The Tyrosine Phosphatase hPTPRβ Controls the Early Signals and Dopaminergic Cells Viability via the P2X7 Receptor
Abstract
:1. Introduction
2. Results
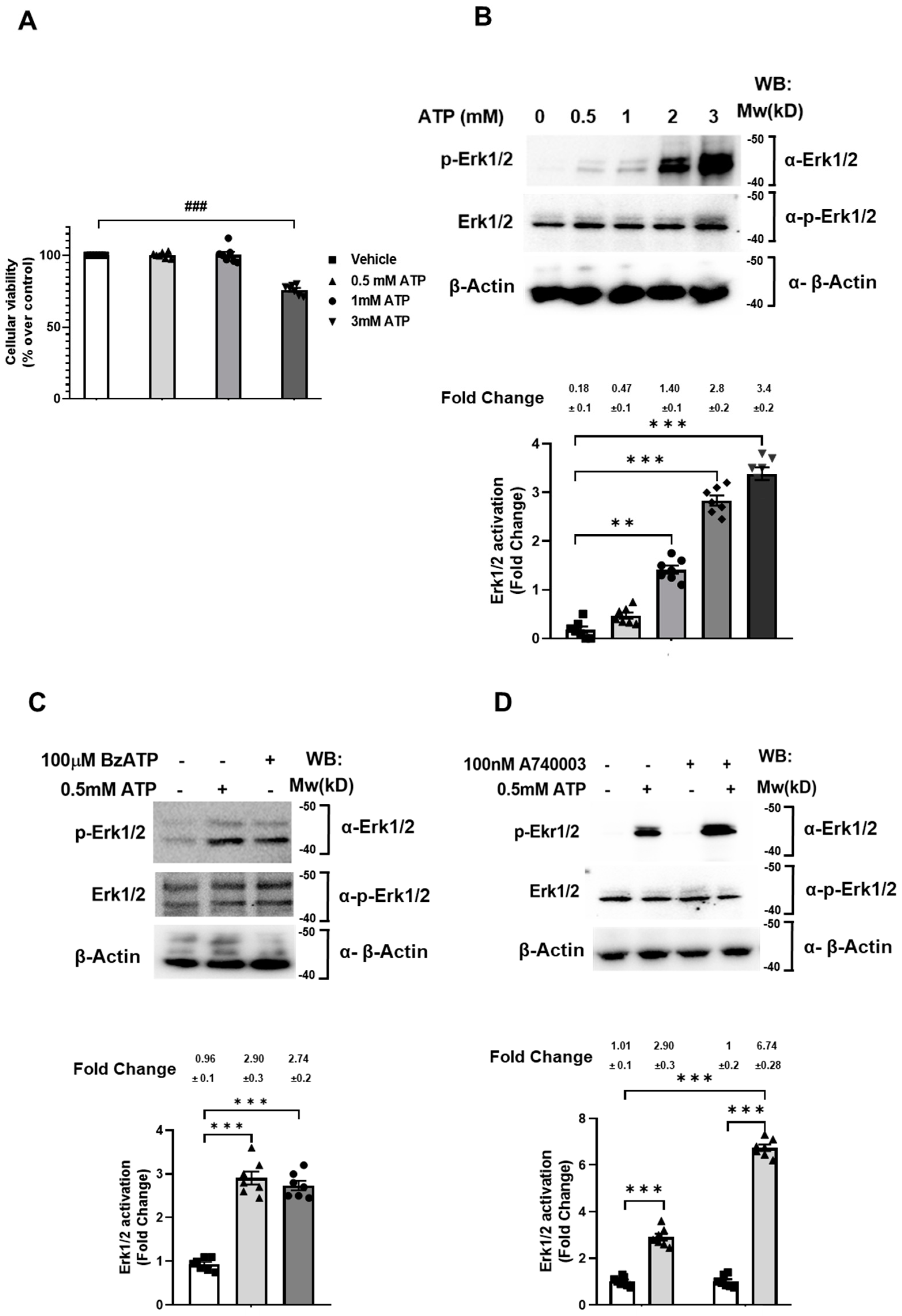
2.1. 0.5 mM ATP Mediates Erk1/2 Phosphorylation via the P2X7 Receptor without Affecting Cell Viability
2.2. P2X7 Receptor Specific Control of Ras Activation in SN4741 Cells
2.3. P2X7 Receptor-Specific Control of Ras Activation Is via the Ca2+-Calmodulin/RasGRF1 Signaling Pathway
2.4. Tyrosine Phosphatases Regulate Ras/Erk Activation and Control SN4741 Cell Viability via the P2X7 Receptor
2.5. PTPRβ Regulates Ras/Erk Activation and Controls SN4741 Cell Viability via the P2X7 Receptor
3. Discussion
4. Experimental Procedures
4.1. Reagents
4.2. Cell Culture and Transfections
4.3. Ras, Rap1, Rho, Rac1, and Cdc42 Activation Assay
4.4. Cell Viability Assay
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Approval
Abbreviations
References
- Draganov, D.; Lee, P.P. Purinergic Signaling Within the Tumor Microenvironment. Adv. Exp. Med. Biol. 2021, 1270, 73–87. [Google Scholar]
- Markwardt, F. Human P2X7 receptors-Properties of single ATP-gated ion channels. Biochem. Pharmacol. 2020, 114307. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, L.; Zheng, G. Expression and function of P2 receptors in hematopoietic stem and progenitor cells. Stem. Cell Investig. 2015, 2, 14. [Google Scholar] [PubMed]
- Agostinho, P.; Madeira, D.; Dias, L.; Simões, A.P.; Cunha, R.A.; Canas, P.M. Purinergic signaling orchestrating neuron-glia communication. Pharmacol. Res. 2020, 162, 105253. [Google Scholar] [CrossRef] [PubMed]
- Andrejew, R.; Oliveira-Giacomelli, Á.; Ribeiro, D.E.; Glaser, T.; Arnaud-Sampaio, V.F.; Lameu, C.; Ulrich, H. The P2X7 Receptor: Central Hub of Brain Diseases. Front. Mol. Neurosci. 2020, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Illes, P. P2X7 Receptors Amplify CNS Damage in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 5996. [Google Scholar] [CrossRef]
- Crabbé, M.; Van der Perren, A.; Bollaerts, I.; Kounelis, S.; Baekelandt, V.; Bormans, G.; Casteels, C.; Moons, L.; Van Laere, K. Increased P2X7 Receptor Binding is Associated with Neuroinflammation in Acute but Not Chronic Rodent Models for Parkinson’s Disease. Front. Neurosci. 2019, 13, 799. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.H.; Xie, X.; Luo, X.G.; Shang, H.; He, Z.Y. Inhibiting purinergic P2X7 receptors with the antagonist brilliant blue G is neuroprotective in an intranigral lipopolysaccharide animal model of Parkinson′s disease. Mol. Med. Rep. 2017, 15, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Calzaferri, F.; Ruiz-Ruiz, C.; De Diego, A.M.G.; De Pascual, R.; Méndez-López, I.; Cano-Abad, M.F.; Maneu, V.; Ríos, C.D.L.; Gandía, L.; García, A.G. The purinergic P2X7 receptor as a potential drug target to combat neuroinflammation in neurodegenerative diseases. Med. Res. Rev. 2020, 40, 2427–2465. [Google Scholar] [CrossRef]
- Monif, M.; Burnstock, G.; Williams, D.A. Microglia: Proliferation and activation driven by the P2X7 receptor. Int. J. Biochem. Cell Biol. 2010, 42, 1753–1756. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Culbert, A.A.; Evans, N.A.; Chessell, I.; Davis, J.B.; Richardson, J.C. P2X(7) receptors on microglial cells mediate injury to cortical neurons in vitro. Glia 2006, 54, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Carmo, M.; Menezes, A.P.F.; Nunes, A.C.L.; Pliássova, A.; Rolo, A.; Palmeira, C.; Cunha, R.A.; Canas, P.; Andrade, G. The P2X7 receptor antagonist Brilliant Blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology 2014, 81, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Dutta, G.; Zhang, P.; Liu, B. The lipopolysaccharide Parkinson’s disease animal model: Mechanistic studies and drug discovery. Fundam. Clin. Pharmacol. 2008, 22, 453–464. [Google Scholar] [CrossRef]
- Oliveira-Giacomelli, Á.; Albino, C.M.; de Souza, H.D.N.; Corrêa-Velloso, J.; Santos, A.P.D.J.; Baranova, J.; Ulrich, H. P2Y6 and P2X7 Receptor Antagonism Exerts Neuroprotective/ Neuroregenerative Effects in an Animal Model of Parkinson’s Disease. Front. Cell Neurosci. 2019, 13, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tufekci, K.U.; Genc, S.; Genc, K. The endotoxin-induced neuroinflammation model of Parkinson′s disease. Parkinsons Dis. 2011, 2011, 487450. [Google Scholar] [PubMed] [Green Version]
- KKim, M.; Jiang, L.-H.; Wilson, H.; North, R.; Surprenant, A. Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J. 2001, 20, 6347–6358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamsky, K.; Schilling, J.; Garwood, J.; Faissner, A.; Peles, E. Glial tumor cell adhesion is mediated by binding of the FNIII domain of receptor protein tyrosine phosphatase beta (RPTPbeta) to tenascin C. Oncogene 2001, 20, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Lenertz, L.Y.; Gavala, M.L.; Zhu, Y.; Bertics, P.J. Transcriptional control mechanisms associated with the nucleotide receptor P2X7, a critical regulator of immunologic, osteogenic, and neurologic functions. Immunol. Res. 2011, 50, 22–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, L.; Pan, C.; He, S.-M.; Lang, B.; Gao, G.-D.; Wang, X.-L.; Wang, Y. The Ras Superfamily of Small GTPases in Non-neoplastic Cerebral Diseases. Front. Mol. Neurosci. 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sastre, A.A.; Montoro, M.L.; Gálvez-Martín, P.; Lacerda, H.; Lucia, A.; Llavero, F.; Zugaza, J. Small GTPases of the Ras and Rho Families Switch on/off Signaling Pathways in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 6312. [Google Scholar] [CrossRef] [PubMed]
- Nakhaeirad, S.; Haghighi, F.; Nouri, P.; Adariani, S.R.; Lissy, J.; Jasemi, N.S.K.; Dvorsky, R.; Ahmadian, M.R. Structural fingerprints, interactions, and signaling networks of RAS family proteins beyond RAS isoforms. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 130–156. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, K.; Serchov, T.; Mann, S.A.; Dietzel, I.D.; Heumann, R. Enhancement of dopaminergic properties and protection mediated by neuronal activation of Ras in mouse ventral mesencephalic neurones. Eur. J. Neurosci. 2007, 25, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Heumann, R.; Moratalla, R.; Herrero, M.T.; Chakrabarty, K.; Drucker-Colín, R.; Garcia-Montes, J.R.; Simola, N.; Morelli, M. Dyskinesia in Parkinson’s disease: Mechanisms and current non-pharmacological interventions. J. Neurochem. 2014, 130, 472–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourne, H.R.; Sanders, D.A.; McCormick, F. The GTPase superfamily: Conserved structure and molecular mechanism. Nature 1991, 349, 117–127. [Google Scholar] [CrossRef]
- Son, J.H.; Chun, H.S.; Joh, T.H.; Cho, S.; Conti, B.; Lee, J.W. Neuroprotection and neuronal differentiation studies using substantia nigra dopaminergic cells derived from transgenic mouse embryos. J. Neurosci. 1999, 19, 10–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, D.; Los, M.; Bauer, M.K.; Vandenabeele, P.; Wesselborg, S.; Schulze-Osthoff, K. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett. 1999, 447, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K. Microglial activation by purines and pyrimidines. Glia 2002, 40, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Le Feuvre, R.; Brough, D.; Rothwell, N. Extracellular ATP and P2X7 receptors in neurodegeneration. Eur. J. Pharmacol. 2002, 447, 261–269. [Google Scholar] [CrossRef]
- Le Stunff, H.; Auger, R.; Kanellopoulos, J.; Raymond, M.-N. The Pro-451 to Leu polymorphism within the C-terminal tail of P2X7 receptor impairs cell death but not phospholipase D activation in murine thymocytes. J. Biol. Chem. 2004, 279, 16918–16926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, D.-J.; Kim, J.; Jung, S.-Y.; Song, R.; Noh, J.-H.; Park, Y.; Ryu, S.H.; Kim, J.-H.; Kong, Y.-Y.; Chung, J.-M.; et al. Extracellular ATP mediates necrotic cell swelling in SN4741 dopaminergic neurons through P2X7 receptors. J. Biol. Chem. 2007, 282, 37350–37358. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.; Alapat, D.; Wen, X.; Timo, K.; Burstein, D.; Lisanti, M.; Shears, S.; Kohtz, D.S. Ectopic expression of murine diphosphoinositol polyphosphate phosphohydrolase 1 attenuates signaling through the ERK1/2 pathway. Cell Signal. 2004, 16, 1045–1059. [Google Scholar] [CrossRef]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef] [PubMed]
- Donnelly-Roberts, D.L.; Jarvis, M.F. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br. J. Pharmacol. 2007, 151, 571–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturani, E.; Abbondio, A.; Branduardi, P.; Ferrari, C.; Zippel, R.; Martegani, E.; Vanoni, M.E.; Denis-Donini, S. The Ras Guanine nucleotide Exchange Factor CDC25Mm is present at the synaptic junction. Exp. Cell Res. 1997, 235, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, F.S.; Falzoni, S.; Adinolfi, E.; Ferrari, D.; Di Virgilio, F. The P2X7 receptor is a key modulator of aerobic glycolysis. Cell Death Dis. 2012, 3, e370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farnsworth, C.L.; Freshney, N.W.; Rosen, L.B.; Ghosh, A.; Greenberg, M.E.; Feig, L.A. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature 1995, 376, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Chen, X.-Q.; Tian, X.; Chen, L.; Wu, Y.-X.; Huang, D.; Yi, H.-L.; Yi, C.-L.; Li, C.-Y. P2X3, but not P2X1, receptors mediate ATP-activated current in neurons innervating tooth-pulp. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Prasai, P.; Stefos, G.C.; Becker, W. Extracellular ATP activates NFAT-dependent gene expression in neuronal PC12 cells via P2X receptors. BMC Neurosci. 2011, 12, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, A.; Arimura, Y.; Manago, Y.; Nishikawa, K.; Aoki, K.; Wada, E.; Suzuki, Y.; Osaka, H.; Setsuie, R.; Sakurai, M.; et al. Parkin potentiates ATP-induced currents due to activation of P2X receptors in PC12 cells. J. Cell Physiol. 2006, 209, 172–182. [Google Scholar] [CrossRef]
- Gündüz, D.; Troidl, C.; Tanislav, C.; Rohrbach, S.; Hamm, C.; Aslam, M. Role of PI3K/Akt and MEK/ERK Signalling in cAMP/Epac-Mediated Endothelial Barrier Stabilisation. Front. Physiol. 2019, 10, 1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birukova, A.A.; Smurova, K.; Birukov, K.G.; Kaibuchi, K.; Garcia, J.G.; Verin, A.D. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc. Res. 2004, 67, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Minichiello, L. TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 2009, 10, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Belcheva, M.M.; Coscia, C.J. Diversity of G protein-coupled receptor signaling pathways to ERK/MAP kinase. Neurosignals 2002, 11, 34–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, J.C. Regulation and Function of the RasGRP Family of Ras Activators in Blood Cells. Genes Cancer 2011, 2, 320–334. [Google Scholar] [CrossRef]
- Baines, C.P.; Zhang, J.; Wang, G.W.; Zheng, Y.T.; Xiu, J.X.; Cardwell, E.M.; Bolli, R.; Ping, P. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: Enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ. Res. 2002, 90, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Jiang, H.; Yang, F.; Nakaso, K.; Feng, J. Parkin protects dopaminergic neurons against microtubule-depolymerizing toxins by attenuating microtubule-associated protein kinase activation. J. Biol. Chem. 2009, 284, 4009–4017. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, S.; Schnellmann, R.G. A death-promoting role for extracellular signal-regulated kinase. J. Pharmacol. Exp. Ther. 2006, 319, 991–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Rusnak, M.; Luedtke, R.R.; Sidhu, A. D1 dopamine receptor mediates dopamine-induced cytotoxicity via the ERK signal cascade. J. Biol. Chem. 2004, 279, 39317–39330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagda, R.K.; Zhu, J.; Kulich, S.M.; Chu, C.T. Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress: Implications for Parkinson’s disease. Autophagy 2008, 4, 770–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.-H.; Kulich, S.M.; Oury, T.D.; Chu, C. Cytoplasmic aggregates of phosphorylated extracellular signal-regulated protein kinases in Lewy body diseases. Am. J. Pathol. 2002, 161, 2087–2098. [Google Scholar] [CrossRef] [Green Version]
- Deuel, T.F. Anaplastic lymphoma kinase: “Ligand Independent Activation” mediated by the PTN/RPTPbeta/zeta signaling pathway. Biochim. Biophys. Acta 2013, 1834, 2219–2223. [Google Scholar] [CrossRef]
- Mathivet, T.; Mazot, P.; Vigny, M. In contrast to agonist monoclonal antibodies, both C-terminal truncated form and full length form of Pleiotrophin failed to activate vertebrate ALK (anaplastic lymphoma kinase)? Cell Signal. 2007, 19, 2434–2443. [Google Scholar] [CrossRef]
- Moog-Lutz, C.; Degoutin, J.; Gouzi, J.Y.; Frobert, Y.; Carvalho, N.B.-D.; Bureau, J.; Créminon, C.; Vigny, M. Activation and inhibition of anaplastic lymphoma kinase receptor tyrosine kinase by monoclonal antibodies and absence of agonist activity of pleiotrophin. J. Biol. Chem. 2005, 280, 26039–26048. [Google Scholar] [CrossRef] [Green Version]
- Perez-Pinera, P.; Zhang, W.; Chang, Y.; Vega, J.A.; Deuel, T.F. Anaplastic lymphoma kinase is activated through the pleiotrophin/receptor protein-tyrosine phosphatase beta/zeta signaling pathway: An alternative mechanism of receptor tyrosine kinase activation. J. Biol. Chem. 2007, 282, 28683–28690. [Google Scholar] [CrossRef] [Green Version]
- Degoutin, J.; Vigny, M.; Gouzi, J.Y. ALK activation induces Shc and FRS2 recruitment: Signaling and phenotypic outcomes in PC12 cells differentiation. FEBS Lett. 2007, 581, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Motegi, A.; Fujimoto, J.; Kotani, M.; Sakuraba, H.; Yamamoto, T. ALK receptor tyrosine kinase promotes cell growth and neurite outgrowth. J. Cell Sci. 2004, 117 Pt 15, 3319–3329. [Google Scholar] [CrossRef] [Green Version]
- Palmer, R.H.; Vernersson, E.; Grabbe, C.; Hallberg, B. Anaplastic lymphoma kinase: Signalling in development and disease. Biochem. J. 2009, 420, 345–361. [Google Scholar] [CrossRef] [Green Version]
- Souttou, B.; Carvalho, N.B.-D.; Raulais, D.; Vigny, M. Activation of anaplastic lymphoma kinase receptor tyrosine kinase induces neuronal differentiation through the mitogen-activated protein kinase pathway. J. Biol. Chem. 2001, 276, 9526–9531. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Dankowski, A.; Hagg, T. Protein tyrosine phosphatase inhibition reduces degeneration of dopaminergic substantia nigra neurons and projections in 6-OHDA treated adult rats. Eur. J. Neurosci. 2007, 25, 1332–1340. [Google Scholar] [CrossRef]
- Llavero, F.; Artaso, A.; Lacerda, H.M.; Parada, L.A.; Zugaza, J.L. Lck/PLCgamma control migration and proliferation of interleukin (IL)-2-stimulated T cells via the Rac1 GTPase/glycogen phosphorylase pathway. Cell Signal. 2016, 28, 1713–1724. [Google Scholar] [CrossRef] [Green Version]
- Llavero, F.; Montoro, M.L.; Sastre, A.A.; Fernández-Moreno, D.; Lacerda, H.M.; Parada, L.A.; Lucia, A.; Zugaza, J.L. Epidermal growth factor receptor controls glycogen phosphorylase in T cells through small GTPases of the RAS family. J. Biol. Chem. 2019, 294, 4345–4358. [Google Scholar] [CrossRef]
- Llavero, F.; Urzelai, B.; Osinalde, N.; Gálvez, P.; Lacerda, H.M.; Parada, L.A.; Zugaza, J.L. Guanine nucleotide exchange factor alphaPIX leads to activation of the Rac 1 GTPase/glycogen phosphorylase pathway in interleukin (IL)-2-stimulated T cells. J. Biol. Chem. 2015, 290, 9171–9182. [Google Scholar] [CrossRef] [Green Version]
- Arrizabalaga, O.; Lacerda, H.M.; Zubiaga, A.M.; Zugaza, J.L. Rac1 Protein Regulates Glycogen Phosphorylase Activation and Controls Interleukin (IL)-2-dependent T Cell Proliferation. J. Biol. Chem. 2012, 287, 11878–11890. [Google Scholar] [CrossRef] [Green Version]





| Drug Name | Activity | Treatment | Concentration |
|---|---|---|---|
| ATP | Agonist of P2X receptors | 5 min | 500 µM |
| BzATP | Specific agonist of P2X7 receptor | 10 min | 100 µM |
| A-740003 | Specific antagonist of P2X7 receptor | 30 min | 100 nM |
| GF109203X hydrochloride | PKC inhibitor | 1 h | 1 µM |
| Na3VO4 | Tyrosine phosphatase inhibitor | 20 min | 1 mM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal, F.L.; Luque Montoro, M.; Arrazola Sastre, A.; Lacerda, H.M.; Zugaza, J.L. The Tyrosine Phosphatase hPTPRβ Controls the Early Signals and Dopaminergic Cells Viability via the P2X7 Receptor. Int. J. Mol. Sci. 2021, 22, 12936. https://doi.org/10.3390/ijms222312936
Bernal FL, Luque Montoro M, Arrazola Sastre A, Lacerda HM, Zugaza JL. The Tyrosine Phosphatase hPTPRβ Controls the Early Signals and Dopaminergic Cells Viability via the P2X7 Receptor. International Journal of Molecular Sciences. 2021; 22(23):12936. https://doi.org/10.3390/ijms222312936
Chicago/Turabian StyleBernal, Francisco Llavero, Miriam Luque Montoro, Alazne Arrazola Sastre, Hadriano M. Lacerda, and José Luis Zugaza. 2021. "The Tyrosine Phosphatase hPTPRβ Controls the Early Signals and Dopaminergic Cells Viability via the P2X7 Receptor" International Journal of Molecular Sciences 22, no. 23: 12936. https://doi.org/10.3390/ijms222312936







