Current Status of Biomarkers in Anti-N-Methyl-D-Aspartate Receptor Encephalitis
Abstract
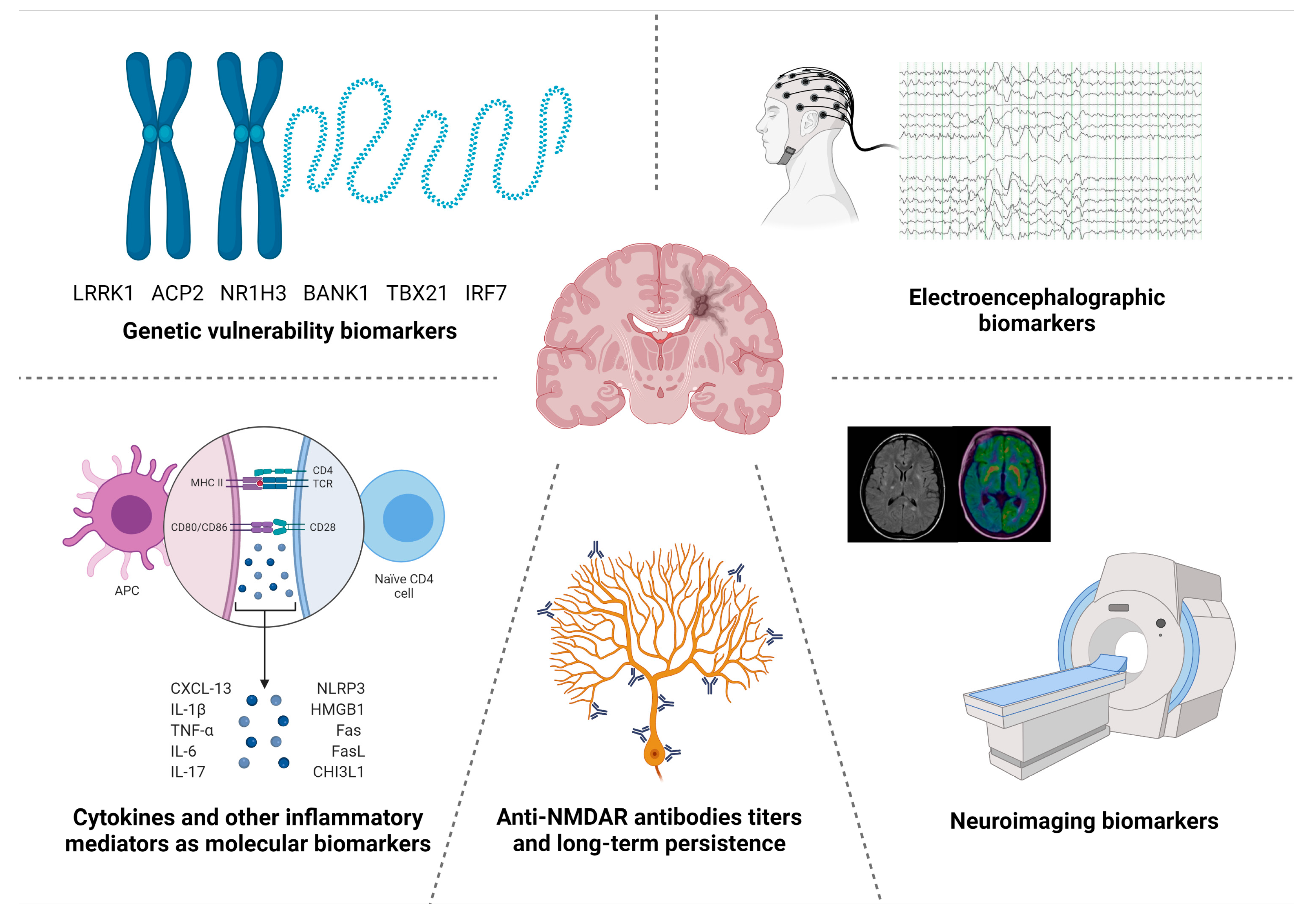
:1. Introduction
2. An Overview of Anti-NMDAR Encephalitis
3. Clinical and Paraclinical Features as Markers of Anti-NMDAR Encephalitis
3.1. Clinical Predictors
3.2. Neuroimaging Biomarkers
3.3. Electroencephalographic Biomarkers
4. Molecular Biomarkers in Anti-NMDAR Encephalitis
4.1. Blood and CSF Soluble Biomarkers
4.1.1. Anti-NMDAR Antibodies
4.1.2. Cytokines
4.1.3. Other Molecular Biomarkers
4.2. Genetic Susceptibility Biomarkers
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hébert, J.; Riche, B.; Vogrig, A.; Muñiz-Castrillo, S.; Joubert, B.; Picard, G.; Rogemond, V.; Psimaras, D.; Alentorn, A.; Berzero, G.; et al. Epidemiology of paraneoplastic neurologic syndromes and autoimmune encephalitides in France. Neurol. Neuroimmunol. Neuroinflammation 2020, 7, e883. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, J.; Armangué, T.; Planagumà, J.; Radosevic, M.; Mannara, F.; Leypoldt, F.; Geis, C.; Lancaster, E.; Titulaer, M.J.; Rosenfeld, M.R.; et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: Mechanisms and models. Lancet Neurol. 2019, 18, 1045–1057. [Google Scholar] [CrossRef]
- Orphanet Rare Diseases Anti-NMDAR Encephalitis. Available online: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=ES&Expert=217253 (accessed on 7 November 2021).
- Orphanet Rare Diseases Definition. Available online: https://www.orpha.net/consor/cgi-bin/Disease.php?lng=EN (accessed on 7 November 2021).
- Kempf, L.; Goldsmith, J.C.; Temple, R. Challenges of developing and conducting clinical trials in rare disorders. Am. J. Med. Genet. Part A 2018, 176, 773–783. [Google Scholar] [CrossRef]
- Atkinson, A.J.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Schooley, R.T.; Spilker, B.A.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar]
- FDA Biomarker Definition. Available online: https://www.fda.gov/drugs/biomarker-qualification-program/about-biomarkers-and-qualification#what-is (accessed on 7 November 2021).
- Parikh, N.I.; Vasan, R.S. Assessing the clinical utility of biomarkers in medicine. Biomark. Med. 2007, 1, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K.; Ferner, R.E. Biomarkers—a general review. Curr. Protoc. Pharmacol. 2017, 2017, 9.23.1–9.23.17. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B. Biomarkers as drug development tools: Discovery, validation, qualification and use. Nat. Rev. Rheumatol. 2018, 14, 354–362. [Google Scholar] [CrossRef]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools). 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK326791/ (accessed on 8 November 2021).
- Balu, R.; Mccracken, L.; Lancaster, E.; Graus, F.; Dalmau, J.; Titulaer, M.J. A score that predicts 1-year functional status in patients with anti-NMDA receptor encephalitis. Neurology 2019, 92, E244–E252. [Google Scholar] [CrossRef] [Green Version]
- Dalmau, J.; Geis, C.; Graus, F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol. Rev. 2017, 97, 839–887. [Google Scholar] [CrossRef]
- Nosadini, M.; Eyre, M.; Molteni, E.; Thomas, T.; Irani, S.R.; Dalmau, J.; Dale, R.C.; Lim, M.; Anlar, B.; Armangue, T.; et al. Use and Safety of Immunotherapeutic Management of N -Methyl- d -Aspartate Receptor Antibody Encephalitis: A Meta-analysis. JAMA Neurol. 2021, 1–12. [Google Scholar] [CrossRef]
- Titulaer, M.J.; McCracken, L.; Gabilondo, I.; Armangué, T.; Glaser, C.; Iizuka, T.; Honig, L.S.; Benseler, S.M.; Kawachi, I.; Martinez-Hernandez, E.; et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: An observational cohort study. Lancet Neurol. 2013, 12, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Favier, M.; Joubert, B.; Picard, G.; Rogemond, V.; Thomas, L.; Rheims, S.; Bailhache, M.; Villega, F.; Pédespan, J.M.; Berzero, G.; et al. Initial clinical presentation of young children with N-methyl-D-aspartate receptor encephalitis. Eur. J. Paediatr. Neurol. 2018, 22, 404–411. [Google Scholar] [CrossRef]
- Viaccoz, A.; Desestret, V.; Ducray, F.; Picard, G.; Cavillon, G.; Rogemond, V.; Antoine, J.C.; Delattre, J.Y.; Honnorat, J. Clinical specificities of adult male patients with NMDA receptor antibodies encephalitis. Neurology 2014, 82, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, J.; Lancaster, E.; Martinez-Hernandez, E.; Rosenfeld, M.R.; Balice-Gordon, R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011, 10, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Dalmau, J.; Tüzün, E.; Wu, H.; Masjuan, J.; Rossi, J.E.; Voloschin, A.; Baehring, J.M.; Shimazaki, H.; Koide, R.; King, D.; et al. Paraneoplastic Anti-N-methyl-D-aspartate Receptor Encephalitis Associated with Ovarian Teratoma. Ann. Neurol. 2007, 61, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Chefdeville, A.; Treilleux, I.; Mayeur, M.E.; Couillault, C.; Picard, G.; Bost, C.; Mokhtari, K.; Vasiljevic, A.; Meyronet, D.; Rogemond, V.; et al. Immunopathological characterization of ovarian teratomas associated with anti-N-methyl-D-aspartate receptor encephalitis. Acta Neuropathol. Commun. 2019, 7, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavaliere, E.; Nosadini, M.; Pelizza, M.F.; Ventura, G.; Toldo, I.; Sartori, S. Anti-NMDAR encephalitis preceded by non-herpetic central nervous system infection: Systematic literature review and first case of tick-borne encephalitis triggering anti-NMDAR encephalitis. J. Neuroimmunol. 2019, 332, 1–7. [Google Scholar] [CrossRef]
- Armangue, T.; Spatola, M.; Vlagea, A.; Mattozzi, S.; Cárceles-Cordon, M.; Martinez-Heras, E.; Llufriu, S.; Muchart, J.; Erro, M.E.; Abraira, L.; et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: A prospective observational study and retrospective analysis. Lancet Neurol. 2018, 17, 760–772. [Google Scholar] [CrossRef] [Green Version]
- Vogrig, A.; Muñiz-Castrillo, S.; Desestret, V.; Joubert, B.; Honnorat, J. Pathophysiology of paraneoplastic and autoimmune encephalitis: Genes, infections, and checkpoint inhibitors. Ther. Adv. Neurol. Disord. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Salovin, A.; Glanzman, J.; Roslin, K.; Armangue, T.; Lynch, D.R.; Panzer, J.A. Anti-NMDA receptor encephalitis and nonencephalitic HSV-1 infection. Neurol. Neuroimmunol. NeuroInflammation 2018, 5, 4–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwenkenbecher, P.; Skripuletz, T.; Lange, P.; Dürr, M.; Konen, F.F.; Möhn, N.; Ringelstein, M.; Menge, T.; Friese, M.A.; Melzer, N.; et al. Intrathecal Antibody Production against Epstein-Barr, Herpes Simplex, and Other Neurotropic Viruses in Autoimmune Encephalitis. Neurol. Neuroimmunol. Neuroinflammation 2021, 8, e1062. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Titulaer, M.J.; Balu, R.; Benseler, S.; Bien, C.G.; Cellucci, T.; Cortese, I.; Dale, R.C.; Gelfand, J.M.; Geschwind, M.; et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016, 15, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Dalmau, J.; Gleichman, A.J.; Hughes, E.G.; Rey, J.; Rossi, E.; Peng, X.; Lai, M.; Dessain, S.K.; Rosenfeld, M.R.; Balice-Gordon, R.; et al. Articles Anti-NMDA-receptor encephalitis: Case series and analysis of the eff ects of antibodies. Lancet Neurol. 2008, 7, 1091–1098. [Google Scholar] [CrossRef] [Green Version]
- Hughes, E.G.; Peng, X.; Gleichman, A.J.; Lai, M.; Zhou, L.; Tsou, R.; Parsons, T.D.; Lynch, D.R.; Dalmau, J.; Balice-Gordon, R.J. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J. Neurosci. 2010, 30, 5866–5875. [Google Scholar] [CrossRef] [Green Version]
- Planagumà, J.; Leypoldt, F.; Mannara, F.; Gutiérrez-Cuesta, J.; Martín-García, E.; Aguilar, E.; Titulaer, M.J.; Petit-Pedrol, M.; Jain, A.; Balice-Gordon, R.; et al. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain 2015, 138, 94–109. [Google Scholar] [CrossRef]
- Mikasova, L.; De Rossi, P.; Bouchet, D.; Georges, F.; Rogemond, V.; Didelot, A.; Meissirel, C.; Honnorat, J.; Groc, L. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain 2012, 135, 1606–1621. [Google Scholar] [CrossRef] [Green Version]
- Dale, R.C.; Pillai, S.; Brilot, F. Cerebrospinal fluid CD19+ B-cell expansion in N-methyl-D-aspartate receptor encephalitis. Dev. Med. Child Neurol. 2013, 55, 191–193. [Google Scholar] [CrossRef]
- Hachiya, Y.; Uruha, A.; Kasai-Yoshida, E.; Shimoda, K.; Satoh-Shirai, I.; Kumada, S.; Kurihara, E.; Suzuki, K.; Ohba, A.; Hamano, S.; et al. Rituximab ameliorates anti-N-methyl-d-aspartate receptor encephalitis by removal of short-lived plasmablasts. J. Neuroimmunol. 2013, 265, 128–130. [Google Scholar] [CrossRef]
- Malviya, M.; Barman, S.; Golombeck, K.S.; Planagumà, J.; Mannara, F.; Strutz-Seebohm, N.; Wrzos, C.; Demir, F.; Baksmeier, C.; Steckel, J.; et al. NMDAR encephalitis: Passive transfer from man to mouse by a recombinant antibody. Ann. Clin. Transl. Neurol. 2017, 4, 768–783. [Google Scholar] [CrossRef] [Green Version]
- Bien, C.G.; Vincent, A.; Barnett, M.H.; Becker, A.J.; Blümcke, I.; Graus, F.; Jellinger, K.A.; Reuss, D.E.; Ribalta, T.; Schlegel, J.; et al. Immunopathology of autoantibody-associated encephalitides: Clues for pathogenesis. Brain 2012, 135, 1622–1638. [Google Scholar] [CrossRef] [Green Version]
- Camdessanché, J.P.; Streichenberger, N.; Cavillon, G.; Rogemond, V.; Jousserand, G.; Honnorat, J.; Convers, P.; Antoine, J.C. Brain immunohistopathological study in a patient with anti-NMDAR encephalitis. Eur. J. Neurol. 2011, 18, 929–931. [Google Scholar] [CrossRef]
- Martinez-Hernandez, E.; Horvath, J.; Shiloh-Malawsky, Y.; Sangha, N.; Martinez-Lage, M.; Dalmau, J. Analysis of Complement and Plasma Cells in the Brain of Patients with Anti-NMDAR Encephalitis. Neurology 2011, 77, 589–593. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Lee, T.S.; Shin, W.Y.; Lee, S.H.; Shin, R.H.; Kim, Y.D.; Kim, S.; Lim, A.J.; Moom, J.; Park, K.I.; et al. Teratoma Removal, Steroid, IVIG, Rituximab and Tocilizumab (T-SIRT) in Anti-NMDAR Encephalitis. Neurotherapeutics 2020, 18, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Sell, J.; Haselmann, H.; Hallermann, S.; Hust, M.; Geis, C. Autoimmune encephalitis: Novel therapeutic targets at the preclinical level. Expert Opin. Ther. Targets 2021, 25, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Ratuszny, D.; Skripuletz, T.; Wegner, F.; Groß, M.; Falk, C.; Jacobs, R.; Ruschulte, H.; Stangel, M.; Sühs, K.W. Case Report: Daratumumab in a Patient With Severe Refractory Anti-NMDA Receptor Encephalitis. Front. Neurol. 2020, 11, 1635. [Google Scholar] [CrossRef]
- Behrendt, V.; Krogias, C.; Reinacher-Schick, A.; Gold, R.; Kleiter, I. Bortezomib treatment for patients with anti-N-methyl-D-aspartate receptor encephalitis. JAMA Neurol. 2016, 73, 1251–1253. [Google Scholar] [CrossRef] [Green Version]
- Keddie, S.; Crisp, S.J.; Blackaby, J.; Cox, A.; Coles, A.; Hart, M.; Church, A.J.; Vincent, A.; Zandi, M.; Lunn, M.P. Plasma cell depletion with bortezomib in the treatment of refractory N-methyl-d-aspartate (NMDA) receptor antibody encephalitis. Rational developments in neuroimmunological treatment. Eur. J. Neurol. 2018, 25, 1384–1388. [Google Scholar] [CrossRef]
- Titulaer, M.J.; McCracken, L.; Gabilondo, I.; Iizuka, T.; Kawachi, I.; Bataller, L.; Torrents, A.; Dalmau, J. Late-onset anti-NMDA receptor encephalitis. Neurology 2013, 81, 1058–1063. [Google Scholar] [CrossRef] [Green Version]
- Irani, S.R.; Bera, K.; Waters, P.; Zuliani, L.; Maxwell, S.; Zandi, M.S.; Friese, M.A.; Galea, I.; Kullmann, D.M.; Beeson, D.; et al. N-methyl-d-aspartate antibody encephalitis: Temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010, 133, 1655–1667. [Google Scholar] [CrossRef] [PubMed]
- Titulaer, M.J.; Höftberger, R.; Iizuka, T.; Leypoldt, F.; McCracken, L.; Cellucci, T.; Benson, L.A.; Shu, H.; Irioka, T.; Hirano, M.; et al. Overlapping demyelinating syndromes and anti-N-methyl-D-aspartate receptor encephalitis. Ann. Neurol. 2014, 75, 411–428. [Google Scholar] [CrossRef]
- Zhang, T.; Duan, Y.; Ye, J.; Xu, W.; Shu, N.; Wang, C.; Li, K.; Liu, Y. Brain MRI characteristics of patients with anti-n-methyl-daspartate receptor encephalitis and their associations with 2-year clinical outcome. Am. J. Neuroradiol. 2018, 39, 824–829. [Google Scholar] [CrossRef] [Green Version]
- Bacchi, S.; Franke, K.; Wewegama, D.; Needham, E.; Patel, S.; Menon, D. Magnetic resonance imaging and positron emission tomography in anti-NMDA receptor encephalitis: A systematic review. J. Clin. Neurosci. 2018, 52, 54–59. [Google Scholar] [CrossRef]
- Heine, J.; Prüss, H.; Bartsch, T.; Ploner, C.J.; Paul, F.; Finke, C. Imaging of autoimmune encephalitis—Relevance for clinical practice and hippocampal function. Neuroscience 2015, 309, 68–83. [Google Scholar] [CrossRef]
- Iizuka, T.; Kaneko, J.; Tominaga, N.; Someko, H.; Nakamura, M.; Ishima, D.; Kitamura, E.; Masuda, R.; Oguni, E.; Yanagisawa, T.; et al. Association of progressive cerebellar atrophy with long-term outcome in patients with Anti-N-Methyl-D-Aspartate receptor encephalitis. JAMA Neurol. 2016, 73, 706–713. [Google Scholar] [CrossRef] [Green Version]
- Finke, C.; Kopp, U.A.; Pajkert, A.; Behrens, J.R.; Leypoldt, F.; Wuerfel, J.T.; Ploner, C.J.; Prüss, H.; Paul, F. Structural Hippocampal Damage Following Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Biol. Psychiatry 2016, 79, 727–734. [Google Scholar] [CrossRef]
- Peer, M.; Prüss, H.; Ben-Dayan, I.; Paul, F.; Arzy, S.; Finke, C. Functional connectivity of large-scale brain networks in patients with anti-NMDA receptor encephalitis: An observational study. Lancet Psychiatry 2017, 4, 768–774. [Google Scholar] [CrossRef]
- Leypoldt, F.; Buchert, R.; Kleiter, I.; Marienhagen, J.; Gelderblom, M.; Magnus, T.; Dalmau, J.; Gerloff, C.; Lewerenz, J. Fluorodeoxyglucose positron emission tomography in anti-N-methyl-D- aspartate receptor encephalitis: Distinct pattern of disease. J. Neurol. Neurosurg. Psychiatry 2012, 83, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.C.; Tseng, J.R.; Wu, C.L.; Su, F.C.; Weng, W.C.; Hsu, C.C.; Chang, K.H.; Wu, C.F.; Hsiao, I.T.; Lin, C.P. Different FDG-PET metabolic patterns of anti-AMPAR and anti-NMDAR encephalitis: Case report and literature review. Brain Behav. 2020, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Rosch, R.E.; Wright, S.; Cooray, G.; Papadopoulou, M.; Goyal, S.; Lim, M.; Vincent, A.; Upton, A.L.; Baldeweg, T.; Friston, K.J. NMDA-receptor antibodies alter cortical microcircuit dynamics. Proc. Natl. Acad. Sci. USA 2018, 115, E9916–E9925. [Google Scholar] [CrossRef] [Green Version]
- Gillinder, L.; Warren, N.; Hartel, G.; Dionisio, S.; O’Gorman, C. EEG findings in NMDA encephalitis—A systematic review. Seizure 2019, 65, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, S.E.; Pargeon, K.; Frechette, E.S.; Hirsch, L.J.; Dalmau, J.; Friedman, D. Extreme delta brush; A unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology 2012, 79, 1094–1100. [Google Scholar] [CrossRef] [Green Version]
- Jeannin-Mayer, S.; André-Obadia, N.; Rosenberg, S.; Boutet, C.; Honnorat, J.; Antoine, J.C.; Mazzola, L. EEG analysis in anti-NMDA receptor encephalitis: Description of typical patterns. Clin. Neurophysiol. 2019, 130, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Van Sonderen, A.; Arends, S.; Tavy, D.L.J.; Bastiaansen, A.E.M.; De Bruijn, M.A.A.M.; Schreurs, M.W.J.; Smitt, P.A.E.S.; Titulaer, M.J. Predictive value of electroencephalography in anti-NMDA receptor encephalitis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Nathoo, N.; Anderson, D.; Jirsch, J. Extreme Delta Brush in Anti-NMDAR Encephalitis Correlates with Poor Functional Outcome and Death. Front. Neurol. 2021, 12, 686521. [Google Scholar] [CrossRef] [PubMed]
- Steriade, C.; Hantus, S.; Moosa, A.N.V.; Rae-Grant, A.D. Extreme delta—With or without brushes: A potential surrogate marker of disease activity in anti-NMDA-receptor encephalitis. Clin. Neurophysiol. 2018, 129, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Gofshteyn, J.S.; Yeshokumar, A.K.; Jette, N.; Thakur, K.T.; Luche, N.; Yozawitz, E.; Varnado, S.; Klenofsky, B.; Tuohy, M.C.; Ankam, J.; et al. Clinical and electrographic features of persistent seizures and status epilepticus associated with anti-NMDA receptor encephalitis (anti-NMDARE). Epileptic Disord. 2020, 22, 739–751. [Google Scholar] [CrossRef]
- Foff, E.P.; Taplinger, D.; Suski, J.; Lopes, M.B.S.; Quigg, M. EEG Findings May Serve as a Potential Biomarker for Anti-NMDA Receptor Encephalitis. Clin. EEG Neurosci. 2017, 48, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Blackman, G.; Kumar, K.; Hanrahan, J.G.; Dalrymple, A.; Mullatti, N.; Moran, N.; Valentin, A.; Gibson, L.; Pollak, T.A.; David, A. Quantitative EEG as a Prognostic Tool in Suspected Anti-N-Methyl-D-Aspartate Receptor Antibody Encephalitis. J. Clin. Neurophysiol. 2021. [Google Scholar] [CrossRef]
- Jiang, N.; Guan, H.; Lu, Q.; Ren, H.; Peng, B. Features and prognostic value of quantitative electroencephalogram changes in critically ill and non-critically ill anti-NMDAR encephalitis patients: A pilot study. Front. Neurol. 2018, 9, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Wolters, J.E.J.; Van Breda, S.G.; Kleinjans, J.C.; De Kok, T.M. Development of novel tools for the in vitro investigation of drug-induced liver injury. Expert Opin. Drug Metab. Toxicol. 2015, 11, 1523–1537. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, M.P.; Diamandis, E.P.; Blasutig, I.M. The long journey of cancer biomarkers from the bench to the clinic. Clin. Chem. 2013, 59, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Hara, M.; Martinez-Hernandez, E.; Ariño, H.; Armangué, T.; Spatola, M.; Petit-Pedrol, M.; Saiz, A.; Rosenfeld, M.R.; Graus, F.; Dalmau, J. Clinical and pathogenic significance of IgG, IgA, and IgM antibodies against the NMDA receptor. Neurology 2018, 90, E1386–E1394. [Google Scholar] [CrossRef]
- Gresa-Arribas, N.; Titulaer, M.J.; Torrents, A.; Aguilar, E.; McCracken, L.; Leypoldt, F.; Gleichman, A.J.; Balice-Gordon, R.; Rosenfeld, M.R.; Lynch, D.; et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: A retrospective study. Lancet Neurol. 2014, 13, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Desestret, V.; Chefdeville, A.; Viaccoz, A.; Bost, C.; Ducray, F.; Picard, G.; Rogemond, V.; Chaffois, M.O.; Blanc, C.; Bardel, C.; et al. CSF IgA NMDAR antibodies are potential biomarkers for teratomas in anti-NMDAR encephalitis. Neurol. Neuroimmunol. NeuroInflammation 2015, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-García, R.; Muñoz-Sánchez, G.; Naranjo, L.; Guasp, M.; Sabater, L.; Saiz, A.; Dalmau, J.; Graus, F.; Martinez-Hernandez, E. Limitations of a Commercial Assay as Diagnostic Test of Autoimmune Encephalitis. Front. Immunol. 2021, 12, 691536. [Google Scholar] [CrossRef]
- Hirohata, S.; Tanaka, K. Differential expression of antibodies to NMDA receptor in anti-NMDA receptor encephalitis and in neuropsychiatric systemic lupus erythematosus. Lupus Sci. Med. 2019, 6, 1–6. [Google Scholar] [CrossRef]
- Hansen, H.C.; Klingbeil, C.; Dalmau, J.; Li, W.; Weißbrich, B.; Wandinger, K.P. P. Persistent intrathecal antibody synthesis 15 years after recovering from anti-n-methyl-d-aspartate receptor encephalitis. Arch. Neurol. 2013, 70, 117–119. [Google Scholar] [CrossRef] [Green Version]
- Pranzatelli, M.R. Advances in biomarker-guided therapy for pediatric- and adult-onset neuroinflammatory disorders: Targeting chemokines/cytokines. Front. Immunol. 2018, 9, 557. [Google Scholar] [CrossRef]
- Leypoldt, F.; Höftberger, R.; Titulaer, M.J.; Armangue, T.; Gresa-Arribas, N.; Jahn, H.; Rostásy, K.; Schlumberger, W.; Meyer, T.; Wandinger, K.P.; et al. Investigations on CXCL13 in anti-N-methyl-D-aspartate receptor encephalitis: A potential biomarker of treatment response. JAMA Neurol. 2015, 72, 180–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liba, Z.; Kayserova, J.; Elisak, M.; Marusic, P.; Nohejlova, H.; Hanzalova, J.; Komarek, V.; Sediva, A. Anti-N-methyl-D-aspartate receptor encephalitis: The clinical course in light of the chemokine and cytokine levels in cerebrospinal fluid. J. Neuroinflammation 2016, 13, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, J.I.; Lee, S.T.; Moon, J.; Jung, K.H.; Sunwoo, J.S.; Lim, J.A.; Kim, T.J.; Shin, Y.W.; Lee, K.J.; Jun, J.S.; et al. Distinct intrathecal interleukin-17/interleukin-6 activation in anti-N-methyl-D-aspartate receptor encephalitis. J. Neuroimmunol. 2016, 297, 141–147. [Google Scholar] [CrossRef]
- Kothur, K.; Wienholt, L.; Mohammad, S.S.; Tantsis, E.M.; Pillai, S.; Britton, P.N.; Jones, C.A.; Angiti, R.R.; Barnes, E.H.; Schlub, T.; et al. Utility of CSF cytokine/chemokines as markers of active intrathecal inflammation: Comparison of demyelinating, anti-NMDAR and enteroviral encephalitis. PLoS ONE 2016, 11, e0161656. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Chen, L.; Chen, B.; Cai, Y.; Li, P.; Yan, L.; Zeng, D. Th17 cells were recruited and accumulated in the cerebrospinal fluid and correlated with the poor prognosis of anti-NMDAR encephalitis. Acta Biochim. Biophys. Sin. 2018, 50, 1266–1273. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Kang, W.; Peng, G.; Yu, D.; Ma, Q.; Li, Y.; Zhao, Y.; Li, L.; Dai, F.; et al. Cytokines/Chemokines: Potential Biomarkers for Non-paraneoplastic Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Front. Neurol. 2020, 11, 582296. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Liu, X.N.; Li, X.; Zhang, X.; Quan, C.; Chen, X.J. Raised cerebrospinal fluid BAFF and APRIL levels in anti-N-methyl-D-aspartate receptor encephalitis: Correlation with clinical outcome. J. Neuroimmunol. 2017, 305, 84–91. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Zheng, D.; Wang, Z.; Pan, S.; Yin, J.; Wang, H. Elevated serum and cerebrospinal fluid CD138 in patients with anti-N-methyl-d-aspartate receptor encephalitis. Front. Mol. Neurosci. 2019, 12, 116. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, B.; Pei, S.; Zheng, D.; Wang, Z.; Ji, T.; Pan, S.; Shen, H.Y.; Wang, H. Higher CSF Levels of NLRP3 Inflammasome Is Associated with Poor Prognosis of Anti-N-methyl-D-Aspartate Receptor Encephalitis. Front. Immunol. 2019, 10, 905. [Google Scholar] [CrossRef] [PubMed]
- Ai, P.; Zhang, X.; Xie, Z.; Liu, G.; Liu, X.; Pan, S.; Wang, H. The HMGB1 is increased in CSF of patients with an Anti-NMDAR encephalitis. Acta Neurol. Scand. 2018, 137, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ai, P.; Zheng, D.; Jiang, Y.; Liu, X.; Pan, S.; Wang, H. Cerebrospinal fluid pentraxin 3 and CD40 ligand in anti-N-menthyl-D-aspartate receptor encephalitis. J. Neuroimmunol. 2018, 315, 40–44. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Yin, M.; Zhao, J.; Lu, F.; Wang, Z.; Yu, X.; Wang, S.; Zheng, D.; Wang, H. High Level of Soluble CD146 In Cerebrospinal Fluid Might be a Biomarker of Severity of Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Front. Immunol. 2021, 12, 680424. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, C.; Zhang, Y.; Sun, R.; Wang, H.; Li, G.; Zhang, J. Elevated CHI3L1 and OPN levels in patients with anti-N-methyl-D-aspartate receptor encephalitis. J. Neuroimmunol. 2019, 334, 577005. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.W.; Pan, S.Y.; Xie, W.; Shen, H.Y.; Wang, H.H. Elevated soluble Fas and FasL in cerebrospinal fluid and serum of patients with anti-N-methyl-D-aspartate receptor encephalitis. Front. Neurol. 2018, 9, 904. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Ding, Y.; Zheng, D.; Wang, Z.; Pan, S.; Ji, T.; Shen, H.Y.; Wang, H. Elevation of YKL-40 in the CSF of anti-NMDAR encephalitis patients is associated with poor prognosis. Front. Neurol. 2018, 9, 727. [Google Scholar] [CrossRef] [Green Version]
- Gaetani, L.; Blennow, K.; Calabresi, P.; Di Filippo, M.; Parnetti, L.; Zetterberg, H. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 2019, 90, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gu, Y.; An, H.; Zhou, Z.; Zheng, D.; Wang, Z.; Wen, Z.; Shen, H.Y.; Wang, Q.; Wang, H. Cerebrospinal fluid light and heavy neurofilament level increased in anti-N-methyl-d-aspartate receptor encephalitis. Brain Behav. 2019, 9, e01354. [Google Scholar] [CrossRef] [Green Version]
- Muñiz-Castrillo, S.; Vogrig, A.; Honnorat, J. Associations between HLA and autoimmune neurological diseases with autoantibodies. Autoimmun. Highlights 2020, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Lee, S.T.; Moon, J.; Sunwoo, J.S.; Byun, J.I.; Lim, J.A.; Shin, Y.W.; Jun, J.S.; Lee, H.S.; Lee, W.J.; et al. Anti-LGI1 encephalitis is associated with unique HLA subtypes. Ann. Neurol. 2017, 81, 183–192. [Google Scholar] [CrossRef] [PubMed]
- van Sonderen, A.; Roelen, D.L.; Stoop, J.A.; Verdijk, R.M.; Haasnoot, G.W.; Thijs, R.D.; Wirtz, P.W.; Schreurs, M.W.J.; Claas, F.H.J.; Sillevis Smitt, P.A.E.; et al. Anti-LGI1 encephalitis is strongly associated with HLA-DR7 and HLA-DRB4. Ann. Neurol. 2017, 81, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Binks, S.; Varley, J.; Lee, W.; Makuch, M.; Elliott, K.; Gelfand, J.M.; Jacob, S.; Leite, M.I.; Maddison, P.; Chen, M.; et al. Distinct HLA associations of LGI1 and CASPR2-antibody diseases. Brain 2018, 141, 2263–2271. [Google Scholar] [CrossRef]
- Muñiz-Castrillo, S.; Haesebaert, J.; Thomas, L.; Vogrig, A.; Pinto, A.L.; Picard, G.; Blanc, C.; Do, L.D.; Joubert, B.; Berzero, G.; et al. Clinical and Prognostic Value of Immunogenetic Characteristics in Anti-LGI1 Encephalitis. Neurol. Neuroimmunol. Neuroinflammation 2021, 8, e974. [Google Scholar] [CrossRef]
- Muñiz-Castrillo, S.; Joubert, B.; Elsensohn, M.H.; Pinto, A.L.; Saint-Martin, M.; Vogrig, A.; Picard, G.; Rogemond, V.; Dubois, V.; Tamouza, R.; et al. Anti-CASPR2 clinical phenotypes correlate with HLA and immunological features. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1076–1084. [Google Scholar] [CrossRef]
- Mueller, S.H.; Färber, A.; Prüss, H.; Melzer, N.; Golombeck, K.S.; Kümpfel, T.; Thaler, F.; Elisak, M.; Lewerenz, J.; Kaufmann, M.; et al. Genetic predisposition in anti-LGI1 and anti-NMDA receptor encephalitis. Ann. Neurol. 2018, 83, 863–869. [Google Scholar] [CrossRef]
- Shu, Y.; Qiu, W.; Zheng, J.; Sun, X.; Yin, J.; Yang, X.; Yue, X.; Chen, C.; Deng, Z.; Li, S.; et al. HLA class II allele DRB1∗16:02 is associated with anti-NMDAR encephalitis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 652–658. [Google Scholar] [CrossRef]
- Tietz, A.K.; Angstwurm, K.; Baumgartner, T.; Doppler, K.; Eisenhut, K.; Elišák, M.; Franke, A.; Golombeck, K.S.; Handreka, R.; Kaufmann, M.; et al. Genome-wide association study identifies two new loci associated with anti-NMDAR encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Guo, J.; Ma, X.; Yan, Y.; Wang, Y.; Chen, C.; Sun, X.; Wang, H.; Yin, J.; Long, Y.; et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is associated with IRF7, BANK1 and TBX21 polymorphisms in two populations. Eur. J. Neurol. 2021, 28, 595–601. [Google Scholar] [CrossRef]
- Armangue, T.; Baucells, B.J.; Vlagea, A.; Petit-Pedrol, M.; Esteve-Solé, A.; Deyà-Martínez, A.; Ruiz-García, R.; Juan, M.; Pérez de Diego, R.; Dalmau, J.; et al. Toll-like receptor 3 deficiency in autoimmune encephalitis post-herpes simplex encephalitis. Neurol. Neuroimmunol. Neuroinflammation 2019, 6, 4–6. [Google Scholar] [CrossRef] [Green Version]

| Probable Anti-NMDAR Encephalitis |
Diagnosis can be made when all three of the following criteria have been met:
|
| Definite anti-NMDAR encephalitis |
| Diagnosis can be made in the presence of one or more of the six major groups of symptoms and IgG GluN1 antibodies *, after reasonable exclusion of other disorders. |
| Type of Biomarker | Findings | Molecule | References |
|---|---|---|---|
| Clinical activity | Acute phase | CXCL-13 | [73,74,75,76,77,78] |
| BAFF and APRIL | [79] | ||
| IFN-γ | [74,78,80] | ||
| TNF-α | [74,76,78,80] | ||
| CXCL-10 | [74,76,78] | ||
| CCL-22 | [77,78] | ||
| IL-1β | [77,81] | ||
| IL-6 | [75,76,77,81,82] | ||
| IL-7 | [74,78] | ||
| IL-10 | [76,78] | ||
| IL-17A | [74,75,77,81,82,83] | ||
| NLRP3 | [81] | ||
| CD146 | [84] | ||
| Elevated for months after the acute phase | IFN-γ, TNF-α, CXCL-10, IL-7, IL-17A | [74] | |
| Relapses | CXCL-13 | [73,76] | |
| CXCL-10 | [76] | ||
| IL-17A | [77] | ||
| Clinical severity | CXCL-13 | [76] | |
| CXCL-10 | [76,78] | ||
| CCL-22 | [77,78] | ||
| IL-6 | [77,78,81,83] | ||
| IL-10 | [78] | ||
| IL-17A | [81,83] | ||
| CHI3L1 | [85] | ||
| OPN | [85] | ||
| CD138 | [80] | ||
| CD40L | [83] | ||
| PTX3 | [83] | ||
| sFas and sFasL | [86] | ||
| Inflammatory activity | CSF antibody titers | CXCL-13 | [73] |
| Pleocytosis | CXCL-13, CXCL-10 | [74] | |
| Treatment response | Limited response | IL-17A | [77] |
| Outcomes | Poor long-term outcomes | CXCL-13 | [73] |
| BAFF and APRIL | [79] | ||
| CXCL-10 | [76] | ||
| IL-17A | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciano-Petersen, N.L.; Cabezudo-García, P.; Muñiz-Castrillo, S.; Honnorat, J.; Serrano-Castro, P.J.; Oliver-Martos, B. Current Status of Biomarkers in Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Int. J. Mol. Sci. 2021, 22, 13127. https://doi.org/10.3390/ijms222313127
Ciano-Petersen NL, Cabezudo-García P, Muñiz-Castrillo S, Honnorat J, Serrano-Castro PJ, Oliver-Martos B. Current Status of Biomarkers in Anti-N-Methyl-D-Aspartate Receptor Encephalitis. International Journal of Molecular Sciences. 2021; 22(23):13127. https://doi.org/10.3390/ijms222313127
Chicago/Turabian StyleCiano-Petersen, Nicolás Lundahl, Pablo Cabezudo-García, Sergio Muñiz-Castrillo, Jérôme Honnorat, Pedro Jesús Serrano-Castro, and Begoña Oliver-Martos. 2021. "Current Status of Biomarkers in Anti-N-Methyl-D-Aspartate Receptor Encephalitis" International Journal of Molecular Sciences 22, no. 23: 13127. https://doi.org/10.3390/ijms222313127
APA StyleCiano-Petersen, N. L., Cabezudo-García, P., Muñiz-Castrillo, S., Honnorat, J., Serrano-Castro, P. J., & Oliver-Martos, B. (2021). Current Status of Biomarkers in Anti-N-Methyl-D-Aspartate Receptor Encephalitis. International Journal of Molecular Sciences, 22(23), 13127. https://doi.org/10.3390/ijms222313127








