Viral Transduction of Human Rod Opsin or Channelrhodopsin Variants to Mouse ON Bipolar Cells Does Not Impact Retinal Anatomy or Cause Measurable Death in the Targeted Cells
Abstract
1. Introduction
2. Results
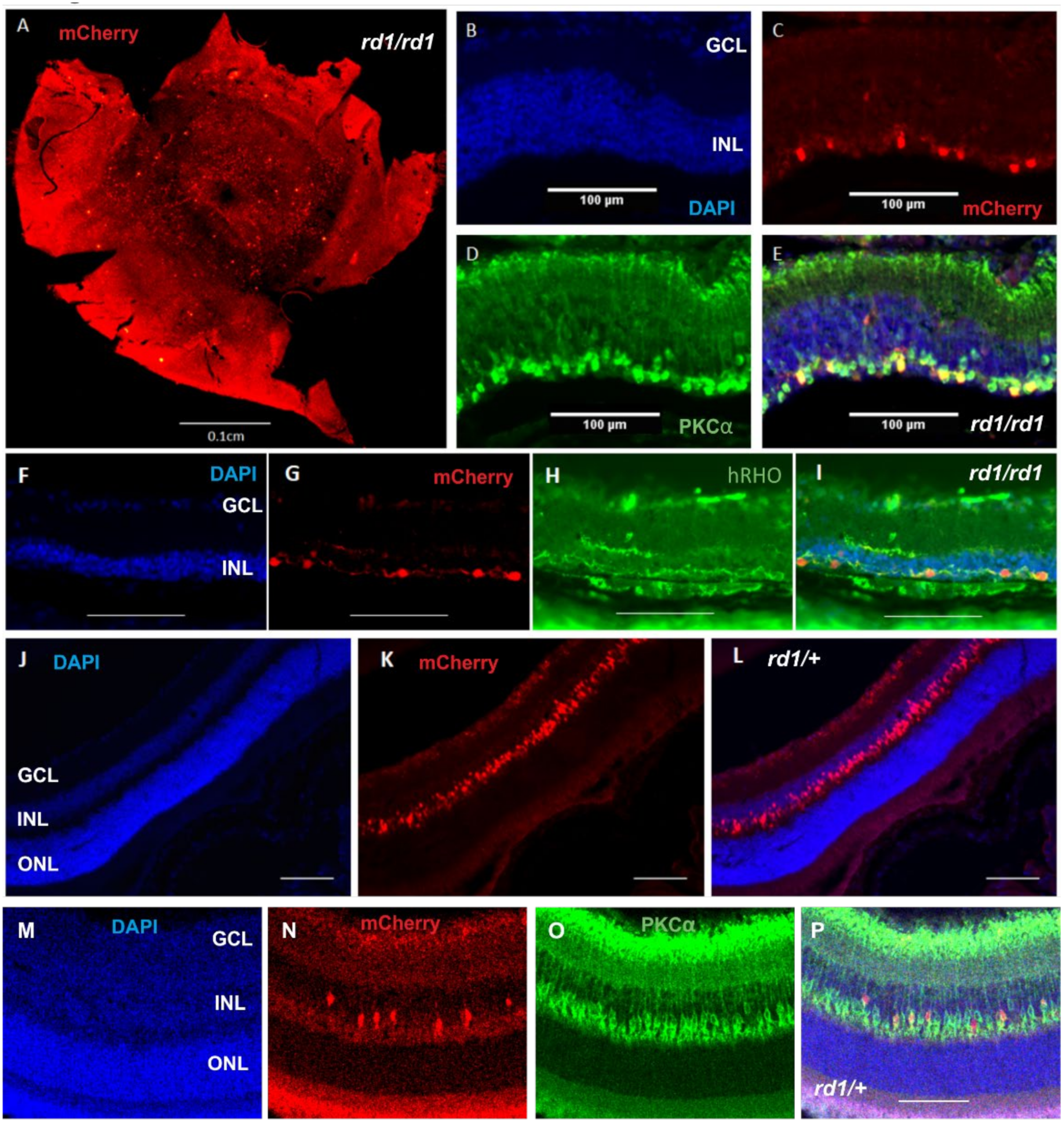
2.1. Expression of Human Rod Opsin in ON Bipolar Cells Using the Grm6cre Mouse
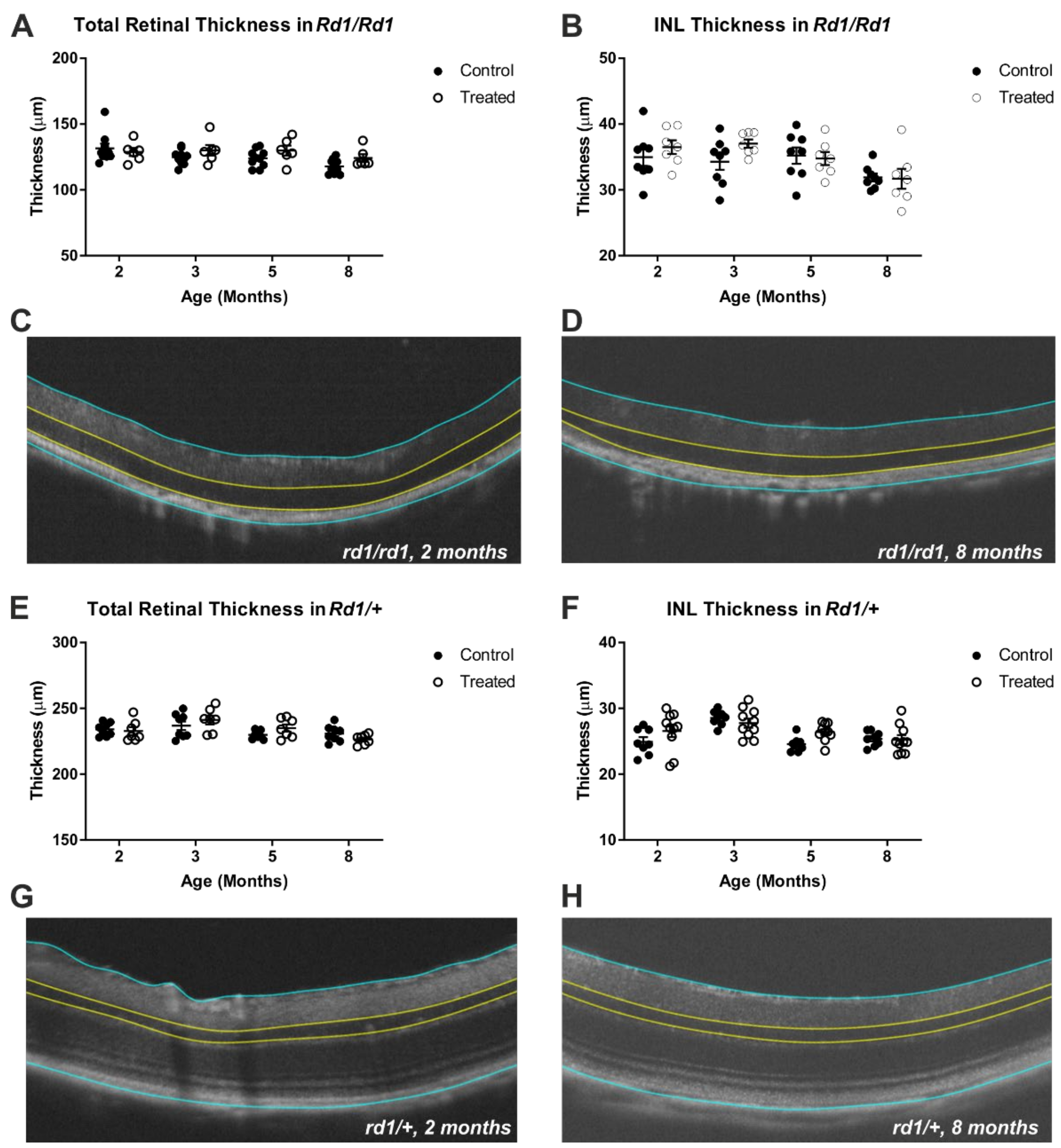
2.2. Gross Retinal Anatomy Is Not Impaired by Viral Delivery of Rod Opsin to ON Bipolar Cells
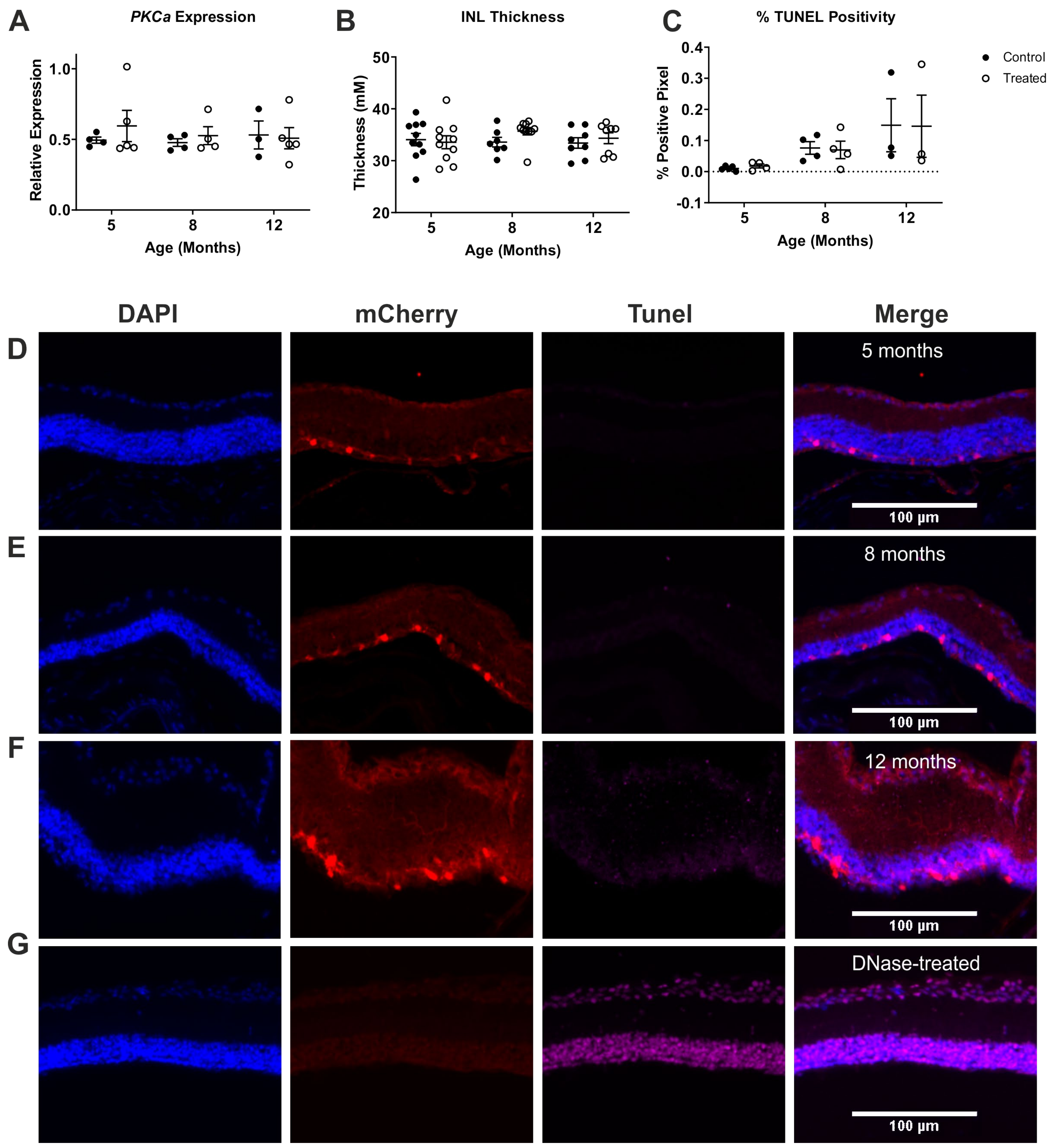
2.3. ON Bipolar Cell Survival Is Not Impacted by Viral Delivery of Rod Opsin
2.4. Expression of Channelrhodopsin Variants Does Not Impact Gross Retinal Anatomy or Bipolar Cell Number
3. Discussion
4. Methods
4.1. Mice
4.2. Immunohistochemistry
4.3. PCR
4.4. Optical Coherence Tomography
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santos, A.; Humayun, M.S.; de Juan, E.; Greenburg, R.J.; Marsh, M.J.; Klock, I.B.; Milam, A.H. Preservation of the Inner Retina in Retinitis Pigmentosa. A morphometric analysis. Arch. Ophthalmol. 1997, 115, 511–515. [Google Scholar] [CrossRef]
- Berry, M.H.; Holt, A.; Salari, A.; Veit, J.; Visel, M.; Levitz, J.; Aghi, K.; Gaub, B.M.; Sivyer, B.; Flannery, J.G.; et al. Restoration of high-sensitivity and adapting vision with a cone opsin. Nat. Commun. 2019, 10, 1221. [Google Scholar] [CrossRef] [PubMed]
- Gaub, B.M.; Berry, M.H.; Holt, A.E.; Isacoff, E.Y.; Flannery, J.G. Optogenetic Vision Restoration Using Rhodopsin for Enhanced Sensitivity. Mol. Ther. 2015, 23, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Macé, E.; Caplette, R.; Marre, O.; Sengupta, A.; Chaffiol, A.; Barbe, P.; Desrosiers, M.; Bamberg, E.; Sahel, J.-A.; Picaud, S.; et al. Targeting Channelrhodopsin-2 to ON-bipolar Cells With Vitreally Administered AAV Restores ON and OFF Visual Responses in Blind Mice. Mol. Ther. 2015, 23, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Chaffiol, A.; Caplette, R.; Jaillard, C.; Brazhnikova, E.; Desrosiers, M.; Dubus, E.; Duhamel, L.; Macé, E.; Marre, O.; Benoit, P.; et al. A New Promoter Allows Optogenetic Vision Restoration with Enhanced Sensitivity in Macaque Retina. Mol. Ther. 2017, 25, 2546–2560. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, M.; Pielecka-Fortuna, J.; Löwel, S.; Kleinlogel, S. Restoring the ON Switch in Blind Retinas: Opto-mGluR6, a Next-Generation, Cell-Tailored Optogenetic Tool. PLoS Biol. 2015, 13, e1002143. [Google Scholar] [CrossRef]
- Bi, A.; Cui, J.; Ma, Y.-P.; Olshevskaya, E.; Pu, M.; Dizhoor, A.; Pan, Z.-H. Ectopic Expression of a Microbial-Type Rhodopsin Restores Visual Responses in Mice with Photoreceptor Degeneration. Neuron 2006, 50, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Perny, M.; Muri, L.; Dawson, H.; Kleinlogel, S. Chronic activation of the D156A point mutant of Channelrhodopsin-2 signals apoptotic cell death: The good and the bad. Cell Death Dis. 2016, 7, e2447. [Google Scholar] [CrossRef]
- Xiong, W.; Wu, D.M.; Xue, Y.; Wang, S.K.; Chung, M.J.; Ji, X.; Cepko, C.L. AAV cis-regulatory sequences are correlated with ocular toxicity. Proc. Natl. Acad. Sci. USA 2019, 116, 5785–5794. [Google Scholar] [CrossRef] [PubMed]
- Busskamp, V.; Duebel, J.; Balya, D.; Fradot, M.; Viney, T.J.; Siegert, S.; Groner, A.C.; Cabuy, E.; Forster, V.; Seeliger, M.; et al. Genetic Reactivation of Cone Photoreceptors Restores Visual Responses in Retinitis Pigmentosa. Science 2010, 329, 413–417. [Google Scholar] [CrossRef]
- Zhang, Y.; Ivanova, E.; Bi, A.; Pan, Z.-H. Ectopic Expression of Multiple Microbial Rhodopsins Restores ON and OFF Light Responses in Retinas with Photoreceptor Degeneration. J. Neurosci. 2009, 29, 9186–9196. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; Eleftheriou, C.; Allen, A.; Milosavljevic, N.; Pienaar, A.; Bedford, R.; Davis, K.E.; Bishop, P.N.; Lucas, R. Restoration of Vision with Ectopic Expression of Human Rod Opsin. Curr. Biol. 2015, 25, 2111–2122. [Google Scholar] [CrossRef]
- Kleinlogel, S.; Feldbauer, K.; Dempski, R.E.; Fotis, H.; Wood, P.G.; Bamann, C.; Bamberg, E. Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nat. Neurosci. 2011, 14, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 2003, 100, 13940–13945. [Google Scholar] [CrossRef] [PubMed]
- Ganjawala, T.H.; Lu, Q.; Fenner, M.D.; Abrams, G.W.; Pan, Z.-H. Improved CoChR Variants Restore Visual Acuity and Contrast Sensitivity in a Mouse Model of Blindness under Ambient Light Conditions. Mol. Ther. 2019, 27, 1195–1205. [Google Scholar] [CrossRef]
- Lin, J.; Knutsen, P.M.; Muller, A.; Kleinfeld, D.; Tsien, R.Y. ReaChR: A red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 2013, 16, 1499–1508. [Google Scholar] [CrossRef]
- Van Wyk, M.; Hulliger, E.C.; Girod, L.; Ebneter, A.; Kleinlogel, S. Present Molecular Limitations of ON-Bipolar Cell Targeted Gene Therapy. Front. Neurosci. 2017, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Lagali, P.S.; Balya, D.; Awatramani, G.B.; Münch, T.; Kim, D.; Busskamp, V.; Cepko, C.L.; Roska, B. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat. Neurosci. 2008, 11, 667–675. [Google Scholar] [CrossRef]
- Ochakovski, G.A.; Bartz-Schmidt, K.U.; Fischer, M.D. Retinal Gene Therapy: Surgical Vector Delivery in the Translation to Clinical Trials. Front. Neurosci. 2017, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.P.; Dinculescu, A.; Li, Q.; Deng, W.-T.; Pang, J.-J.; Min, S.-H.; Chiodo, V.; Neeley, A.W.; Govindasamy, L.; Bennett, A.; et al. Novel Properties of Tyrosine-mutant AAV2 Vectors in the Mouse Retina. Mol. Ther. 2011, 19, 293–301. [Google Scholar] [CrossRef]
- Hickey, D.G.; Edwards, T.; Barnard, A.R.; Singh, M.S.; De Silva, S.; McClements, M.E.; Flannery, J.; Hankins, M.W.; MacLaren, R. Tropism of engineered and evolved recombinant AAV serotypes in the rd1 mouse and ex vivo primate retina. Gene Ther. 2017, 24, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Morgans, C.W.; Zhang, J.; Jeffrey, B.G.; Nelson, S.M.; Burke, N.S.; Duvoisin, R.M.; Brown, R.L. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc. Natl. Acad. Sci. USA 2009, 106, 19174–19178. [Google Scholar] [CrossRef]
- Nomura, A.; Shigemoto, R.; Nakamura, Y.; Okamoto, N.; Mizuno, N.; Nakanishi, S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell 1994, 77, 361–369. [Google Scholar] [CrossRef]
- Masu, M.; Iwakabe, H.; Tagawa, Y.; Miyoshi, T.; Yamashita, M.; Fukuda, Y.; Nakanishi, S. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell 1995, 80, 757–765. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Fujimoto, J.G. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Niyadurupola, N.; Sidaway, P.; Osborne, A.; Broadway, D.C.; Sanderson, J. The development of human organotypic retinal cultures (HORCs) to study retinal neurodegeneration. Br. J. Ophthalmol. 2011, 95, 720–726. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greferath, U.; Grünert, U.; Wässle, H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J. Comp. Neurol. 1990, 301, 433–442. [Google Scholar] [CrossRef]
- Negishi, K.; Kato, S.; Teranishi, T. Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci. Lett. 1988, 94, 247–252. [Google Scholar] [CrossRef]
- Shen, Y.; Luo, X.; Liu, S.; Shen, Y.; Nawy, S.; Shen, Y. Rod bipolar cells dysfunction occurs before ganglion cells loss in excitotoxin-damaged mouse retina. Cell Death Dis. 2019, 10, 905. [Google Scholar] [CrossRef]
- Litvinchuk, A.; Wan, Y.-W.; Swartzlander, D.B.; Chen, F.; Cole, A.; Propson, N.E.; Wang, Q.; Zhang, B.; Liu, Z.; Zheng, H. Complement C3aR Inactivation Attenuates Tau Pathology and Reverses an Immune Network Deregulated in Tauopathy Models and Alzheimer’s Disease. Neuron 2018, 100, 1337–1353.e5. [Google Scholar] [CrossRef]
- Hulliger, E.C.; Hostettler, S.M.; Kleinlogel, S. Empowering Retinal Gene Therapy with a Specific Promoter for Human Rod and Cone ON-Bipolar Cells. Mol. Ther. Methods Clin. Dev. 2020, 17, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [PubMed]


| ARP Fwd | CGACCTGGAAGTCCAACTAC |
| ARP Rev | ATCTGCTGCATCTGCTTG |
| GAPDH Fwd | TGCACCACCAACTGCTTAG |
| GAPDH Rev | GATGCAGGGATGATGTTC |
| PSMB2 Fwd | AAATGCGGAATGGATATGAATTG |
| PSMB2 Rev | GAAGACAGTCAGCCAGGTT |
| mCherry Fwd | GATAACATGGCCATCATCAAGGA |
| mCherry Rev | CGTGGCCGTTCACGGAG |
| PKCa/PRKCA Fwd | CGGAAGCCCTACCTTCTGTG |
| PKCa/PRKCA Rev | TCTCGTACCGTGACGTGGAG |
| hRHO Fwd | CCTGCTCAACCTAGCCGTG |
| hRHO Rev | AGGGCAATTTCACCGCCCA |
| IBA1/AIF1 Fwd | CAGACTGCCAGCCTAAGACA |
| IBA1/AIF1 Rev | AGGAATTGCTTGTTGATCCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wright, P.; Rodgers, J.; Wynne, J.; Bishop, P.N.; Lucas, R.J.; Milosavljevic, N. Viral Transduction of Human Rod Opsin or Channelrhodopsin Variants to Mouse ON Bipolar Cells Does Not Impact Retinal Anatomy or Cause Measurable Death in the Targeted Cells. Int. J. Mol. Sci. 2021, 22, 13111. https://doi.org/10.3390/ijms222313111
Wright P, Rodgers J, Wynne J, Bishop PN, Lucas RJ, Milosavljevic N. Viral Transduction of Human Rod Opsin or Channelrhodopsin Variants to Mouse ON Bipolar Cells Does Not Impact Retinal Anatomy or Cause Measurable Death in the Targeted Cells. International Journal of Molecular Sciences. 2021; 22(23):13111. https://doi.org/10.3390/ijms222313111
Chicago/Turabian StyleWright, Phillip, Jessica Rodgers, Jonathan Wynne, Paul N. Bishop, Robert J. Lucas, and Nina Milosavljevic. 2021. "Viral Transduction of Human Rod Opsin or Channelrhodopsin Variants to Mouse ON Bipolar Cells Does Not Impact Retinal Anatomy or Cause Measurable Death in the Targeted Cells" International Journal of Molecular Sciences 22, no. 23: 13111. https://doi.org/10.3390/ijms222313111
APA StyleWright, P., Rodgers, J., Wynne, J., Bishop, P. N., Lucas, R. J., & Milosavljevic, N. (2021). Viral Transduction of Human Rod Opsin or Channelrhodopsin Variants to Mouse ON Bipolar Cells Does Not Impact Retinal Anatomy or Cause Measurable Death in the Targeted Cells. International Journal of Molecular Sciences, 22(23), 13111. https://doi.org/10.3390/ijms222313111








