Salt-Sensitive Hypertension in GR+/− Rats Is Accompanied with Dysregulation in Adrenal Soluble Epoxide Hydrolase and Polyunsaturated Fatty Acid Pathways
Abstract
:1. Introduction
2. Results
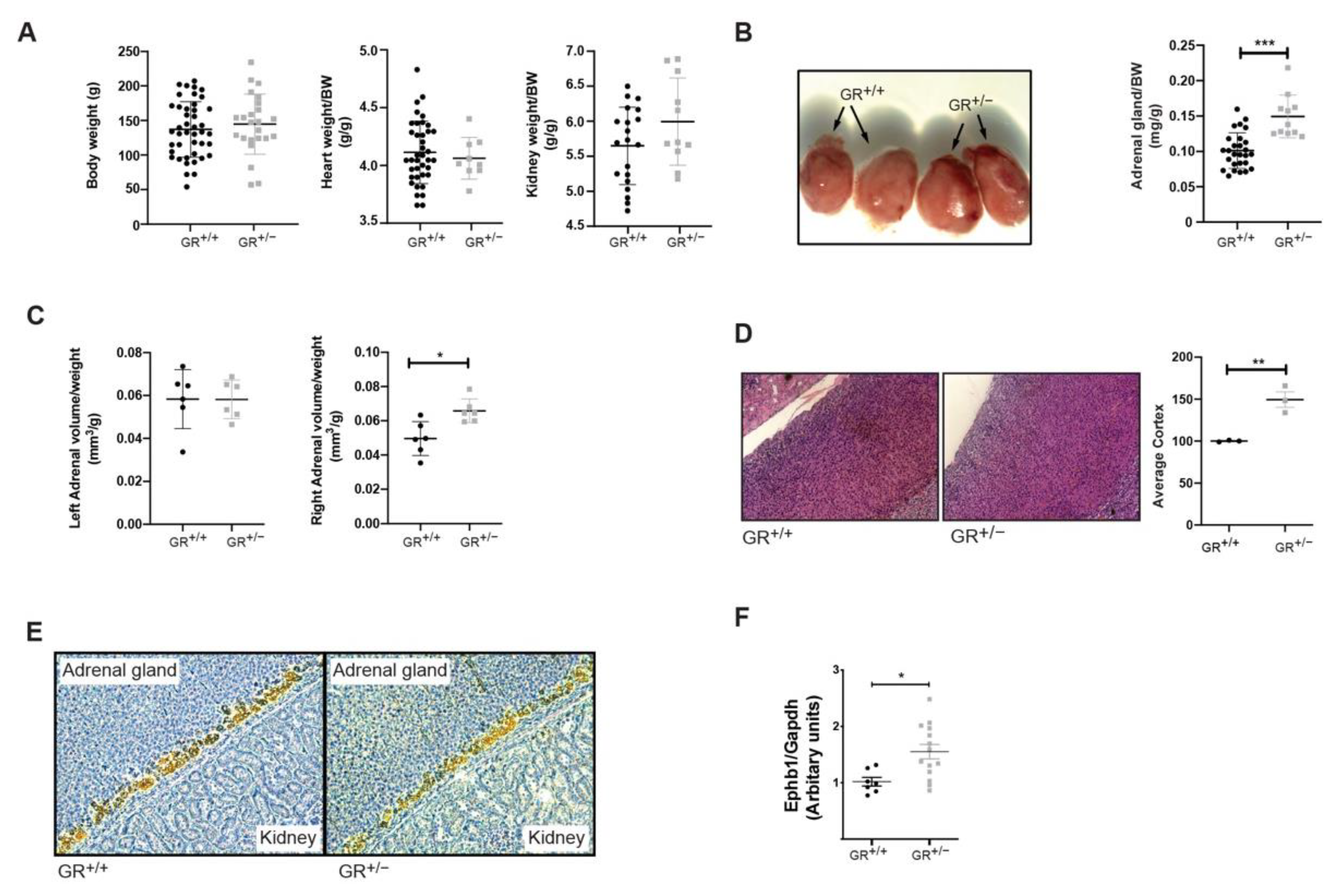
2.1. GR+/− Rats Develop Hormonal Disturbances and Adrenal Gland Hyperplasia
2.2. GR+/− Rats Developed Salt-Sensitive Hypertension
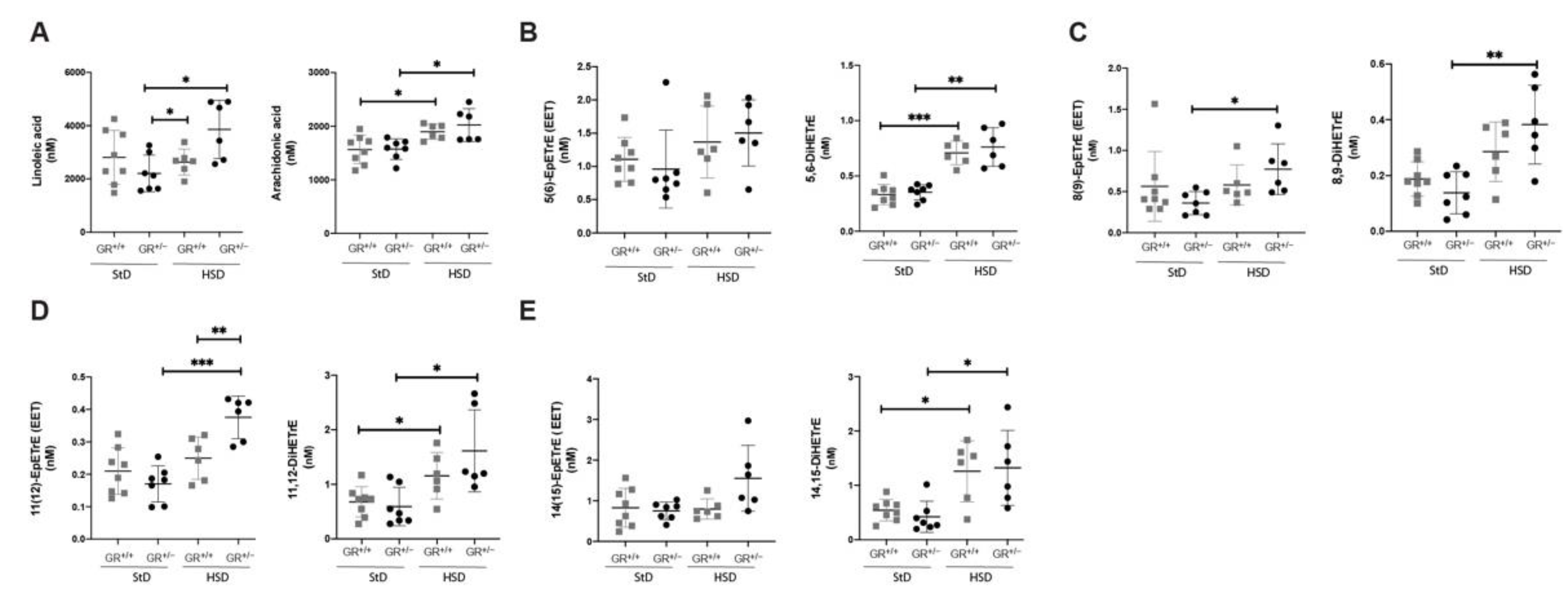
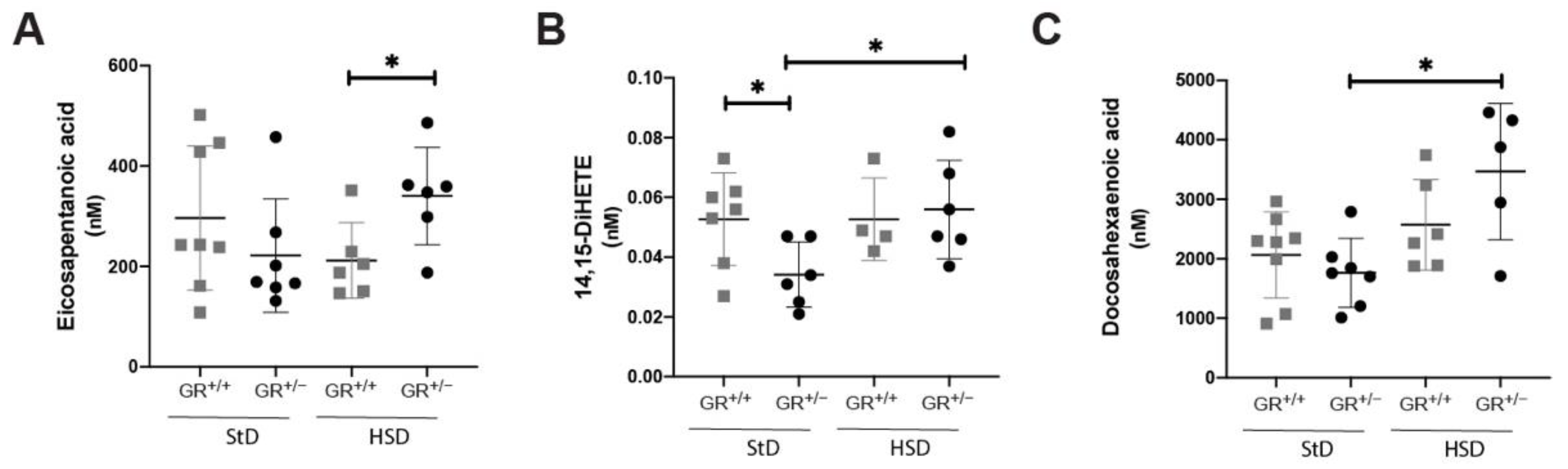
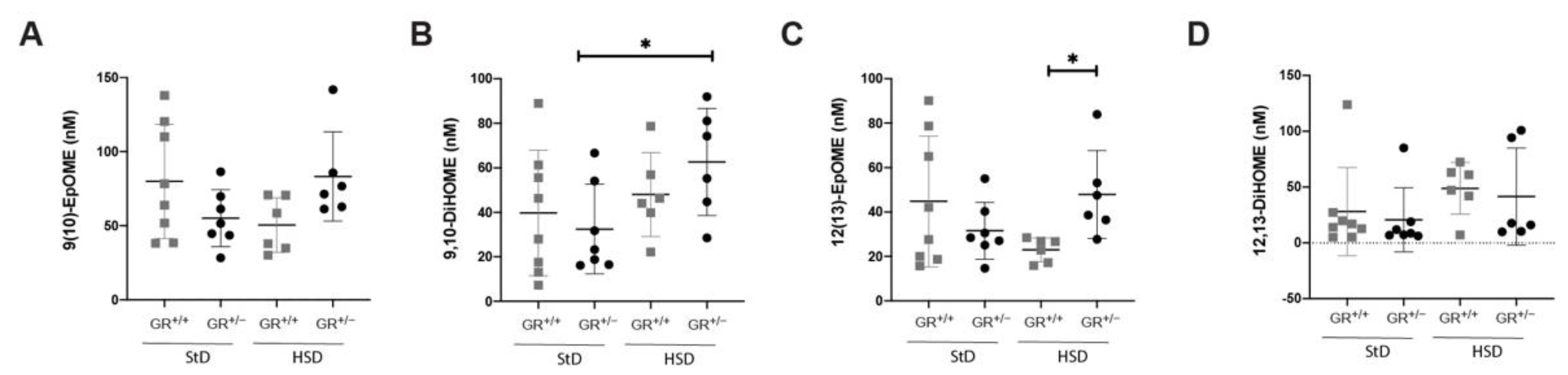
2.3. Salt-Sensitive Hypertension in GR+/− Rats Is Associated with Changes within the Fatty Acids Metabolism
3. Discussion
4. Materials and Methods
4.1. Rats
4.2. Metabolic Cages Studies
4.3. PCR
4.4. Isolation of Rat Embryonic Fibroblasts (REFs)
4.5. Western Blot Analysis
4.6. Histology
4.7. Steroid Profile Analysis
4.8. Blood Pressure
4.9. Echography
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Baid, S.; Nieman, L.K. Glucocorticoid excess and hypertension. Curr. Hypertens. Rep. 2004, 6, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Glucocorticoids. Chem. Immunol. Allergy 2014, 100, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, J.A.; Brown, M.A.; Kelly, J.J.; Williamson, P.M. Mechanisms of cortisol-induced hypertension in humans. Steroids 1995, 60, 76–80. [Google Scholar] [CrossRef]
- Dallman, M.F.; Strack, A.M.; Akana, S.F.; Bradbury, M.J.; Hanson, E.S.; Scribner, K.A.; Smith, M. Feast and famine: Critical role of glucocorticoids with insulin in daily energy flow. Front. Neuroendocrinol. 1993, 14, 303–347. [Google Scholar] [CrossRef] [PubMed]
- Raff, H.; Sharma, S.T.; Nieman, L.K. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr. Physiol. 2014, 4, 739–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, V.; Heyliger, A.; Maekawa, T.; Sasano, H.; Carrick, K.; Woodruff, S.; Rabaglia, J.; Auchus, R.J.; Ghayee, H.K. Benign adrenal adenomas secreting excess mineralocorticoids and glucocorticoids. Endocrinol. Diabetes Metab. Case Rep. 2013, 2013, 130042. [Google Scholar] [CrossRef]
- Vicennati, V.; Repaci, A.; di Dalmazi, G.; Rinaldi, E.; Golfieri, R.; Giampalma, E.; Minni, F.; Marrano, N.; Santini, D.; Pasquali, R. Combined aldosterone and cortisol secretion by adrenal incidentaloma. Int. J. Surg. Pathol. 2012, 20, 316–319. [Google Scholar] [CrossRef]
- Arlt, W.; Lang, K.; Sitch, A.J.; Dietz, A.S.; Rhayem, Y.; Bancos, I.; Feuchtinger, A.; Chortis, V.; Gilligan, L.C.; Ludwig, P.; et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Lu, N.Z.; Wardell, S.E.; Burnstein, K.L.; Defranco, D.; Fuller, P.J.; Giguere, V.; Hochberg, R.B.; McKay, L.; Renoir, J.M.; Weigel, N.L.; et al. International union of pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: Glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol. Rev. 2006, 58, 782–797. [Google Scholar] [CrossRef]
- Chrousos, G.P.; Vingerhoeds, A.; Brandon, D.; Eil, C.; Pugeat, M.; De Vroede, M.; Loriaux, D.L.; Lipsett, M.B. Primary cortisol resistance in man. A glucocorticoid receptor-mediated disease. J. Clin. Investig. 1982, 69, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Kino, T.; Chrousos, G.P. Glucocorticoid and mineralocorticoid resistance/hypersensitivity syndromes. J. Endocrinol. 2001, 169, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Karl, M.; Lamberts, S.W.; Detera-Wadleigh, S.D.; Encio, I.J.; Stratakis, C.A.; Hurley, D.M.; Accili, D.; Chrousos, G.P. Familial glucocorticoid resistance caused by a splice site deletion in the human glucocorticoid receptor gene. J. Clin. Endocrinol. Metab. 1993, 76, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, S.W.; Koper, J.W.; Biemond, P.; den Holder, F.H.; de Jong, F.H. Cortisol receptor resistance: The variability of its clinical presentation and response to treatment. J. Clin. Endocrinol. Metab. 1992, 74, 313–321. [Google Scholar] [CrossRef]
- Charmandari, E.; Kino, T.; Ichijo, T.; Chrousos, G.P. Generalized glucocorticoid resistance: Clinical aspects, molecular mechanisms, and implications of a rare genetic disorder. J. Clin. Endocrinol. Metab. 2008, 93, 1563–1572. [Google Scholar] [CrossRef] [Green Version]
- McMahon, S.K.; Pretorius, C.J.; Ungerer, J.P.; Salmon, N.J.; Conwell, L.S.; Pearen, M.A.; Batch, J.A. Neonatal complete generalized glucocorticoid resistance and growth hormone deficiency caused by a novel homozygous mutation in Helix 12 of the ligand binding domain of the glucocorticoid receptor gene (NR3C1). J. Clin. Endocrinol. Metab. 2010, 95, 297–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nader, N.; Bachrach, B.E.; Hurt, D.E.; Gajula, S.; Pittman, A.; Lescher, R.; Kino, T. A novel point mutation in helix 10 of the human glucocorticoid receptor causes generalized glucocorticoid resistance by disrupting the structure of the ligand-binding domain. J. Clin. Endocrinol. Metab. 2010, 95, 2281–2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitellius, G.; Fagart, J.; Delemer, B.; Amazit, L.; Ramos, N.; Bouligand, J.; Le Billan, F.; Castinetti, F.; Guiochon-Mantel, A.; Trabado, S.; et al. Three novel heterozygous point mutations of NR3C1 causing glucocorticoid resistance. Hum. Mutat. 2016, 37, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Bouligand, J.; Delemer, B.; Hecart, A.C.; Meduri, G.; Viengchareun, S.; Amazit, L.; Trabado, S.; Feve, B.; Guiochon-Mantel, A.; Young, J.; et al. Familial glucocorticoid receptor haploinsufficiency by non-sense mediated mRNA decay, adrenal hyperplasia and apparent mineralocorticoid excess. PLoS ONE 2010, 5, e13563. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Blendy, J.A.; Monaghan, A.P.; Krieglstein, K.; Schmid, W.; Aguzzi, A.; Fantuzzi, G.; Hummler, E.; Unsicker, K.; Schutz, G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995, 9, 1608–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridder, S.; Chourbaji, S.; Hellweg, R.; Urani, A.; Zacher, C.; Schmid, W.; Zink, M.; Hortnagl, H.; Flor, H.; Henn, F.A.; et al. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J. Neurosci. 2005, 25, 6243–6250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michailidou, Z.; Carter, R.N.; Marshall, E.; Sutherland, H.G.; Brownstein, D.G.; Owen, E.; Cockett, K.; Kelly, V.; Ramage, L.; Al-Dujaili, E.A.; et al. Glucocorticoid receptor haploinsufficiency causes hypertension and attenuates hypothalamic-pituitary-adrenal axis and blood pressure adaptions to high-fat diet. FASEB J. 2008, 22, 3896–3907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivy, J.R.; Evans, L.C.; Moorhouse, R.; Richardson, R.V.; Al-Dujaili, E.A.S.; Flatman, P.W.; Kenyon, C.J.; Chapman, K.E.; Bailey, M.A. Renal and blood pressure response to a high-salt diet in mice with reduced global expression of the glucocorticoid receptor. Front. Physiol. 2018, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D. Epoxyeicosanoids in hypertension. Physiol. Res. 2019, 68, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Roman, R.J. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 2002, 82, 131–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, W.B.; Deeter, C.; Gauthier, K.M.; Ingraham, R.H.; Falck, J.R.; Li, P.L. 14,15-Dihydroxyeicosatrienoic acid relaxes bovine coronary arteries by activation of K(Ca) channels. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1656–H1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falck, J.R.; Krishna, U.M.; Reddy, Y.K.; Kumar, P.S.; Reddy, K.M.; Hittner, S.B.; Deeter, C.; Sharma, K.K.; Gauthier, K.M.; Campbell, W.B. Comparison of vasodilatory properties of 14,15-EET analogs: Structural requirements for dilation. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H337–H349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Xu, F.; Huse, L.M.; Morisseau, C.; Draper, A.J.; Newman, J.W.; Parker, C.; Graham, L.; Engler, M.M.; Hammock, B.D.; et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ. Res. 2000, 87, 992–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imig, J.D.; Zhao, X.; Capdevila, J.H.; Morisseau, C.; Hammock, B.D. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension 2002, 39, 690–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponce de Leon, V.; Merillat, A.M.; Tesson, L.; Anegon, I.; Hummler, E. Generation of TALEN-mediated GRdim knock-in rats by homologous recombination. PLoS ONE 2014, 9, e88146. [Google Scholar] [CrossRef]
- Brennan, C.H.; Chittka, A.; Barker, S.; Vinson, G.P. Eph receptors and zonation in the rat adrenal cortex. J. Endocrinol. 2008, 198, 185–191. [Google Scholar] [CrossRef]
- Cicala, M.V.; Mantero, F. Hypertension in Cushing’s syndrome: From pathogenesis to treatment. Neuroendocrinology 2010, 92 (Suppl. S1), 44–49. [Google Scholar] [CrossRef]
- Tripathi, N.; Paliwal, S.; Sharma, S.; Verma, K.; Gururani, R.; Tiwari, A.; Verma, A.; Chauhan, M.; Singh, A.; Kumar, D.; et al. Discovery of novel soluble epoxide hydrolase inhibitors as potent vasodilators. Sci. Rep. 2018, 8, 14604. [Google Scholar] [CrossRef] [Green Version]
- Luther, J.M.; Wei, D.S.; Ghoshal, K.; Peng, D.; Adler, G.K.; Turcu, A.F.; Nian, H.; Yu, C.; Solorzano, C.C.; Pozzi, A.; et al. Treatment of primary aldosteronism increases plasma epoxyeicosatrienoic acids. Hypertension 2021, 77, 1323–1331. [Google Scholar] [CrossRef]
- Nicolaides, N.C.; Charmandari, E. Glucocorticoid resistance. Exp. Suppl. 2019, 111, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P.; Detera-Wadleigh, S.D.; Karl, M. Syndromes of glucocorticoid resistance. Ann. Intern. Med. 1993, 119, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doggrell, S.A.; Brown, L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc. Res. 1998, 39, 89–105. [Google Scholar] [CrossRef]
- Vitellius, G.; Trabado, S.; Hoeffel, C.; Bouligand, J.; Bennet, A.; Castinetti, F.; Decoudier, B.; Guiochon-Mantel, A.; Lombes, M.; Delemer, B.; et al. Significant prevalence of NR3C1 mutations in incidentally discovered bilateral adrenal hyperplasia: Results of the French MUTA-GR Study. Eur. J. Endocrinol. 2018, 178, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Tatsi, C.; Xekouki, P.; Nioti, O.; Bachrach, B.; Belyavskaya, E.; Lyssikatos, C.; Stratakis, C.A. A novel mutation in the glucocorticoid receptor gene as a cause of severe glucocorticoid resistance complicated by hypertensive encephalopathy. J. Hypertens. 2019, 37, 1475–1481. [Google Scholar] [CrossRef]
- Vitellius, G.; Delemer, B.; Caron, P.; Chabre, O.; Bouligand, J.; Pussard, E.; Trabado, S.; Lombes, M. Impaired 11beta-hydroxysteroid dehydrogenase type 2 in glucocorticoid-resistant patients. J. Clin. Endocrinol. Metab. 2019, 104, 5205–5216. [Google Scholar] [CrossRef] [PubMed]
- Mitani, F.; Suzuki, H.; Hata, J.; Ogishima, T.; Shimada, H.; Ishimura, Y. A novel cell layer without corticosteroid-synthesizing enzymes in rat adrenal cortex: Histochemical detection and possible physiological role. Endocrinology 1994, 135, 431–438. [Google Scholar] [CrossRef]
- Mitani, F.; Mukai, K.; Miyamoto, H.; Suematsu, M.; Ishimura, Y. The undifferentiated cell zone is a stem cell zone in adult rat adrenal cortex. Biochim. Biophys. Acta 2003, 1619, 317–324. [Google Scholar] [CrossRef]
- Peters, B.; Clausmeyer, S.; Obermuller, N.; Woyth, A.; Kranzlin, B.; Gretz, N.; Peters, J. Specific regulation of StAR expression in the rat adrenal zona glomerulosa. An in situ hybridization study. J. Histochem. Cytochem. 1998, 46, 1215–1221. [Google Scholar] [CrossRef] [Green Version]
- McNeill, H.; Whitworth, E.; Vinson, G.P.; Hinson, J.P. Distribution of extracellular signal-regulated protein kinases 1 and 2 in the rat adrenal and their activation by angiotensin II. J. Endocrinol. 2005, 187, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Aiba, M.; Fujibayashi, M. Alteration of subcapsular adrenocortical zonation in humans with aging: The progenitor zone predominates over the previously well-developed zona glomerulosa after 40 years of age. J. Histochem. Cytochem. 2011, 59, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P. The role of 11beta-hydroxysteroid dehydrogenase type 2 in human hypertension. Biochim. Biophys. Acta 2010, 1802, 1178–1187. [Google Scholar] [CrossRef]
- Cowley, A.W., Jr.; Yang, C.; Zheleznova, N.N.; Staruschenko, A.; Kurth, T.; Rein, L.; Kumar, V.; Sadovnikov, K.; Dayton, A.; Hoffman, M.; et al. Evidence of the importance of Nox4 in production of hypertension in dahl salt-sensitive rats. Hypertension 2016, 67, 440–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibowitz, A.; Volkov, A.; Voloshin, K.; Shemesh, C.; Barshack, I.; Grossman, E. Melatonin prevents kidney injury in a high salt diet-induced hypertension model by decreasing oxidative stress. J. Pineal Res. 2016, 60, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K.; Jin, D.; Takai, S.; Iwanaga, K. Involvement of Vanin-1 in ameliorating effect of oxidative renal tubular injury in dahl-salt sensitive rats. Int. J. Mol. Sci. 2019, 20, 4481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.; Chen, S.; Wang, Y.; Liu, J.; Yao, Q.; Huang, Y.; Li, H.; Zhu, M.; Wang, S.; Li, L.; et al. Down-regulated CBS/H2S pathway is involved in high-salt-induced hypertension in Dahl rats. Nitric Oxide 2015, 46, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Thunhorst, R.L.; Johnson, A.K. Thirst and salt appetite responses in young and old Brown Norway rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R317–R327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thunhorst, R.L.; Beltz, T.G.; Johnson, A.K. Effects of aging on mineralocorticoid-induced salt appetite in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1498–R1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.J.; Kriska, T.; Gauthier, K.M.; Falck, J.R.; Campbell, W.B. Effect of angiotensin II and ACTH on adrenal blood flow in the male rat adrenal gland in vivo. Endocrinology 2018, 159, 217–226. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Lu, M.; Guan, Y.F.; Zhu, Y.; Wang, Y. Renal over expression of soluble epoxide hydrolase in rat with hypertension induced by high-salt. Beijing Da Xue Xue Bao Yi Xue Ban 2010, 42, 126–130. [Google Scholar]
- Nayeem, M.A.; Zeldin, D.C.; Boegehold, M.A.; Falck, J.R. Salt modulates vascular response through adenosine A(2A) receptor in eNOS-null mice: Role of CYP450 epoxygenase and soluble epoxide hydrolase. Mol. Cell. Biochem. 2011, 350, 101–111. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Wang, C.; Zhu, Y.; Ai, D. Soluble epoxide hydrolase: A potential target for metabolic diseases. J. Diabetes 2016, 8, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Capdevila, J.H.; Wei, S.; Yan, J.; Karara, A.; Jacobson, H.R.; Falck, J.R.; Guengerich, F.P.; DuBois, R.N. Cytochrome P-450 arachidonic acid epoxygenase. Regulatory control of the renal epoxygenase by dietary salt loading. J. Biol. Chem. 1992, 267, 21720–21726. [Google Scholar] [CrossRef]
- Makita, K.; Takahashi, K.; Karara, A.; Jacobson, H.R.; Falck, J.R.; Capdevila, J.H. Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J. Clin. Investig. 1994, 94, 2414–2420. [Google Scholar] [CrossRef] [PubMed]
- Ivy, J.R.; Carter, R.N.; Zhao, J.F.; Buckley, C.; Urquijo, H.; Rog-Zielinska, E.A.; Panting, E.; Hrabalkova, L.; Nicholson, C.; Agnew, E.J.; et al. Glucocorticoids regulate mitochondrial fatty acid oxidation in fetal cardiomyocytes. J. Physiol. 2021, 599, 4901–4924. [Google Scholar] [CrossRef] [PubMed]
- Honetschlagerova, Z.; Huskova, Z.; Vanourkova, Z.; Sporkova, A.; Kramer, H.J.; Hwang, S.H.; Tsai, H.J.; Hammock, B.D.; Imig, J.D.; Cervenka, L.; et al. Renal mechanisms contributing to the antihypertensive action of soluble epoxide hydrolase inhibition in Ren-2 transgenic rats with inducible hypertension. J. Physiol. 2011, 589, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, B.N.; Montani, J.P. The time course of salt-induced hypertension, and why it matters. Int. J. Obes. 2008, 32 (Suppl. S6), S35–S47. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strajhar, P.; Schmid, Y.; Liakoni, E.; Dolder, P.C.; Rentsch, K.M.; Kratschmar, D.V.; Odermatt, A.; Liechti, M.E. Acute effects of lysergic acid diethylamide on circulating steroid levels in healthy subjects. J. Neuroendocrinol. 2016, 28, 12374. [Google Scholar] [CrossRef]
- Wiesel, P.; Mazzolai, L.; Nussberger, J.; Pedrazzini, T. Two-kidney, one clip and one-kidney, one clip hypertension in mice. Hypertension 1997, 29, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Favreau, J.T.; Nguyen, B.T.; Gao, I.; Yu, P.; Tao, M.; Schneiderman, J.; Gaudette, G.R.; Ozaki, C.K. Murine ultrasound imaging for circumferential strain analyses in the angiotensin II abdominal aortic aneurysm model. J. Vasc. Surg. 2012, 56, 462–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanderriele, P.-E.; Wang, Q.; Mérillat, A.-M.; Ino, F.; Aeschlimann, G.; Ehret, X.; Ancin Del Olmo, D.; Ponce de León, V.; Scholl, U.I.; Winter, D.V.; et al. Salt-Sensitive Hypertension in GR+/− Rats Is Accompanied with Dysregulation in Adrenal Soluble Epoxide Hydrolase and Polyunsaturated Fatty Acid Pathways. Int. J. Mol. Sci. 2021, 22, 13218. https://doi.org/10.3390/ijms222413218
Vanderriele P-E, Wang Q, Mérillat A-M, Ino F, Aeschlimann G, Ehret X, Ancin Del Olmo D, Ponce de León V, Scholl UI, Winter DV, et al. Salt-Sensitive Hypertension in GR+/− Rats Is Accompanied with Dysregulation in Adrenal Soluble Epoxide Hydrolase and Polyunsaturated Fatty Acid Pathways. International Journal of Molecular Sciences. 2021; 22(24):13218. https://doi.org/10.3390/ijms222413218
Chicago/Turabian StyleVanderriele, Paul-Emmanuel, Qing Wang, Anne-Marie Mérillat, Frédérique Ino, Gilles Aeschlimann, Xavier Ehret, David Ancin Del Olmo, Verónica Ponce de León, Ute I. Scholl, Denise V. Winter, and et al. 2021. "Salt-Sensitive Hypertension in GR+/− Rats Is Accompanied with Dysregulation in Adrenal Soluble Epoxide Hydrolase and Polyunsaturated Fatty Acid Pathways" International Journal of Molecular Sciences 22, no. 24: 13218. https://doi.org/10.3390/ijms222413218





