Designing Cardiovascular Implants Taking in View the Endothelial Basement Membrane
Abstract
:1. Introduction
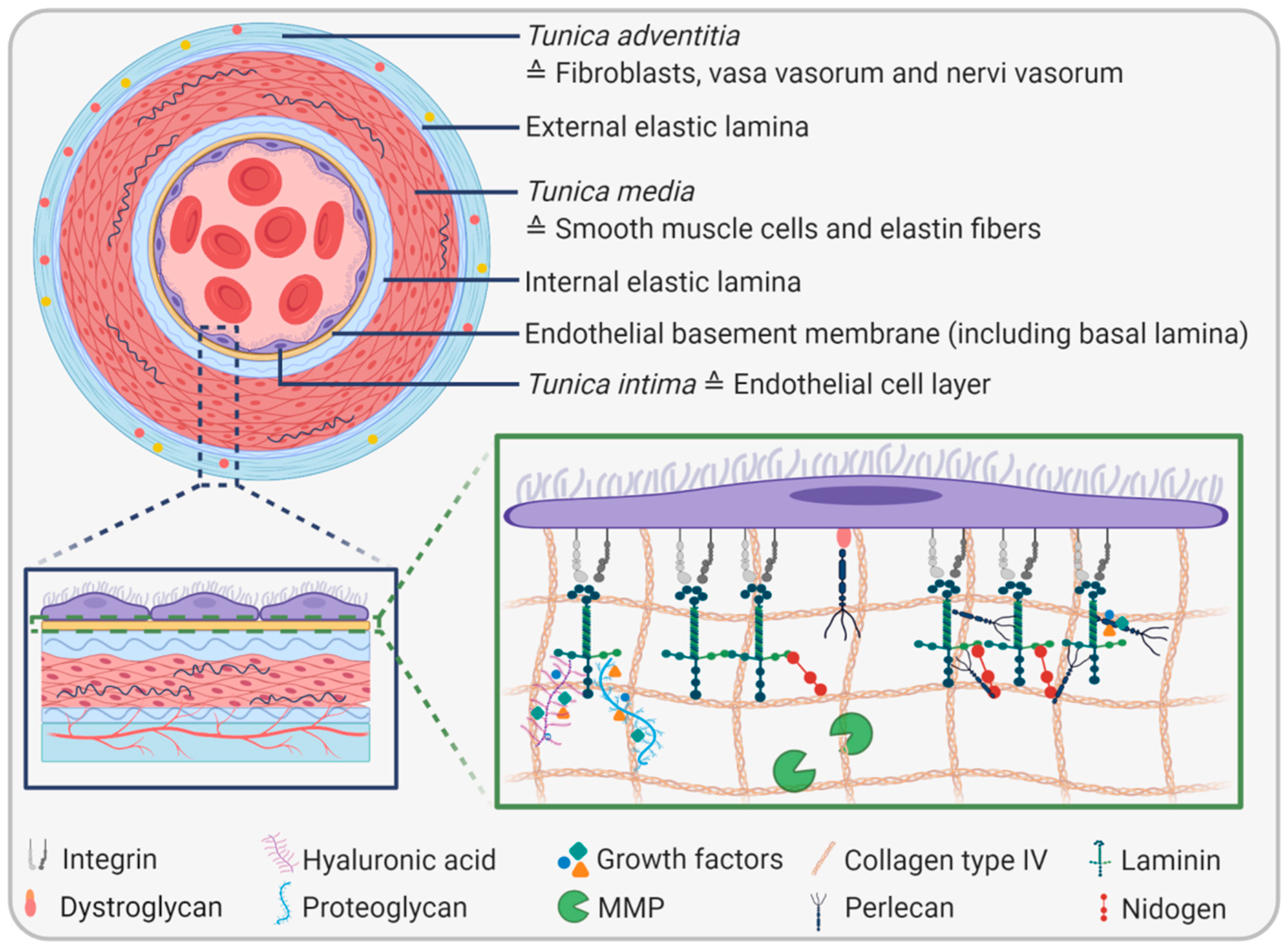
2. The Endothelial Basement Membrane
2.1. EBM Composition and Biophysical Properties
2.2. EBM Functions
2.3. EBM Interactions with the Luminal Side
2.4. EBM Interactions with the Abluminal Side
3. Strategies to Generate or Mimic Endothelial Basement Membranes
3.1. Decellularization of Natural Blood Vessels
3.2. Bioinstructive Implant Surfaces for Endothelial Basement Membrane Formation In Vivo
3.3. Tissue-Engineered Endothelial Basement Membranes
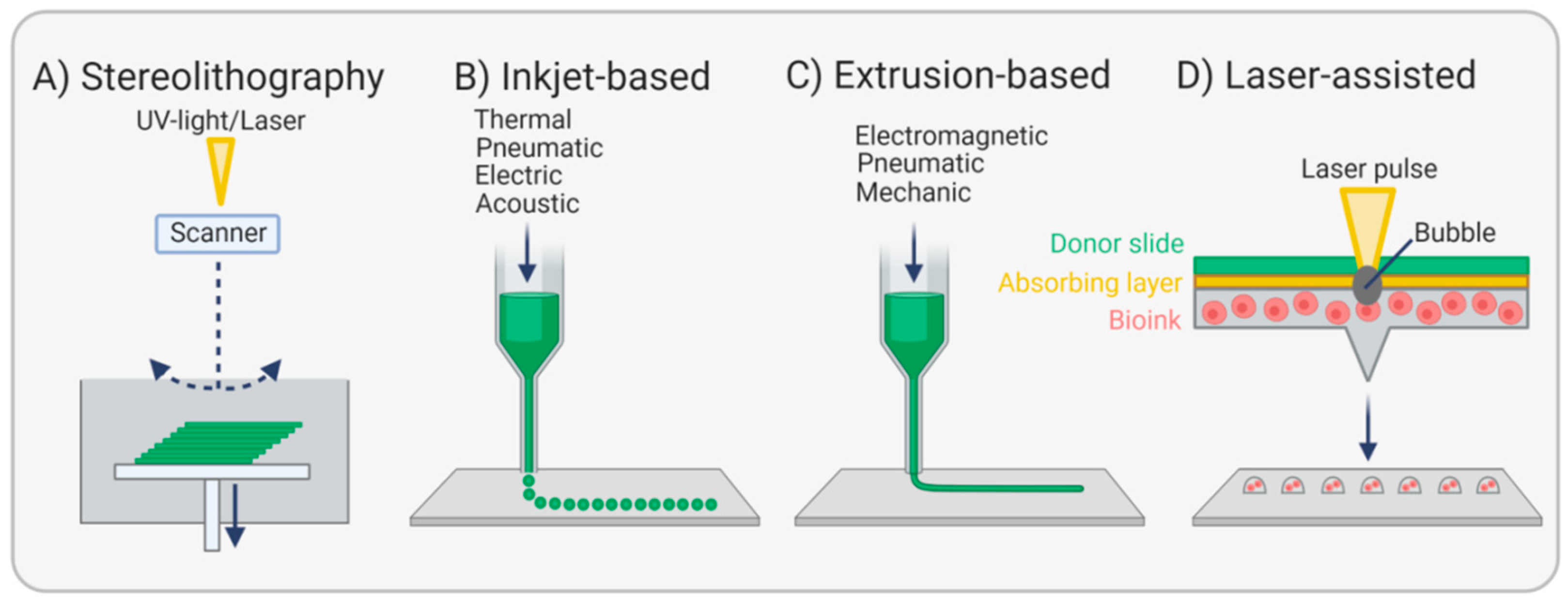
3.4. Bioprinting Endothelial Basement Membranes
4. Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 30 September 2021).
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft—Past, Present, and Future. Tissue Eng. Part B 2016, 22, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Bordenave, L.; Fernandez, P.; Rémy-Zolghadri, M.; Villars, S.; Daculsi, R.; Midy, D. In vitro endothelialized ePTFE prostheses: Clinical update 20 years after the first realization. Clin. Hemorheol. Microcirc. 2005, 33, 227–234. [Google Scholar]
- Cahill, P.A.; Redmond, E.M. Vascular endothelium—Gatekeeper of vessel health. Atherosclerosis 2016, 248, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baluk, P.; Morikawa, S.; Haskell, A.; Mancuso, M.; McDonald, D.M. Abnormalities of Basement Membrane on Blood Vessels and Endothelial Sprouts in Tumors. Am. J. Pathol. 2003, 163, 1801–1815. [Google Scholar] [CrossRef] [Green Version]
- Post, A.; Wang, E.; Cosgriff-Hernandez, E. A Review of Integrin-Mediated Endothelial Cell Phenotype in the Design of Cardiovascular Devices. Ann. Biomed. Eng. 2019, 47, 366–380. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef]
- Randles, M.J.; Humphries, M.J.; Lennon, R. Proteomic definitions of basement membrane composition in health and disease. Matrix Biol. 2017, 57–58, 12–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, C.B. Rethinking glomerular basement membrane thickening in diabetic nephropathy: Adaptive or pathogenic? Am. J. Physiol. Ren. Physiol. 2016, 311, F831–F843. [Google Scholar] [CrossRef] [Green Version]
- Witjas, F.M.R.; van den Berg, B.M.; van den Berg, C.W.; Engelse, M.A.; Rabelink, T.J. Concise Review: The Endothelial Cell Extracellular Matrix Regulates Tissue Homeostasis and Repair. Stem Cells Transl. Med. 2019, 8, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Pozzi, A.; Yurchenco, P.D.; Lozzo, R.V. The nature and biology of basement membranes. Matrix Biol. 2017, 57–58, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Di Russo, J.; Luik, A.L.; Yousif, L.; Budny, S.; Oberleithner, H.; Hofschroer, V.; Klingauf, J.; van Bavel, E.; Bakker, E.N.; Hellstrand, P.; et al. Endothelial basement membrane laminin 511 is essential for shear stress response. EMBO J. 2017, 36, 183–201. [Google Scholar] [CrossRef]
- Song, J.; Zhang, X.L.; Buscher, K.; Wang, Y.; Wang, H.Y.; Di Russo, J.; Li, L.X.; Lutke-Enking, S.; Zarbock, A.; Stadtmann, A.; et al. Endothelial Basement Membrane Laminin 511 Contributes to Endothelial Junctional Tightness and Thereby Inhibits Leukocyte Transmigration. Cell Rep. 2017, 18, 1256–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nybo, T.; Dieterich, S.; Gamon, L.F.; Chuang, C.Y.; Hammer, A.; Hoefler, G.; Malle, E.; Rogowska-Wrzesinska, A.; Davies, M.J. Chlorination and oxidation of the extracellular matrix protein laminin and basement membrane extracts by hypochlorous acid and myeloperoxidase. Redox Biol. 2019, 20, 496–513. [Google Scholar] [CrossRef]
- Lorentzen, L.G.; Chuang, C.Y.; Rogowska-Wrzesinska, A.; Davies, M.J. Identification and quantification of sites of nitration and oxidation in the key matrix protein laminin and the structural consequences of these modifications. Redox Biol. 2019, 24, 101226. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.C.; Blantz, R.C. Biophysics of Glomerular Filtration. Compr. Physiol. 2012, 2, 1671–1699. [Google Scholar] [CrossRef]
- Ganesan, M.K.; Finsterwalder, R.; Leb, H.; Resch, U.; Neumüller, K.; de Martin, R.; Petzelbauer, P. Three-Dimensional Coculture Model to Analyze the Cross Talk Between Endothelial and Smooth Muscle Cells. Tissue Eng. Part C 2017, 23, 38–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubbiotti, M.A.; Neill, T.; Iozzo, R.V. A current view of perlecan in physiology and pathology: A mosaic of functions. Matrix Biol. 2017, 57–58, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Steiner, E.; Enzmann, G.U.; Lyck, R.; Lin, S.; Rüegg, M.A.; Kröger, S.; Engelhardt, B. The heparan sulfate proteoglycan agrin contributes to barrier properties of mouse brain endothelial cells by stabilizing adherens junctions. Cell Tissue Res. 2014, 358, 465–479. [Google Scholar] [CrossRef] [Green Version]
- Katt, M.E.; Linville, R.M.; Mayo, L.N.; Xu, Z.S.; Searson, P.C. Functional brain-specific microvessels from iPSC-derived human brain microvascular endothelial cells: The role of matrix composition on monolayer formation. Fluids Barriers CNS 2018, 15, 7. [Google Scholar] [CrossRef]
- Glentis, A.; Gurchenkov, V.; Matic Vignjevic, D. Assembly, heterogeneity, and breaching of the basement membranes. Cell Adhes. Migr. 2014, 8, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Böse, K.; Nischt, R.; Page, A.; Bader, B.L.; Paulsson, M.; Smyth, N. Loss of nidogen-1 and -2 results in syndactyly and changes in limb development. J. Biol. Chem. 2006, 281, 39620–39629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Löffek, S.; Schilling, O.; Franzke, C.-W. Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Manzaneque, J.C.; Fernández-Rodríguez, R.; Rodríguez-Baena, F.J.; Iruela-Arispe, M.L. ADAMTS proteases in vascular biology. Matrix Biol. 2015, 44–46, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Yousif, L.F.; Di Russo, J.; Sorokin, L. Laminin isoforms in endothelial and perivascular basement membranes. Cell Adhes. Migr. 2013, 7, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, M.S.; Chuang, C.Y.; Melrose, J.; Davies, M.J.; Iozzo, R.V.; Whitelock, J.M. The role of vascular-derived perlecan in modulating cell adhesion, proliferation and growth factor signaling. Matrix Biol. 2014, 35, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, S.; Banda, O.A.; Farino, C.J.; Sperduto, J.L.; Keller, K.A.; Taitano, R.; Slater, J.H. Biofabrication Strategies and Engineered In Vitro Systems for Vascular Mechanobiology. Adv. Healthc. Mater. 2020, 9, 1901255. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Scott, H.A.; Monickaraj, F.; Xu, J.; Ardekani, S.; Nitta, C.F.; Cabrera, A.; McGuire, P.G.; Mohideen, U.; Das, A.; et al. Basement membrane stiffening promotes retinal endothelial activation associated with diabetes. FASEB J. 2016, 30, 601–611. [Google Scholar] [CrossRef]
- Gupta, P.; Moses, J.C.; Mandal, B.B. Surface Patterning and Innate Physicochemical Attributes of Silk Films Concomitantly Govern Vascular Cell Dynamics. ACS Biomater. Sci. Eng. 2019, 5, 933–949. [Google Scholar] [CrossRef]
- Hallmann, R.; Horn, N.; Selg, M.; Wendler, O.; Pausch, F.; Sorokin, L.M. Expression and function of laminins in the embryonic and mature vasculature. Physiol. Rev. 2005, 85, 979–1000. [Google Scholar] [CrossRef] [Green Version]
- Ohta, R.; Niwa, A.; Taniguchi, Y.; Suzuki, N.M.; Toga, J.; Yagi, E.; Saiki, N.; Nishinaka-Arai, Y.; Okada, C.; Watanabe, A.; et al. Laminin-guided highly efficient endothelial commitment from human pluripotent stem cells. Sci. Rep. 2016, 6, 35680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, H.; Yamashita, M.; Hashita, T.; Iwao, T.; Matsunaga, T. Laminin 221 fragment is suitable for the differentiation of human induced pluripotent stem cells into brain microvascular endothelial-like cells with robust barrier integrity. Fluids Barriers CNS 2020, 17, 25. [Google Scholar] [CrossRef]
- Wong, L.; Kumar, A.; Gabela-Zuniga, B.; Chua, J.; Singh, G.; Happe, C.L.; Engler, A.J.; Fan, Y.; McCloskey, K.E. Substrate stiffness directs diverging vascular fates. Acta Biomater. 2019, 96, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.; Multhaupt, H.A.B.; Pocock, R.; Couchman, J.R. Cell-extracellular matrix and cell-cell adhesion are linked by syndecan-4. Matrix Biol. 2017, 60–61, 57–69. [Google Scholar] [CrossRef]
- Cavalheiro, R.P.; Lima, M.A.; Jarrouge-Boucas, T.R.; Viana, G.M.; Lopes, C.C.; Coulson-Thomas, V.J.; Dreyfuss, J.L.; Yates, E.A.; Tersariol, I.L.S.; Nader, H.B. Coupling of vinculin to F-actin demands Syndecan-4 proteoglycan. Matrix Biol. 2017, 63, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, H.; Ninomiya, H.; Kitamura, Y.; Fujiwara, K.; Masaki, T. Vascular endothelial cells that express dystroglycan are involved in angiogenesis. J. Cell Sci. 2002, 115, 1487–1496. [Google Scholar] [CrossRef]
- Biswas, S.; Watters, J.; Bachay, G.; Varshney, S.; Hunter, D.D.; Hu, H.Y.; Brunken, W.J. Laminin-dystroglycan signaling regulates retinal arteriogenesis. FASEB J. 2018, 32, 6261–6273. [Google Scholar] [CrossRef]
- Galvagni, F.; Nardi, F.; Maida, M.; Bernardini, G.; Vannuccini, S.; Petraglia, F.; Santucci, A.; Orlandini, M. CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration adhesion and migration. Oncotarget 2016, 7, 10090–10103. [Google Scholar] [CrossRef] [Green Version]
- Jayadev, R.; Sherwood, D.R. Basement membranes. Curr. Biol. 2017, 27, R207–R211. [Google Scholar] [CrossRef] [Green Version]
- Lieleg, O.; Baumgartel, R.M.; Bausch, A.R. Selective Filtering of Particles by the Extracellular Matrix: An Electrostatic Bandpass. Biophys. J. 2009, 97, 1569–1577. [Google Scholar] [CrossRef] [Green Version]
- Chew, C.; Lennon, R. Basement Membrane Defects in Genetic Kidney Diseases. Front. Pediatr. 2018, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Voisin, M.-B.; Pröbstl, D.; Nourshargh, S. Venular Basement Membranes Ubiquitously Express Matrix Protein Low-Expression Regions: Characterization in Multiple Tissues and Remodeling during Inflammation. Am. J. Pathol. 2010, 176, 482–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihara, J.; Ishihara, A.; Fukunaga, K.; Sasaki, K.; White, M.J.V.; Briquez, P.S.; Hubbell, J.A. Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat. Commun. 2018, 9, 2163. [Google Scholar] [CrossRef] [Green Version]
- Arnaoutova, I.; George, J.; Kleinman, H.K.; Benton, G. The endothelial cell tube formation assay on basement membrane turns 20: State of the science and the art. Angiogenesis 2009, 12, 267–274. [Google Scholar] [CrossRef]
- Pozzi, A.; Zent, R. Regulation of endothelial cell functions by basement membrane- and arachidonic acid-derived products. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 254–272. [Google Scholar] [CrossRef]
- Davis, G.E.; Senger, D.R. Endothelial extracellular matrix: Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 2005, 97, 1093–1107. [Google Scholar] [CrossRef] [Green Version]
- Park-Windhol, C.; D’Amore, P.A. Disorders of Vascular Permeability. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 251–281. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.Y.; Rigor, R.R. Chapter 4: The Endothelial Barrier. In Regulation of Endothelial Barrier Function; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Su, G.; Atakilit, A.; Li, J.T.; Wu, N.; Bhattacharya, M.; Zhu, J.; Shieh, J.E.; Li, E.; Chen, R.; Sun, S.; et al. Absence of integrin αvβ3 enhances vascular leak in mice by inhibiting endothelial cortical actin formation. Am. J. Respir. Crit. Care Med. 2012, 185, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Pulous, F.E.; Grimsley-Myers, C.M.; Kansal, S.; Kowalczyk, A.P.; Petrich, B.G. Talin-Dependent Integrin Activation Regulates VE-Cadherin Localization and Endothelial Cell Barrier Function. Circ. Res. 2019, 124, 891–903. [Google Scholar] [CrossRef]
- Alghisi, G.C.; Ponsonnet, L.; Rüegg, C. The integrin antagonist cilengitide activates alphaVbeta3, disrupts VE-cadherin localization at cell junctions and enhances permeability in endothelial cells. PLoS ONE 2009, 4, e4449. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Ehling, M.; Kato, K.; Kanai, K.; van Lessen, M.; Frye, M.; Zeuschner, D.; Nakayama, M.; Vestweber, D.; Adams, R.H. Integrin β1 controls VE-cadherin localization and blood vessel stability. Nat. Commun. 2015, 6, 6429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakanpaa, L.; Kiss, E.A.; Jacquemet, G.; Miinalainen, I.; Lerche, M.; Guzmán, C.; Mervaala, E.; Eklund, L.; Ivaska, J.; Saharinen, P. Targeting β1-integrin inhibits vascular leakage in endotoxemia. Proc. Natl. Acad. Sci. USA 2018, 115, E6467–E6476. [Google Scholar] [CrossRef] [Green Version]
- Yurdagul, A., Jr.; Orr, A.W. Blood Brothers: Hemodynamics and Cell-Matrix Interactions in Endothelial Function. Antioxid. Redox Signal. 2016, 25, 415–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izawa, Y.; Gu, Y.H.; Osada, T.; Kanazawa, M.; Hawkins, B.T.; Koziol, J.A.; Papayannopoulou, T.; Spatz, M.; del Zoppo, G.J. beta 1-integrin-matrix interactions modulate cerebral microvessel endothelial cell tight junction expression and permeability. J. Cereb. Blood Flow Metab. 2018, 38, 641–658. [Google Scholar] [CrossRef]
- Moore, D.H.; Ruska, H. The fine structure of capillaries and small arteries. J. Biophys. Biochem. Cytol. 1957, 3, 457–462. [Google Scholar] [CrossRef] [Green Version]
- Straub, A.C.; Zeigler, A.C.; Isakson, B.E. The myoendothelial junction: Connections that deliver the message. Physiology 2014, 29, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-J.; Kwok, C.; Juan, C.-C.; Hsu, Y.-P.; Shih, K.-C.; Chen, C.-C.; Ho, L.-T. Angiotensin II enhances endothelin-1-induced vasoconstriction through upregulating endothelin type A receptor. Biochem. Biophys. Res. Commun. 2014, 451, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Lilly, B. We Have Contact: Endothelial Cell-Smooth Muscle Cell Interactions. Physiology 2014, 29, 234–241. [Google Scholar] [CrossRef] [Green Version]
- McCallinhart, P.E.; Biwer, L.A.; Clark, O.E.; Isakson, B.E.; Lilly, B.; Trask, A.J. Myoendothelial Junctions of Mature Coronary Vessels Express Notch Signaling Proteins. Front. Physiol. 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Heberlein, K.R.; Straub, A.C.; Isakson, B.E. The myoendothelial junction: Breaking through the matrix? Microcirculation 2009, 16, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Pogoda, K.; Kameritsch, P. Molecular regulation of myoendothelial gap junctions. Curr. Opin. Pharmacol. 2019, 45, 16–22. [Google Scholar] [CrossRef]
- Xu, J.; Yang, G.M.; Li, T.; Liu, L.M. Myoendothelial gap junctions mediate regulation of angiopoietin-2-induced vascular hyporeactivity after hypoxia through connexin 43-gated cAMP transfer. Am. J. Physiol. Cell Physiol. 2017, 313, C262–C273. [Google Scholar] [CrossRef] [PubMed]
- Sowa, G. Caveolae, Caveolins, Cavins, and Endothelial Cell Function: New Insights. Front. Physiol. 2012, 2, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-Engineered Grafts from Human Decellularized Extracellular Matrices: A Systematic Review and Future Perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Boer, U.; Hurtado-Aguilar, L.G.; Klingenberg, M.; Lau, S.; Jockenhoevel, S.; Haverich, A.; Wilhelmi, M. Effect of Intensified Decellularization of Equine Carotid Arteries on Scaffold Biomechanics and Cytotoxicity. Ann. Biomed. Eng. 2015, 43, 2630–2641. [Google Scholar] [CrossRef]
- Lin, C.H.; Hsia, K.; Ma, H.; Lee, H.; Lu, J.H. In Vivo Performance of Decellularized Vascular Grafts: A Review Article. Int. J. Mol. Sci. 2018, 19, 2101. [Google Scholar] [CrossRef] [Green Version]
- Madden, R.; Lipkowitz, G.; Benedetto, B.; Kurbanov, A.; Miller, M.; Bow, L. Decellularized cadaver vein allografts used for hemodialysis access do not cause allosensitization or preclude kidney transplantation. Am. J. Kidney Dis. 2002, 40, 1240–1243. [Google Scholar] [CrossRef]
- Fischer, K.; Kraner-Scheiber, S.; Petersen, B.; Rieblinger, B.; Buermann, A.; Flisikowska, T.; Flisikowski, K.; Christan, S.; Edlinger, M.; Baars, W.; et al. Efficient production of multi-modified pigs for xenotransplantation by ‘combineering’, gene stacking and gene editing. Sci. Rep. 2016, 6, 29081. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.; Tang, X.H.; Gong, T.; Ye, W.; Pan, C.J.; Ding, H.Y.; Luo, X.; Li, X.; Wang, Q.M. Surface Modification with ECM-Inspired SDF-1 alpha/Laminin-Loaded Nanocoating for Vascular Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 30373–30386. [Google Scholar] [CrossRef]
- Anderson, D.E.J.; Truong, K.P.; Hagen, M.W.; Yim, E.K.F.; Hinds, M.T. Biomimetic modification of poly(vinyl alcohol): Encouraging endothelialization and preventing thrombosis with antiplatelet monotherapy. Acta Biomater. 2019, 86, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Radke, D.; Jia, W.; Sharma, D.; Fena, K.; Wang, G.; Goldman, J.; Zhao, F. Tissue Engineering at the Blood-Contacting Surface: A Review of Challenges and Strategies in Vascular Graft Development. Adv. Healthc. Mater. 2018, 7, e1701461. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; McKenzie, T.G.; O’Connor, A.J.; Qiao, G.G.; Heath, D.E. Nano-scale clustering of integrin-binding ligands regulates endothelial cell adhesion, migration, and endothelialization rate: Novel materials for small diameter vascular graft applications. J. Mater. Chem. B 2017, 5, 5942–5953. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Yao, D.Y.; Niu, Y.M.; Liu, H.F.; Fan, Y.B. Surface Modification of Multiple Bioactive Peptides to Improve Endothelialization of Vascular Grafts. Macromol. Biosci. 2019, 19, 1800368. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Li, Q.L.; Chen, J.L.; Luo, R.F.; Maitz, M.F.; Huang, N. Immobilization of serum albumin and peptide aptamer for EPC on polydopamine coated titanium surface for enhanced in-situ self-endothelialization. Mater. Sci. Eng. C 2016, 60, 219–229. [Google Scholar] [CrossRef]
- Wang, D.F.; Xu, Y.Y.; Wang, L.X.; Wang, X.F.; Yan, S.J.; Yilmaz, G.; Li, Q.; Turng, L.S. Long-term nitric oxide release for rapid endothelialization in expanded polytetrafluoroethylene small-diameter artificial blood vessel grafts. Appl. Surf. Sci. 2020, 507, 145028. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, L.C.; Ren, X.K.; Guo, J.T.; Xia, S.H.; Zhang, W.C.; Feng, Y.K. Co-immobilization of ACH(11) antithrombotic peptide and CAG cell-adhesive peptide onto vascular grafts for improved hemocompatibility and endothelialization. Acta Biomater. 2019, 97, 344–359. [Google Scholar] [CrossRef]
- Hao, D.K.; Fan, Y.H.; Xiao, W.W.; Liu, R.W.; Pivetti, C.; Walimbe, T.; Guo, F.Z.; Zhang, X.K.; Farmer, D.L.; Wang, F.S.; et al. Rapid endothelialization of small diameter vascular grafts by a bioactive integrin-binding ligand specifically targeting endothelial progenitor cells and endothelial cells. Acta Biomater. 2020, 108, 178–193. [Google Scholar] [CrossRef]
- Liao, S.R.; He, Q.; Yang, L.; Liu, S.; Zhang, Z.; Guidoin, R.; Fu, Q.; Xie, X.Y. Toward endothelialization via vascular endothelial growth factor immobilization on cell-repelling functional polyurethanes. J. Biomed. Mater. Res. Part B 2019, 107, 965–977. [Google Scholar] [CrossRef]
- Dai, W.W.; Guo, H.F.; Qian, D.H.; Qin, Z.X.; Lei, Y.; Hou, X.Y.; Wen, C. Improving endothelialization by the combined application of polyethylene glycol coated cerium oxide nanoparticles and VEGF in electrospun polyurethane scaffolds. J. Mater. Chem. B 2017, 5, 1053–1061. [Google Scholar] [CrossRef]
- Li, B.-c.; Chang, H.; Ren, K.-f.; Ji, J. Substrate-mediated delivery of gene complex nanoparticles via polydopamine coating for enhancing competitiveness of endothelial cells. Colloids Surf. B 2016, 147, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Chen, Y.; Tang, W.; Zhang, N.; Li, Z.; Liu, Z.; Yu, B.; Xu, F.-J. Reduction-Responsive Nucleic Acid Delivery Systems To Prevent In-Stent Restenosis in Rabbits. ACS Appl. Mater. Interfaces 2019, 11, 28307–28316. [Google Scholar] [CrossRef]
- Schulz, C.; Krüger-Genge, A.; Jung, F.; Lendlein, A. Aptamer supported in vitro endothelialization of poly(ether imide) films. Clin. Hemorheol. Microcirc. 2020, 75, 201–217. [Google Scholar] [CrossRef]
- Deng, J.C.; Yuan, S.H.; Li, X.; Wang, K.B.; Xie, L.X.; Li, N.; Wang, J.; Huang, N. Heparin/DNA aptamer co-assembled multifunctional catecholamine coating for EPC capture and improved hemocompatibility of vascular devices. Mater. Sci. Eng. C 2017, 79, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Wawrzynska, M.; Kraskiewicz, H.; Paprocka, M.; Krawczenko, A.; Bielawska-Pohl, A.; Bialy, D.; Roleder, T.; Wojakowski, W.; O’Connor, I.B.; Duda, M.; et al. Functionalization with a VEGFR2-binding antibody fragment leads to enhanced endothelialization of a cardiovascular stent in vitro and in vivo. J. Biomed. Mater. Res. Part B 2020, 108, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, H.P.; Wang, M.A.; Li, X.X.; Yin, H.H. Surface Coating of Polytetrafluoroethylene with Extracellular Matrix and Anti-CD34 Antibodies Facilitates Endothelialization and Inhibits Platelet Adhesion Under Sheer Stress. Tissue Eng. Regener. Med. 2017, 14, 359–370. [Google Scholar] [CrossRef]
- L’Heureux, N.; Pâquet, S.; Labbé, R.; Germain, L.; Auger, F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998, 12, 47–56. [Google Scholar] [CrossRef] [PubMed]
- L’Heureux, N.; Dusserre, N.; Konig, G.; Victor, B.; Keire, P.; Wight, T.N.; Chronos, N.A.; Kyles, A.E.; Gregory, C.R.; Hoyt, G.; et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat. Med. 2006, 12, 361–365. [Google Scholar] [CrossRef]
- McAllister, T.N.; Maruszewski, M.; Garrido, S.A.; Wystrychowski, W.; Dusserre, N.; Marini, A.; Zagalski, K.; Fiorillo, A.; Avila, H.; Manglano, X.; et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: A multicentre cohort study. Lancet 2009, 373, 1440–1446. [Google Scholar] [CrossRef]
- Wystrychowski, W.; McAllister, T.N.; Zagalski, K.; Dusserre, N.; Cierpka, L.; L’Heureux, N. First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. J. Vasc. Surg. 2014, 60, 1353–1357. [Google Scholar] [CrossRef] [Green Version]
- Magnan, L.; Labrunie, G.; Marais, S.; Rey, S.; Dusserre, N.; Bonneu, M.; Lacomme, S.; Gontier, E.; L’Heureux, N. Characterization of a Cell-Assembled extracellular Matrix and the effect of the devitalization process. Acta Biomater. 2018, 82, 56–67. [Google Scholar] [CrossRef]
- Carrabba, M.; Madeddu, P. Current Strategies for the Manufacture of Small Size Tissue Engineering Vascular Grafts. Front. Bioeng. Biotechnol. 2018, 6, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peck, M.; Gebhart, D.; Dusserre, N.; McAllister, T.N.; L’Heureux, N. The Evolution of Vascular Tissue Engineering and Current State of the Art. Cells Tissues Organs 2012, 195, 144–158. [Google Scholar] [CrossRef] [Green Version]
- Magnan, L.; Labrunie, G.; Fénelon, M.; Dusserre, N.; Foulc, M.-P.; Lafourcade, M.; Svahn, I.; Gontier, E.; Vélez, V.; Jaime, H.; et al. Human textiles: A cell-synthesized yarn as a truly “bio” material for tissue engineering applications. Acta Biomater. 2020, 105, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, U.; Tonooka, Y.; Koretake, R.; Akimoto, T.; Gonda, Y.; Saito, J.; Umemura, M.; Fujita, T.; Sakuma, S.; Arai, F.; et al. Arterial graft with elastic layer structure grown from cells. Sci. Rep. 2017, 7, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bornstädt, D.V.; Wang, H.; Paulsen, M.J.; Goldstone, A.B.; Eskandari, A.; Thakore, A.; Stapleton, L.; Steele, A.N.; Truong, V.N.; Jaatinen, K.; et al. Rapid Self-Assembly of Bioengineered Cardiovascular Bypass Grafts From Scaffold-Stabilized, Tubular Bilevel Cell Sheets. Circulation 2018, 138, 2130–2144. [Google Scholar] [CrossRef]
- Weinberg, C.B.; Bell, E. A blood vessel model constructed from collagen and cultured vascular cells. Science 1986, 231, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Dahl, S.L.M.; Kypson, A.P.; Lawson, J.H.; Blum, J.L.; Strader, J.T.; Li, Y.L.; Manson, R.J.; Tente, W.E.; DiBernardo, L.; Hensley, M.T.; et al. Readily Available Tissue-Engineered Vascular Grafts. Sci. Transl. Med. 2011, 3, 68ra69. [Google Scholar] [CrossRef]
- Kirkton, R.D.; Santiago-Maysonet, M.; Lawsonl, J.H.; Tente, W.E.; Dahl, S.L.M.; Niklason, L.E.; Prichard, H.L. Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci. Transl. Med. 2019, 11, eaau6934. [Google Scholar] [CrossRef]
- Gui, L.Q.; Boyle, M.J.; Kamin, Y.M.; Huang, A.H.; Starcher, B.C.; Miller, C.A.; Vishnevetsky, M.J.; Niklason, L.E. Construction of Tissue-Engineered Small-Diameter Vascular Grafts in Fibrin Scaffolds in 30 Days. Tissue Eng. Part A 2014, 20, 1499–1507. [Google Scholar] [CrossRef] [Green Version]
- Niklason, L.E.; Lawson, J.H. Bioengineered human blood vessels. Science 2020, 370, eaaw8682. [Google Scholar] [CrossRef]
- Zeng, J.; Sasaki, N.; Correia, C.R.; Mano, J.F.; Matsusaki, M. Fabrication of Artificial Nanobasement Membranes for Cell Compartmentalization in 3D Tissues. Small 2020, 16, 1907434. [Google Scholar] [CrossRef]
- Palchesko, R.N.; Funderburgh, J.L.; Feinberg, A.W. Engineered Basement Membranes for Regenerating the Corneal Endothelium. Adv. Healthc. Mater. 2016, 5, 2942–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davoudi, P.; Assadpour, S.; Derakhshan, M.A.; Ai, J.; Solouk, A.; Ghanbari, H. Biomimetic modification of polyurethane-based nanofibrous vascular grafts: A promising approach towards stable endothelial lining. Mater. Sci. Eng. C 2017, 80, 213–221. [Google Scholar] [CrossRef]
- Yu, C.L.; Xing, M.Y.; Sun, S.B.; Guan, G.P.; Wang, L. In vitro evaluation of vascular endothelial cell behaviors on biomimetic vascular basement membranes. Colloids Surf. B 2019, 182, 110381. [Google Scholar] [CrossRef]
- Zhao, Q.L.; Cui, H.Q.; Wang, J.; Chen, H.X.; Wang, Y.L.; Zhang, L.D.; Du, X.M.; Wang, M. Regulation Effects of Biomimetic Hybrid Scaffolds on Vascular Endothelium Remodeling. ACS Appl. Mater. Interfaces 2018, 10, 23583–23594. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Cui, H.; Chen, H.; Wang, Y.; Du, X. Programmed Shape-Morphing Scaffolds Enabling Facile 3D Endothelialization. Adv. Funct. Mater. 2018, 28, 1801027. [Google Scholar] [CrossRef]
- Chen, M.; Li, L.; Xia, L.; Zhang, F.; Jiang, S.; Hu, H.; Li, X.; Wang, H. Temperature Responsive Shape-Memory Scaffolds with Circumferentially Aligned Nanofibers for Guiding Smooth Muscle Cell Behavior. Macromol. Biosci. 2020, 20, e1900312. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.W.; UVP Inc. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 4575330, 11 March 1986. [Google Scholar]
- Miri, A.K.; Khalilpour, A.; Cecen, B.; Maharjan, S.; Shin, S.R.; Khademhosseini, A. Multiscale bioprinting of vascularized models. Biomaterials 2019, 198, 204–216. [Google Scholar] [CrossRef]
- Tomasina, C.; Bodet, T.; Mota, C.; Moroni, L.; Camarero-Espinosa, S. Bioprinting Vasculature: Materials, Cells and Emergent Techniques. Materials 2019, 12, 2701. [Google Scholar] [CrossRef] [Green Version]
- Papaioannou, T.G.; Manolesou, D.; Dimakakos, E.; Tsoucalas, G.; Vavuranakis, M.; Tousoulis, D. 3D Bioprinting Methods and Techniques: Applications on Artificial Blood Vessel Fabrication. Acta Cardiol. Sin. 2019, 35, 284–289. [Google Scholar] [CrossRef]
- Sun, W.; Starly, B.; Daly, A.C.; Burdick, J.A.; Groll, J.; Skeldon, G.; Shu, W.; Sakai, Y.; Shinohara, M.; Nishikawa, M.; et al. The bioprinting roadmap. Biofabrication 2020, 12, 022002. [Google Scholar] [CrossRef] [PubMed]
- Sasmal, P.; Datta, P.; Wu, Y.; Ozbolat, I.T. 3D bioprinting for modelling vasculature. Microphysiol. Syst. 2018, 2, 9. [Google Scholar] [CrossRef]
- Jeong, H.J.; Nam, H.; Jang, J.; Lee, S.J. 3D Bioprinting Strategies for the Regeneration of Functional Tubular Tissues and Organs. Bioengineering 2020, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Ng, H.Y.; Lee, K.-X.A.; Kuo, C.-N.; Shen, Y.-F. Bioprinting of artificial blood vessels. Int. J. Bioprint. 2018, 4, 140. [Google Scholar] [CrossRef] [PubMed]
- Masaeli, E.; Forster, V.; Picaud, S.; Karamali, F.; Nasr-Esfahani, M.H.; Marquette, C. Tissue engineering of retina through high resolution 3-dimensional inkjet bioprinting. Biofabrication 2020, 12, 025006. [Google Scholar] [CrossRef]
- Huber, B.; Engelhardt, S.; Meyer, W.; Kruger, H.; Wenz, A.; Schonhaar, V.; Tovar, G.E.M.; Kluger, P.J.; Borchers, K. Blood-Vessel Mimicking Structures by Stereolithographic Fabrication of Small Porous Tubes Using Cytocompatible Polyacrylate Elastomers, Biofunctionalization and Endothelialization. J. Funct. Biomater. 2016, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Kim, H.; Kim, B.S.; Kong, J.S.; Lee, J.Y.; Park, B.W.; Chae, S.; Kim, J.; Ban, K.; Jang, J.; et al. Tissue-engineering of vascular grafts containing endothelium and smooth-muscle using triple-coaxial cell printing. Appl. Phys. Rev. 2019, 6, 041402. [Google Scholar] [CrossRef]
- Zhou, Y.; Gui, Q.Y.; Yu, W.Y.; Liao, S.L.; He, Y.L.; Tao, X.L.; Yu, Y.; Wang, Y.P. Interfacial Diffusion Printing: An Efficient Manufacturing Technique for Artificial Tubular Grafts. ACS Biomater. Sci. Eng. 2019, 5, 6311–6318. [Google Scholar] [CrossRef] [Green Version]
- Freeman, S.; Ramos, R.; Chando, P.A.; Zhou, L.X.; Reeser, K.; Jin, S.; Soman, P.; Ye, K.M. A bioink blend for rotary 3D bioprinting tissue engineered small-diameter vascular constructs. Acta Biomater. 2019, 95, 152–164. [Google Scholar] [CrossRef]
- Tamay, D.G.; Dursun Usal, T.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2019, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Zilla, P.; Deutsch, M.; Bezuidenhout, D.; Davies, N.H.; Pennel, T. Progressive Reinvention or Destination Lost? Half a Century of Cardiovascular Tissue Engineering. Front. Cardiovasc. Med. 2020, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Pashuck, E.T.; Duchet, B.J.R.; Hansel, C.S.; Maynard, S.A.; Chow, L.W.; Stevens, M.M. Controlled Sub-Nanometer Epitope Spacing in a Three-Dimensional Self-Assembled Peptide Hydrogel. ACS Nano 2016, 10, 11096–11104. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, S.; Gossen, M.; Lendlein, A. Designing Cardiovascular Implants Taking in View the Endothelial Basement Membrane. Int. J. Mol. Sci. 2021, 22, 13120. https://doi.org/10.3390/ijms222313120
Lau S, Gossen M, Lendlein A. Designing Cardiovascular Implants Taking in View the Endothelial Basement Membrane. International Journal of Molecular Sciences. 2021; 22(23):13120. https://doi.org/10.3390/ijms222313120
Chicago/Turabian StyleLau, Skadi, Manfred Gossen, and Andreas Lendlein. 2021. "Designing Cardiovascular Implants Taking in View the Endothelial Basement Membrane" International Journal of Molecular Sciences 22, no. 23: 13120. https://doi.org/10.3390/ijms222313120
APA StyleLau, S., Gossen, M., & Lendlein, A. (2021). Designing Cardiovascular Implants Taking in View the Endothelial Basement Membrane. International Journal of Molecular Sciences, 22(23), 13120. https://doi.org/10.3390/ijms222313120





