Long-Term Effects of Neural Precursor Cell Transplantation on Secondary Injury Processes and Functional Recovery after Severe Cervical Contusion-Compression Spinal Cord Injury
Abstract
:1. Introduction
2. Results
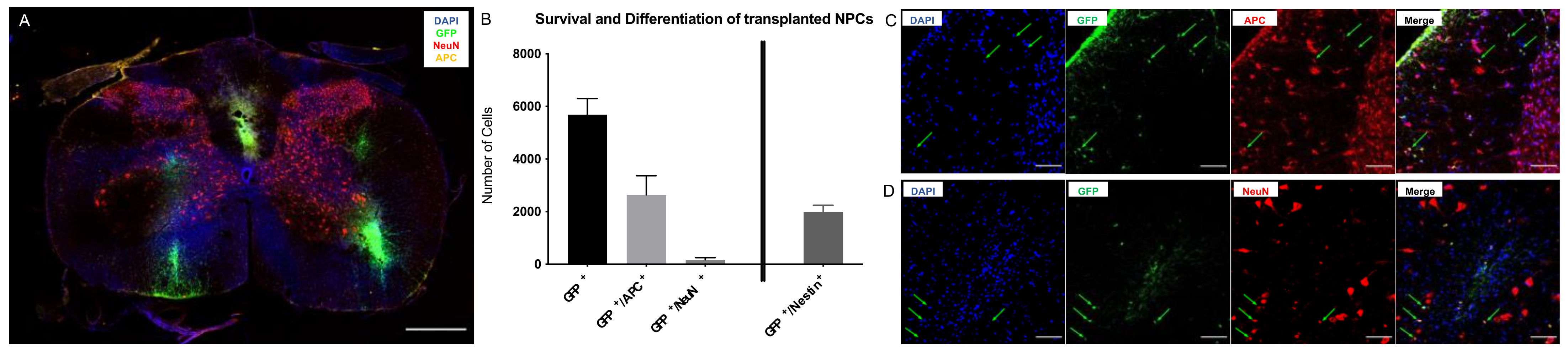
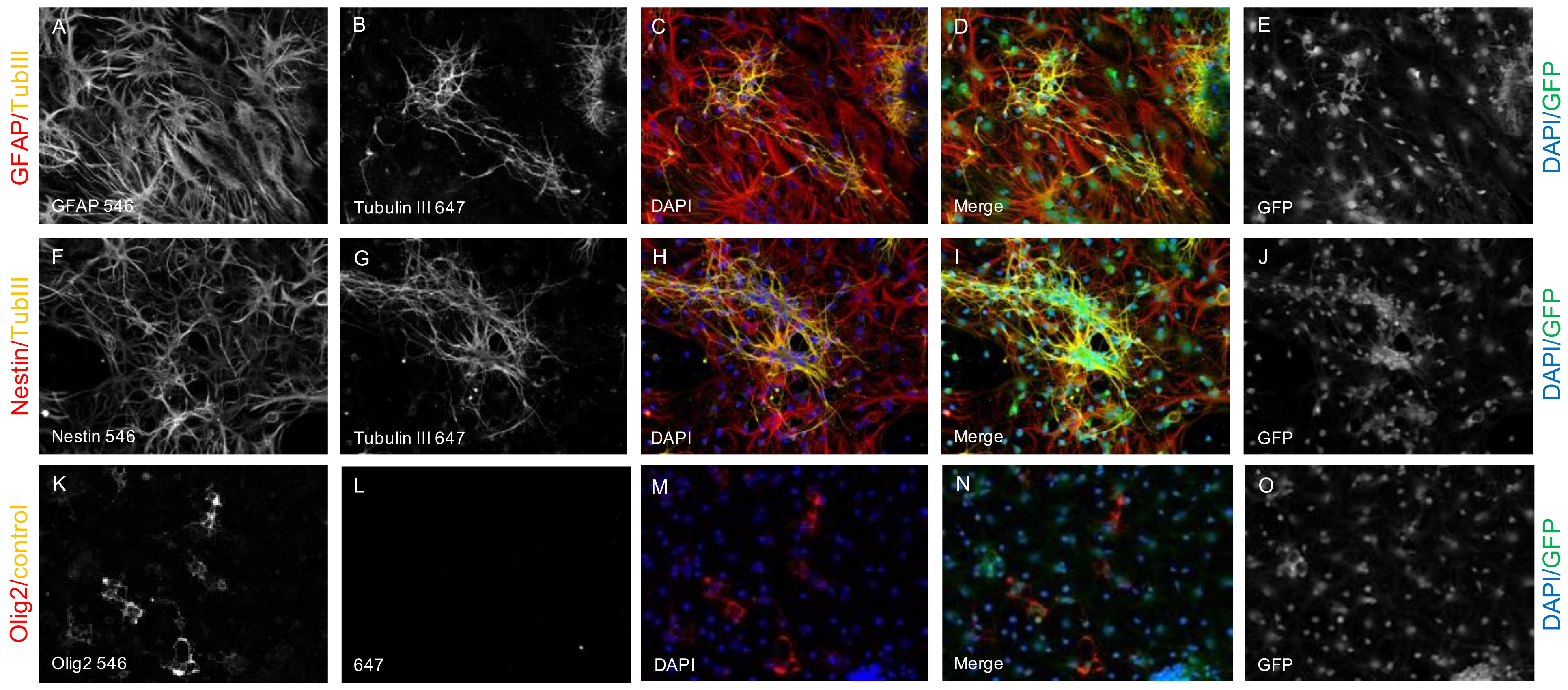
2.1. Survival and Differentiation of NPCs
2.2. Spared Motor Neurons and Myelination
2.3. Preservation or Regeneration of Descending and Ascending Spinal Tracts
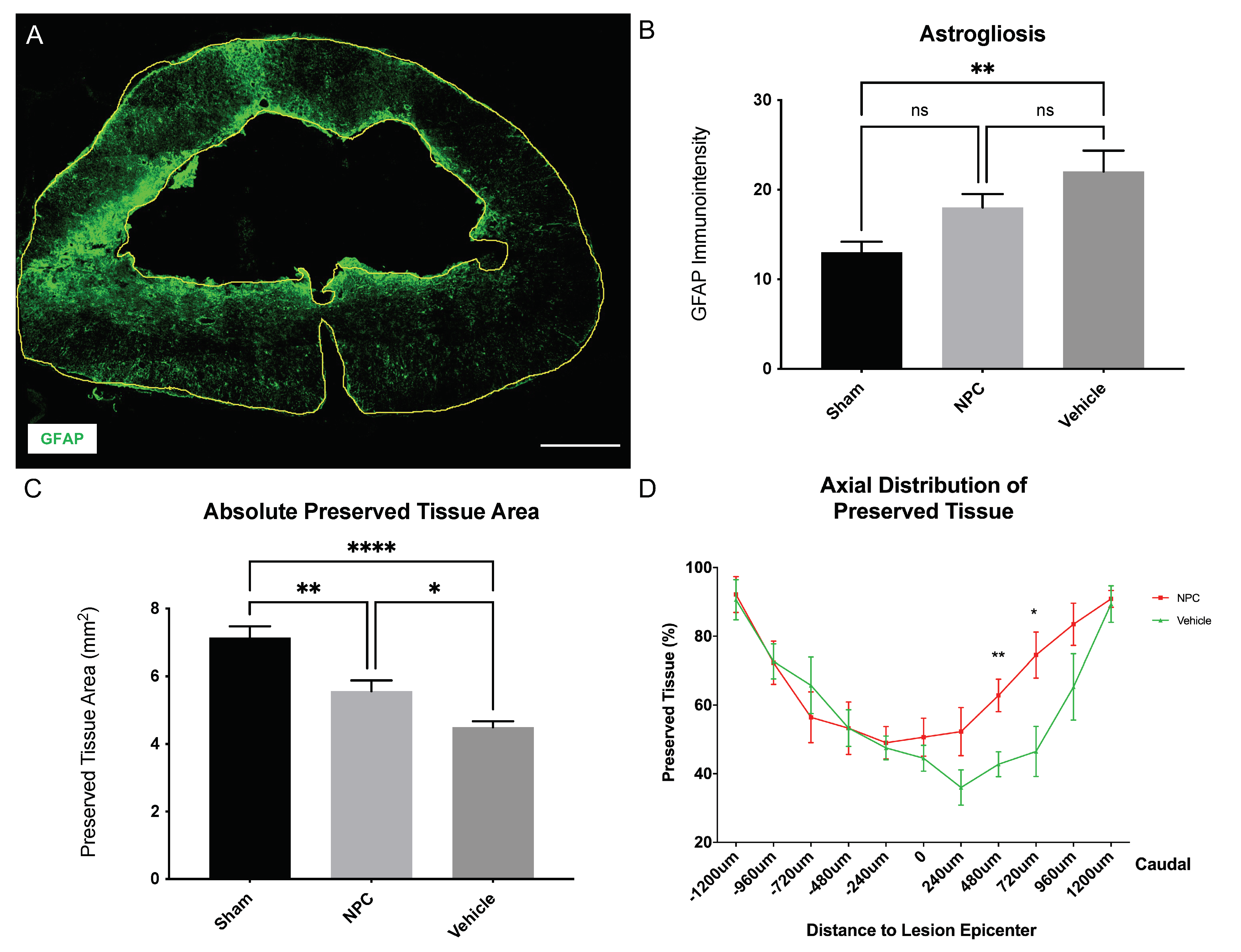
2.4. Astrogliosis and Tissue Preservation
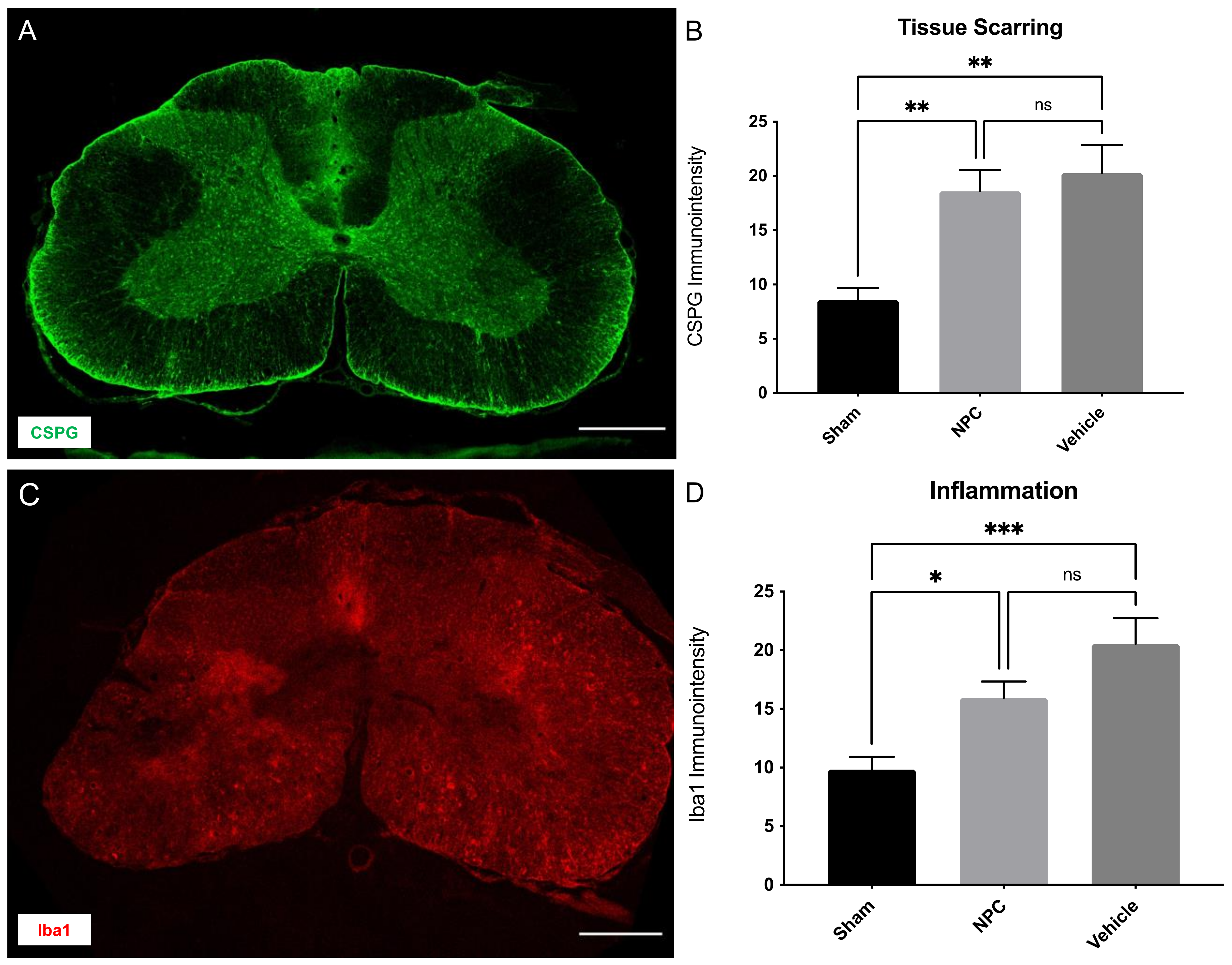
2.5. Tissue Scarring and Inflammation
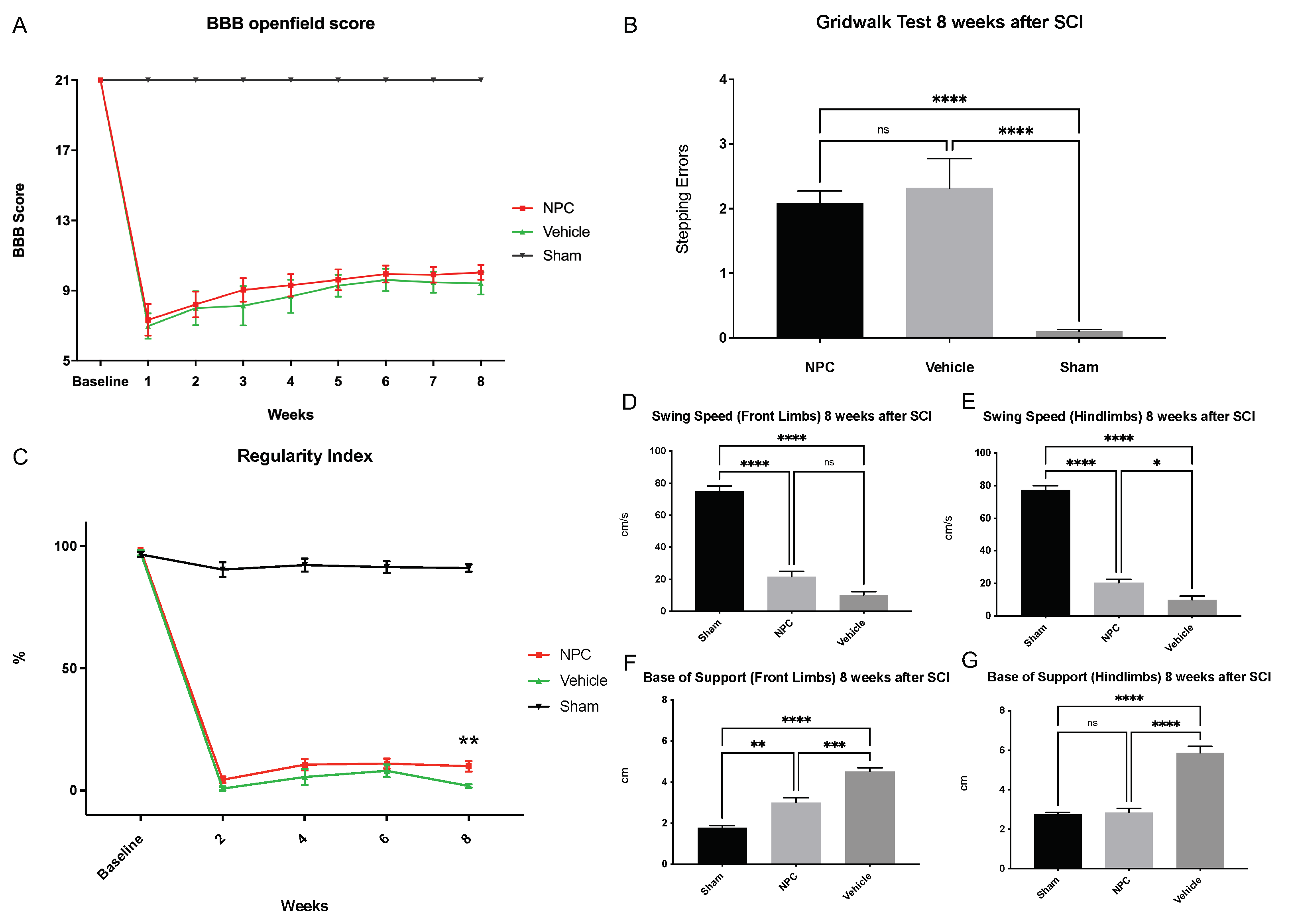
2.6. Functional Recovery
3. Discussion
4. Materials and Methods
4.1. Isolation and Cultivation of NPCs
4.2. Animals, Experimental Groups and Study Design
4.3. Spinal Cord Injury and NPC-Transplantation
4.4. Behavioral Assessment
4.5. Retrograde Fiber Labeling and Manganese-Enhanced MR-Imaging
4.6. Animal Perfusion and Tissue Processing
4.7. Immunofluorescence Staining
4.8. Imaging Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| APC | Adenomatous polyposis coli |
| BBB | Basso–Beattie–Bresnahan locomotor rating scale |
| bFGF | Basic fibroblast growth factor |
| BOS | Base of support |
| ChaT | Choline acetyltransferase |
| CSPG | Chondroitin sulfate proteoglycan |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| EGF | Epidermal growth factor |
| FG | FluoroGold |
| GF | Growth factor |
| GFAP | Glial fibrillary acidic protein |
| Iba1 | Ionized calcium binding adaptor molecule 1 |
| MBP | Myelin basic protein |
| MEMRI | Manganese-enhanced magnetic resonance imaging |
| MRI | Magnetic resonance imaging |
| NeuN | Neuronal nuclei |
| NPC | Neuronal precursor cell |
| Olig2 | Oligodendrocyte transcription factor |
| PBS | Phosphate buffered saline |
| PDGF-AA | Platelet-derived growth factor AA |
| RI | Regularity index |
| ROI | Region of interest |
| SCI | Spinal cord injury |
| SEM | Standard error of the mean |
| SNR | Signal-to-noise ratio |
| SS | Swing speed |
| TubIII | βIII-Tubulin |
References
- Furlan, J.C.; Sakakibara, B.M.; Miller, W.C.; Krassioukov, A.V. Global incidence and prevalence of traumatic spinal cord injury. Can. J. Neurol. Sci. 2013, 40, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, L.H.; Fehlings, M.G. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine 2001, 26, S2–S12. [Google Scholar] [CrossRef]
- Furlan, J.C.; Fehlings, M.G. The impact of age on mortality, impairment, and disability among adults with acute traumatic spinal cord injury. J. Neurotrauma 2009, 26, 1707–1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, K.D. Targeting recovery: Priorities of the spinal cord-injured population. J. Neurotrauma 2004, 21, 1371–1383. [Google Scholar] [CrossRef]
- Anderson, K.D.; Sharp, K.G.; Steward, O. Bilateral cervical contusion spinal cord injury in rats. Exp. Neurol. 2009, 220, 9–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tator, C.H. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995, 5, 407–413. [Google Scholar] [CrossRef]
- Wu, J.; Pajoohesh-Ganji, A.; Stoica, B.A.; Dinizo, M.; Guanciale, K.; Faden, A.I. Delayed expression of cell cycle proteins contributes to astroglial scar formation and chronic inflammation after rat spinal cord contusion. J. Neuroinflamm. 2012, 9, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.; Wang, Y.; Graham, L.; McHale, K.; Gao, M.; Wu, D.; Brock, J.; Blesch, A.; Rosenzweig, E.S.; Havton, L.A.; et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012, 150, 1264–1273. [Google Scholar] [CrossRef] [Green Version]
- Meletis, K.; Barnabé-Heider, F.; Carlén, M.; Evergren, E.; Tomilin, N.; Shupliakov, O.; Frisén, J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008, 6, e182. [Google Scholar] [CrossRef]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef]
- Tetzlaff, W.; Okon, E.B.; Karimi-abdolrezaee, S.; Hill, C.E.; Sparling, J.S.; Plemel, J.R.; Plunet, W.T.; Tsai, E.C.; Baptiste, D.; Smithson, L.J.; et al. A systematic review of cellular transplantation therapies for spinal cord injury. J. Neurotrauma 2011, 28, 1611–1682. [Google Scholar] [CrossRef]
- Quertainmont, R.; Cantinieaux, D.; Botman, O.; Sid, S.; Schoenen, J.; Franzen, R. Mesenchymal stem cell graft improves recovery after spinal cord injury in adult rats through neurotrophic and pro-angiogenic actions. PLoS ONE 2012, 7, e39500. [Google Scholar] [CrossRef]
- Nakajima, H.; Uchida, K.; Guerrero, A.R.; Watanabe, S.; Sugita, D.; Takeura, N.; Yoshida, A.; Long, G.; Wright, K.T.; Johnson, W.E.B.; et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J. Neurotrauma 2012, 29, 1614–1625. [Google Scholar] [CrossRef] [Green Version]
- Karimi-Abdolrezaee, S.; Eftekharpour, E.; Wang, J.; Morshead, C.M.; Fehlings, M.G. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J. Neurosci. 2006, 26, 3377–3389. [Google Scholar] [CrossRef]
- Hawryluk, G.W.J.; Spano, S.; Chew, D.; Wang, S.; Erwin, M.; Chamankhah, M.; Forgione, N.; Fehlings, M.G. An examination of the mechanisms by which neural precursors augment recovery following spinal cord injury: A key role for remyelination. Cell Transpl. 2014, 23, 365–380. [Google Scholar] [CrossRef] [Green Version]
- Sankavaram, S.R.; Hakim, R.; Covacu, R.; Frostell, A.; Neumann, S.; Svensson, M.; Brundin, L. Adult Neural Progenitor Cells Transplanted into Spinal Cord Injury Differentiate into Oligodendrocytes, Enhance Myelination, and Contribute to Recovery. Stem Cell Rep. 2019, 12, 950–966. [Google Scholar] [CrossRef] [Green Version]
- Yousefifard, M.; Rahimi-Movaghar, V.; Nasirinezhad, F.; Baikpour, M.; Safari, S.; Saadat, S.; Moghadas Jafari, A.; Asady, H.; Razavi Tousi, S.M.T.T.; Hosseini, M. Neural stem/progenitor cell transplantation for spinal cord injury treatment; A systematic review and meta-analysis. Neuroscience 2016, 322, 377–397. [Google Scholar] [CrossRef] [Green Version]
- Curtis, E.; Martin, J.R.; Gabel, B.; Sidhu, N.; Rzesiewicz, T.K.; Mandeville, R.; Van Gorp, S.; Leerink, M.; Tadokoro, T.; Marsala, S.; et al. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell 2018, 22, 941–950.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvestro, S.; Bramanti, P.; Trubiani, O.; Mazzon, E. Stem cells therapy for spinal cord injury: An overview of clinical trials. Int. J. Mol. Sci. 2020, 21, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilcox, J.T.; Satkunendrarajah, K.; Zuccato, J.A.; Nassiri, F.; Fehlings, M.G. Neural precursor cell transplantation enhances functional recovery and reduces astrogliosis in bilateral compressive/contusive cervical spinal cord injury. Stem Cells Transl. Med. 2014, 3, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.K.; Soril, L.J.J.J.; Bacon, M.; Beattie, M.S.; Blesch, A.; Bresnahan, J.C.; Bunge, M.B.; Dunlop, S.A.; Fehlings, M.G.; Ferguson, A.R.; et al. Demonstrating efficacy in preclinical studies of cellular therapies for spinal cord injury—How much is enough? Exp. Neurol. 2013, 248, 30–44. [Google Scholar] [CrossRef]
- Kim, B.G.; Hwang, D.H.; Lee, S.I.; Kim, E.J.; Kim, S.U. Stem cell-based cell therapy for spinal cord injury. Cell Transpl. 2007, 16, 355–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siebert, J.R.; Osterhout, D.J. The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J. Neurochem. 2011, 119, 176–188. [Google Scholar] [CrossRef]
- Barnabé-Heider, F.; Frisén, J. Stem cells for spinal cord repair. Cell Stem Cell 2008, 3, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.K.; Choi, K.H.; Yoo, J.Y.; Kim, D.Y.; Kim, S.J.; Jeon, S.R. A Phase III Clinical Trial Showing Limited Efficacy of Autologous Mesenchymal Stem Cell Therapy for Spinal Cord Injury. Neurosurgery 2016, 78, 436–447. [Google Scholar] [CrossRef] [Green Version]
- Levi, A.D.; Anderson, K.D.; Okonkwo, D.O.; Park, P.; Bryce, T.; Kurpad, S.N.; Aarabi, B.; Hsieh, J.; Gant, K. Clinical Outcomes from a Multi-Center Study of Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury. J. Neurotrauma 2019, 36, 891–902. [Google Scholar] [CrossRef]
- Lammertse, D.P.; Jones, L.A.T.; Charlifue, S.B.; Kirshblum, S.C.; Apple, D.F.; Ragnarsson, K.T.; Falci, S.P. Autologous incubated macrophage therapy in acute, complete spinal cord injury: Results of the phase 2 randomized controlled multicenter trial. Spinal Cord 2012, 50, 661–671. [Google Scholar] [CrossRef]
- MM, S.; MK, T.; HS, K. The Extent of Myelin Pathology Differs Following Contusion and Transection Spinal Cord Injury. J. Neurotrauma 2007, 24, 1631–1646. [Google Scholar]
- Kwon, B.K.; Hillyer, J.; Tetzlaff, W. Translational research in spinal cord injury: A survey of opinion from the SCI community. J. Neurotrauma 2010, 27, 21–33. [Google Scholar] [CrossRef]
- Iwasaki, M.; Wilcox, J.T.; Nishimura, Y.; Zweckberger, K.; Suzuki, H.; Wang, J.; Liu, Y.; Karadimas, S.K.; Fehlings, M.G. Synergistic effects of self-assembling peptide and neural stem/progenitor cells to promote tissue repair and forelimb functional recovery in cervical spinal cord injury. Biomaterials 2014, 35, 2617–2629. [Google Scholar] [CrossRef]
- Moonen, G.; Satkunendrarajah, K.; Wilcox, J.T.; Badner, A.; Mothe, A.; Foltz, W.; Fehlings, M.G.; Tator, C.H. A New Acute Impact-Compression Lumbar Spinal Cord Injury Model in the Rodent. J. Neurotrauma 2016, 33, 278–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simard, J.M.; Tsymbalyuk, O.; Keledjian, K.; Ivanov, A.; Ivanova, S.; Gerzanich, V. Comparative effects of glibenclamide and riluzole in a rat model of severe cervical spinal cord injury. Exp. Neurol. 2012, 233, 566–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zweckberger, K.; Ahuja, C.S.; Liu, Y.; Wang, J.; Fehlings, M.G. Self-assembling peptides optimize the post-traumatic milieu and synergistically enhance the effects of neural stem cell therapy after cervical spinal cord injury. Acta Biomater. 2016, 42, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Abdolrezaee, S.; Eftekharpour, E.; Wang, J.; Schut, D.; Fehlings, M.G. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J. Neurosci. 2010, 30, 1657–1676. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, A.; Tsuji, O.; Shibata, S.; Nori, S.; Takano, M.; Kobayashi, Y.; Takahashi, Y.; Fujiyoshi, K.; Hara, C.M.; Miyawaki, A.; et al. Significance of remyelination by neural stem/progenitor cells transplanted into the injured spinal cord. Stem Cells 2011, 29, 1983–1994. [Google Scholar] [CrossRef]
- Anwar, M.A.; Al Shehabi, T.S.; Eid, A.H. Inflammogenesis of secondary spinal cord injury. Front. Cell. Neurosci. 2016, 10, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Parr, A.M.; Kulbatski, I.; Zahir, T.; Wang, X.; Yue, C.; Keating, A.; Tator, C.H. Transplanted adult spinal cord-derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience 2008, 155, 760–770. [Google Scholar] [CrossRef]
- Lepore, A.C.; Han, S.S.W.; Tyler-Polsz, C.J.; Cai, J.; Rao, M.S.; Fischer, I. Differential fate of multipotent and lineage-restricted neural precursors following transplantation into the adult CNS. Neuron Glia Biol. 2004, 1, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Keirstead, H.S.; Nistor, G.; Bernal, G.; Totoiu, M.; Cloutier, F.; Sharp, K.; Steward, O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 2005, 25, 4694–4705. [Google Scholar] [CrossRef]
- Anderson, A.J.; Haus, D.L.; Hooshmand, M.J.; Perez, H.; Sontag, C.J.; Cummings, B.J.; Gross, B.; Cell, S.; Road, H.S.; Irvine, U.C. Achieving stable human stem cell engraftment and survival in the CNS. Regen. Med. 2012, 6, 367–406. [Google Scholar] [CrossRef] [Green Version]
- Lachapelle, F.; Avellana-Adalid, V.; Nait-Oumesmar, B.; Baron-Van Evercooren, A. Fibroblast growth factor-2 (FGF-2) and platelet-derived growth factor AB (PDGF AB) promote adult SVZ-derived oligodendrogenesis in vivo. Mol. Cell. Neurosci. 2002, 20, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Shitaka, Y.; Saito, H. The effect of basic fibroblast growth factor (bFGF) and nerve growth factor (NGF) on the survival of septal neurons transplanted into the third ventricle in rats. Jpn. J. Pharmacol. 1994, 64, 27–33. [Google Scholar] [CrossRef]
- Vescovi, A.L.; Reynolds, B.A.; Fraser, D.D.; Weiss, S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron 1993, 11, 951–966. [Google Scholar] [CrossRef]
- Bottai, D.; Cigognini, D.; Madaschi, L.; Adami, R.; Nicora, E.; Menarini, M.; Di Giulio, A.M.; Gorio, A. Embryonic stem cells promote motor recovery and affect inflammatory cell infiltration in spinal cord injured mice. Exp. Neurol. 2010, 223, 452–463. [Google Scholar] [CrossRef]
- Sharp, J.; Frame, J.; Siegenthaler, M.; Nistor, G.; Keirstead, H.S. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells 2010, 28, 152–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawryluk, G.W.J.; Mothe, A.; Wang, J.; Wang, S.; Tator, C.; Fehlings, M.G. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 2012, 21, 2222–2238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younsi, A.; Zheng, G.; Scherer, M.; Riemann, L.; Zhang, H.; Tail, M.; Hatami, M.; Skutella, T.; Unterberg, A.; Zweckberger, K. Treadmill training improves survival and differentiation of transplanted neural precursor cells after cervical spinal cord injury. Stem Cell Res. 2020, 45, 101812. [Google Scholar] [CrossRef]
- Hofstetter, C.P.; Holmström, N.A.V.; Lilja, J.A.; Schweinhardt, P.; Hao, J.; Spenger, C.; Wiesenfeld-Hallin, Z.; Kurpad, S.N.; Frisén, J.; Olson, L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat. Neurosci. 2005, 8, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Abematsu, M.; Tsujimura, K.; Yamano, M.; Saito, M.; Kohno, K.; Kohyama, J.; Namihira, M.; Komiya, S.; Nakashima, K. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J. Clin. Investig. 2010, 120, 3255–3266. [Google Scholar] [CrossRef] [Green Version]
- Nori, S.; Okada, Y.; Yasuda, A.; Tsuji, O.; Takahashi, Y.; Kobayashi, Y.; Fujiyoshi, K.; Koike, M.; Uchiyama, Y.; Ikeda, E.; et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 16825–16830. [Google Scholar] [CrossRef] [Green Version]
- Butt, A.M.; Hornby, M.F.; Kirvell, S.; Berry, M. Platelet-derived growth factor delays oligodendrocyte differentiation and axonal myelination in vivo in the anterior medullary velum of the developing rat. J. Neurosci. Res. 1997, 48, 588–596. [Google Scholar] [CrossRef]
- Hu, J.-G.; Fu, S.-L.; Wang, Y.-X.; Li, Y.; Jiang, X.-Y.; Wang, X.-F.; Qiu, M.-S.; Lu, P.-H.; Xu, X.-M. Platelet-derived growth factor-AA mediates oligodendrocyte lineage differentiation through activation of extracellular signal-regulated kinase signaling pathway. Neuroscience 2008, 151, 138–147. [Google Scholar] [CrossRef]
- Stronati, E.; Conti, R.; Cacci, E.; Cardarelli, S.; Biagioni, S.; Poiana, G. Extracellular vesicle-induced differentiation of neural stem progenitor cells. Int. J. Mol. Sci. 2019, 20, 3691. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.L.; Nistor, G.; Wyatt, T.; Yin, H.Z.; Poole, A.J.; Weiss, J.H.; Gardener, M.J.; Dijkstra, S.; Fischer, D.F.; Keirstead, H.S. Histological and functional benefit following transplantation of motor neuron progenitors to the injured rat spinal cord. PLoS ONE 2010, 5, e11852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, Y.; Sawamoto, K.; Miyata, T.; Miyao, S.; Watanabe, M.; Nakamura, M.; Bregman, B.S.; Koike, M.; Uchiyama, Y.; Toyama, Y.; et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J. Neurosci. Res. 2002, 69, 925–933. [Google Scholar] [CrossRef]
- Lee, K.-Z.; Lane, M.A.; Dougherty, B.J.; Mercier, L.M.; Sandhu, M.S.; Sanchez, J.C.; Reier, P.J.; Fuller, D.D. Intraspinal transplantation and modulation of donor neuron electrophysiological activity. Exp. Neurol. 2014, 251, 47–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younsi, A.; Zheng, G.; Scherer, M.; Riemann, L.; Zhang, H.; Tail, M.; Hatami, M.; Skutella, T.; Unterberg, A.; Zweckberger, K. Three Growth Factors Induce Proliferation and Differentiation of Neural Precursor Cells In Vitro and Support Cell-Transplantation after Spinal Cord Injury In Vivo. Stem Cells Int. 2020, 2020. [Google Scholar] [CrossRef]
- Hernandez, M.; Patzig, J.; Mayoral, S.R.; Costa, K.D.; Chan, J.R.; Casaccia, P. Mechanostimulation Promotes Nuclear and Epigenetic Changes in Oligodendrocytes. J. Neurosci. 2016, 36, 806–813. [Google Scholar] [CrossRef] [Green Version]
- Hines, J.H.; Ravanelli, A.M.; Schwindt, R.; Scott, E.K.; Appel, B. Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 2015, 18, 683–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic spinal cord injury—Repair and regeneration. Clin. Neurosurg. 2017, 80, S22–S90. [Google Scholar] [CrossRef]
- Riemann, L.; Younsi, A.; Scherer, M.; Zheng, G.; Skutella, T.; Unterberg, A.W.; Zweckberger, K. Transplantation of neural precursor cells attenuates chronic immune environment in cervical spinal cord injury. Front. Neurol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, D.J.; Popovich, P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef] [Green Version]
- Hamers, F.P.; Lankhorst, A.J.; van Laar, T.J.; Veldhuis, W.B.; Gispen, W.H. Automated quantitative gait analysis during overground locomotion in the rat: Its application to spinal cord contusion and transection injuries. J. Neurotrauma 2001, 18, 187–201. [Google Scholar] [CrossRef]
- Schaal, S.M.; Kitay, B.M.; Cho, K.S.; Lo, T.P.; Barakat, D.J.; Marcillo, A.E.; Sanchez, A.R.; Andrade, C.M.; Pearse, D.D. Schwann cell transplantation improves reticulospinal axon growth and forelimb strength after severe cervical spinal cord contusion. Cell Transplant. 2007, 16, 207–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, A.R.; Irvine, K.A.; Gensel, J.C.; Nielson, J.L.; Lin, A.; Ly, J.; Segal, M.R.; Ratan, R.R.; Bresnahan, J.C.; Beattie, M.S. Derivation of Multivariate Syndromic Outcome Metrics for Consistent Testing across Multiple Models of Cervical Spinal Cord Injury in Rats. PLoS ONE 2013, 8, e59712. [Google Scholar] [CrossRef]
- Whishaw, I.Q.; Pellis, S.M.; Gorny, B.; Kolb, B.; Tetzlaff, W. Proximal and distal impairments in rat forelimb use in reaching follow unilateral pyramidal tract lesions. Behav. Brain Res. 1993, 56, 59–76. [Google Scholar] [CrossRef]
- Alluin, O.; Karimi-Abdolrezaee, S.; Delivet-Mongrain, H.; Leblond, H.; Fehlings, M.G.; Rossignol, S. Kinematic study of locomotor recovery after spinal cord clip compression injury in rats. J. Neurotrauma 2011, 28, 1963–1981. [Google Scholar] [CrossRef]
- Stokes, B.T.; Reier, P.J. Fetal grafts alter chronic behavioral outcome after contusion damage to the adult rat spinal cord. Exp. Neurol. 1992, 116, 1–12. [Google Scholar] [CrossRef]
- Behrmann, D.L.; Bresnahan, J.C.; Beattie, M.S.; Shah, B.R. Spinal Cord Injury Produced by Consistent Mechanical Displacement of the Cord in Rats: Behavioral and Histologic Analysis. J. Neurotrauma 1992, 9, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, S.; Nishimura, S.; Shinozaki, M.; Takano, M.; Konomi, T.; Tsuji, O.; Nagoshi, N.; Toyama, Y.; Liu, M.; Okano, H.; et al. The Amelioration of Pain-Related Behavior in Mice with Chronic Spinal Cord Injury Treated with Neural Stem/Progenitor Cell Transplantation Combined with Treadmill Training. J. Neurotrauma 2018, 35, 2561–2571. [Google Scholar] [CrossRef]
- Liu, M.; Li, K.; Wang, Y.; Zhao, G.; Jiang, J. Stem Cells in the Treatment of Neuropathic Pain: Research Progress of Mechanism. Stem Cells Int. 2020, 2020, 8861251. [Google Scholar] [CrossRef] [PubMed]
- Zweckberger, K.; Liu, Y.; Wang, J.; Forgione, N.; Fehlings, M.G. Synergetic use of neural precursor cells and self-assembling peptides in experimental cervical spinal cord injury. J. Vis. Exp. 2015, 96, e52105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Younsi, A.; Zheng, G.; Tail, M.; Harms, A.-K.; Roth, J.; Hatami, M.; Skutella, T.; Unterberg, A.; Zweckberger, K. Sonic Hedgehog modulates the inflammatory response and improves functional recovery after spinal cord injury in a thoracic contusion-compression model. Eur. Spine J. 2021, 30, 1509–1520. [Google Scholar] [CrossRef]
- BASSO, D.M.; BEATTIE, M.S.; BRESNAHAN, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Isu, T.; Iizuka, T.; Iwasaki, Y.; Nagashima, M.; Akino, M.; Abe, H. Spinal cord herniation associated with an intradural spinal arachnoid cyst diagnosed by magnetic resonance imaging. Neurosurgery 1991, 29, 137–139. [Google Scholar] [CrossRef]
- Metz, G.A.S.; Merkler, D.; Dietz, V.; Schwab, M.E.; Fouad, K. Efficient testing of motor function in spinal cord injured rats. Brain Res. 2000, 883, 165–177. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, H.; Satkunendrarajah, K.; Yao, G.S.; Bayon, Y.; Fehlings, M.G. A self-assembling peptide reduces glial scarring, attenuates post-traumatic inflammation and promotes neurological recovery following spinal cord injury. Acta Biomater. 2013, 9, 8075–8088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Michaelis, T.; Frahm, J. Mapping of retinal projections in the living rat using high-resolution 3D gradient-echo MRI with Mn2+-induced contrast. Magn. Reson. Med. 2001, 46, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Narita, K.; Kawasaki, F.; Kita, H. Mn and Mg influxes through Ca channels of motor nerve terminals are prevented by verapamil in frogs. Brain Res. 1990, 510, 289–295. [Google Scholar] [CrossRef]
- Sloot, W.N.; Gramsbergen, J.B. Axonal transport of manganese and its relevance to selective neurotoxicity in the rat basal ganglia. Brain Res. 1994, 657, 124–132. [Google Scholar] [CrossRef]
- Pautler, R.G.; Mongeau, R.; Jacobs, R.E. In vivo trans-synaptic tract tracing from the murine striatum and amygdala utilizing manganese enhanced MRI (MEMRI). Magn. Reson. Med. 2003, 50, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M.T.; Márton, G.; Pajer, K.; Hartmann, J.; Walder, N.; Rossmann, M.; Parzer, P.; Redl, H.; Nógrádi, A.; Stieltjes, B. Monitoring of Short-Term Erythropoietin Therapy in Rats with Acute Spinal Cord Injury Using Manganese-Enhanced Magnetic Resonance Imaging. J. Neuroimaging 2015, 25, 582–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walder, N.; Petter-Puchner, A.H.; Brejnikow, M.; Redl, H.; Essig, M.; Stieltjes, B. Manganese enhanced magnetic resonance imaging in a contusion model of spinal cord injury in rats: Correlation with motor function. Investig. Radiol. 2008, 43, 277–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stieltjes, B.; Klussmann, S.; Bock, M.; Umathum, R.; Mangalathu, J.; Letellier, E.; Rittgen, W.; Edler, L.; Krammer, P.H.; Kauczor, H.-U.; et al. Manganese-enhanced magnetic resonance imaging for in vivo assessment of damage and functional improvement following spinal cord injury in mice. Magn. Reson. Med. 2006, 55, 1124–1131. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younsi, A.; Zheng, G.; Riemann, L.; Scherer, M.; Zhang, H.; Tail, M.; Hatami, M.; Skutella, T.; Unterberg, A.; Zweckberger, K. Long-Term Effects of Neural Precursor Cell Transplantation on Secondary Injury Processes and Functional Recovery after Severe Cervical Contusion-Compression Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 13106. https://doi.org/10.3390/ijms222313106
Younsi A, Zheng G, Riemann L, Scherer M, Zhang H, Tail M, Hatami M, Skutella T, Unterberg A, Zweckberger K. Long-Term Effects of Neural Precursor Cell Transplantation on Secondary Injury Processes and Functional Recovery after Severe Cervical Contusion-Compression Spinal Cord Injury. International Journal of Molecular Sciences. 2021; 22(23):13106. https://doi.org/10.3390/ijms222313106
Chicago/Turabian StyleYounsi, Alexander, Guoli Zheng, Lennart Riemann, Moritz Scherer, Hao Zhang, Mohamed Tail, Maryam Hatami, Thomas Skutella, Andreas Unterberg, and Klaus Zweckberger. 2021. "Long-Term Effects of Neural Precursor Cell Transplantation on Secondary Injury Processes and Functional Recovery after Severe Cervical Contusion-Compression Spinal Cord Injury" International Journal of Molecular Sciences 22, no. 23: 13106. https://doi.org/10.3390/ijms222313106
APA StyleYounsi, A., Zheng, G., Riemann, L., Scherer, M., Zhang, H., Tail, M., Hatami, M., Skutella, T., Unterberg, A., & Zweckberger, K. (2021). Long-Term Effects of Neural Precursor Cell Transplantation on Secondary Injury Processes and Functional Recovery after Severe Cervical Contusion-Compression Spinal Cord Injury. International Journal of Molecular Sciences, 22(23), 13106. https://doi.org/10.3390/ijms222313106





