An Update on Graphene-Based Nanomaterials for Neural Growth and Central Nervous System Regeneration
Abstract
:1. Introduction
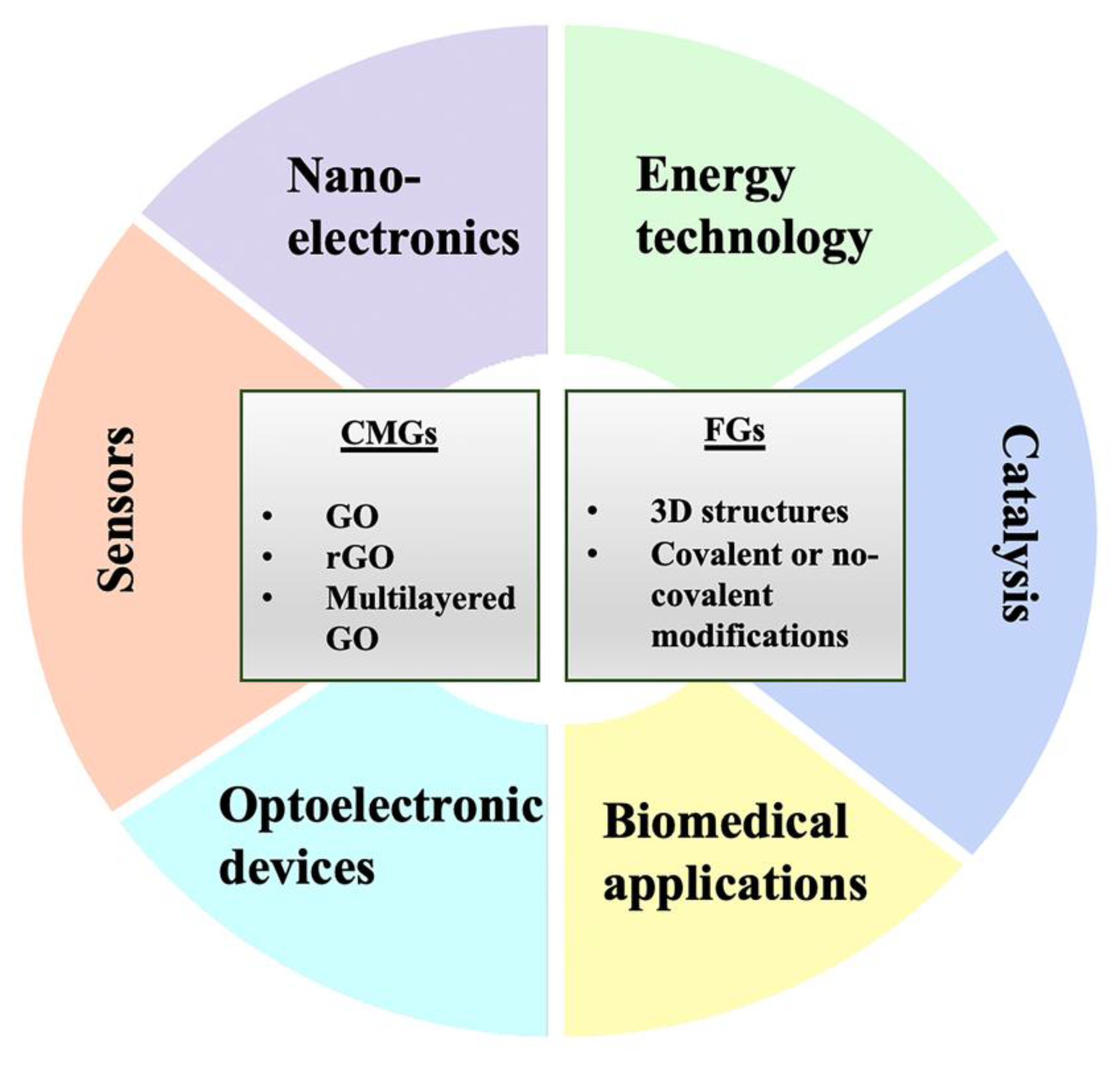
2. Graphene and Its Chemical Derivatives
3. Biomedical Applications
4. Graphene-Based Nanomaterials for Neural Growth and Central Nervous System Regeneration
5. Crucial Aspects of Biocompatibility and Toxicity Evaluation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, C.; Li, Y.; Tjong, S. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syama, S.; Mohanan, P.V. Safety and Biocompatibility of Graphene: A New Generation Nanomaterial for Biomedical Application. Int. J. Biol. Macromol. 2016, 86, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on Graphene and Its Derivatives: Synthesis Methods and Potential Industrial Implementation. J. Taiwan Inst. Chem. Eng. 2019, 98, 163–180. [Google Scholar] [CrossRef]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-Based Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [Green Version]
- Foo, M.E.; Gopinath, S.C.B. Feasibility of Graphene in Biomedical Applications. Biomed. Pharmacother. 2017, 94, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Yoon, H.; Kwon, O.S. Graphene-Based Nanoelectronic Biosensors. J. Ind. Eng. Chem. 2016, 38, 13–22. [Google Scholar] [CrossRef]
- Ghuge, D.; Shirode, A.; Kadam, V. Graphene: A Comprehensive Review. Curr. Drug Targets 2017, 18, 724–733. [Google Scholar] [CrossRef]
- Lu, N.; Wang, L.; Lv, M.; Tang, Z.; Fan, C. Graphene-Based Nanomaterials in Biosystems. Nano Res. 2019, 12, 247–264. [Google Scholar] [CrossRef]
- Tupone, M.G.; d’Angelo, M.; Castelli, V.; Catanesi, M.; Benedetti, E.; Cimini, A. A State-of-the-Art of Functional Scaffolds for 3D Nervous Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 639765. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Xiao, X.; Li, J.; Zhao, Z.; Yu, D.; Li, Q. Self-Assembled Graphene-Based Architectures and Their Applications. Adv. Sci. 2018, 5, 1700626. [Google Scholar] [CrossRef]
- Cong, H.-P.; Chen, J.-F.; Yu, S.-H. Graphene-Based Macroscopic Assemblies and Architectures: An Emerging Material System. Chem. Soc. Rev. 2014, 43, 7295–7325. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.V.; Ménard-Moyon, C.; Verma, S.; Bianco, A. Graphene-Based Nanomaterials for Nanobiotechnology and Biomedical Applications. Nanomedicine 2013, 8, 1669–1688. [Google Scholar] [CrossRef]
- Sagadevan, S.; Shahid, M.M.; Yiqiang, Z.; Oh, W.-C.; Soga, T.; Anita Lett, J.; Alshahateet, S.F.; Fatimah, I.; Waqar, A.; Paiman, S.; et al. Functionalized Graphene-Based Nanocomposites for Smart Optoelectronic Applications. Nanotechnol. Rev. 2021, 10, 605–635. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Zhang, F.; Liang, H.; Feng, X. Nanocomposites and Macroscopic Materials: Assembly of Chemically Modified Graphene Sheets. Chem. Soc. Rev. 2012, 41, 6160. [Google Scholar] [CrossRef]
- Shirvalilou, S.; Khoei, S.; Khoee, S.; Mahdavi, S.R.; Raoufi, N.J.; Motevalian, M.; Karimi, M.Y. Enhancement Radiation-Induced Apoptosis in C6 Glioma Tumor-Bearing Rats via PH-Responsive Magnetic Graphene Oxide Nanocarrier. J. Photochem. Photobiol. B Biol. 2020, 205, 111827. [Google Scholar] [CrossRef]
- Yang, S.; Feng, X.; Ivanovici, S.; Müllen, K. Fabrication of Graphene-Encapsulated Oxide Nanoparticles: Towards High-Performance Anode Materials for Lithium Storage. Angew. Chem. Int. Ed. 2010, 49, 8408–8411. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Wang, Z.; Dong, X.C.; Chen, P.; Lou, X.W. (David) Graphene-Wrapped TiO2 Hollow Structures with Enhanced Lithium Storage Capabilities. Nanoscale 2011, 3, 2158. [Google Scholar] [CrossRef]
- Nayak, J.K.; Parhi, P.; Jha, R. Graphene Oxide Encapsulated Gold Nanoparticle Based Stable Fibre Optic Sucrose Sensor. Sens. Actuators B Chem. 2015, 221, 835–841. [Google Scholar] [CrossRef]
- Cruz, S.; Girão, A.; Gonçalves, G.; Marques, P. Graphene: The Missing Piece for Cancer Diagnosis? Sensors 2016, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.; Kostarelos, K.; Prato, M.; Bianco, A. Biocompatibility and Biodegradability of 2D Materials: Graphene and Beyond. Chem. Commun. 2019, 55, 5540–5546. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Dynamic Microenvironments: The Fourth Dimension. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Solís-Fernández, P.; Bissett, M.; Ago, H. Synthesis, Structure and Applications of Graphene-Based 2D Heterostructures. Chem. Soc. Rev. 2017, 46, 4572–4613. [Google Scholar] [CrossRef] [Green Version]
- Choi, T.Y.; Hwang, B.-U.; Kim, B.-Y.; Trung, T.Q.; Nam, Y.H.; Kim, D.-N.; Eom, K.; Lee, N.-E. Stretchable, Transparent, and Stretch-Unresponsive Capacitive Touch Sensor Array with Selectively Patterned Silver Nanowires/Reduced Graphene Oxide Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 18022–18030. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Zhang, M.; Liu, M.; Wong, I.Y.; Hurt, R.H. Ultrastretchable Graphene-Based Molecular Barriers for Chemical Protection, Detection, and Actuation. ACS Nano 2018, 12, 234–244. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of Graphene and Its Applications: A Review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Bagheri, S. Graphene Supported Heterogeneous Catalysts: An Overview. Int. J. Hydrog. Energy 2015, 40, 948–979. [Google Scholar] [CrossRef]
- Yang, Y.; Asiri, A.M.; Tang, Z.; Du, D.; Lin, Y. Graphene Based Materials for Biomedical Applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- Ioniţă, M.; Vlăsceanu, G.M.; Watzlawek, A.A.; Voicu, S.I.; Burns, J.S.; Iovu, H. Graphene and Functionalized Graphene: Extraordinary Prospects for Nanobiocomposite Materials. Compos. Part B Eng. 2017, 121, 34–57. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-Graphene Oxide for Cellular Imaging and Drug Delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Jiao, G.; Gao, L.; Jin, P.; Li, X. The Preparation and Drug Delivery of a Graphene–Carbon Nanotube–Fe3O4 Nanoparticle Hybrid. J. Mater. Chem. B 2013, 1, 2658. [Google Scholar] [CrossRef]
- Hoseini-Ghahfarokhi, M.; Mirkiani, S.; Mozaffari, N.; Abdolahi Sadatlu, M.A.; Ghasemi, A.; Abbaspour, S.; Akbarian, M.; Farjadain, F.; Karimi, M. Applications of Graphene and Graphene Oxide in Smart Drug/Gene Delivery: Is the World Still Flat? Int. J. Nanomed. 2020, 15, 9469–9496. [Google Scholar] [CrossRef] [PubMed]
- Herranz, F.; Almarza, E.; Rodríguez, I.; Salinas, B.; Rosell, Y.; Desco, M.; Bulte, J.W.; Ruiz-Cabello, J. The Application of Nanoparticles in Gene Therapy and Magnetic Resonance Imaging. Microsc. Res. Tech. 2011, 74, 577–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, M.; Liu, Y.; Chen, T.; Du, F.; Zhao, X.; Xiong, C.; Zhou, Y. Is Graphene a Promising Nano-Material for Promoting Surface Modification of Implants or Scaffold Materials in Bone Tissue Engineering? Tissue Eng. Part B Rev. 2014, 20, 477–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinescu, S.; Ionita, M.; Ignat, S.-R.; Costache, M.; Hermenean, A. Graphene Oxide Enhances Chitosan-Based 3D Scaffold Properties for Bone Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 5077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.-L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G.; et al. Graphene for Controlled and Accelerated Osteogenic Differentiation of Human Mesenchymal Stem Cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef] [Green Version]
- Dias, D.; Vale, A.C.; Cunha, E.P.F.; Paiva, M.; Reis, R.L.; Vaquette, C.; Alves, N.M. 3D -printed Cryomilled Poly(Ε-caprolactone)/Graphene Composite Scaffolds for Bone Tissue Regeneration. J Biomed. Mater. Res. 2021, 109, 961–972. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly(Propylene Fumarate)/Polyethylene Glycol-Modified Graphene Oxide Nanocomposites for Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 17902–17914. [Google Scholar] [CrossRef]
- Farshid, B.; Lalwani, G.; Mohammadi, M.S.; Sankaran, J.S.; Patel, S.; Judex, S.; Simonsen, J.; Sitharaman, B. Two-dimensional Graphene Oxide-reinforced Porous Biodegradable Polymeric Nanocomposites for Bone Tissue Engineering. J. Biomed. Mater. Res. 2019, 107, 1143–1153. [Google Scholar] [CrossRef]
- Diez-Pascual, A. Tissue Engineering Bionanocomposites Based on Poly(Propylene Fumarate). Polymers 2017, 9, 260. [Google Scholar] [CrossRef] [Green Version]
- Lalwani, G.; Henslee, A.M.; Farshid, B.; Lin, L.; Kasper, F.K.; Qin, Y.-X.; Mikos, A.G.; Sitharaman, B. Two-Dimensional Nanostructure-Reinforced Biodegradable Polymeric Nanocomposites for Bone Tissue Engineering. Biomacromolecules 2013, 14, 900–909. [Google Scholar] [CrossRef] [Green Version]
- Ciriza, J.; Saenz del Burgo, L.; Gurruchaga, H.; Borras, F.E.; Franquesa, M.; Orive, G.; Hernández, R.M.; Pedraz, J.L. Graphene Oxide Enhances Alginate Encapsulated Cells Viability and Functionality While Not Affecting the Foreign Body Response. Drug Deliv. 2018, 25, 1147–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, G.; Kim, S.-W.; Park, J.; Park, J.; Kim, S.; Kim, Y.S.; Ahn, Y.; Jung, D.-W.; Williams, D.R.; Lee, J.Y. Anti-Oxidant Activity Reinforced Reduced Graphene Oxide/Alginate Microgels: Mesenchymal Stem Cell Encapsulation and Regeneration of Infarcted Hearts. Biomaterials 2019, 225, 119513. [Google Scholar] [CrossRef]
- Krueger, E.; Chang, A.N.; Brown, D.; Eixenberger, J.; Brown, R.; Rastegar, S.; Yocham, K.M.; Cantley, K.D.; Estrada, D. Graphene Foam as a Three-Dimensional Platform for Myotube Growth. ACS Biomater. Sci. Eng. 2016, 2, 1234–1241. [Google Scholar] [CrossRef]
- Ameri, S.K.; Singh, P.K.; D’Angelo, R.; Stoppel, W.; Black, L.; Sonkusale, S.R. Three Dimensional Graphene Scaffold for Cardiac Tissue Engineering and In-Situ Electrical Recording. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 4201–4203. [Google Scholar]
- Wang, J.; Cui, C.; Nan, H.; Yu, Y.; Xiao, Y.; Poon, E.; Yang, G.; Wang, X.; Wang, C.; Li, L.; et al. Graphene Sheet-Induced Global Maturation of Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells. ACS Appl. Mater. Interfaces 2017, 9, 25929–25940. [Google Scholar] [CrossRef]
- Shin, S.R.; Aghaei-Ghareh-Bolagh, B.; Dang, T.T.; Topkaya, S.N.; Gao, X.; Yang, S.Y.; Jung, S.M.; Oh, J.H.; Dokmeci, M.R.; Tang, X.S.; et al. Cell-Laden Microengineered and Mechanically Tunable Hybrid Hydrogels of Gelatin and Graphene Oxide. Adv. Mater. 2013, 25, 6385–6391. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Kannan, R.; Ramachandran, B.; Moorthy, G.; Suguna, L.; Muthuvijayan, V. Development of Reduced Graphene Oxide (RGO)-Isabgol Nanocomposite Dressings for Enhanced Vascularization and Accelerated Wound Healing in Normal and Diabetic Rats. J. Colloid Interface Sci. 2018, 517, 251–264. [Google Scholar] [CrossRef]
- Zlotorynski, E. Stretching Chromatin Promotes Transcription. Nat. Rev. Mol. Cell Biol. 2016, 17, 610. [Google Scholar] [CrossRef]
- Montanez-Sauri, S.I.; Beebe, D.J.; Sung, K.E. Microscale Screening Systems for 3D Cellular Microenvironments: Platforms, Advances, and Challenges. Cell. Mol. Life Sci. 2015, 72, 237–249. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Shi, H.; Wu, H.; Zhang, Y.; Wang, X.; Wu, D.; An, L.; Yang, S. Prostate Cancer Targeted Multifunctionalized Graphene Oxide for Magnetic Resonance Imaging and Drug Delivery. Carbon 2016, 107, 87–99. [Google Scholar] [CrossRef]
- Younis, M.R.; He, G.; Lin, J.; Huang, P. Recent Advances on Graphene Quantum Dots for Bioimaging Applications. Front. Chem. 2020, 8, 424. [Google Scholar] [CrossRef]
- Bourrier, A.; Shkorbatova, P.; Bonizzato, M.; Rey, E.; Barraud, Q.; Courtine, G.; Othmen, R.; Reita, V.; Bouchiat, V.; Delacour, C. Monolayer Graphene Coating of Intracortical Probes for Long-Lasting Neural Activity Monitoring. Adv. Healthc. Mater. 2019, 8, 1801331. [Google Scholar] [CrossRef]
- Mohd Firdaus, R.; Berrada, N.; Desforges, A.; Mohamed, A.R.; Vigolo, B. From 2D Graphene Nanosheets to 3D Graphene-based Macrostructures. Chem. Asian J. 2020, 15, 2902–2924. [Google Scholar] [CrossRef]
- Catanesi, M.; Panella, G.; Benedetti, E.; Fioravanti, G.; Perrozzi, F.; Ottaviano, L.; Leandro, L.D.; Ardini, M.; Giansanti, F.; d’Angelo, M.; et al. YAP/TAZ Mechano-Transduction as the Underlying Mechanism of Neuronal Differentiation Induced by Reduced Graphene Oxide. Nanomedicine 2018, 13, 3091–3106. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Mo, X.; Wang, H. Reduced Graphene Oxide-Encapsulated Microfiber Patterns Enable Controllable Formation of Neuronal-Like Networks. Adv. Mater. 2020, 32, 2004555. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, H.B.; Kim, Y.; Jeong, J.R.; Lee, M.H.; Kang, K. Neurite Guidance on Laser-Scribed Reduced Graphene Oxide. Nano Lett. 2018, 18, 7421–7427. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Abouei, E.; Hatamie, S.; Ghasemi, E. Accelerated Differentiation of Neural Stem Cells into Neurons on Ginseng-Reduced Graphene Oxide Sheets. Carbon 2014, 66, 395–406. [Google Scholar] [CrossRef]
- Convertino, D.; Fabbri, F.; Mishra, N.; Mainardi, M.; Cappello, V.; Testa, G.; Capsoni, S.; Albertazzi, L.; Luin, S.; Marchetti, L.; et al. Graphene Promotes Axon Elongation through Local Stall of Nerve Growth Factor Signaling Endosomes. Nano Lett. 2020, 20, 3633–3641. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, J.; Sim, S.H.; Sung, M.G.; Kim, K.S.; Hong, B.H.; Hong, S. Enhanced Differentiation of Human Neural Stem Cells into Neurons on Graphene. Adv. Mater. 2011, 23, H263–H267. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Gao, S.; Song, Q.; Huang, R.; Wang, L.; Liu, L.; Dai, J.; Tang, M.; Cheng, G. Three-Dimensional Graphene Foam as a Biocompatible and Conductive Scaffold for Neural Stem Cells. Sci. Rep. 2013, 3, 1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Q.; Zhang, Y.; Chen, X.; Li, H.; Cheng, L.; Zhu, W.; Zhang, Z.; Tang, M.; Liu, W.; Wang, H.; et al. Three-Dimensional Graphene Enhances Neural Stem Cell Proliferation Through Metabolic Regulation. Front. Bioeng. Biotechnol. 2020, 7, 436. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gu, J.; Wu, H.; Zhu, G.; Feng, D.; Li, Y.; Guo, W.; Tian, K.; Gao, G.; Gao, L. Pentazocine Protects SN4741 Cells Against MPP+-Induced Cell Damage via Up-Regulation of the Canonical Wnt/β-Catenin Signaling Pathway. Front. Aging Neurosci. 2017, 9, 196. [Google Scholar] [CrossRef]
- Rawat, S.; Jain, K.G.; Gupta, D.; Raghav, P.K.; Chaudhuri, R.; Pinky; Shakeel, A.; Arora, V.; Sharma, H.; Debnath, D.; et al. Graphene Nanofiber Composites for Enhanced Neuronal Differentiation of Human Mesenchymal Stem Cells. Nanomedicine 2021, 16, 1963–1982. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, N.; Thakur, V.; Chattopadhyay, M.; Joddar, B. The Efficacy of Graphene Foams for Culturing Mesenchymal Stem Cells and Their Differentiation into Dopaminergic Neurons. Stem Cells Int. 2018, 2018, 3410168. [Google Scholar] [CrossRef] [PubMed]
- Magaz, A.; Li, X.; Gough, J.E.; Blaker, J.J. Graphene Oxide and Electroactive Reduced Graphene Oxide-Based Composite Fibrous Scaffolds for Engineering Excitable Nerve Tissue. Mater. Sci. Eng. C 2021, 119, 111632. [Google Scholar] [CrossRef]
- Rodriguez-Losada, N.; Wendelbob, R.; Ocaña, M.C.; Casares, A.D.; Guzman de Villoría, R.; Aguirre Gomez, J.A.; Arraez, M.A.; Gonzalez-Alegre, P.; Medina, M.A.; Arenas, E.; et al. Graphene Oxide and Reduced Derivatives, as Powder or Film Scaffolds, Differentially Promote Dopaminergic Neuron Differentiation and Survival. Front. Neurosci. 2020, 14, 570409. [Google Scholar] [CrossRef]
- Smidt, M.P.; Asbreuk, C.H.J.; Cox, J.J.; Chen, H.; Johnson, R.L.; Burbach, J.P.H. A Second Independent Pathway for Development of Mesencephalic Dopaminergic Neurons Requires Lmx1b. Nat. Neurosci. 2000, 3, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Smidt, M.P.; Smits, S.M.; Bouwmeester, H.; Hamers, F.P.T.; van der Linden, A.J.A.; Hellemons, A.J.C.G.M.; Graw, J.; Burbach, J.P.H. Early Developmental Failure of Substantia Nigra Dopamine Neurons in Mice Lacking the Homeodomain Gene Pitx3. Development 2004, 131, 1145–1155. [Google Scholar] [CrossRef] [Green Version]
- Arenas, E.; Denham, M.; Villaescusa, J.C. How to Make a Midbrain Dopaminergic Neuron. Development 2015, 142, 1918–1936. [Google Scholar] [CrossRef] [Green Version]
- Durso, M.; Borrachero-Conejo, A.I.; Bettini, C.; Treossi, E.; Scidà, A.; Saracino, E.; Gazzano, M.; Christian, M.; Morandi, V.; Tuci, G.; et al. Biomimetic Graphene for Enhanced Interaction with the External Membrane of Astrocytes. J. Mater. Chem. B 2018, 6, 5335–5342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradhan, K.; Das, G.; Khan, J.; Gupta, V.; Barman, S.; Adak, A.; Ghosh, S. Neuro-Regenerative Choline-Functionalized Injectable Graphene Oxide Hydrogel Repairs Focal Brain Injury. ACS Chem. Neurosci. 2019, 10, 1535–1543. [Google Scholar] [CrossRef]
- Simpson, L.A.; Eng, J.J.; Hsieh, J.T.C.; Wolfe, D.L.; The Spinal Cord Injury Rehabilitation Evidence (SCIRE) Research Team. The Health and Life Priorities of Individuals with Spinal Cord Injury: A Systematic Review. J. Neurotrauma 2012, 29, 1548–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhavan, O.; Ghaderi, E.; Shirazian, S.A.; Rahighi, R. Rolled Graphene Oxide Foams as Three-Dimensional Scaffolds for Growth of Neural Fibers Using Electrical Stimulation of Stem Cells. Carbon 2016, 97, 71–77. [Google Scholar] [CrossRef]
- Domínguez-Bajo, A.; González-Mayorga, A.; Guerrero, C.R.; Palomares, F.J.; García, R.; López-Dolado, E.; Serrano, M.C. Myelinated Axons and Functional Blood Vessels Populate Mechanically Compliant RGO Foams in Chronic Cervical Hemisected Rats. Biomaterials 2019, 192, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Qi, Z.; Li, Q.; Ma, Y.; Fu, C.; Zheng, S.; Kong, W.; Liu, Q.; Yang, X. Graphene Oxide-PLGA Hybrid Nanofibres for the Local Delivery of IGF-1 and BDNF in Spinal Cord Repair. Artif. Cells Nanomed. Biotechnol. 2019, 47, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Song, J.; Zhao, X.; Chen, W.; Ouyang, Y.; Yuan, W.; Fan, C. 3D Fabrication with Integration Molding of a Graphene Oxide/Polycaprolactone Nanoscaffold for Neurite Regeneration and Angiogenesis. Adv. Sci. 2018, 5, 1700499. [Google Scholar] [CrossRef]
- Fang, X.; Guo, H.; Zhang, W.; Fang, H.; Li, Q.; Bai, S.; Zhang, P. Reduced Graphene Oxide–GelMA–PCL Hybrid Nanofibers for Peripheral Nerve Regeneration. J. Mater. Chem. B 2020, 8, 10593–10601. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Y.; Chen, L.; Zhu, T.; Ye, K.; Jia, C.; Wang, H.; Zhu, M.; Fan, C.; Mo, X. In Vitro and In Vivo Studies of Electroactive Reduced Graphene Oxide-Modified Nanofiber Scaffolds for Peripheral Nerve Regeneration. Acta Biomater. 2019, 84, 98–113. [Google Scholar] [CrossRef]
- Domínguez-Bajo, A.; González-Mayorga, A.; López-Dolado, E.; Munuera, C.; García-Hernández, M.; Serrano, M.C. Graphene Oxide Microfibers Promote Regenerative Responses after Chronic Implantation in the Cervical Injured Spinal Cord. ACS Biomater. Sci. Eng. 2020, 6, 2401–2414. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.C.P.; Soares, E.S.; de Jesus, M.B.; Ceragioli, H.J.; Ferreira, M.S.; Catharino, R.R.; da Cruz-Höfling, M.A. Reduced Graphene Oxide Induces Transient Blood–Brain Barrier Opening: An in Vivo Study. J. Nanobiotechnol. 2015, 13, 78. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Shen, H.; Mao, J.; Zhang, L.; Jiang, Z.; Sun, T.; Lan, Q.; Zhang, Z. Transferrin Modified Graphene Oxide for Glioma-Targeted Drug Delivery: In Vitro and in Vivo Evaluations. ACS Appl. Mater. Interfaces 2013, 5, 6909–6914. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yoo, J.M.; Hwang, H.; Lee, J.; Lee, S.H.; Yun, S.P.; Park, M.J.; Lee, M.; Choi, S.; Kwon, S.H.; et al. Graphene Quantum Dots Prevent α-Synucleinopathy in Parkinson’s Disease. Nat. Nanotech. 2018, 13, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.C.P.; Soares, E.S.; de Jesus, M.B.; Ceragioli, H.J.; Batista, Â.G.; Nyúl-Tóth, Á.; Molnár, J.; Wilhelm, I.; Maróstica, M.R.; Krizbai, I.; et al. PEGylation of Reduced Graphene Oxide Induces Toxicity in Cells of the Blood–Brain Barrier: An In Vitro and In Vivo Study. Mol. Pharm. 2016, 13, 3913–3924. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Star, A.; Vidal, S. Sweet Carbon Nanostructures: Carbohydrate Conjugates with Carbon Nanotubes and Graphene and Their Applications. Chem. Soc. Rev. 2013, 42, 4532–4542. [Google Scholar] [CrossRef]
- Moschetta, M.; Chiacchiaretta, M.; Cesca, F.; Roy, I.; Athanassiou, A.; Benfenati, F.; Papadopoulou, E.L.; Bramini, M. Graphene Nanoplatelets Render Poly(3-Hydroxybutyrate) a Suitable Scaffold to Promote Neuronal Network Development. Front. Neurosci. 2021, 15, 731198. [Google Scholar] [CrossRef]
- Moschetta, M.; Lee, J.; Rodrigues, J.; Podestà, A.; Varvicchio, O.; Son, J.; Lee, Y.; Kim, K.; Lee, G.; Benfenati, F.; et al. Hydrogenated Graphene Improves Neuronal Network Maturation and Excitatory Transmission. Adv. Biol. 2021, 5, 2000177. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Sun, M.; Li, Y.; Guo, Z.; Li, H. Reduced Graphene Oxide-Coated Electrospun Fibre: Effect of Orientation, Coverage and Electrical Stimulation on Schwann Cells Behavior. J. Mater. Chem. B 2021, 9, 2656–2665. [Google Scholar] [CrossRef]
- Yang, B.; Wang, P.-B.; Mu, N.; Ma, K.; Wang, S.; Yang, C.-Y.; Huang, Z.-B.; Lai, Y.; Feng, H.; Yin, G.-F.; et al. Graphene Oxide-Composited Chitosan Scaffold Contributes to Functional Recovery of Injured Spinal Cord in Rats. Neural Regen. Res. 2021, 16, 1829. [Google Scholar] [CrossRef]
- Lee, T.-H.; Yen, C.-T.; Hsu, S. Preparation of Polyurethane-Graphene Nanocomposite and Evaluation of Neurovascular Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 597–609. [Google Scholar] [CrossRef]
- Agarwal, G.; Kumar, N.; Srivastava, A. Highly Elastic, Electroconductive, Immunomodulatory Graphene Crosslinked Collagen Cryogel for Spinal Cord Regeneration. Mater. Sci. Eng. C 2021, 118, 111518. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chang, J.-J.; Yung, M.-C.; Huang, W.-C.; Chen, S.-Y. Spontaneously Micropatterned Silk/Gelatin Scaffolds with Topographical, Biological, and Electrical Stimuli for Neuronal Regulation. ACS Biomater. Sci. Eng. 2020, 6, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Y.; Yang, Y.; Wang, A.; Huang, C.; Zhao, Z.; Li, P.; Liu, M.; Fan, Y. Aligned Graphene/Silk Fibroin Conductive Fibrous Scaffolds for Guiding Neurite Outgrowth in Rat Spinal Cord Neurons. J. Biomed. Mater. Res. 2021, 109, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, C.; Jia, Z.; Zhao, Z.; Xiao, X.; Wang, A.; Li, P.; Guan, X.; Zhou, G.; Fan, Y. Promotion of Neuronal Guidance Growth by Aminated Graphene Oxide via Netrin-1/Deleted in Colorectal Cancer Signaling. ACS Chem. Neurosci. 2020, 11, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Smart Electrospun Nanofibers Containing PCL/Gelatin/Graphene Oxide for Application in Nerve Tissue Engineering. Mater. Sci. Eng. C 2019, 103, 109768. [Google Scholar] [CrossRef] [PubMed]
- Qin, E.C.; Kandel, M.E.; Liamas, E.; Shah, T.B.; Kim, C.; Kaufman, C.D.; Zhang, Z.J.; Popescu, G.; Gillette, M.U.; Leckband, D.E.; et al. Graphene Oxide Substrates with N-Cadherin Stimulates Neuronal Growth and Intracellular Transport. Acta Biomater. 2019, 90, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Agrawal, A.; Murali, K.; Varshney, R.; Beniwal, S.; Manhas, S.; Roy, P.; Lahiri, D. Differential Neural Cell Adhesion and Neurite Outgrowth on Carbon Nanotube and Graphene Reinforced Polymeric Scaffolds. Mater. Sci. Eng. C 2019, 97, 539–551. [Google Scholar] [CrossRef]
- Liu, X.; Miller, A.L.; Park, S.; Waletzki, B.E.; Zhou, Z.; Terzic, A.; Lu, L. Functionalized Carbon Nanotube and Graphene Oxide Embedded Electrically Conductive Hydrogel Synergistically Stimulates Nerve Cell Differentiation. ACS Appl. Mater. Interfaces 2017, 9, 14677–14690. [Google Scholar] [CrossRef]
- Liu, X.; Miller II, A.L.; Park, S.; Waletzki, B.E.; Terzic, A.; Yaszemski, M.J.; Lu, L. Covalent Crosslinking of Graphene Oxide and Carbon Nanotube into Hydrogels Enhances Nerve Cell Responses. J. Mater. Chem. B 2016, 4, 6930–6941. [Google Scholar] [CrossRef]
- Feng, Z.-Q.; Yan, K.; Shi, C.; Xu, X.; Wang, T.; Li, R.; Dong, W.; Zheng, J. Neurogenic Differentiation of Adipose Derived Stem Cells on Graphene-Based Mat. Mater. Sci. Eng. C 2018, 90, 685–692. [Google Scholar] [CrossRef]
- Martín, C.; Merino, S.; González-Domínguez, J.M.; Rauti, R.; Ballerini, L.; Prato, M.; Vázquez, E. Graphene Improves the Biocompatibility of Polyacrylamide Hydrogels: 3D Polymeric Scaffolds for Neuronal Growth. Sci. Rep. 2017, 7, 10942. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Guiney, L.M.; Chang, C.H.; Mansukhani, N.D.; Ji, Z.; Wang, X.; Liao, Y.-P.; Jiang, W.; Sun, B.; Hersam, M.C.; et al. Surface Oxidation of Graphene Oxide Determines Membrane Damage, Lipid Peroxidation, and Cytotoxicity in Macrophages in a Pulmonary Toxicity Model. ACS Nano 2018, 12, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Lammel, T.; Boisseaux, P.; Fernández-Cruz, M.-L.; Navas, J.M. Internalization and Cytotoxicity of Graphene Oxide and Carboxyl Graphene Nanoplatelets in the Human Hepatocellular Carcinoma Cell Line Hep G2. Part Fibre Toxicol. 2013, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Pelin, M.; Fusco, L.; Martín, C.; Sosa, S.; Frontiñán-Rubio, J.; González-Domínguez, J.M.; Durán-Prado, M.; Vázquez, E.; Prato, M.; Tubaro, A. Graphene and Graphene Oxide Induce ROS Production in Human HaCaT Skin Keratinocytes: The Role of Xanthine Oxidase and NADH Dehydrogenase. Nanoscale 2018, 10, 11820–11830. [Google Scholar] [CrossRef] [Green Version]
- Kurapati, R.; Martìn, C.; Palermo, V.; Nishina, Y.; Bianco, A. Biodegradation of Graphene Materials Catalyzed by Human Eosinophil Peroxidase. Faraday Discuss. 2021, 227, 189–203. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Shirazian, S.A. Near Infrared Laser Stimulation of Human Neural Stem Cells into Neurons on Graphene Nanomesh Semiconductors. Colloids Surf. B Biointerfaces 2015, 126, 313–321. [Google Scholar] [CrossRef]
- Akhavan, O.; Abdolahad, M.; Esfandiar, A.; Mohatashamifar, M. Photodegradation of Graphene Oxide Sheets by TiO2 Nanoparticles after a Photocatalytic Reduction. J. Phys. Chem. C 2010, 114, 12955–12959. [Google Scholar] [CrossRef]
- Machnicki, C.E.; Fu, F.; Jing, L.; Chen, P.-Y.; Wong, I.Y. Mechanochemical Engineering of 2D Materials for Multiscale Biointerfaces. J. Mater. Chem. B 2019, 7, 6293–6309. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, X.; Sun, J.; Zhou, Q. Specific Nanotoxicity of Graphene Oxide during Zebrafish Embryogenesis. Nanotoxicology 2015, 10, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of Graphene-Family Nanoparticles: A General Review of the Origins and Mechanisms. Part Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Graphene-Based Scaffold | Biomedical Applications | Main Results | References |
|---|---|---|---|
| rGO encapsulated on poly (l-lactic acid-co-caprolactone) microfibers (PLCL) | Development of 3D neural networks | Neurite outgrowth and formation of orientated neuronal-like networks | [58] |
| Laser-Scribed rGO | Generation of micropatterned in vitro neuronal networks | Adhesion and survival of rat primary neurons and, at the same time, guide the subsequent elongation of neurites | [59] |
| Ginseng-rGO sheets | Neural stem cell (NSC) differentiation | Accelerated differentiation of neural stem cells into neurons | [60] |
| 3D-graphene foam | NSC proliferation and cell fate decision | Enhancement of neural stem cell proliferation through metabolic regulation | [64] |
| electrospun polycaprolactone (PCL) and graphene (G) nanocomposite | MSCs differentiation | Enhancement of differentiation of MSCs into dopaminergic neurons | [66] |
| Silk/GO micro/nano-fibrous scaffold | Nerve regeneration | Enhancement of metabolic activity, Neuronoma NG108-15 cells proliferation and neurite outgrowth | [68] |
| GO, full reduced (FRGO), and partially reduced (PRGO) powder and film scaffold | Neuron differentiation and survival | Promotion of DA differentiation and prevention of DA cell loss | [69] |
| Choline-Functionalized Injectable GO Hydrogel | Neural regeneration and brain injury repair | Promotion of neurite outgrowth, stabilization of microtubule networks, and enhancement of neural markers expression | [74] |
| 3D porous rGO foams scaffold | Neural repair | Ingrowth of myelinated vGlut2+ axons within rGO scaffolds | [77] |
| GO-PLGA hybrid nanofibres | Spinal cord repair | Enhancement of neuronal proliferation and differentiation in vitro, and NSCs protection from oxidative stress | [78] |
| GO/Polycaprolactone nanoscaffold | Neurite regeneration | Promotion of functional and morphological recovery in peripheral nerve regeneration | [79] |
| rGO-GelMA-PCL hybrid nanofibers | Peripheral nerve regeneration | Promotion of both sensory/motor nerve regeneration and functional recovery in rats | [80] |
| rGO-coated ApF/PLCL (AP/RGO) scaffold | Peripheral nerve regeneration | Enhancement of SC migration, proliferation, and myelination in vitro and promotion of nerve regeneration in vivo | [81] |
| poly(3-hydroxybutyrate) [P(3HB)]/graphene nanoplateletes composite | Neuronal network development | Promotion of neuronal growth and maturation | [88] |
| Hydrogenated Graphene | Neuronal regeneration and electrical sensing/recording. | Promotion of neuronal adhesion and network maturation and modulation of neuronal activity | [89] |
| rGO-coated polycaprolactone fibrous scaffold | Nerve regeneration | Higher level of proliferation and nerve growth factor (NGF) expression of Schwann cells | [90] |
| Chitosan-graphene oxide scaffold | Nerve regeneration | Recovery of neurological function after spinal cord injury | [91] |
| Silk/Gelatin scaffold | Nerve regeneration | Increase in neuronal adhesion, differentiation, and neurite elongation | [94] |
| Polyurethane-Graphene Nanocomposite | Neural tissue engineering | Increase in neurovascular regeneration and peripheral nerve regeneration | [92] |
| Graphene collagen cryogel scaffold | Neural tissue regeneration | Neuronal differentiation; immune-modulatory secretion; cellular growth and migration on organotypic culture of spinal cord | [93] |
| Graphene/silk fibroin scaffold | Neural tissue engineering | Neurite outgrowth | [95] |
| Aminated graphene oxide (NH2-GO) scaffold | Nervous tissue regeneration | Induction of neurite elongation and increase in branches in cortical neurons | [96] |
| Electrospun PCL/gelatin/graphene nanofibrous mats | Nerve tissue engineering | Increase in PC12 cells attachment and proliferation | [97] |
| N-cadherin-graphene oxide-based scaffold | Neuron development and regeneration | Stimulation of neuronal growth and intracellular transport | [98] |
| Graphene nanoplatelets (GNPs) and multiwalled carbon nanotubes (MWCNTs) and chitosan scaffold | Neural cell regeneration | Differential neural cell adhesion and neurite outgrowth | [99] |
| 3D-Printed PCL/rGO Conductive Scaffold | Neural tissue engineering | Neural differentiation | [39] |
| Collagen-coated 3D graphene foam (GF) | Neural tissue engineering | Differentiation into dopaminergic neurons from MSC | [67] |
| rGOaCNTpega-OPF-MTAC composite hydrogel | Nerve regeneration | Enhancement proliferation and spreading of PC12 cells; stimulation of neurite development | [100] |
| GOa-CNTpega-oligo(polyethylene glycol fumarate) (OPF) hydrogel | Neural tissue engineering | Increase in electrical conductivity; stimulation of neurite development | [101] |
| GO and rGO mat | Neural tissue engineering | Neurogenic differentiation | [102] |
| Graphene-Polyacrylamide Hydrogel | Tissue engineering | Development of synaptic activity | [103] |
| Notes | References | |
|---|---|---|
| Advantages | ||
| Biocompatibility of some GBMs | Since they interact with cells, tissue and organs, harmful effects should be avoided. | [1,30,63,82] |
| Easy functionalization | GBMs can be adapted using covalent or no-covalent modifications and assembled with organic or inorganic molecules | [14,31] |
| Ability to pass barriers | Graphene nanoparticles can improve the penetration of drugs through BBB | [18,32] |
| Malleability | Materials can fold in different kinds of shapes and topography | [111] |
| Application in tissue regeneration | Two-dimensional and three-dimensional structures are suitable for cells adhesion, growth and differentiation, supporting tissue repair | [36,48,49,62] |
| Limitations | ||
| Toxicity of some nanomaterials | Chemical features, functionalization and doses could influence the safety of these compounds. | [104,112] |
| Biodegradation | The clearance and elimination from the body represent another concern related to biocompatibility and safety, especially for long-term exposure. | [108,109,110] |
| Route of administration | These compounds exert different degrees of toxicological effects depending on the routes of administration | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tupone, M.G.; Panella, G.; d’Angelo, M.; Castelli, V.; Caioni, G.; Catanesi, M.; Benedetti, E.; Cimini, A. An Update on Graphene-Based Nanomaterials for Neural Growth and Central Nervous System Regeneration. Int. J. Mol. Sci. 2021, 22, 13047. https://doi.org/10.3390/ijms222313047
Tupone MG, Panella G, d’Angelo M, Castelli V, Caioni G, Catanesi M, Benedetti E, Cimini A. An Update on Graphene-Based Nanomaterials for Neural Growth and Central Nervous System Regeneration. International Journal of Molecular Sciences. 2021; 22(23):13047. https://doi.org/10.3390/ijms222313047
Chicago/Turabian StyleTupone, Maria Grazia, Gloria Panella, Michele d’Angelo, Vanessa Castelli, Giulia Caioni, Mariano Catanesi, Elisabetta Benedetti, and Annamaria Cimini. 2021. "An Update on Graphene-Based Nanomaterials for Neural Growth and Central Nervous System Regeneration" International Journal of Molecular Sciences 22, no. 23: 13047. https://doi.org/10.3390/ijms222313047
APA StyleTupone, M. G., Panella, G., d’Angelo, M., Castelli, V., Caioni, G., Catanesi, M., Benedetti, E., & Cimini, A. (2021). An Update on Graphene-Based Nanomaterials for Neural Growth and Central Nervous System Regeneration. International Journal of Molecular Sciences, 22(23), 13047. https://doi.org/10.3390/ijms222313047









