 41 µm | Innovative ophthalmic insert composed of hyaluronan (HA) nanofibers for the dual delivery of an antioxidant (ferulic acid, FA) and an antimicrobial peptide (ε-polylysine, ε-PL) antibacterial activity: Pseudomonas aeruginosa and Staphylococcus aureus | [99] |
| FA-loaded G400E200-0 and G400E200-C hydrogels Hydrogels functionalized with the nitrogenous base cytosine for the controlled uptake and release of transferulic acid (TA) | FRIR strong band at 1655 cm−1 (amide carbonyl group) | ↑ Accumulation of FA in cornea and sclera tissues | [100] |
| Polymeric nanoparticles (NPs) consisting of polylactic acid (NPA) and poly(lactic-co-glycolic acid) (NPB) | FA-NPAs: Size: 178 nm PDI: 0.056 Zeta potential: −33.7 mV FA-NPBs: Size: 219 nm PDI: 0.207 Zeta potential: −23.80 | Promising carriers for ocular drug delivery | [101] |
| Self-microemulsifying drug delivery system: FA-loaded SMEDDS | Droplet size: 15.24 nm | Oral bioavailability: 185.96% Higher distribution in the brain and enhanced serotonin levels in the brain Extended the sleep time by 2-fold and has good stability | [102] |
| Zein-casein-lysine protein-FA-nanoparticles | Size: 199 nm Zeta potential: −26 mV | Modulate the intestinal permeability of FA Prolonged FA release safe profile against Caco-2 and HT29-MTX cells | [104] |
| Combination FA and ZnO-NPs | No data | Significant improvement in the elevated serum creatinine and BUN and MDA concentrations and expression of TNF-α, Bax and caspase-3 in kidney tissues Rise in the creatinine clearance, the activities of catalase (CAT) and superoxide dismutase (SOD) and the expression of HO-1, HIF-1α genes and proliferation marker (ki67) in kidney tissues | [105] |
| PFA–DOX NPs nanoparticles of poly(ferulic acid) containing doxorubicin | No data | Accumulation and retention at the tumor site Superior tumor suppression. Improving safety Reduced the physical toxicity of free DOX | [106] |
| FA-grafted curdlan conjugate (Cur-D-g-FA) | Zeta potential: −22.57–(−34.87) mV | Favorable bioaccessibility of BC in vitro oxidation stability | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stompor-Gorący, M.; Machaczka, M. Recent Advances in Biological Activity, New Formulations and Prodrugs of Ferulic Acid. Int. J. Mol. Sci. 2021, 22, 12889. https://doi.org/10.3390/ijms222312889
Stompor-Gorący M, Machaczka M. Recent Advances in Biological Activity, New Formulations and Prodrugs of Ferulic Acid. International Journal of Molecular Sciences. 2021; 22(23):12889. https://doi.org/10.3390/ijms222312889
Chicago/Turabian StyleStompor-Gorący, Monika, and Maciej Machaczka. 2021. "Recent Advances in Biological Activity, New Formulations and Prodrugs of Ferulic Acid" International Journal of Molecular Sciences 22, no. 23: 12889. https://doi.org/10.3390/ijms222312889
APA StyleStompor-Gorący, M., & Machaczka, M. (2021). Recent Advances in Biological Activity, New Formulations and Prodrugs of Ferulic Acid. International Journal of Molecular Sciences, 22(23), 12889. https://doi.org/10.3390/ijms222312889







