Metabolome Profiling of Heat Priming Effects, Senescence, and Acclimation of Bread Wheat Induced by High Temperatures at Different Growth Stages
Abstract
:1. Introduction
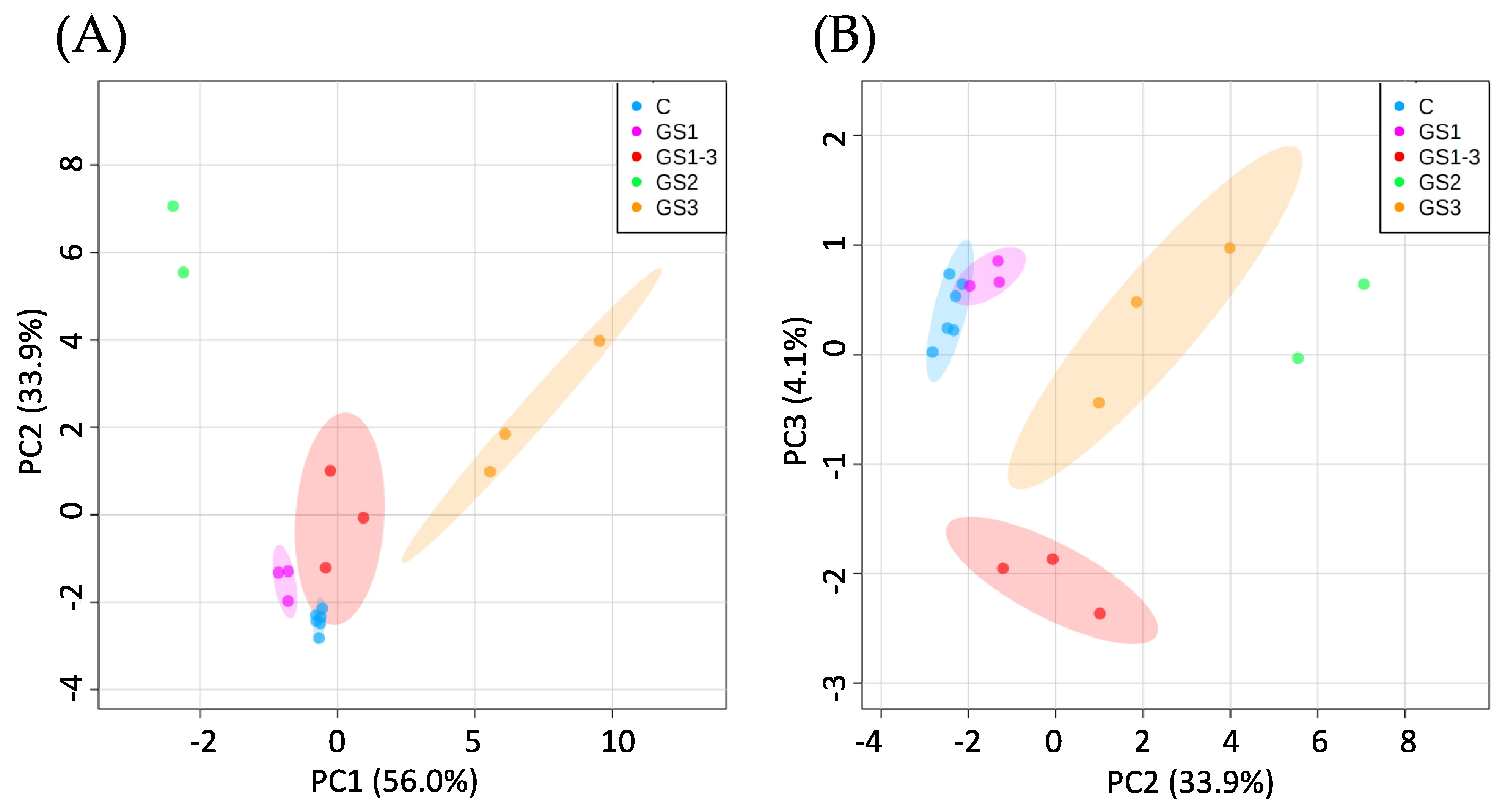
2. Results
2.1. Analysis of Metabolite Trends under Different High-Temperature Timings
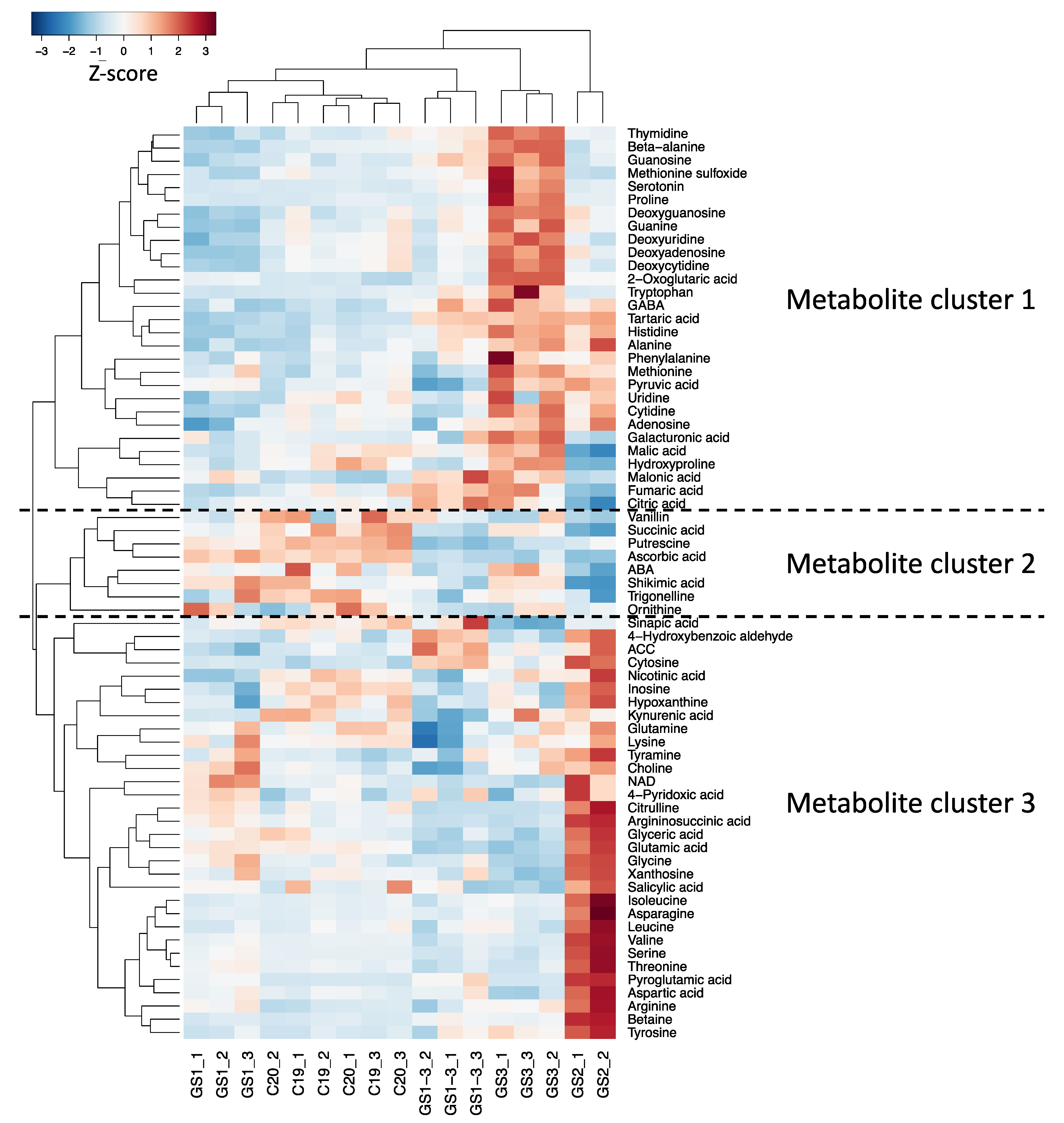
2.2. Clustering and Heat Map Based on the Metabolite Response in Each High-Temperature Treatment
2.3. Metabolites That Differed Significantly from the Control and Growth Stage-Specific Behavior
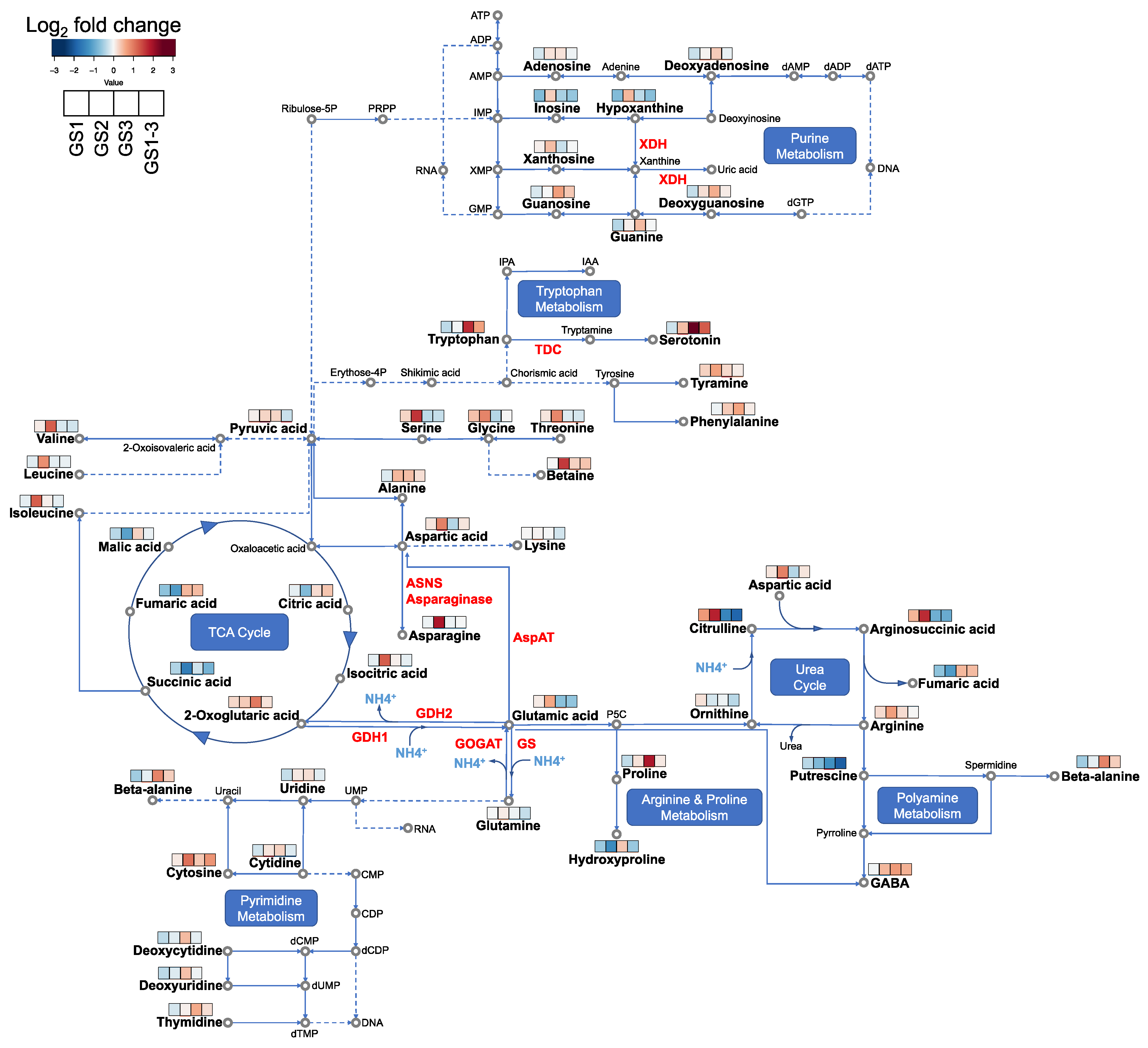
2.4. Enrichment Analysis of Differentially Accumulated Metabolites Corresponding to Stage-Specific Heat Stress
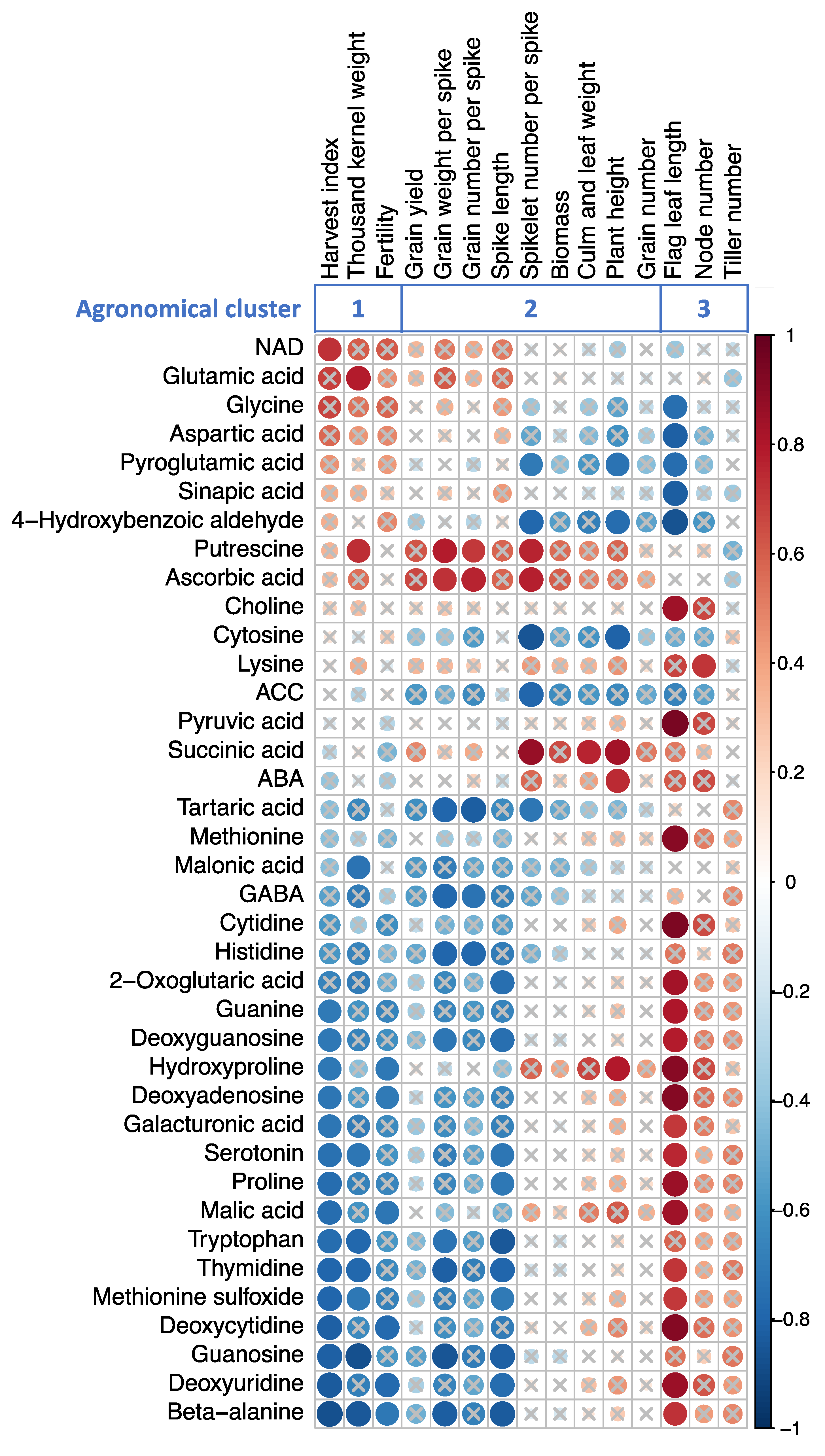
2.5. Correlation with the Altered Metabolite Concentrations and Agronomic Traits
3. Discussion
3.1. Metabolites Involved in Senescence Can Explain Heat Priming and Acclimation
3.2. Metabolites Involved in Ammonia Recycling and Urea Cycle Indicate Recovery from Heat Stress
3.3. Metabolites Can Explain Agronomic Traits Alteration by Heat Stress
4. Materials and Methods
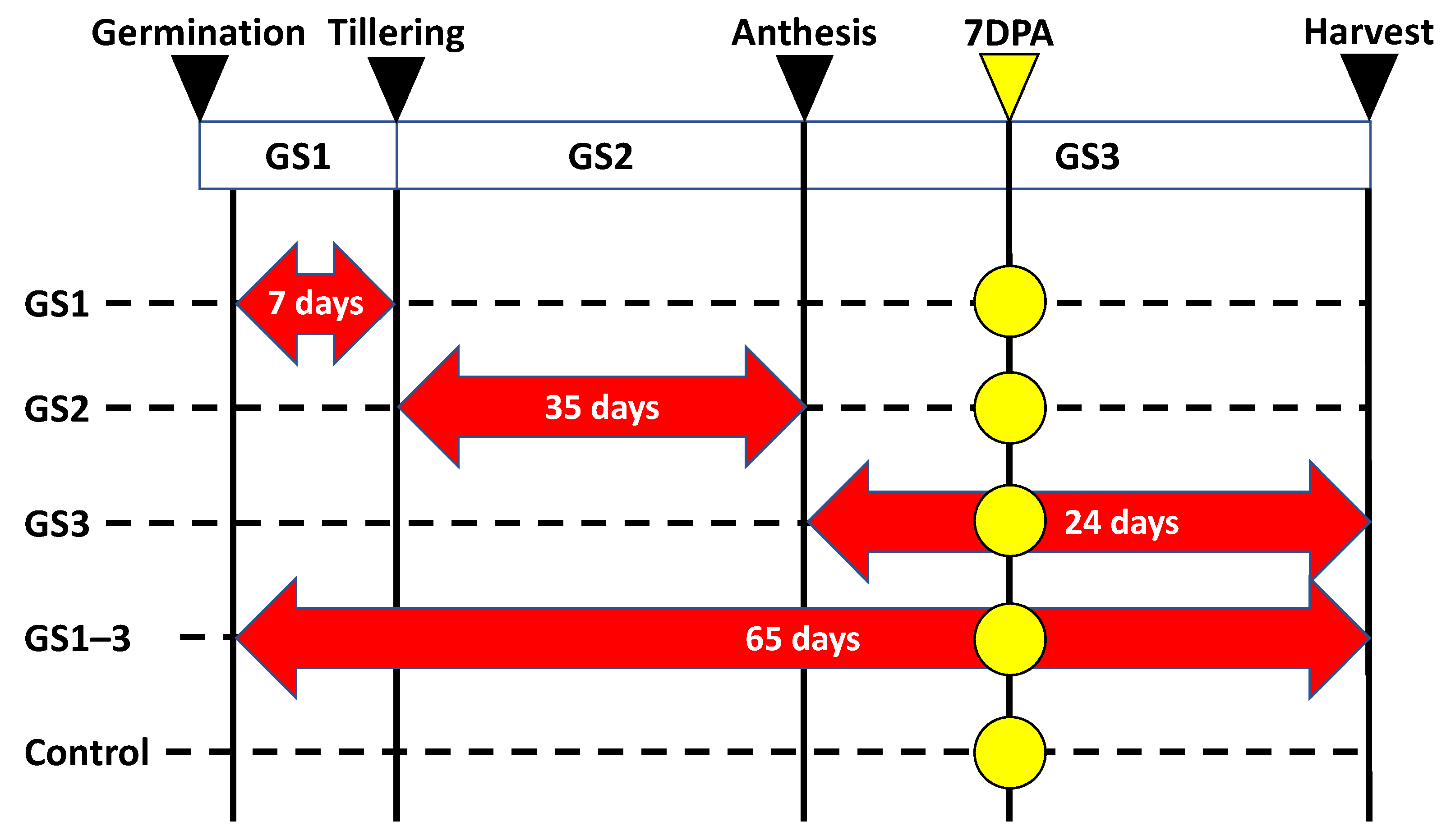
4.1. Plant Material and Growth Conditions
4.2. Metabolite Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Itam, M.; Mega, R.; Tadano, S.; Abdelrahman, M.; Matsunaga, S.; Yamasaki, Y.; Akashi, K.; Tsujimoto, H. Metabolic and Physiological Responses to Progressive Drought Stress in Bread Wheat. Sci. Rep. 2020, 10, 17189. [Google Scholar] [CrossRef]
- Tian, H.; Lam, S.M.; Shui, G. Metabolomics, a Powerful Tool for Agricultural Research. Int. J. Mol. Sci. 2016, 17, 1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itam, M.; Abdelrahman, M.; Yamasaki, Y.; Mega, R.; Gorafi, Y.; Akashi, K.; Tsujimoto, H. Aegilops tauschii Introgressions Improve Physio-Biochemical Traits and Metabolite Plasticity in Bread Wheat under Drought Stress. Agronomy 2020, 10, 1588. [Google Scholar] [CrossRef]
- Yuan, L.; Tang, L.; Zhu, S.; Hou, J.; Chen, G.; Liu, F.; Liu, S.; Wang, C. Influence of Heat Stress on Leaf Morphology and Nitrogen-Carbohydrate Metabolisms in Two Wucai (Brassica Campestris L.) Genotypes. Acta Soc. Bot. Pol. 2017, 86. [Google Scholar] [CrossRef]
- Glaubitz, U.; Erban, A.; Kopka, J.; Hincha, D.K.; Zuther, E. High Night Temperature Strongly Impacts TCA Cycle, Amino Acid and Polyamine Biosynthetic Pathways in Rice in a Sensitivity-Dependent Manner. J. Exp. Bot. 2015, 66, 6385–6397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Lay, P.; Isaure, M.P.; Sarry, J.E.; Kuhn, L.; Fayard, B.; le Bail, J.L.; Bastien, O.; Garin, J.; Roby, C.; Bourguignon, J. Metabolomic, Proteomic and Biophysical Analyses of Arabidopsis Thaliana Cells Exposed to a Caesium Stress. Influence of Potassium Supply. Biochimie 2006, 88, 1533–1547. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Reig, M.; Jaumot, J.; Piña, B.; Moyano, E.; Galceran, M.T.; Tauler, R. Metabolomic Analysis of the Effects of Cadmium and Copper Treatment in Oryza Sativa L. Using Untargeted Liquid Chromatography Coupled to High Resolution Mass Spectrometry and All-Ion Fragmentation. Metallomics 2017, 9, 660–675. [Google Scholar] [CrossRef]
- Shulaev, V.; Cortes, D.; Miller, G.; Mittler, R. Metabolomics for Plant Stress Response. Physiol. Plant 2008, 132, 199–208. [Google Scholar] [CrossRef]
- Guo, R.; Yang, Z.; Li, F.; Yan, C.; Zhong, X.; Liu, Q.; Xia, X.; Li, H.; Zhao, L. Comparative Metabolic Responses and Adaptive Strategies of Wheat (Triticum Aestivum) to Salt and Alkali Stress. BMC Plant Biol. 2015, 15, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyneke, E.; Watanabe, M.; Erban, A.; Duan, G.; Buchner, P.; Walther, D.; Kopka, J.; Hawkesford, M.J.; Hoefgen, R. Characterization of the Wheat Leaf Metabolome during Grain Filling and under Varied N-Supply. Front. Plant Sci. 2017, 8, 2048. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.L.; Palmer, L.; Roessner, U.; Stangoulis, J. Genotypic Variation in the Root and Shoot Metabolite Profiles of Wheat (Triticum Aestivum L.) Indicate Sustained, Preferential Carbon Allocation as a Potential Mechanism in Phosphorus Efficiency. Front. Plant Sci. 2019, 0, 995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsunaga, S.; Yamasaki, Y.; Toda, Y.; Mega, R.; Akashi, K.; Tsujimoto, H. Stage-Specific Characterization of Physiological Response to Heat Stress in the Wheat Cultivar Norin 61. Int. J. Mol. Sci. 2021, 22, 6942. [Google Scholar] [CrossRef]
- Law, R.D.; Crafts-Brandner, S.J. Inhibition and Acclimation of Photosynthesis to Heat Stress Is Closely Correlated with Activation of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase. Plant Physiol. 1999, 120, 173–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witte, C.-P.; Herde, M. Nucleotide Metabolism in Plants. Plant Physiol. 2020, 182, 63–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashihara, H.; Stasolla, C.; Fujimura, T.; Crozier, A. Purine Salvage in Plants. Phytochemistry 2018, 147, 89–124. [Google Scholar] [CrossRef]
- Avila-Ospina, L.; Clément, G.; Masclaux-Daubresse, C. Metabolite Profiling for Leaf Senescence in Barley Reveals Decreases in Amino Acids and Glycolysis Intermediates. Agronomy 2017, 7, 15. [Google Scholar] [CrossRef]
- Hesberg, C.; Hänsch, R.; Mendel, R.R.; Bittner, F. Tandem Orientation of Duplicated Xanthine Dehydrogenase Genes from Arabidopsis Thaliana: Differential gene expression and enzyme activities. J. Biol. Chem. 2004, 279, 13547–13554. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, A.; Sakamoto, S.; Takahashi, M.; Morikawa, H.; Sakamoto, A. The RNAi-Mediated Silencing of Xanthine Dehydrogenase Impairs Growth and Fertility and Accelerates Leaf Senescence in Transgenic Arabidopsis Plants. Plant Cell Physiol. 2007, 48, 1484–1495. [Google Scholar] [CrossRef]
- Watanabe, S.; Nakagawa, A.; Izumi, S.; Shimada, H.; Sakamoto, A. RNA Interference-Mediated Suppression of Xanthine Dehydrogenase Reveals the Role of Purine Metabolism in Drought Tolerance in Arabidopsis. FEBS Lett. 2010, 584, 1181–1186. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Kang, K.; Lee, K.; Back, K. Characterization of Tryptamine 5-Hydroxylase and Serotonin Synthesis in Rice Plants. Plant Cell Rep. 2007, 26, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Pelagio-Flores, R.; Ortíz-Castro, R.; Méndez-Bravo, A.; Macías-Rodríguez, L.; López-Bucio, J. Serotonin, a Tryptophan-Derived Signal Conserved in Plants and Animals, Regulates Root System Architecture Probably Acting as a Natural Auxin Inhibitor in Arabidopsis Thaliana. Plant Cell Physiol. 2011, 52, 490–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.; Kim, Y.-S.; Park, S.; Back, K. Senescence-Induced Serotonin Biosynthesis and Its Role in Delaying Senescence in Rice Leaves. Plant Physiol. 2009, 150, 1380. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, A.; Hashimoto, Y.; Tanaka, C.; Dubouzet, J.G.; Nakao, T.; Matsuda, F.; Nishioka, T.; Miyagawa, H.; Wakasa, K. The Tryptophan Pathway Is Involved in the Defense Responses of Rice against Pathogenic Infection via Serotonin Production. Plant J. 2008, 54, 481–495. [Google Scholar] [CrossRef]
- Shin, H.; Oh, S.; Arora, R.; Kim, D. Proline accumulation in response to high temperature in winter-acclimated shoots of Prunus persica: A response associated with growth resumption or heat stress? Canadian J. Plant Sci. 2016, 96, 630–638. [Google Scholar] [CrossRef] [Green Version]
- Blume, C.; Ost, J.; Mühlenbruch, M.; Peterhänsel, C.; Laxa, M. Low CO2 Induces Urea Cycle Intermediate Accumulation in Arabidopsis Thaliana. PLoS ONE 2019, 14, e0210342. [Google Scholar] [CrossRef]
- Liang, C.-G.; Chen, L.-P.; Wang, Y.; Liu, J.; Xu, G.-L.; Li, T. High Temperature at Grain-Filling Stage Affects Nitrogen Metabolism Enzyme Activities in Grains and Grain Nutritional Quality in Rice. Rice Sci. 2011, 18, 210–216. [Google Scholar] [CrossRef]
- Oliveira, I.C.; Brenner, E.; Chiu, J.; Hsieh, M.-H.; Kouranov, A.; Lam, H.-M.; Shin, M.J.; Coruzzi, G. Metabolite and Light Regulation of Metabolism in Plants: Lessons from the Study of a Single Biochemical Pathway. Braz. J. Med. Biol. Res. 2001, 34, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Morot-Gaudry, J.-F.; Job, D.; Lea, P.J. Amino Acid Metabolism. In Plant Nitrogen; Springer: Berlin/Heidelberg, Germany, 2001; pp. 167–211. [Google Scholar] [CrossRef]
- Glutamine, Glutamate, Aspartate, and Asparagine Are Central Regulators of Nitrogen Assimilation, Metabolism, and Transport. Available online: https://biocyclopedia.com/index/plant_pathways/glutamine_glutamate_aspartate_and_asparagine.php (accessed on 15 October 2021).
- Bauwe, H.; Hagemann, M.; Fernie, A.R. Photorespiration: Players, Partners and Origin. Trends Plant Sci. 2010, 15, 330–336. [Google Scholar] [CrossRef]
- Voss, I.; Sunil, B.; Scheibe, R.; Raghavendra, A.S. Emerging Concept for the Role of Photorespiration as an Important Part of Abiotic Stress Response. Plant Biol. 2013, 15, 713–722. [Google Scholar] [CrossRef]
- Timm, S.; Florian, A.; Wittmiß, M.; Jahnke, K.; Hagemann, M.; Fernie, A.R.; Bauwe, H. Serine Acts as a Metabolic Signal for the Transcriptional Control of Photorespiration-Related Genes in Arabidopsis. Plant Physiol. 2013, 162, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Hu, Y.; Wang, C.; Liu, W.; Ma, G.; Han, Q.; Ma, D. Effects of High Temperature and Drought Stress on the Expression of Gene Encoding Enzymes and the Activity of Key Enzymes Involved in Starch Biosynthesis in Wheat Grains. Front. Plant Sci. 2019, 10, 1414. [Google Scholar] [CrossRef]
- Yang, H.; Gu, X.; Ding, M.; Lu, W.; Lu, D. Heat Stress during Grain Filling Affects Activities of Enzymes Involved in Grain Protein and Starch Synthesis in Waxy Maize. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bechtel, D.B.; Wilson, J.D. Amyloplast Formation and Starch Granule Development in Hard Red Winter Wheat. Cereal Chem. J. 2003, 80, 175–183. [Google Scholar] [CrossRef]
- Guo, G.; Lv, D.; Yan, X.; Subburaj, S.; Ge, P.; Li, X.; Hu, Y.; Yan, Y. Proteome Characterization of Developing Grains in Bread Wheat Cultivars (Triticum Aestivum L.). BMC Plant Biol. 2012, 12, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Duan, G.; Huang, Z.; Lin, H. The Role of Polyamines in the Ontogeny of Higher Plants. Acta Agric. Boreali Occident. Sin. 2006, 15, 190–194. [Google Scholar] [CrossRef]
- Duan, G. Effect of Speridine on Protein Contents and Protease during Senescence of Fxicsed Wheat Leaves. J. Sichuan Teach. Coll. 2000, 21, 44–47. [Google Scholar] [CrossRef]
- Shao, C.G.; Wang, H.; Yu-Fen, B.I. Relationship between Endogenous Polyamines and Tolerance in Medicago Sativa L.under Heat Stress. Acta Agrestia Sin. 2015, 23, 1214–1219. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X. Effect of Temperature on Endogenous Polyamine Content in Leaves of Chinese Kale Seedlings. J. South. China Agric. Univ. 2002, 23, 9–12. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Savka, M.A.; Hudson, A.O. The Synthesis and Role of β-Alanine in Plants. Front. Plant Sci. 2019, 10, 921. [Google Scholar] [CrossRef] [Green Version]
- Acevedo, E.; Silva, P.; Silva, H. Wheat Growth and Physiology. FAO Corp. Doc. Repos. 2009, 4, 1–31. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A Decimal Code for the Growth Stages of Cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
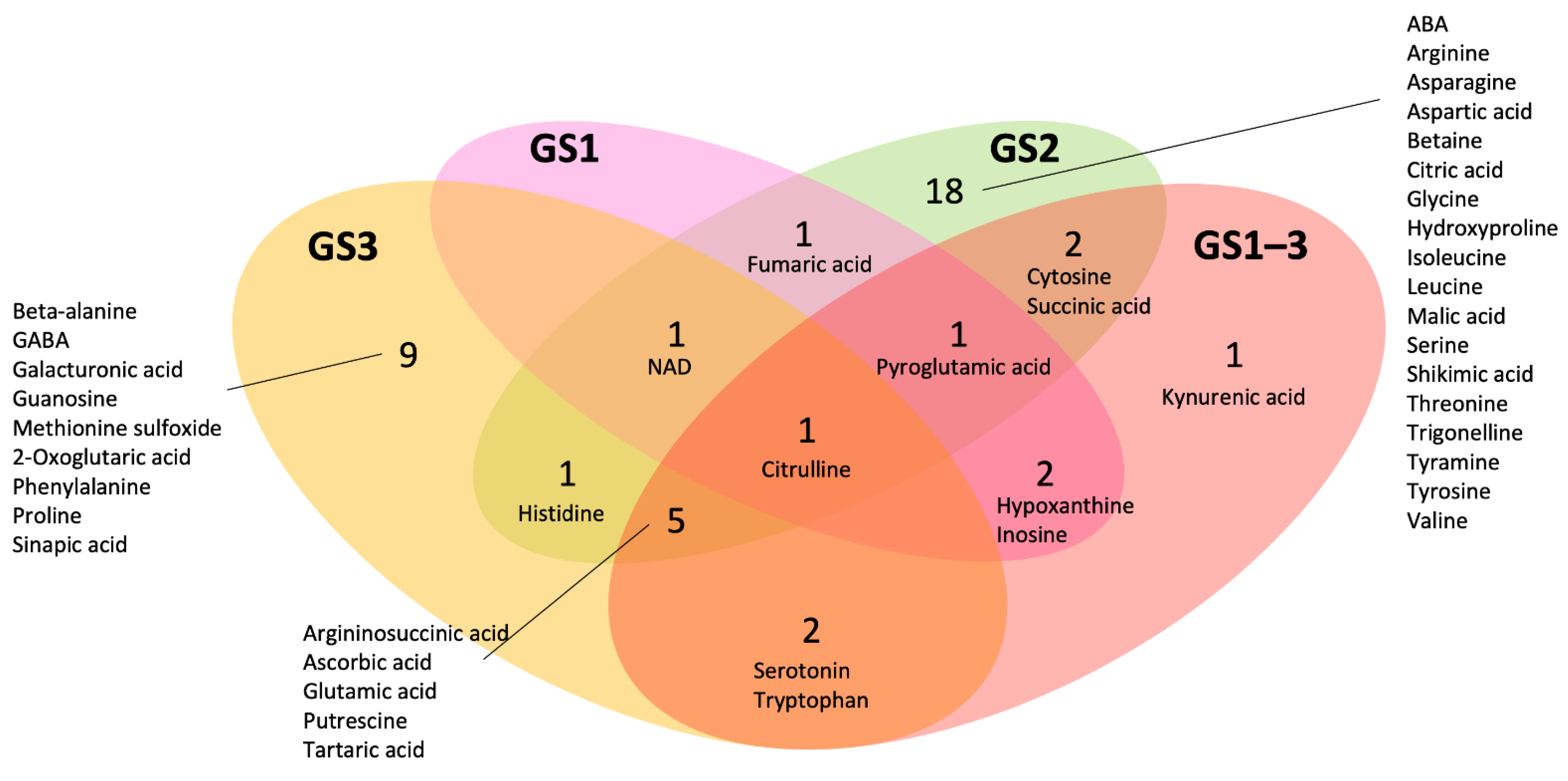

| Metabolites | GS1 | GS2 | GS3 | GS 1–3 | Metabolites | GS1 | GS2 | GS3 | GS 1–3 |
|---|---|---|---|---|---|---|---|---|---|
| ABA | 0.92 | 0.63 | 1.09 | 0.76 | Kynurenic acid | 0.78 | 0.98 | 1.01 | 0.63 |
| ACC | 0.84 | 1.36 | 1.07 | 1.38 | Leucine * | 0.92 | 1.78 | 0.93 | 0.95 |
| Adenosine | 0.88 | 1.12 | 1.13 | 0.97 | Lysine * | 1.02 | 1.03 | 1.00 | 0.85 |
| Alanine * | 0.91 | 1.39 | 1.36 | 1.14 | Malic acid | 0.77 | 0.49 | 1.22 | 0.95 |
| Arginine * | 1.16 | 1.61 | 1.17 | 1.01 | Malonic acid | 1.19 | 1.01 | 1.30 | 1.33 |
| Argininosuccinic acid * | 1.42 | 3.26 | 0.55 | 0.52 | Methionine * | 1.08 | 1.20 | 1.40 | 0.91 |
| Ascorbic acid | 1.04 | 0.48 | 0.59 | 0.60 | Methionine sulfoxide * | 0.68 | 0.73 | 1.89 | 1.16 |
| Asparagine * | 0.97 | 3.78 | 0.96 | 1.00 | NAD | 2.07 | 2.30 | 0.57 | 0.75 |
| Aspartic acid * | 1.10 | 1.93 | 0.76 | 1.10 | Nicotinic acid | 0.82 | 1.10 | 0.98 | 0.86 |
| Beta-alanine * | 0.70 | 0.93 | 1.90 | 1.23 | Ornithine * | 1.12 | 0.87 | 0.97 | 0.77 |
| Betaine | 1.00 | 3.00 | 1.22 | 1.31 | 2-Oxoglutaric acid | 1.18 | 1.25 | 2.01 | 1.11 |
| Choline | 1.12 | 1.13 | 1.06 | 0.89 | Phenylalanine * | 0.96 | 1.26 | 1.53 | 1.06 |
| Citric acid | 0.93 | 0.60 | 1.16 | 1.30 | Proline * | 0.80 | 1.12 | 3.78 | 1.09 |
| Citrulline * | 1.66 | 3.45 | 0.37 | 0.28 | Putrescine | 0.77 | 0.59 | 0.41 | 0.26 |
| Cytidine | 0.86 | 1.11 | 1.22 | 0.90 | 4-Pyridoxic acid | 1.15 | 1.25 | 1.00 | 1.09 |
| Cytosine | 1.08 | 2.14 | 1.27 | 1.70 | Pyroglutamic acid * | 2.25 | 8.55 | 0.98 | 3.15 |
| Deoxyadenosine | 0.83 | 1.03 | 1.27 | 0.95 | Pyruvic acid | 1.04 | 1.21 | 1.20 | 0.83 |
| Deoxycytidine | 0.78 | 0.92 | 1.37 | 0.94 | Salicylic acid | 0.98 | 1.36 | 0.70 | 0.89 |
| Deoxyguanosine | 0.82 | 1.13 | 1.46 | 1.06 | Serine * | 1.20 | 3.10 | 0.79 | 0.81 |
| Deoxyuridine | 0.79 | 0.90 | 1.32 | 0.97 | Serotonin | 0.84 | 1.36 | 8.69 | 2.43 |
| Fumaric acid | 0.65 | 0.46 | 1.40 | 1.37 | Shikimic acid | 1.19 | 0.25 | 1.07 | 0.69 |
| GABA * | 0.97 | 1.39 | 1.66 | 1.44 | Sinapic acid | 0.80 | 0.77 | 0.43 | 1.08 |
| Galacturonic acid | 1.00 | 0.82 | 1.71 | 1.10 | Succinic acid | 0.75 | 0.35 | 0.85 | 0.56 |
| Glutamic acid * | 1.06 | 1.53 | 0.63 | 0.65 | Tartaric acid | 0.80 | 2.24 | 2.14 | 1.99 |
| Glutamine * | 1.00 | 1.06 | 0.96 | 0.82 | Threonine * | 1.10 | 1.87 | 0.90 | 0.87 |
| Glyceric acid | 1.02 | 1.45 | 0.78 | 0.82 | Thymidine | 0.87 | 1.05 | 1.49 | 1.14 |
| Glycine * | 1.33 | 1.88 | 0.76 | 1.02 | Trigonelline | 0.85 | 0.59 | 0.88 | 0.72 |
| Guanine | 0.76 | 1.05 | 1.38 | 1.00 | Tryptophan * | 0.79 | 0.98 | 3.23 | 1.57 |
| Guanosine | 0.88 | 0.99 | 1.60 | 1.29 | Tyramine | 1.22 | 1.56 | 1.19 | 1.06 |
| Histidine * | 0.84 | 1.54 | 1.78 | 1.37 | Tyrosine * | 1.01 | 1.87 | 1.27 | 1.11 |
| 4-Hydroxybenzaldehyde | 0.95 | 1.42 | 0.89 | 1.29 | Uridine | 0.81 | 1.09 | 1.14 | 0.92 |
| Hydroxyproline * | 0.68 | 0.39 | 1.28 | 0.68 | Valine * | 1.06 | 2.39 | 0.89 | 0.86 |
| Hypoxanthine | 0.60 | 1.39 | 0.79 | 0.62 | Vanillin | 0.85 | 0.73 | 0.84 | 0.90 |
| Inosine | 0.61 | 1.25 | 0.72 | 0.66 | Xanthosine | 1.04 | 1.35 | 0.83 | 1.00 |
| Isoleucine * | 0.93 | 2.39 | 1.04 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsunaga, S.; Yamasaki, Y.; Mega, R.; Toda, Y.; Akashi, K.; Tsujimoto, H. Metabolome Profiling of Heat Priming Effects, Senescence, and Acclimation of Bread Wheat Induced by High Temperatures at Different Growth Stages. Int. J. Mol. Sci. 2021, 22, 13139. https://doi.org/10.3390/ijms222313139
Matsunaga S, Yamasaki Y, Mega R, Toda Y, Akashi K, Tsujimoto H. Metabolome Profiling of Heat Priming Effects, Senescence, and Acclimation of Bread Wheat Induced by High Temperatures at Different Growth Stages. International Journal of Molecular Sciences. 2021; 22(23):13139. https://doi.org/10.3390/ijms222313139
Chicago/Turabian StyleMatsunaga, Sachiko, Yuji Yamasaki, Ryosuke Mega, Yusuke Toda, Kinya Akashi, and Hisashi Tsujimoto. 2021. "Metabolome Profiling of Heat Priming Effects, Senescence, and Acclimation of Bread Wheat Induced by High Temperatures at Different Growth Stages" International Journal of Molecular Sciences 22, no. 23: 13139. https://doi.org/10.3390/ijms222313139
APA StyleMatsunaga, S., Yamasaki, Y., Mega, R., Toda, Y., Akashi, K., & Tsujimoto, H. (2021). Metabolome Profiling of Heat Priming Effects, Senescence, and Acclimation of Bread Wheat Induced by High Temperatures at Different Growth Stages. International Journal of Molecular Sciences, 22(23), 13139. https://doi.org/10.3390/ijms222313139






