Effects of Intermittent Hypoxia on Cytokine Expression Involved in Insulin Resistance
Abstract
:1. Introduction
2. Intermittent Hypoxia and Cytokines
2.1. Intermittent Hypoxia and Hepatokines
2.2. Intermittent Hypoxia and Adipokines
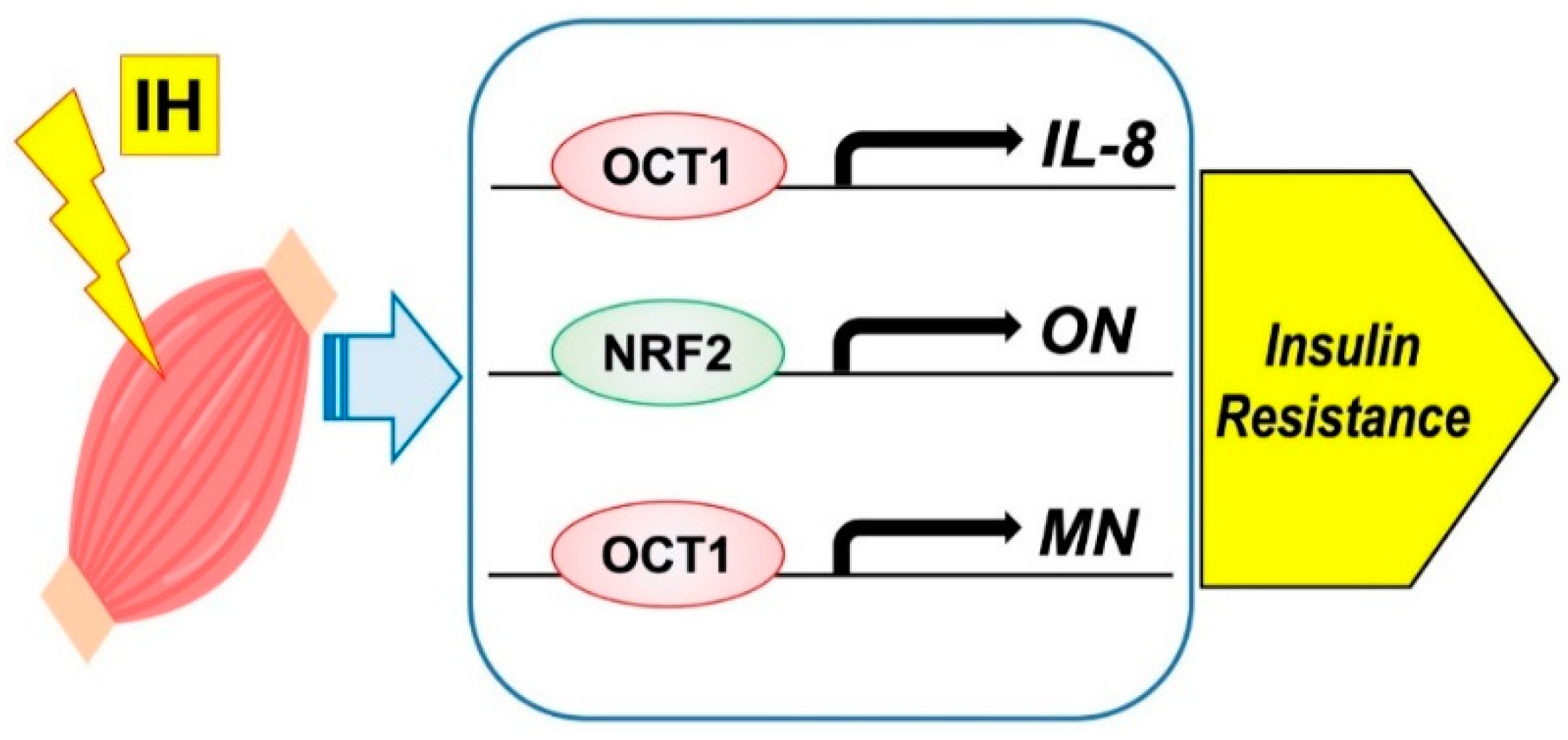
2.3. Intermittent Hypoxia and Myokines
3. Differences in Experimental IH Conditions In Vitro and In Vivo
4. Intermittent Hypoxia and Inflammatory Cytokines
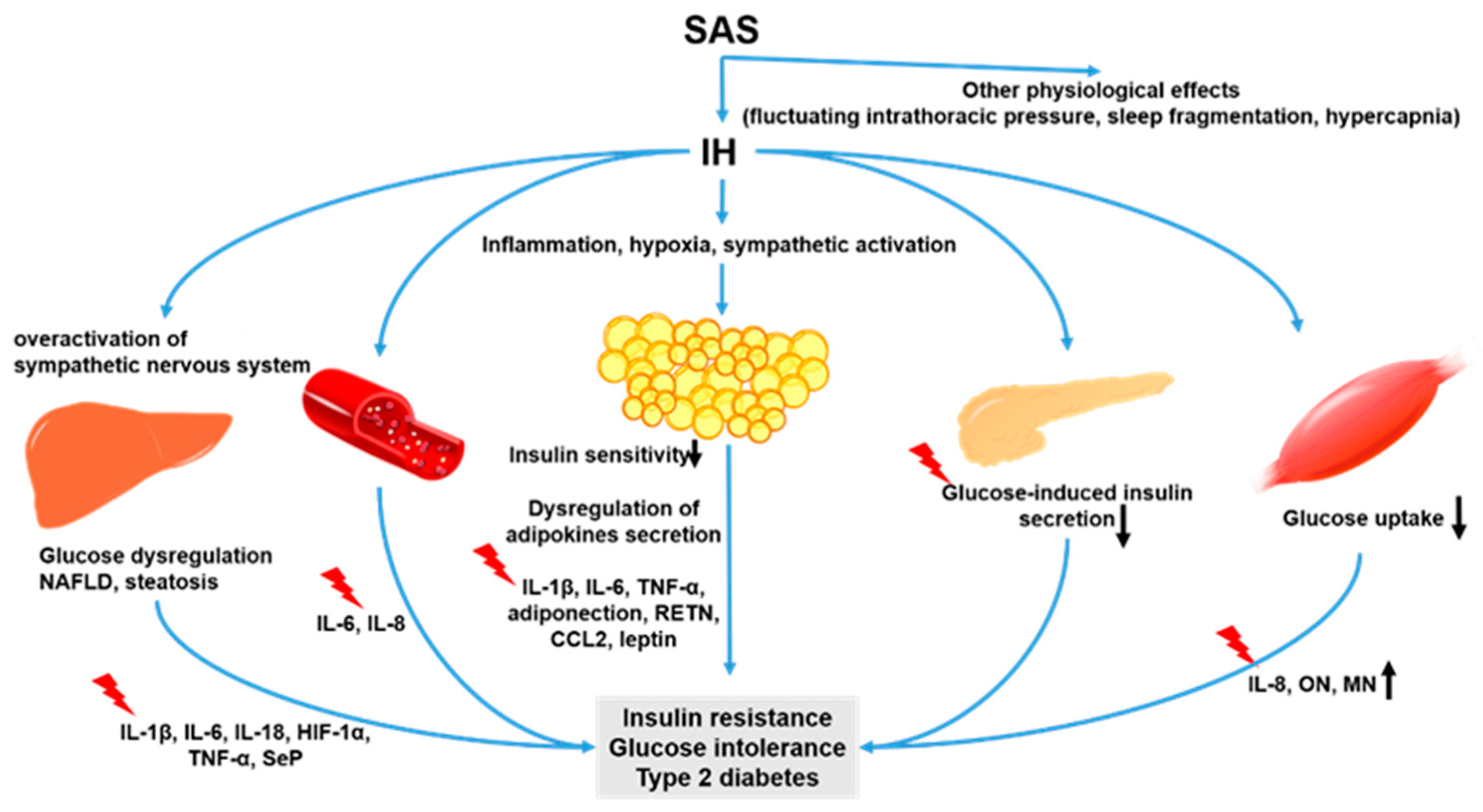
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADIPOQ | Adiponectin |
| ANGPTL | Angiopoietin-like protein |
| AHSG | Alpha-2-HS-glycoprotein |
| CCL2 | C-C motif chemokine ligand 2 |
| C/EBP | CCAAT/enhancer binding protein |
| CHOP | C/EBP homologous protein |
| DM | Diabetes mellitus |
| FFA | Free fatty acid |
| FGF21 | Fibroblast growth factor |
| HGF | Hepatocyte growth factor |
| HIF | Hypoxia-inducible factor |
| HIP/PAP | Hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein |
| IH | Intermittent hypoxia |
| IL | Interleukin |
| I/R | Ischemia-reperfusion |
| LECT | Leukocyte cell-derived chemotaxin |
| LEP | Leptin |
| MAPK | Mitogen-activated protein kinase |
| miRNA | micro RNA |
| MN | Myonectin |
| NAFLD | Nonalcoholic fatty liver disease |
| NASH | Nonalcoholic steatohepatitis |
| NF-κB | Nuclear factor-κB |
| OCT1 | Octamer DNA binding transcription factor-1 |
| ON | Osteonectin |
| OSAS | Obstructive sleep apnea syndrome |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PPAR | Peroxisome proliferator-activated receptor |
| Reg | Regenerating gene |
| ROS | Reactive oxygen species |
| SAS | Sleep apnea syndrome |
| SeP | Selenoprotein P |
| SH | Sustained hypoxia |
| TNF-α | Tumor necrosis factor-α |
| UCP-1 | Uncoupling protein-1 |
| VEGF | Vascular endothelial growth factor |
| WD | Western diet |
References
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Mesarwi, O.A.; Loomba, R.; Malhotra, A. Obstructive sleep apnea, hypoxia, and nonalcoholic fatty liver disease. Am. J. Respir. Crit. Care Med. 2019, 199, 830–841. [Google Scholar] [CrossRef]
- Kyotani, Y.; Takasawa, S.; Yoshizumi, M. Proliferative pathways of vascular smooth muscle cells in response to intermittent hypoxia. Int. J. Mol. Sci. 2019, 20, 2706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchiyama, T.; Ota, H.; Itaya-Hironaka, A.; Shobatake, R.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Makino, M.; Kimura, H.; Takeda, M.; Ohbayashi, C.; et al. Up-regulation of selenoprotein P and HIP/PAP mRNAs in hepatocytes by intermittent hypoxia via down-regulation of miR-203. Biochem. Biophys. Rep. 2017, 11, 130–137. [Google Scholar] [CrossRef]
- Uchiyama, T.; Itaya-Hironaka, A.; Yamauchi, A.; Makino, M.; Sakuramoto-Tsuchida, S.; Shobatake, R.; Ota, H.; Takeda, M.; Ohbayashi, C.; Takasawa, S. Intermittent hypoxia up-regulates CCL2, RETN, and TNFα mRNAs in adipocytes via down-regulation of miR-452. Int. J. Mol. Sci. 2019, 20, 1960. [Google Scholar] [CrossRef] [Green Version]
- Ota, H.; Fujita, Y.; Yamauchi, M.; Muro, S.; Kimura, H.; Takasawa, S. Relationship between intermittent hypoxia and Type 2 diabetes in sleep apnea syndrome. Int. J. Mol. Sci. 2019, 20, 4756. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Dos Santos, A.R.; de Oliveira Zanuso, B.; Miola, V.F.B.; Barbalho, S.M.; Santos Bueno, P.C.; Flato, U.A.P.; Detregiachi, C.R.P.; Buchaim, D.V.; Buchaim, R.L.; Tofano, R.J.; et al. Adipokines, myokines, and hepatokines: Crosstalk and metabolic repercussions. Int. J. Mol. Sci. 2021, 22, 2639. [Google Scholar] [CrossRef]
- Lee, J.J.; Sundar, K.M. Evaluation and management of adults with obstructive sleep apnea syndrome. Lung 2021, 199, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Tasali, E.; Ip, M.S. Obstructive sleep apnea and metabolic syndrome: Alterations in glucose metabolism and inflammation. Proc. Am. Thorac. Soc. 2008, 5, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, N.M.; Beamer, B.A. Alterations in glucose disposal in sleep-disordered breathing. Am. J. Respir. Crit. Care Med. 2009, 179, 235–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinke, C.; Bevans-Fonti, S.; Drager, L.F.; Shin, M.K.; Polotsky, V.Y. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J. Appl. Physiol. 2011, 111, 881–890. [Google Scholar] [CrossRef] [Green Version]
- de Lima, F.F.; Mazzotti, D.R.; Tufik, S.; Bittencourt, L. The role inflammatory response genes in obstructive sleep apnea syndrome: A review. Sleep Breath. 2016, 20, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, K.J.; Austin, D.; Skatrud, J.B.; Young, T. Association of sleep apnea and type II diabetes: A population-based study. Am. J. Respir. Crit. Care Med. 2005, 172, 1590–1595. [Google Scholar] [CrossRef] [Green Version]
- Tasali, E.; Mokhlesi, B.; Van Cauter, E. Obstructive sleep apnea and type 2 diabetes: Interacting epidemics. Chest 2008, 133, 496–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, S. Adipose tissue inflammation by intermittent hypoxia: Mechanistic link between obstructive sleep apnoea and metabolic dysfunction. J. Physiol. 2017, 595, 2423–2430. [Google Scholar] [CrossRef] [Green Version]
- Michalek-Zrabkowska, M.; Macek, P.; Martynowicz, H.; Gac, P.; Mazur, G.; Grzeda, M.; Poreba, R. Obstructive sleep apnea as a risk factor of insulin resistance in nondiabetic adults. Life 2021, 11, 50. [Google Scholar] [CrossRef]
- Song, S.O.; He, K.; Narla, R.R.; Kang, H.G.; Ryu, H.U.; Boyko, E.J. Metabolic consequences of obstructive sleep apnea especially pertaining to diabetes mellitus and insulin sensitivity. Diabetes Metab. J. 2019, 43, 144–155. [Google Scholar] [CrossRef]
- Akasaka, J.; Naruse, K.; Sado, T.; Uchiyama, T.; Makino, M.; Yamauchi, A.; Ota, H.; Sakuramoto-Tsuchida, S.; Itaya-Hironaka, A.; Takasawa, S.; et al. Involvement of receptor for advanced glycation endproducts in hypertensive disorders of pregnancy. Int. J. Mol. Sci. 2019, 20, 5462. [Google Scholar] [CrossRef] [Green Version]
- Muraki, I.; Tanigawa, T.; Yamagishi, K.; Sakurai, S.; Ohira, T.; Imano, H.; Kitamura, A.; Kiyama, M.; Sato, S.; Shimamoto, T.; et al. Nocturnal intermittent hypoxia and the development of type 2 diabetes: The circulatory risk in communities study (CIRCS). Diabetologia 2010, 53, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Briançon-Marjollet, A.; Monneret, D.; Henri, M.; Joyeux-Faure, M.; Totoson, P.; Cachot, S.; Faure, P.; Godin-Ribuot, D. Intermittent hypoxia in obese Zucker rats: Cardiometabolic and inflammatory effects. Exp. Physiol. 2016, 101, 1432–1442. [Google Scholar] [CrossRef] [Green Version]
- Ota, H.; Tamaki, S.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Morioka, T.; Takasawa, S.; Kimura, H. Attenuation of glucose-induced insulin secretion by intermittent hypoxia via down-regulation of CD38. Life Sci. 2012, 90, 206–211. [Google Scholar] [CrossRef]
- Kyotani, Y.; Ota, H.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Zhao, J.; Ozawa, K.; Nagayama, K.; Ito, S.; Takasawa, S.; et al. Intermittent hypoxia induces the proliferation of rat vascular smooth muscle cell with the increases in epidermal growth factor family and erbB2 receptor. Exp. Cell Res. 2013, 319, 3042–3050. [Google Scholar] [CrossRef]
- Ota, H.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Miyaoka, T.; Fujimura, T.; Tsujinaka, H.; Yoshimoto, K.; Nakagawara, K.; Tamaki, S.; et al. Pancreatic β cell proliferation by intermittent hypoxia via up-regulation of Reg family genes and HGF gene. Life Sci. 2013, 93, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Shobatake, R.; Itaya-Hironaka, A.; Yamauchi, A.; Makino, M.; Sakuramoto-Tsuchida, S.; Uchiyama, T.; Ota, H.; Takahashi, N.; Ueno, S.; Sugie, K.; et al. Intermittent hypoxia up-regulates gene expressions of peptide YY (PYY), glucagon-like peptide-1 (GLP-1), and neurotensin (NTS) in enteroendocrine cells. Int. J. Mol. Sci. 2019, 20, 1849. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zou, J.; Li, X.; Zhao, X.; Zou, J.; Liu, S.; Meng, L.; Qian, Y.; Xu, H.; Yi, H.; et al. Effect of the interaction between obstructive sleep apnea and lipoprotein(a) on insulin resistance: A large-scale cross-sectional study. J. Diabetes Res. 2019, 2019, 9583286. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsu, P.Y.; Hsiao, C.C.; Lin, M.C. Epigenetics: A potential mechanism involved in the pathogenesis of various adverse consequences of obstructive sleep apnea. Int. J. Mol. Sci. 2019, 20, 2937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Feijóo-Bandín, S.; Aragón-Herrera, A.; Moraña-Fernández, S.; Anido-Varela, L.; Tarazón, E.; Roselló-Lletí, E.; Portolés, M.; Moscoso, I.; Gualillo, O.; González-Juanatey, J.R.; et al. Adipokines and inflammation: Focus on cardiovascular diseases. Int. J. Mol. Sci. 2020, 21, 7711. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; Arnaud, C.; Fitzpatrick, S.F.; Gaucher, J.; Tamisier, R.; Pépin, J.L. Adipose tissue as a key player in obstructive sleep apnoea. Eur. Respir. Rev. 2019, 28, 190006. [Google Scholar] [CrossRef]
- Takasawa, S.; Shobatake, R.; Itaya-Hironaka, A.; Makino, M.; Sakuramoto-Tsuchida, S.; Uchiyama, T.; Ota, H.; Yamauchi, A. Up-regulation of IL-8, osteonectin, and myonectin mRNAs by intermittent hypoxia via OCT1- and NRF2-mediated mechanisms in skeletal muscle cells. Diabetologia 2020, 63 (Suppl. S1), 453. [Google Scholar]
- Shobatake, R.; Takasawa, K.; Ota, H.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Uchiyama, T.; Makino, M.; Sugie, K.; Takasawa, S.; et al. Up-regulation of POMC and CART mRNAs by intermittent hypoxia via GATA transcription factors in human neuronal cells. Int. J. Biochem. Cell Biol. 2018, 95, 100–107. [Google Scholar] [CrossRef]
- Maniaci, A.; Iannella, G.; Cocuzza, S.; Vicini, C.; Magliulo, G.; Ferlito, S.; Cammaroto, G.; Meccariello, G.; De Vito, A.; Nicolai, A.; et al. Oxidative stress and inflammation biomarker expression in obstructive sleep apnea patients. J. Clin. Med. 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meagher, R.B.; Ambati, S.; Cheng, H.; Ma, P.; Phillips, B.G. Patients with obstructive sleep apnea have altered levels of four cytokines associated with cardiovascular and kidney disease, but near normal levels with airways therapy. Nat. Sci. Sleep 2021, 13, 457–466. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsu, P.Y.; Su, M.C.; Chin, C.H.; Liou, C.W.; Wang, T.Y.; Lin, Y.Y.; Lee, C.P.; Lin, M.C.; Hsiao, C.C. miR-21-5p under-expression in patients with obstructive sleep apnea modulates intermittent hypoxia with re-oxygenation-induced-cell apoptosis and cytotoxicity by targeting pro-inflammatory TNF-α-TLR4 signaling. Int. J. Mol. Sci. 2020, 21, 999. [Google Scholar] [CrossRef] [Green Version]
- In, S.M.; Park, D.Y.; Lee, K.I.; Gu, G.; Kim, H.J. The effects of intermittent hypoxia on human nasal mucosa. Sleep Breath. 2021, 25, 1453–1460. [Google Scholar] [CrossRef]
- Du, Y.; Wang, X.; Li, L.; Hao, W.; Zhang, H.; Li, Y.; Qin, Y.; Nie, S.; Christopher, T.A.; Lopez, B.L.; et al. miRNA-mediated suppression of a cardioprotective cardiokine as a novel mechanism exacerbating post-MI remodeling by sleep breathing disorders. Circ. Res. 2020, 126, 212–228. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Ota, H.; Kimura, Y.; Takasawa, S. Effects of intermittent hypoxia on pulmonary vascular and systemic diseases. Int. J. Environ. Res. Public Health 2019, 16, 3101. [Google Scholar] [CrossRef] [Green Version]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 268–279. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Clement, K.; Pepin, J.L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism 2016, 65, 1124–1135. [Google Scholar] [CrossRef] [Green Version]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Nobili, V.; Cutrera, R.; Liccardo, D.; Pavone, M.; Devito, R.; Giorgio, V.; Verrillo, E.; Baviera, G.; Musso, G. Obstructive sleep apnea syndrome affects liver histology and inflammatory cell activation in pediatric nonalcoholic fatty liver disease, regardless of obesity/insulin resistance. Am. J. Respir. Crit. Care Med. 2014, 189, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.H.; Kim, I.K.; Lee, H.I.; Joo, H.; Lim, J.U.; Lee, J.; Lee, S.H.; Moon, H.S. Chronic intermittent hypoxia induces liver fibrosis in mice with diet-induced obesity via TLR4/MyD88/MAPK/NF-kB signaling pathways. Biochem. Biophys. Res. Commun. 2017, 490, 349–355. [Google Scholar] [CrossRef]
- Jullian-Desayes, I.; Trzepizur, W.; Boursier, J.; Joyeux-Faure, M.; Bailly, S.; Benmerad, M.; Le Vaillant, M.; Jaffre, S.; Pigeanne, T.; Bizieux-Thaminy, A.; et al. Obstructive sleep apnea, chronic obstructive pulmonary disease and NAFLD: An individual participant data meta-analysis. Sleep Med. 2021, 77, 357–364. [Google Scholar] [CrossRef]
- Savransky, V.; Bevans, S.; Nanayakkara, A.; Li, J.; Smith, P.L.; Torbenson, M.S.; Polotsky, V.Y. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G871–G877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Zhao, Y.; Guo, Y.J.; Zhao, Y.S.; Liu, H.; Ren, J.; Li, J.R.; Ji, E.S. A rapid juvenile murine model of nonalcoholic steatohepatitis (NASH): Chronic intermittent hypoxia exacerbates western diet-induced NASH. Life Sci. 2021, 276, 119403. [Google Scholar] [CrossRef]
- Paschetta, E.; Belci, P.; Alisi, A.; Liccardo, D.; Cutrera, R.; Musso, G.; Nobili, V. OSAS-related inflammatory mechanisms of liver injury in nonalcoholic fatty liver disease. Mediat. Inflamm. 2015, 2015, 815721. [Google Scholar] [CrossRef]
- Mesarwi, O.A.; Moya, E.A.; Zhen, X.; Gautane, M.; Zhao, H.; Wegbrans Giró, P.; Alshebli, M.; McCarley, K.E.; Breen, E.C.; Malhotra, A. Hepatocyte HIF-1 and intermittent hypoxia independently impact liver fibrosis in murine NAFLD. Am. J. Respir. Cell Mol. Biol. 2021, 65, 390–402. [Google Scholar] [CrossRef]
- Barros, D.; García-Río, F. Obstructive sleep apnea and dyslipidemia: From animal models to clinical evidence. Sleep 2019, 42, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Cody, S.O.; Potthoff, M.J. Hepatokines and metabolism: Deciphering communication from the liver. Mol. Metab. 2021, 44, 101138. [Google Scholar] [CrossRef]
- Misu, H. Identification of hepatokines involved in pathology of type 2 diabetes and obesity. Endocr. J. 2019, 66, 659–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burk, R.F.; Hill, K.E. Selenoprotein P: An extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu. Rev. Nutr. 2005, 25, 215–235. [Google Scholar] [CrossRef]
- Takasawa, S. Regenerating gene (REG) product and its potential clinical usage. Expert Opin. Ther. Targets 2016, 20, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Terazono, K.; Yamamoto, H.; Takasawa, S.; Shiga, K.; Yonemura, Y.; Tochino, Y.; Okamoto, H. A novel gene activated in regenerating islets. J. Biol. Chem. 1988, 263, 2111–2114. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Takasawa, S. Okamoto model for necrosis and its expansions, CD38-cyclic ADP-ribose signal system for intracellular Ca2+ mobilization and Reg ([Regenerating gene] protein)-Reg receptor system for cell regeneration. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2021, 79, 423–461. [Google Scholar] [CrossRef] [PubMed]
- Shervani, N.J.; Takasawa, S.; Uchigata, Y.; Akiyama, T.; Nakagawa, K.; Noguchi, N.; Takada, H.; Takahashi, I.; Yamauchi, A.; Ikeda, T.; et al. Autoantibodies to REG, a beta-cell regeneration factor, in diabetic patients. Eur. J. Clin. Investig. 2004, 34, 752–758. [Google Scholar] [CrossRef]
- Watanabe, T.; Yonemura, Y.; Yonekura, H.; Suzuki, Y.; Miyashita, H.; Sugiyama, K.; Moriizumi, S.; Unno, M.; Tanaka, O.; Kondo, H.; et al. Pancreatic beta-cell replication and amelioration of surgical diabetes by Reg protein. Proc. Natl. Acad. Sci. USA 1994, 91, 3589–3592. [Google Scholar] [CrossRef] [Green Version]
- Lasserre, C.; Christa, L.; Simon, M.T.; Vernier, P.; Bréchot, C. A novel gene (HIP) activated in human primary liver cancer. Cancer Res. 1992, 52, 5089–5095. [Google Scholar] [PubMed]
- Orelle, B.; Keim, V.; Masciotra, L.; Dagorn, J.C.; Iovanna, J.L. Human pancreatitis-associated protein. Messenger RNA cloning and expression in pancreatic diseases. J. Clin. Investig. 1992, 90, 2284–2291. [Google Scholar] [CrossRef] [Green Version]
- Lieu, H.T.; Batteux, F.; Simon, M.T.; Cortes, A.; Nicco, C.; Zavala, F.; Pauloin, A.; Tralhao, J.G.; Soubrane, O.; Weill, B.; et al. HIP/PAP accelerates liver regeneration and protects against acetaminophen injury in mice. Hepatology 2005, 42, 618–626. [Google Scholar] [CrossRef]
- Gironella, M.; Folch-Puy, E.; LeGoffic, A.; Garcia, S.; Christa, L.; Smith, A.; Tebar, L.; Hunt, S.P.; Bayne, R.; Smith, A.J.; et al. Experimental acute pancreatitis in PAP/HIP knock-out mice. Gut 2007, 56, 1091–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, M.T.; Pauloin, A.; Normand, G.; Lieu, H.T.; Mouly, H.; Pivert, G.; Carnot, F.; Tralhao, J.G.; Brechot, C.; Christa, L. HIP/PAP stimulates liver regeneration after partial hepatectomy and combines mitogenic and anti-apoptotic functions through the PKA signaling pathway. FASEB J. 2003, 17, 1441–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, Y.; Itaya-Hironaka, A.; Yamauchi, A.; Makino, M.; Sakuramoto-Tsuchida, S.; Ota, H.; Kawaguchi, R.; Takasawa, S. Intermittent hypoxia upregulates the renin and Cd38 mRNAs in renin-producing cells via the downregulation of miR-203. Int. J. Mol. Sci. 2021, 22, 10127. [Google Scholar] [CrossRef]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or hyperplasia: Dynamics of adipose tissue growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajesh, Y.; Sarkar, D. Association of adipose tissue and adipokines with development of obesity-induced liver cancer. Int. J. Mol. Sci. 2021, 22, 2163. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Gileles-Hillel, A.; Almendros, I.; Khalyfa, A.; Nigdelioglu, R.; Qiao, Z.; Hamanaka, R.B.; Mutlu, G.M.; Akbarpour, M.; Gozal, D. Prolonged exposures to intermittent hypoxia promote visceral white adipose tissue inflammation in a murine model of severe sleep apnea: Effect of normoxic recovery. Sleep 2017, 40, zsw074. [Google Scholar] [CrossRef]
- Poulain, L.; Thomas, A.; Rieusset, J.; Casteilla, L.; Levy, P.; Arnaud, C.; Dematteis, M. Visceral white fat remodelling contributes to intermittent hypoxia-induced atherogenesis. Eur. Respir. J. 2014, 43, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Murphy, A.M.; Thomas, A.; Crinion, S.J.; Kent, B.D.; Tambuwala, M.M.; Fabre, A.; Pepin, J.L.; Roche, H.M.; Arnaud, C.; Ryan, S. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur. Respir. J. 2017, 49, 1601731. [Google Scholar] [CrossRef] [Green Version]
- Prabhakar, N.R.; Kumar, G.K. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir. Physiol. Neurobiol. 2010, 174, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zhao, M.; Wang, Y.; Zhong, F.; Liu, J.; Gao, L.; Zhao, J. Associations between serum free fatty acid levels and incident diabetes in a 3-year cohort study. Diabetes Metab. Syndr. Obes. 2021, 14, 2743–2751. [Google Scholar] [CrossRef]
- Lytrivi, M.; Castell, A.L.; Poitout, V.; Cnop, M. Recent insights into mechanisms of β-cell lipo- and glucolipotoxicity in Type 2 diabetes. J. Mol. Biol. 2020, 432, 1514–1534. [Google Scholar] [CrossRef]
- Benito-Vicente, A.; Jebari-Benslaiman, S.; Galicia-Garcia, U.; Larrea-Sebal, A.; Uribe, K.B.; Martin, C. Molecular mechanisms of lipotoxicity-induced pancreatic β-cell dysfunction. Int. Rev. Cell Mol. Biol. 2021, 359, 357–402. [Google Scholar] [CrossRef]
- Gabryelska, A.; Karuga, F.F.; Szmyd, B.; Białasiewicz, P. HIF-1α as a mediator of insulin resistance, T2DM, and its complications: Potential links with obstructive sleep apnea. Front. Physiol. 2020, 11, 1035. [Google Scholar] [CrossRef]
- Gabryelska, A.; Szmyd, B.; Panek, M.; Szemraj, J.; Kuna, P.; Białasiewicz, P. Serum hypoxia-inducible factor-1α protein level as a diagnostic marker of obstructive sleep apnea. Pol. Arch. Intern. Med. 2020, 130, 158–160. [Google Scholar] [CrossRef] [Green Version]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 2005, 112, 2660–2667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and cycling hypoxia: Drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: A review of the molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar] [CrossRef]
- Reddy, S.; Amutha, A.; Rajalakshmi, R.; Bhaskaran, R.; Monickaraj, F.; Rangasamy, S.; Anjana, R.M.; Abhijit, S.; Gokulakrishnan, K.; Das, A.; et al. Association of increased levels of MCP-1 and cathepsin-D in young onset type 2 diabetes patients (T2DM-Y) with severity of diabetic retinopathy. J. Diabetes Complicat. 2017, 31, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.P.; Chen, N.H.; Lin, Y.; Ko, W.S.; Pang, J.H. Increased MCP-1 gene expression in monocytes of severe OSA patients and under intermittent hypoxia. Sleep Breath. 2016, 20, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Li, J.; Shin, M.K.; Reinke, C.; Aggarwal, N.R.; Jun, J.C.; Bevans-Fonti, S.; Sztalryd, C.; O’Byrne, S.M.; Kroupa, O.; et al. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur. Heart J. 2012, 33, 783–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, M.Q.; Yeung, S.C.; Mak, J.C.W.; Ip, M.S.M. Differential metabolic and inflammatory responses to intermittent hypoxia in substrains of lean and obese C57BL/6 mice. Life Sci. 2019, 238, 116959. [Google Scholar] [CrossRef]
- Venkata, C.; Venkateshiah, S.B. Sleep-disordered breathing during pregnancy. J. Am. Board Fam. Med. 2009, 22, 158–168. [Google Scholar] [CrossRef]
- Pengo, M.F.; Rossi, G.P.; Steier, J. Obstructive sleep apnea, gestational hypertension and preeclampsia: A review of the literature. Curr. Opin. Pulm. Med. 2014, 20, 588–594. [Google Scholar] [CrossRef]
- Dominguez, J.E.; Street, L.; Louis, J. Management of obstructive sleep apnea in pregnancy. Obstet. Gynecol. Clin. N. Am. 2018, 45, 233–247. [Google Scholar] [CrossRef]
- Oh, K.J.; Lee, D.S.; Kim, W.K.; Han, B.S.; Lee, S.C.; Bae, K.H. Metabolic adaptation in obesity and Type II diabetes: Myokines, adipokines and hepatokines. Int. J. Mol. Sci. 2016, 18, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurens, C.; Bergouignan, A.; Moro, C. Exercise-released myokines in the control of energy metabolism. Front. Physiol. 2020, 11, 91. [Google Scholar] [CrossRef]
- Di Felice, V.; Coletti, D.; Seelaender, M. Editorial: Myokines, adipokines, cytokines in muscle pathophysiology. Front. Physiol. 2020, 11, 592856. [Google Scholar] [CrossRef] [PubMed]
- Wedell-Neergaard, A.S.; Lang Lehrskov, L.; Christensen, R.H.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Exercise-induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: A randomized controlled trial. Cell Metab. 2019, 29, 844–855. [Google Scholar] [CrossRef]
- Park, T.J.; Park, A.; Kim, J.; Kim, J.Y.; Han, B.S.; Oh, K.J.; Lee, E.W.; Lee, S.C.; Bae, K.H.; Kim, W.K. Myonectin inhibits adipogenesis in 3T3-L1 preadipocytes by regulating p38 MAPK pathway. BMB Rep. 2021, 54, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Little, H.C.; Rodriguez, S.; Lei, X.; Tan, S.Y.; Stewart, A.N.; Sahagun, A.; Sarver, D.C.; Wong, G.W. Myonectin deletion promotes adipose fat storage and reduces liver steatosis. FASEB J. 2019, 33, 8666–8687. [Google Scholar] [CrossRef]
- Eckel, J. Myokines in metabolic homeostasis and diabetes. Diabetologia 2019, 62, 1523–1528. [Google Scholar] [CrossRef] [Green Version]
- Garneau, L.; Aguer, C. Role of myokines in the development of skeletal muscle insulin resistance and related metabolic defects in type 2 diabetes. Diabetes Metab. 2019, 45, 505–516. [Google Scholar] [CrossRef]
- Atakan, M.M.; Koşar, Ş.N.; Güzel, Y.; Tin, H.T.; Yan, X. The role of exercise, diet, and cytokines in preventing obesity and improving adipose tissue. Nutrients 2021, 13, 1459. [Google Scholar] [CrossRef] [PubMed]
- Otaka, N.; Shibata, R.; Ohashi, K.; Uemura, Y.; Kambara, T.; Enomoto, T.; Ogawa, H.; Ito, M.; Kawanishi, H.; Maruyama, S.; et al. Myonectin is an exercise-induced myokine that protects the heart from ischemia-reperfusion injury. Circ. Res. 2018, 123, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zheng, Y.; Zhai, X.; Zhao, X.; Cai, L. Diabetic inhibition of preconditioning- and postconditioning-mediated myocardial protection against ischemia/reperfusion injury. Exp. Diabetes Res. 2012, 2012, 198048. [Google Scholar] [CrossRef] [Green Version]
- Szabó, M.R.; Pipicz, M.; Csont, T.; Csonka, C. Modulatory effect of myokines on reactive oxygen species in ischemia/reperfusion. Int. J. Mol. Sci. 2020, 21, 9382. [Google Scholar] [CrossRef] [PubMed]
- Maciel, L.; de Oliveira, D.F.; Mesquita, F.; Souza, H.; Oliveira, L.; Christie, M.L.A.; Palhano, F.L.; Campos de Carvalho, A.C.; Nascimento, J.H.M.; Foguel, D. New cardiomyokine reduces myocardial ischemia/reperfusion injury by PI3K-AKT pathway via a putative KDEL-receptor binding. J. Am. Heart Assoc. 2021, 10, e019685. [Google Scholar] [CrossRef]
- Maciel, L.; de Oliveira, D.F.; Verissimo da Costa, G.C.; Bisch, P.M.; Nascimento, J.H.M. Cardioprotection by the transfer of coronary effluent from ischaemic preconditioned rat hearts: Identification of cardioprotective humoral factors. Basic Res. Cardiol. 2017, 112, 52. [Google Scholar] [CrossRef]
- Glembotski, C.C. Functions for the cardiomyokine, MANF, in cardioprotection, hypertrophy and heart failure. J. Mol. Cell. Cardiol. 2011, 51, 512–517. [Google Scholar] [CrossRef] [Green Version]
- Kiji, T.; Dohi, Y.; Takasawa, S.; Okamoto, H.; Nonomura, A.; Taniguchi, S. Activation of regenerating gene Reg in rat and human hearts in response to acute stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H277–H284. [Google Scholar] [CrossRef] [Green Version]
- Takasawa, S.; Itaya-Hironaka, A.; Yamauchi, A.; Makino, M.; Sakuramoto-Tsuchida, S.; Uchiyama, T.; Takeda, Y.; Kyotani, Y.; Ota, H. Up-regulation of regenerating gene IV and hepatocyte growth factor in cardiomyocytes by intermittent hypoxia and its microRNA-mediated mechanism. Diabetes 2021, 70 (Suppl. S1), 378-P. [Google Scholar] [CrossRef]
- Niijima, M.; Kimura, H.; Edo, H.; Shinozaki, T.; Kang, J.; Masuyama, S.; Tatsumi, K.; Kuriyama, T. Manifestation of pulmonary hypertension during REM sleep in obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 1999, 159, 1766–1772. [Google Scholar] [CrossRef]
- Ota, H.; Takasawa, S.; Yamauchi, M.; Yoshikawa, M.; Tomoda, K.; Kimura, H. Intermittent hypoxia in pancreatic beta cells. Pancreat. Disord. Ther. 2015, 5, S5. [Google Scholar] [CrossRef] [Green Version]
- Polotsky, V.Y.; Savransky, V.; Bevans-Fonti, S.; Reinke, C.; Li, J.; Grigoryev, D.N.; Shimoda, L.A. Intermittent and sustained hypoxia induce a similar gene expression profile in human aortic endothelial cells. Physiol. Genom. 2010, 41, 306–314. [Google Scholar] [CrossRef]
- Nanduri, J.; Wang, N.; Yuan, G.; Khan, S.A.; Souvannakitti, D.; Peng, Y.J.; Kumar, G.K.; Garcia, J.A.; Prabhakar, N.R. Intermittent hypoxia degrades HIF-2α via calpains resulting in oxidative stress: Implications for recurrent apnea-induced morbidities. Proc. Natl. Acad. Sci. USA 2009, 106, 1199–1204. [Google Scholar] [CrossRef] [Green Version]
- Magalang, U.J.; Cruff, J.P.; Rajappan, R.; Hunter, M.G.; Patel, T.; Marsh, C.B.; Raman, S.V.; Parinandi, N.L. Intermittent hypoxia suppresses adiponectin secretion by adipocytes. Exp. Clin. Endocrinol. Diabetes 2009, 117, 129–134. [Google Scholar] [CrossRef]
- Badran, M.; Yassin, B.A.; Lin, D.T.S.; Kobor, M.S.; Ayas, N.; Laher, I. Gestational intermittent hypoxia induces endothelial dysfunction, reduces perivascular adiponectin and causes epigenetic changes in adult male offspring. J. Physiol. 2019, 597, 5349–5364. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hai, B.; Niu, X.; Ai, L.; Cao, Y.; Li, R.; Li, Y. Chronic intermittent hypoxia disturbs insulin secretion and causes pancreatic injury via the MAPK signaling pathway. Biochem. Cell Biol. 2017, 95, 415–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almendros, I.; Farré, R.; Planas, A.M.; Torres, M.; Bonsignore, M.R.; Navajas, D.; Montserrat, J.M. Tissue oxygenation in brain, muscle, and fat in a rat model of sleep apnea: Differential effect of obstructive apneas and intermittent hypoxia. Sleep 2011, 34, 1127–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, C.T.; Kent, B.D.; Crinion, S.J.; McNicholas, W.T.; Ryan, S. Human adipocytes are highly sensitive to intermittent hypoxia induced NF-kappaB activity and subsequent inflammatory gene expression. Biochem. Biophys. Res. Commun. 2014, 447, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.E.; Knight, B.; Pastel, E.; McCulloch, L.J.; Patel, B.; Shore, A.C.; Kos, K. Adipose tissue is influenced by hypoxia of obstructive sleep apnea syndrome independent of obesity. Diabetes Metab. 2017, 43, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ai, L.; Hai, B.; Cao, Y.; Li, R.; Li, H.; Li, Y. Tempol alleviates chronic intermittent hypoxia-induced pancreatic injury through repressing inflammation and apoptosis. Physiol. Res. 2019, 68, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Kyotani, Y.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Makino, M.; Takasawa, S.; Yoshizumi, M. Intermittent hypoxia-induced epiregulin expression by IL-6 production in human coronary artery smooth muscle cells. FEBS Open Bio 2018, 8, 868–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchiyama, T.; Ota, H.; Ohbayashi, C.; Takasawa, S. Effects of Intermittent Hypoxia on Cytokine Expression Involved in Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 12898. https://doi.org/10.3390/ijms222312898
Uchiyama T, Ota H, Ohbayashi C, Takasawa S. Effects of Intermittent Hypoxia on Cytokine Expression Involved in Insulin Resistance. International Journal of Molecular Sciences. 2021; 22(23):12898. https://doi.org/10.3390/ijms222312898
Chicago/Turabian StyleUchiyama, Tomoko, Hiroyo Ota, Chiho Ohbayashi, and Shin Takasawa. 2021. "Effects of Intermittent Hypoxia on Cytokine Expression Involved in Insulin Resistance" International Journal of Molecular Sciences 22, no. 23: 12898. https://doi.org/10.3390/ijms222312898
APA StyleUchiyama, T., Ota, H., Ohbayashi, C., & Takasawa, S. (2021). Effects of Intermittent Hypoxia on Cytokine Expression Involved in Insulin Resistance. International Journal of Molecular Sciences, 22(23), 12898. https://doi.org/10.3390/ijms222312898






