Spherical Silica Functionalized by 2-Naphthalene Methanol Luminophores as a Phosphorescence Sensor
Abstract
:1. Introduction
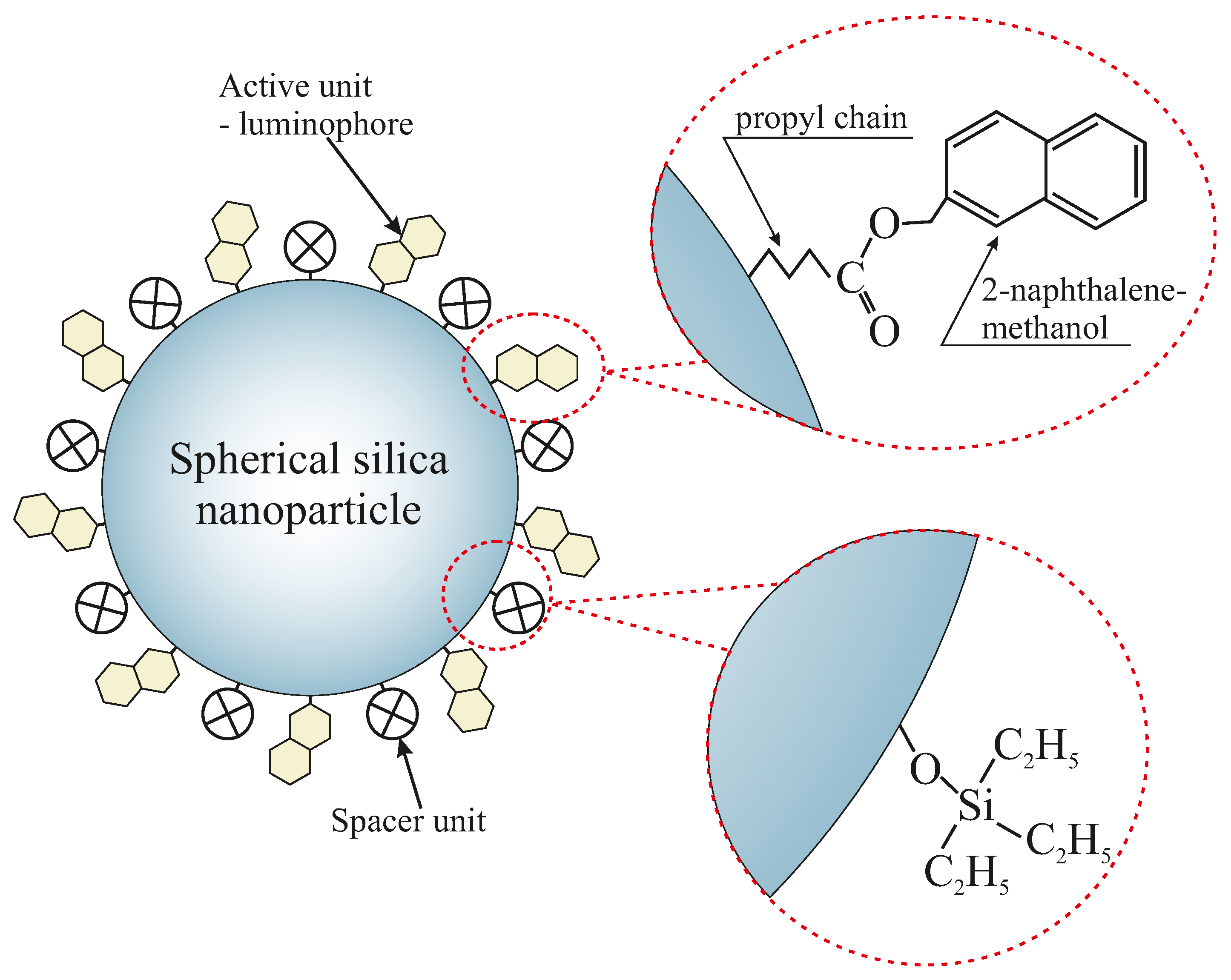
- Sil-S-COONph 1—spherical silica functionalized with 2-naphthalene methanol, containing one spacer unit per single functional group
- Sil-S-COONph 6—spherical silica functionalized with 2-naphthalene methanol, containing six spacer units per single functional group
- Sil-S-COONph 15—spherical silica functionalized with 2-naphthalene methanol, containing 15 spacer units per single functional group
- Sil-S-COOH 1—spherical silica functionalized with propyl carboxylic acid molecules, containing one spacer unit per single functional group
- Sil-S-COOH 6—spherical silica functionalized with propyl carboxylic acid molecules, containing six spacer units per single functional group
- Sil-S-COOH 15—spherical silica functionalized with propyl carboxylic acid molecules, containing 15 spacer units per single functional group.
2. Materials and Methods
2.1. Synthesis of Silica Nanoparticles Functionalized with 2-Naphthalene Methanol
2.2. Characterization Methods
3. Results and Discussion
3.1. Transmission Electron Microscopy Imaging
3.2. X-ray Photoelectron Spectroscopy Measurements
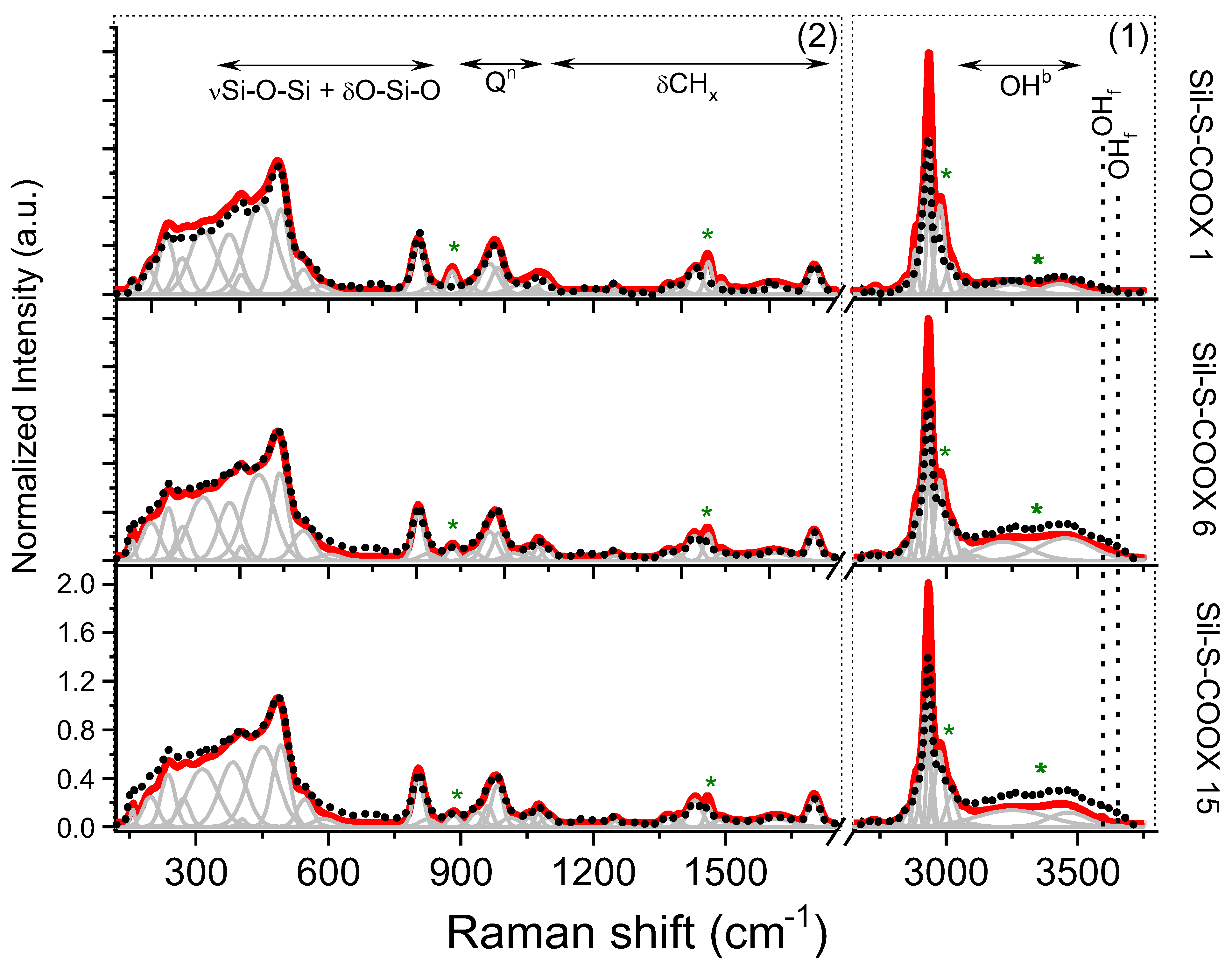
3.3. Raman Spectroscopy
3.4. Triplet State Solvation Dynamics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TEOS | tetraethyl orthosilicate |
| BNTES | butyronitrile triethoxysilane |
| TMSCl | trimethylsilyl chloride |
| Me | methyl groups |
| Et | ethyl units |
References
- Mukherjee, S.; Thilagar, P. Recent advances in purely organic phosphorescent materials. Chem. Commun. 2015, 51, 10988–11003. [Google Scholar] [CrossRef] [PubMed]
- Weigl, P.; Talluto, V.; Walther, T.; Blochowicz, T. Triplet Solvation Dynamics of Hydrogen Bonding Liquids in Confinement. Z. Für. Phys. Chem. 2018, 232, 1017–1039. [Google Scholar] [CrossRef]
- Yoshii, R.; Hirose, A.; Tanaka, K.; Chujo, Y. Functionalization of boron diiminates with unique optical properties: Multicolor tuning of crystallization-induced emission and introduction into the main chain of conjugated polymers. J. Am. Chem. Soc. 2014, 136, 18131–18139. [Google Scholar] [CrossRef] [PubMed]
- Gahlaut, R.; Joshi, H.C.; Joshi, N.K.; Pandey, N.; Arora, P.; Rautela, R.; Suyal, K.; Pant, S. Luminescence characteristics and room temperature phosphorescence of naphthoic acids in polymers. J. Lumin. 2013, 138, 122–128. [Google Scholar] [CrossRef]
- Lee, D.; Bolton, O.; Kim, B.C.; Youk, J.H.; Takayama, S.; Kim, J. Room temperature phosphorescence of metal-free organic materials in amorphous polymer matrices. J. Am. Chem. Soc. 2013, 135, 6325–6329. [Google Scholar] [CrossRef]
- Kwon, M.S.; Lee, D.; Seo, S.; Jung, J.; Kim, J. Tailoring intermolecular interactions for efficient room-temperature phosphorescence from purely organic materials in amorphous polymer matrices. Angew. Chem. 2014, 126, 11359–11363. [Google Scholar] [CrossRef] [Green Version]
- Badiei, A.; Ziarani, G.M. A novel naphthalene-immobilized nanoporous SBA-15 as a highly selective optical sensor for detection of Fe3+ in water. J. Fluoresc. 2015, 25, 1297–1302. [Google Scholar]
- Richert, R. Triplet state solvation dynamics: Basics and applications. J. Chem. Phys. 2000, 113, 8404–8429. [Google Scholar] [CrossRef]
- Weigl, P.; Koestel, D.; Pabst, F.; Gabriel, J.P.; Walther, T.; Blochowicz, T. Local dielectric response in 1-propanol: α-relaxation versus relaxation of mesoscale structures. Phys. Chem. Chem. Phys. 2019, 21, 24778–24786. [Google Scholar] [CrossRef] [Green Version]
- Weigl, P.; Hecksher, T.; Dyre, J.C.; Walther, T.; Blochowicz, T. Identity of the local and macroscopic dynamic elastic responses in supercooled 1-propanol. Phys. Chem. Chem. Phys. 2021, 23, 16537–16541. [Google Scholar] [CrossRef]
- Pabst, F.; Gabriel, J.P.; Bohmer, T.; Weigl, P.; Helbling, A.; Richter, T.; Zourchang, P.; Walther, T.; Blochowicz, T. Generic Structural Relaxation in Supercooled Liquids. J. Phys. Chem. Lett. 2021, 12, 3685–3690. [Google Scholar] [CrossRef] [PubMed]
- Cerveny, S.; Mallamace, F.; Swenson, J.; Vogel, M.; Xu, L. Confined water as model of supercooled water. Chem. Rev. 2016, 116, 7608–7625. [Google Scholar] [CrossRef] [PubMed]
- Stoneman, M.R.; Raicu, V. Dielectric Spectroscopy Based Detection of Specific and Nonspecific Cellular Mechanisms. Sensors 2021, 21, 3177. [Google Scholar] [CrossRef] [PubMed]
- Weigl, P. Sonden für die Triplett-Solvatationsdynamik. Ph.D. Thesis, Technische Universität Darmstadt, Darmstadt, Germany, 2021. [Google Scholar]
- Weigl, P.; Schadt, D.; Weißheit, S.; Thiele, C.M.; Walther, T.; Blochowicz, T. Triplet state solvation dynamics: Extending the accessible timescale by using indole as local probe. Phys. Chem. Chem. Phys. 2021, 23, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, M.; Pastukh, O.; Fedorchuk, A.; Schabikowski, M.; Kowalczyk, P.; Zalasiński, M.; Laskowski, Ł. Nanostructured Silica with Anchoring Units: The 2D Solid Solvent for Molecules and Metal Ions. Int. J. Mol. Sci. 2020, 21, 8137. [Google Scholar] [CrossRef]
- Laskowska, M.; Oyama, M.; Kityk, I.; Marszalek, M.; Dulski, M.; Laskowski, L. Surface functionalization by silver-containing molecules with controlled distribution of functionalities. Appl. Surf. Sci. 2019, 481, 433–436. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Talluto, V.; Blochowicz, T.; Walther, T. A nanosecond regenerative Ti:Sapphire amplifier for the simultaneous generation of 940 nm and of 320 nm pulses. Appl. Phys. B 2016, 122, 122. [Google Scholar] [CrossRef]
- Dulski, M.; Laskowska, M.; Sułowicz, S.; Krzykawski, T.; Pastukh, O.; Zieliński, P.; Pawlik, P.; Nowak, A. The impact of the functionalization of silica mesopores on the structural and biological features of SBA-15. Microporous Mesoporous Mater. 2020, 306, 110453. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database. NIST Standarad Ref. Database 2000, 20. [Google Scholar] [CrossRef]
- Ruangchuay, L.; Schwank, J.; Sirivat, A. Surface degradation of α-naphthalene sulfonate-doped polypyrrole during XPS characterization. Appl. Surf. Sci. 2002, 199, 128–137. [Google Scholar] [CrossRef]
- Post, P.; Wurlitzer, L.; Maus-Friedrichs, W.; Weber, A.P. Characterization and applications of nanoparticles modified in-flight with silica or silica-organic coatings. Nanomaterials 2018, 8, 530. [Google Scholar] [CrossRef] [Green Version]
- Laskowska, M.; Laskowski, L.; Jelonkiewicz, J. SBA-15 mesoporous silica activated by metal ions–Verification of molecular structure on the basis of Raman spectroscopy supported by numerical simulations. J. Mol. Struct. 2015, 1100, 21–26. [Google Scholar] [CrossRef]
- Peszke, J.; Dulski, M.; Nowak, A.; Balin, K.; Zubko, M.; Sułowicz, S.; Nowak, B.; Piotrowska-Seget, Z.; Talik, E.; Wojtyniak, M.; et al. Unique properties of silver and copper silica-based nanocomposites as antimicrobial agents. RSC Adv. 2017, 7, 28092–28104. [Google Scholar] [CrossRef] [Green Version]
- Dulski, M.; Peszke, J.; Włodarczyk, J.; Sułowicz, S.; Piotrowska-Seget, Z.; Dudek, K.; Podwórny, J.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Zubko, M.; et al. Physicochemical and structural features of heat treated silver-silica nanocomposite and their impact on biological properties. Mater. Sci. Eng. C 2019, 103, 109790. [Google Scholar] [CrossRef] [PubMed]
- Dulski, M.; Malarz, K.; Kuczak, M.; Dudek, K.; Matus, K.; Sułowicz, S.; Mrozek-Wilczkiewicz, A.; Nowak, A. An Organic–Inorganic Hybrid Nanocomposite as a Potential New Biological Agent. Nanomaterials 2020, 10, 2551. [Google Scholar] [CrossRef]
- Takahara, S.; Yamamuro, O.; Suga, H. Heat capacities and glass transitions of 1-propanol and 3-methylpentane under pressure. New evidence for the entropy theory. J. Non-Cryst. Solids 1994, 171, 259–270. [Google Scholar] [CrossRef]
- Takemura, T.; Baba, H.; Shindo, Y. Eximer Phosphorescence of Naphthalene in Fluid Solution. Chem. Lett. 1974, 3, 1091–1096. [Google Scholar] [CrossRef]
- Yamamoto, K.; Takemura, T.; Baba, H. Fluorescence and Dual Phosphorescence Spectra of Quinoxaline in Fluid Solution. Bull. Chem. Soc. Jpn. 1978, 51, 729–732. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Tsuneda, T.; Hirao, K. A density-functional study on π-aromatic interaction: Benzene dimer and naphthalene dimer. J. Chem. Phys. 2005, 123, 104307. [Google Scholar] [CrossRef] [PubMed]
- Solov’ev, K.N.; Borisevich, E.A. Intramolecular heavy-atom effect in the photophysics of organic molecules. Phys.-Uspekhi 2005, 48, 231. [Google Scholar] [CrossRef]


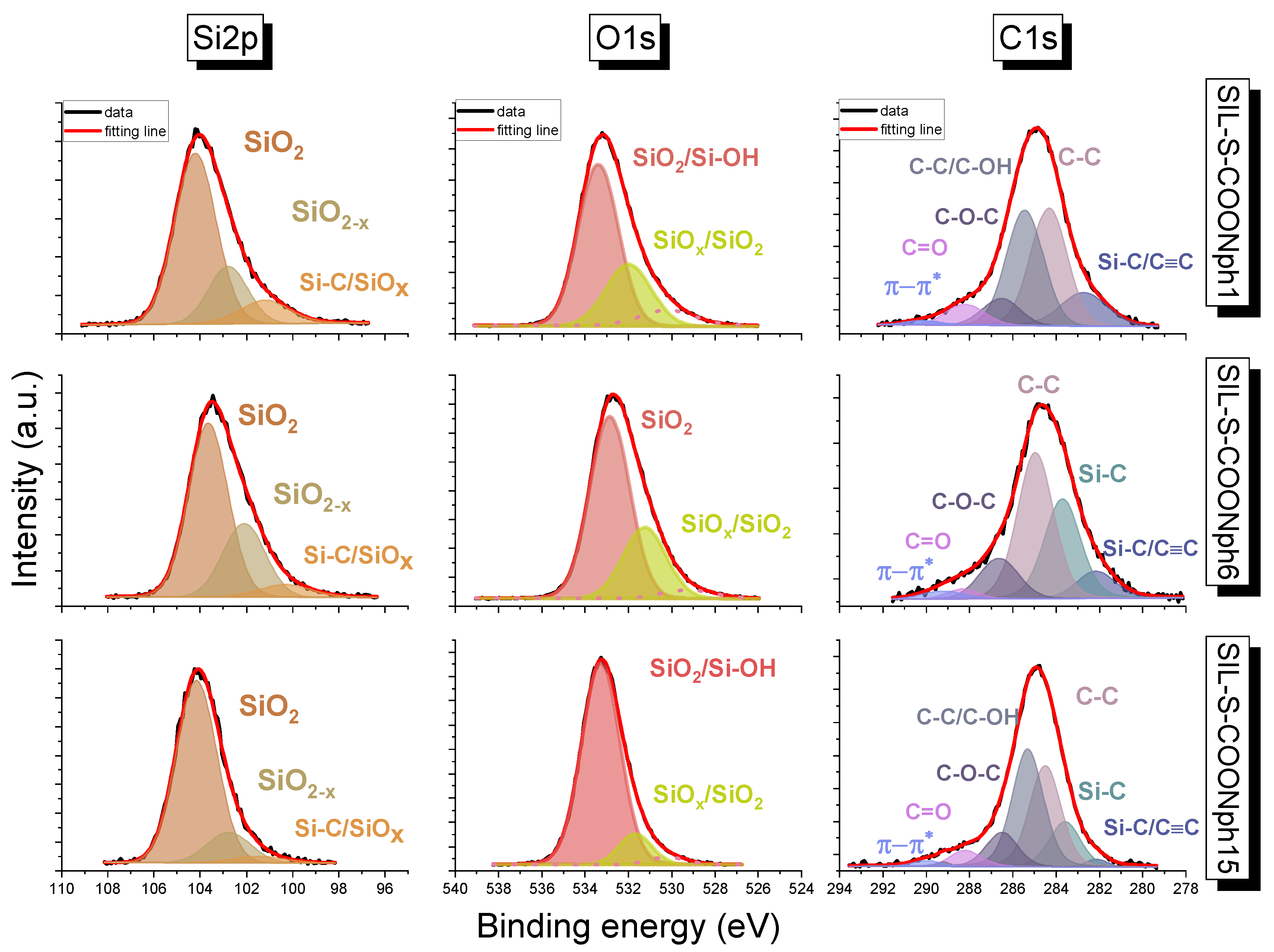



| at% | C1s | O1s | Si2p |
|---|---|---|---|
| Sil-S-COONph 1 | 15.83 | 54.99 | 29.17 |
| Sil-S-COONph 6 | 11.04 | 58.83 | 30.13 |
| Sil-S-COONph 15 | 9.39 | 59.20 | 31.41 |
| at% | C1s | O1s | Si2p |
|---|---|---|---|
| Sil-S-COOH 1 | 25.63 | 49.37 | 25.00 |
| Sil-S-COOH 6 | 30.75 | 46.64 | 22.61 |
| Sil-S-COOH 15 | 19.91 | 51.22 | 28.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskowska, M.; Nowak, A.; Dulski, M.; Weigl, P.; Blochowicz, T.; Laskowski, Ł. Spherical Silica Functionalized by 2-Naphthalene Methanol Luminophores as a Phosphorescence Sensor. Int. J. Mol. Sci. 2021, 22, 13289. https://doi.org/10.3390/ijms222413289
Laskowska M, Nowak A, Dulski M, Weigl P, Blochowicz T, Laskowski Ł. Spherical Silica Functionalized by 2-Naphthalene Methanol Luminophores as a Phosphorescence Sensor. International Journal of Molecular Sciences. 2021; 22(24):13289. https://doi.org/10.3390/ijms222413289
Chicago/Turabian StyleLaskowska, Magdalena, Anna Nowak, Mateusz Dulski, Peter Weigl, Thomas Blochowicz, and Łukasz Laskowski. 2021. "Spherical Silica Functionalized by 2-Naphthalene Methanol Luminophores as a Phosphorescence Sensor" International Journal of Molecular Sciences 22, no. 24: 13289. https://doi.org/10.3390/ijms222413289
APA StyleLaskowska, M., Nowak, A., Dulski, M., Weigl, P., Blochowicz, T., & Laskowski, Ł. (2021). Spherical Silica Functionalized by 2-Naphthalene Methanol Luminophores as a Phosphorescence Sensor. International Journal of Molecular Sciences, 22(24), 13289. https://doi.org/10.3390/ijms222413289








