Safety and Danger Considerations of Novel Treatments for Atopic Dermatitis in Context of Primary Cutaneous Lymphomas
Abstract
1. Introduction
2. Discussion
2.1. New Medications in AD
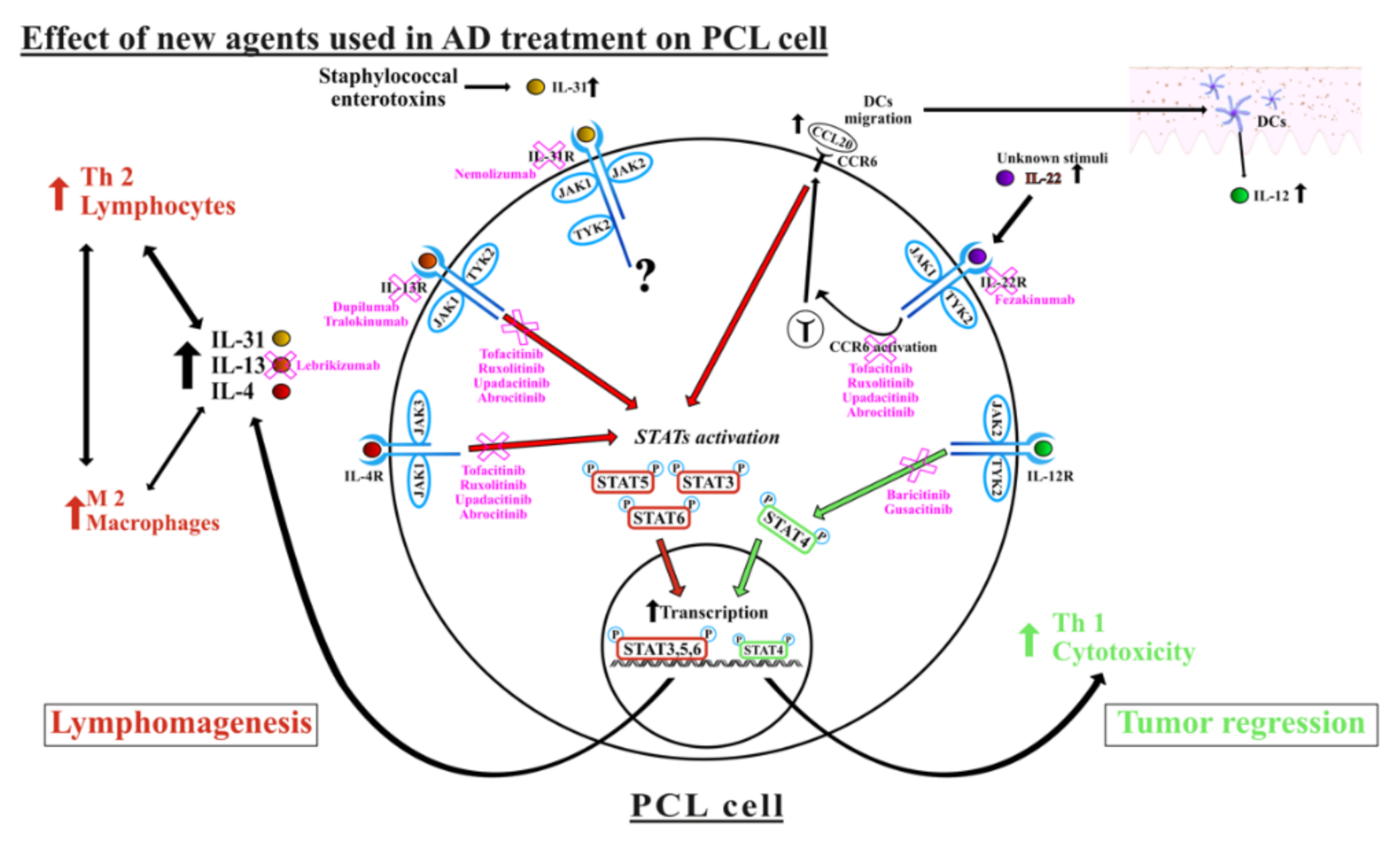
2.2. Role of Interleukin-4 and Interleukin-13 in PCL
2.3. Role of Interleukin-22 in PCL
2.4. Role of Interleukin-31 in PCL
2.5. Role of JAK-STAT Pathways in PCL
2.6. Safety and Danger Concerns of Administering the New Drugs in the Context of PCLs
3. Materials and Methods
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019, 133, 1703–1714. [Google Scholar] [CrossRef]
- Gonzalez, B.R.; Zain, J.; Rosen, S.T.; Querfeld, C. Tumor microenvironment in mycosis fungoides and Sézary syndrome. Curr. Opin. Oncol. 2016, 28, 88–96. [Google Scholar] [CrossRef]
- Yang, N.; Chen, Z.; Zhang, X.; Shi, Y. Novel Targeted Biological Agents for the Treatment of Atopic Dermatitis. BioDrugs 2021 2021, 35, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Traidl, S.; Freimooser, S.; Werfel, T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol. Sel. 2021, 5, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Szalus, K.; Trzeciak, M.; Nowicki, R.J. Jak-stat inhibitors in atopic dermatitis from pathogenesis to clinical trials results. Microorganisms 2020, 8, 1743. [Google Scholar] [CrossRef]
- Hui, R.L.; Lide, W.; Chan, J.; Schottinger, J.; Yoshinaga, M.; Millares, M. Association between exposure to topical tacrolimus or pimecrolimus and cancers. Ann. Pharmacother. 2009, 43, 1956–1963. [Google Scholar] [CrossRef]
- Andersen, L.; Nyeland, M.E.; Nyberg, F. Higher self-reported severity of atopic dermatitis in adults is associated with poorer self-reported health-related quality of life in France, Germany, the U.K. and the U.S.A. Br. J. Dermatol. 2020, 182, 1176–1183. [Google Scholar] [CrossRef]
- Asher, M.I.; Montefort, S.; Björkstén, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Hou, A.; Silverberg, J.I. Secular trends of atopic dermatitis and its comorbidities in United States children between 1997 and 2018. Arch. Dermatol. Res. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wu, J.J.; Han, G. Epidemiology and Characterization of Atopic Dermatitis in East Asian Populations: A Systematic Review. Dermatol. Ther. 2021, 11, 707–717. [Google Scholar] [CrossRef]
- Chello, C.; Carnicelli, G.; Sernicola, A.; Gagliostro, N.; Paolino, G.; Di Fraia, M.; Faina, V.; Muharremi, R.; Grieco, T. Atopic dermatitis in the elderly Caucasian population: Diagnostic clinical criteria and review of the literature. Int. J. Dermatol. 2020, 59, 716–721. [Google Scholar] [CrossRef]
- Barbarot, S.; Auziere, S.; Gadkari, A.; Girolomoni, G.; Puig, L.; Simpson, E.L.; Margolis, D.J.; de Bruin-Weller, M.; Eckert, L. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Kabashima, K.; Bieber, T. Therapeutic pipeline for atopic dermatitis: End of the drought? J. Allergy Clin. Immunol. 2017, 140, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Legendre, L.; Barnetche, T.; Mazereeuw-Hautier, J.; Meyer, N.; Murrell, D.; Paul, C. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2015, 72, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.E.; Schmidt, S.A.J.; Darvalics, B.; Mulick, A.; Abuabara, K.; Wong, A.Y.S.; Sørensen, H.T.; Smeeth, L.; Bhaskaran, K.; Dos Santos Silva, I.; et al. Association between Atopic Eczema and Cancer in England and Denmark. JAMA Dermatol. 2020, 156, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Arellano, F.M.; Arana, A.; Wentworth, C.E.; Fernández-Vidaurre, C.; Schlienger, R.G.; Conde, E. Lymphoma among patients with atopic dermatitis and/or treated with topical immunosuppressants in the United Kingdom. J. Allergy Clin. Immunol. 2009, 123, 1111–1116.e13. [Google Scholar] [CrossRef]
- Miyagaki, T.; Sugaya, M. Erythrodermic cutaneous T-cell lymphoma: How to differentiate this rare disease from atopic dermatitis. J. Dermatol. Sci. 2011, 64, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, T.; Sugaya, M.; Suga, H.; Kamata, M.; Ohmatsu, H.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; Sato, S. IL-22, but not IL-17, Dominant environment in cutaneous T-cell lymphoma. Clin. Cancer Res. 2011, 17, 7529–7538. [Google Scholar] [CrossRef] [PubMed]
- Jackow, C.M.; Cather, J.C.; Hearne, V.; Asano, A.T.; Musser, J.M.; Duvic, M. Association of erythrodermic cutaneous T-cell lymphoma, superantigen- positive Staphylococcus aureus, and oligoclonal T-cell receptor Vβ gene expansion. Blood 1997, 89, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.Q.; Lin, L.; Lin, T.; Hao, F.; Zeng, F.Q.; Bi, Z.G.; Yi, D.; Zhao, B. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: A double-blind multicentre randomized controlled trial. Br. J. Dermatol. 2006, 155, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Vowels, B.R.; Cassin, M.; Vonderheid, E.C.; Rook, A.H. Aberrant cytokine production by sezary syndrome patients: Cytokine secretion pattern resembles murine TH2 cells. J. Investig. Dermatol. 1992, 99, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Vowels, B.R.; Lessin, S.R.; Cassin, M.; Jaworsky, C.; Benoit, B.; Wolfe, J.T.; Rook, A.H. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J. Investig. Dermatol. 1994, 103, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Saed, G.; Fivenson, D.P.; Naidu, Y.; Nickoloff, B.J. Mycosis fungoides exhibits a Th1-type cell-mediated cytokine profile whereas sezary syndrome expresses a Th2-type profile. J. Investig. Dermatol. 1994, 103, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Krueger, J.G. Atopic dermatitis and psoriasis: Two different immune diseases or one spectrum? Curr. Opin. Immunol. 2017, 48, 68–73. [Google Scholar] [CrossRef]
- Bieber, T. Atopic dermatitis. N. Engl. J. Med. 2008, 358, 1483–1494. [Google Scholar] [CrossRef]
- Trzeciak, M.; Olszewska, B.; Sakowicz-Burkiewicz, M.; Sokołowska-Wojdyło, M.; Jankau, J.; Nowicki, R.J.; Pawełczyk, T. Expression Profiles of Genes Encoding Cornified Envelope Proteins in Atopic Dermatitis and Cutaneous T-Cell Lymphomas. Nutrients 2020, 12, 862. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Sugaya, M.; Miyagaki, T.; Ohmatsu, H.; Kawaguchi, M.; Takahashi, N.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; et al. Skin Barrier Dysfunction and Low Antimicrobial Peptide Expression in Cutaneous T-cell Lymphoma. Clin. Cancer Res. 2014, 20, 4339–4348. [Google Scholar] [CrossRef]
- Kopfnagel, V.; Harder, J.; Werfel, T. Expression of antimicrobial peptides in atopic dermatitis and possible immunoregulatory functions. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Saulite, I.; Hoetzenecker, W.; Weidinger, S.; Cozzio, A.; Guenova, E.; Wehkamp, U. Sézary Syndrome and Atopic Dermatitis: Comparison of Immunological Aspects and Targets. BioMed Res. Int. 2016, 2016, 9717530. [Google Scholar] [CrossRef]
- A Study Investigating the Efficacy, Safety, and PK Profile of ANB020 Administered to Adult Subjects with Moderate-to-Severe AD (ATLAS). Available online: https://clinicaltrials.gov/ct2/show/NCT03533751?term=ANB020&cond=Atopic+Dermatitis&rank=1 (accessed on 16 October 2021).
- A Study to Evaluate the Safety and Efficacy of PF-06826647 in Participants with Moderate to Severe Ulcerative Colitis—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04209556?term=PF-06826647&cond=ulcerative+colitis&draw=2&rank=1 (accessed on 16 October 2021).
- NCT03568071—A Study to Assess Efficacy, Safety, Tolerability and Pharmacokinetics (PK)/Pharmacodynamics (PD) of MOR106 in Subjects with Moderate to Severe Atopic Dermatitis. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01660617/full (accessed on 16 October 2021).
- A Study to Test Safety, Tolerability, and the Way the Body Absorbs, Distributes, and Gets Rid of a Study Drug Called MOR106, in Healthy Subjects and in Patients with Moderate to Severe Atopic Dermatitis—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03689829?term=MOR106&cond=Atopic+Dermatitis&draw=2&rank=3 (accessed on 16 October 2021).
- Study of Single and Multiple Doses of ALS-008176 in Healthy Volunteers—Full Text View—ClinicalTrials.gov. Available online: http://www.clinicaltrials.gov/ct2/show/NCT01906164?term=ALS-008176&rank=1 (accessed on 16 October 2021).
- Kang, E.G.; Narayana, P.K.; Pouliquen, I.J.; Lopez, M.C.; Ferreira-Cornwell, M.C.; Getsy, J.A. Efficacy and safety of mepolizumab administered subcutaneously for moderate to severe atopic dermatitis. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 950–953. [Google Scholar] [CrossRef]
- Husein-ElAhmed, H.; Steinhoff, M. Effectiveness of ustekinumab in patients with atopic dermatitis: Analysis of real-world evidence. J. Dermatol. Treat. 2021, in press. [Google Scholar] [CrossRef]
- Weiner, D.M.; Durgin, J.S.; Wysocka, M.; Rook, A.H. The Immunopathogenesis and Immunotherapy of Cutaneous T Cell Lymphoma: Part II, Current and Future Approaches. J. Am. Acad. Dermatol. 2020, 84, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.H.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.A.; Pirozzi, G.; Graham, N.M.H. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev. Clin. Immunol. 2017, 13, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.; de Bruin-Weller, M.; Deleuran, M.; Fargnoli, M.C.; Staumont-Sallé, D.; Hong, C.H.; Sánchez-Carazo, J.; Foley, P.; Seo, S.J.; Msihid, J.; et al. Dupilumab in Adults with Moderate-to-Severe Atopic Dermatitis and Prior Use of Systemic Non-Steroidal Immunosuppressants: Analysis of Four Phase 3 Trials. Dermatol. Ther. 2021, 11, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
- Ultsch, M.; Bevers, J.; Nakamura, G.; Vandlen, R.; Kelley, R.F.; Wu, L.C.; Eigenbrot, C. Structural basis of signaling blockade by anti-IL-13 antibody Lebrikizumab. J. Mol. Biol. 2013, 425, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Blauvelt, A.; Eichenfield, L.F.; Paller, A.S.; Armstrong, A.W.; Drew, J.; Gopalan, R.; Simpson, E.L. Efficacy and Safety of Lebrikizumab, a High-Affinity Interleukin 13 Inhibitor, in Adults With Moderate to Severe Atopic Dermatitis: A Phase 2b Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 411–420. [Google Scholar] [CrossRef]
- Simpson, E.L.; Flohr, C.; Eichenfield, L.F.; Simpson, E.L.; Bieber, T.; Sofen, H.; Taïeb, A.; Owen, R.; Putnam, W.; Castro, M.; et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J. Am. Acad. Dermatol. 2018, 78, 863–871.e11. [Google Scholar] [CrossRef]
- A Study of Lebrikizumab (LY3650150) on Vaccine Response in Adults with Atopic Dermatitis (ADopt-VA)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04626297?term=lebrikizumab&cond=Atopic+Dermatitis&phase=2&draw=2&rank=1 (accessed on 17 August 2021).
- Study to Assess the Safety and Efficacy of Lebrikizumab (LY3650150) in Adolescent Participants with Moderate-to-Severe Atopic Dermatitis—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04250350?term=lebrikizumab&cond=Atopic+Dermatitis&phase=2&draw=2&rank=2 (accessed on 17 August 2021).
- Long-Term Safety and Efficacy Study of Lebrikizumab (LY3650150) in Participants with Moderate-to-Severe Atopic Dermatitis (ADjoin)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04392154?term=lebrikizumab&cond=Atopic+Dermatitis&phase=2&draw=2&rank=3 (accessed on 17 August 2021).
- Evaluation of the Efficacy and Safety of Lebrikizumab (LY3650150) in Moderate to Severe Atopic Dermatitis—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04178967?term=lebrikizumab&cond=Atopic+Dermatitis&phase=2&draw=2&rank=4 (accessed on 17 August 2021).
- Evaluation of the Efficacy and Safety of Lebrikizumab (LY3650150) in Moderate to Severe Atopic Dermatitis (ADvocate1)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04146363?term=lebrikizumab&cond=Atopic+Dermatitis&phase=2&draw=2&rank=5 (accessed on 17 August 2021).
- Safety and Efficacy of Lebrikizumab (LY3650150) in Combination with Topical Corticosteroid in Moderate-to-Severe Atopic Dermatitis—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04250337?term=lebrikizumab&cond=Atopic+Dermatitis&phase=2&draw=2&rank=6 (accessed on 17 August 2021).
- A Study of Lebrikizumab (LY3650150) in Combination with Topical Corticosteroids in Japanese Participants with Moderate-to-Severe Atopic Dermatitis—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04760314?term=lebrikizumab&cond=Atopic+Dermatitis&phase=2&draw=2&rank=7 (accessed on 17 August 2021).
- Popovic, B.; Breed, J.; Rees, D.G.; Gardener, M.J.; Vinall, L.M.K.; Kemp, B.; Spooner, J.; Keen, J.; Minter, R.; Uddin, F.; et al. Structural Characterisation Reveals Mechanism of IL-13-Neutralising Monoclonal Antibody Tralokinumab as Inhibition of Binding to IL-13Rα1 and IL-13Rα2. J. Mol. Biol. 2017, 429, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.P.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br. J. Dermatol. 2021, 184, 437–449. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Toth, D.; Bieber, T.; Alexis, A.F.; Elewski, B.E.; Pink, A.E.; Hijnen, D.; Jensen, T.N.; Bang, B.; Olsen, C.K.; et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: Results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br. J. Dermatol. 2021, 184, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.; Miranda, M.; Castro, M.; Fernandes, I.; Cabral, R.; Santos, A.H.; Fonseca, S.; Rodrigues, J.; Leander, M.; Lau, C.; et al. IL-31 and IL-8 in Cutaneous T-Cell Lymphoma: Looking for Their Role in Itch. Adv. Hematol. 2021, 2021, 5582581. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Brunner, P.M.; Neumann, A.U.; Khattri, S.; Pavel, A.B.; Malik, K.; Singer, G.K.; Baum, D.; Gilleaudeau, P.; Sullivan-Whalen, M.; et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J. Am. Acad. Dermatol. 2018, 78, 872–881.e6. [Google Scholar] [CrossRef]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M. Trial of Nemolizumab and Topical Agents for Atopic Dermatitis with Pruritus. N. Engl. J. Med. 2020, 383, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, T.; Hanifin, J.M.; Furue, M.; Pulka, G.; Mlynarczyk, I.; Wollenberg, A.; Galus, R.; Etoh, T.; Mihara, R.; Yoshida, H.; et al. Anti–Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N. Engl. J. Med. 2017, 376, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.D.; Oussedik, E.; D’Amber, V.; Feldman, S.R. Interleukin-31 pathway and its role in atopic dermatitis: A systematic review. J. Dermatol. Treat. 2017, 28, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Long-Term Safety and Efficacy of Nemolizumab with Moderate-to-Severe Atopic Dermatitis—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03989206?term=nemolizumab&cond=Atopic+Dermatitis&phase=2&draw=2&rank=3 (accessed on 17 August 2021).
- Chovatiya, R.; Paller, A.S. JAK inhibitors in the treatment of atopic dermatitis. J. Allergy Clin. Immunol. 2021, 148, 927–940. [Google Scholar] [CrossRef]
- Chapman, S.; Kwa, M.; Gold, L.S.; Lim, H.W. Janus kinase inhibitors in dermatology: Part, I. A comprehensive review. J. Am. Acad. Dermatol. 2021, in press. [Google Scholar] [CrossRef]
- Simpson, E.L.; Lacour, J.P.; Spelman, L.; Galimberti, R.; Eichenfield, L.F.; Bissonnette, R.; King, B.A.; Thyssen, J.P.; Silverberg, J.I.; Bieber, T.; et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: Results from two randomized monotherapy phase III trials. Br. J. Dermatol. 2020, 183, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Forman, S.; Silverberg, J.I.; Zirwas, M.; Maverakis, E.; Han, G.; Guttman-Yassky, E.; Marnell, D.; Bissonnette, R.; Waibel, J.; et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: Results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J. Am. Acad. Dermatol. 2021, 85, 62–70. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Simpson, E.L.; Thyssen, J.P.; Gooderham, M.; Chan, G.; Feeney, C.; Biswas, P.; Valdez, H.; Dibonaventura, M.; Nduaka, C.; et al. Efficacy and Safety of Abrocitinib in Patients with Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Kabashima, K.; Peris, K.; Silverberg, J.I.; Eichenfield, L.F.; Bieber, T.; Kaszuba, A.; Kolodsick, J.; Yang, F.E.; Gamalo, M.; et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2020, 156, 1333–1343. [Google Scholar] [CrossRef]
- Lilly Lilly and Incyte Announce Top-Line Results from Phase 3 Study (BREEZE-AD4) of Oral Selective JAK Inhibitor Baricitinib in Combination with Topical Corticosteroids in Patients with Moderate to Severe Atopic Dermatitis Not Controlled with Cyclosporine. Available online: https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-announce-top-line-results-phase-3-study-breeze (accessed on 20 October 2021).
- Bieber, T.; Thyssen, J.P.; Reich, K.; Simpson, E.L.; Katoh, N.; Torrelo, A.; De Bruin-Weller, M.; Thaci, D.; Bissonnette, R.; Gooderham, M.; et al. Pooled safety analysis of baricitinib in adult patients with atopic dermatitis from 8 randomized clinical trials. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 476–485. [Google Scholar] [CrossRef]
- Simpson, E.L.; Sinclair, R.; Forman, S.; Wollenberg, A.; Aschoff, R.; Cork, M.; Bieber, T.; Thyssen, J.P.; Yosipovitch, G.; Flohr, C.; et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet 2020, 396, 255–266. [Google Scholar] [CrossRef]
- Bieber, T.; Simpson, E.L.; Silverberg, J.I.; Thaçi, D.; Paul, C.; Pink, A.E.; Kataoka, Y.; Chu, C.-Y.; DiBonaventura, M.; Rojo, R.; et al. Abrocitinib versus Placebo or Dupilumab for Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; de Bruin-Weller, M.; Bieber, T.; Soong, W.; Kabashima, K.; Costanzo, A.; Rosmarin, D.; Lynde, C.; Liu, J.; Gamelli, A.; et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: Week 52 AD Up study results. J. Allergy Clin. Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Teixeira, H.D.; Simpson, E.L.; Papp, K.A.; Pangan, A.L.; Blauvelt, A.; Thaçi, D.; Chu, C.Y.; Hong, C.H.; Katoh, N.; et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): Results from two replicate double-blind, randomised controlled phase 3 trials. Lancet 2021, 397, 2151–2168. [Google Scholar] [CrossRef]
- Blauvelt, A.; Teixeira, H.D.; Simpson, E.L.; Costanzo, A.; De Bruin-Weller, M.; Barbarot, S.; Prajapati, V.H.; Lio, P.; Hu, X.; Wu, T.; et al. Efficacy and Safety of Upadacitinib vs Dupilumab in Adults with Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2021, 157, 1047–1055. [Google Scholar] [CrossRef]
- Open-Label Extension Study of Upadacitinib in Adult Participants with Moderate to Severe Atopic Dermatitis. Available online: https://clinicaltrials.gov/ct2/show/NCT04195698?term=NCT04195698&draw=2&rank=1 (accessed on 21 October 2021).
- A Study to Assess Real-World Use, Safety, and Effectiveness of Oral Upadacitinib in Adult and Adolescent (≥12 Years Old) Participants with Atopic Dermatitis—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05081557?term=upadacitinib&cond=Atopic+Dermatitis&draw=2&rank=6 (accessed on 21 October 2021).
- Papp, K.; Szepietowski, J.C.; Kircik, L.; Toth, D.; Eichenfield, L.F.; Leung, D.Y.M.; Forman, S.B.; Venturanza, M.E.; Sun, K.; Kuligowski, M.E.; et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double-blind studies. J. Am. Acad. Dermatol. 2021, 85, 863–872. [Google Scholar] [CrossRef]
- A Study to Assess the Efficacy and Safety of Ruxolitinib Cream in Children with Atopic Dermatitis (TRuE-AD3)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04921969 (accessed on 21 October 2021).
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Kaino, H.; Nagata, T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J. Am. Acad. Dermatol. 2020, 82, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Efficacy and Safety of Delgocitinib Cream in Adults with Moderate to Severe Chronic Hand Eczema—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04871711?term=delgocitinib&draw=2&rank=9 (accessed on 23 October 2021).
- Efficacy and Safety of Delgocitinib Cream in Adults with Moderate to Severe Chronic Hand Eczema (DELTA 2)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04872101?term=delgocitinib&draw=2&rank=8 (accessed on 23 October 2021).
- Asadullah, K.; Döcke, W.D.; Haeuler, A.; Sterry, W.; Volk, H.D. Progression of mycosis fungoides is associated with increasing cutaneous expression of interleukin-10 mRNA. J. Investig. Dermatol. 1996, 107, 833–837. [Google Scholar] [CrossRef]
- Geskin, L.J.; Viragova, S.; Stolz, D.B.; Fuschiotti, P. Interleukin-13 is overexpressed in cutaneous T-cell lymphoma cells and regulates their proliferation. Blood 2015, 125, 2798–2805. [Google Scholar] [CrossRef]
- Guenova, E.; Watanabe, R.; Teague, J.E.; Desimone, J.A.; Jiang, Y.; Dowlatshahi, M.; Schlapbach, C.; Schaekel, K.; Rook, A.H.; Tawa, M.; et al. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin. Cancer Res. 2013, 19, 3755–3763. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Vonderheid, E.C.; Hess, A.D.; Eischen, C.M.; McGirt, L.Y. Genetic markers associated with progression in early mycosis fungoides. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, T.; Sugaya, M.; Suga, H.; Ohmatsu, H.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; Sato, S. Increased CCL18 expression in patients with cutaneous T-cell lymphoma: Association with disease severity and prognosis. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 24–28. [Google Scholar] [CrossRef]
- Harwix, S.; Zachmann, K.; Neumann, C. T-cell clones from early-stage cutaneous T-cell lymphoma show no polarized Th-1 or Th-2 cytokine profile. Arch. Dermatol. Res. 2000, 292, 1–8. [Google Scholar] [CrossRef]
- Furudate, S.; Fujimura, T.; Kakizaki, A.; Kambayashi, Y.; Asano, M.; Watabe, A.; Aiba, S. The possible interaction between periostin expressed by cancer stroma and tumor-associated macrophages in developing mycosis fungoides. Exp. Dermatol. 2016, 25, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Bang, K.; Lund, M.; Mogensen, S.C.; Thestrup-Pedersen, K. In vitro culture of skin-homing T lymphocytes from inflammatory skin diseases. Exp. Dermatol. 2005, 14, 391–397. [Google Scholar] [CrossRef]
- Yamanaka, K.I.; Clark, R.; Rich, B.; Dowgiert, R.; Hirahara, K.; Hurwitz, D.; Shibata, M.; Mirchandani, N.; Jones, D.A.; Goddard, D.S.; et al. Skin-derived interleukin-7 contributes to the proliferation of lymphocytes in cutaneous T-cell lymphoma. Blood 2006, 107, 2440–2445. [Google Scholar] [CrossRef]
- Furudate, S.; Fujimura, T.; Kakizaki, A.; Hidaka, T.; Asano, M.; Aiba, S. Tumor-associated M2 macrophages in mycosis fungoides acquire immunomodulatory function by interferon alpha and interferon gamma. J. Dermatol. Sci. 2016, 83, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, E.; Corcione, A.; Pistoia, V. The IL-31/IL-31 receptor axis: General features and role in tumor microenvironment. J. Leukoc. Biol. 2017, 102, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, M. Is blocking IL-4 receptor alpha beneficial for patients with mycosis fungoides or Sézary syndrome? J. Dermatol. 2021, 48, e225–e226. [Google Scholar] [CrossRef]
- Vidulich, K.A.; Talpur, R.; Bassett, R.L.; Duvic, M. Overall survival in erythrodermic cutaneous T-cell lymphoma: An analysis of prognostic factors in a cohort of patients with erythrodermic cutaneous T-cell lymphoma. Int. J. Dermatol. 2009, 48, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Talpur, R.; Singh, L.; Daulat, S.; Liu, P.; Seyfer, S.; Trynosky, T.; Wei, W.; Duvic, M. Long term outcomes of 1263 patients with Mycosis fungoides and Sézary syndrome from 1982 to 2009. Clin. Cancer Res. 2012, 18, 5051–5060. [Google Scholar] [CrossRef]
- Papadavid, E.; Economidou, J.; Psarra, A.; Kapsimali, V.; Mantzana, V.; Antoniou, C.; Limas, K.; Stratigos, A.; Stavrianeas, N.; Avgerinou, G.; et al. The relevance of peripheral blood T-helper 1 and 2 cytokine pattern in the evaluation of patients with mycosis fungoides and Sézary syndrome. Br. J. Dermatol. 2003, 148, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, M.; Rubegni, P.; De Aloe, G.; Paulesu, L.; Pasqui, A.L.; Andreassi, L.; Auteri, A.; Fimiani, M. Extracorporeal photochemotherapy restores Th1/Th2 imbalance in patients with early stage cutaneous T-cell lymphoma. Immunology 1997, 92, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Watanabe, R.; Teague, J.E.; Schlapbach, C.; Tawa, M.C.; Adams, N.; Dorosario, A.A.; Chaney, K.S.; Cutler, C.S.; LeBoeuf, N.R.; et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci. Transl. Med. 2012, 4, 117ra7. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H. The role of IL-22 and Th22 cells in human skin diseases. J. Dermatol. Sci. 2013, 72, 3–8. [Google Scholar] [CrossRef]
- Shimauchi, T.; Sasada, K.; Kito, Y.; Mori, T.; Hata, M.; Fujiyama, T.; Ito, T.; Hirakawa, S.; Tokura, Y. CD8+ Sézary syndrome with interleukin-22 production modulated by bacterial sepsis. Br. J. Dermatol. 2013, 168, 881–883. [Google Scholar] [CrossRef]
- Papathemeli, D.; Patsatsi, A.; Papanastassiou, D.; Koletsa, T.; Papathemelis, T.; Avgeros, C.; Pikou, O.; Lazaridou, E.; Georgiou, E. Protein and mrna expression levels of interleukin-17a,-17f and-22 in blood and skin samples of patients with mycosis fungoides. Acta Dermatol. Venereol. 2020, 100, 1–6. [Google Scholar] [CrossRef]
- Ito, M.; Teshima, K.; Ikeda, S.; Kitadate, A.; Watanabe, A.; Nara, M.; Yamashita, J.; Ohshima, K.; Sawada, K.; Tagawa, H. MicroRNA-150 inhibits tumor invasion and metastasis by targeting the chemokine receptor CCR6, in advanced cutaneous T-cell lymphoma. Blood 2014, 123, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, B.; Żawrocki, A.; Lakomy, J.; Karczewska, J.; Gleń, J.; Zabłotna, M.; Malek, M.; Jankau, J.; Lange, M.; Biernat, W.; et al. Mapping signal transducer and activator of transcription (STAT) activity in different stages of mycosis fungoides and Sezary syndrome. Int. J. Dermatol. 2020, 59, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Izykowska, K.; Zawada, M.; Nowicka, K.; Grabarczyk, P.; Braun, F.C.M.; Delin, M.; Möbs, M.; Beyer, M.; Sterry, W.; Schmidt, C.A.; et al. Identification of multiple complex rearrangements associated with deletions in the 6q23-27 region in sézary syndrome. J. Investig. Dermatol. 2013, 133, 2617–2625. [Google Scholar] [CrossRef]
- Fanok, M.H.; Sun, A.; Fogli, L.K.; Narendran, V.; Eckstein, M.; Kannan, K.; Dolgalev, I.; Lazaris, C.; Heguy, A.; Laird, M.E.; et al. Role of Dysregulated Cytokine Signaling and Bacterial Triggers in the Pathogenesis of Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2018, 138, 1116–1125. [Google Scholar] [CrossRef]
- Ikeda, S.; Kitadate, A.; Ito, M.; Abe, F.; Nara, M.; Watanabe, A.; Takahashi, N.; Miyagaki, T.; Sugaya, M.; Tagawa, H. Disruption of CCL20-CCR6 interaction inhibits metastasis of advanced cutaneous T-cell lymphoma. Oncotarget 2016, 7, 13563–13574. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.; Gleń, J.; Rębała, K.; Kowalczyk, A.; Sobjanek, M.; Nowicki, R.; Ruckemann-Dziurdzińska, K.; Sokołowska-Wojdyło, M. Il-31 does not correlate to pruritus related to early stage cutaneous T-cell lymphomas but is involved in pathogenesis of the disease. Acta Derm.-Venereol. 2015, 95, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Maier, E.; Werner, D.; Duschl, A.; Bohle, B.; Horejs-Hoeck, J. Human Th2 but Not Th9 Cells Release IL-31 in a STAT6/NF-κB–Dependent Way. J. Immunol. 2014, 193, 645–654. [Google Scholar] [CrossRef]
- Tracey, L.; Villuendas, R.; Dotor, A.M.; Spiteri, I.; Ortiz, P.; García, J.F.; Rodríguez Peralto, J.L.; Lawler, M.; Piris, M.A. Mycosis fungoides shows concurrent deregulation of multiple genes involved in the TNF signaling pathway: An expression profile study. Blood 2003, 102, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Ohmatsu, H.; Sugaya, M.; Suga, H.; Morimura, S.; Miyagaki, T.; Kai, H.; Kagami, S.; Fujita, H.; Asano, Y.; Tada, Y.; et al. Serum IL-31 levels are increased in patients with cutaneous T-cell lymphoma. Acta Derm. Venereol. 2012, 92, 282–283. [Google Scholar] [CrossRef]
- Singer, E.M.; Shin, D.B.; Nattkemper, L.A.; Benoit, B.M.; Klein, R.S.; Didigu, C.A.; Loren, A.W.; Dentchev, T.; Wysocka, M.; Yosipovitch, G.; et al. IL-31 is produced by the malignant T-Cell population in cutaneous T-Cell lymphoma and correlates with CTCL Pruritus. J. Investig. Dermatol. 2013, 133, 2783–2785. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, B.; Żawrocki, A.; Gleń, J.; Lakomy, J.; Karczewska, J.; Zabłotna, M.; Malek, M.; Jankau, J.; Lange, M.; Biernat, W.; et al. Interleukin-31 is overexpressed in skin and serum in cutaneous T-cell lymphomas but does not correlate to pruritus. Adv. Dermatol. Allergol. 2020, in press. [Google Scholar] [CrossRef]
- Nattkemper, L.A.; Martinez-Escala, M.E.; Gelman, A.B.; Singer, E.M.; Rook, A.H.; Guitart, J.; Yosipovitch, G. Cutaneous T-cell lymphoma and pruritus: The expression of IL-31 and its receptors in the skin. Acta Derm.-Venereol. 2016, 96, 894–898. [Google Scholar] [CrossRef]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemeny, L.; Alenius, H.; Dieu-Nosjean, M.C.; Meller, S.; Rieker, J.; et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 117, 411–417. [Google Scholar] [CrossRef]
- Möbs, M.; Gryzik, S.; Haidar, A.; Humme, D.; Beyer, M.; Vandersee, S. Analysis of the IL-31 pathway in mycosis fungoides and sézary syndrome. Arch. Dermatol. Res. 2015, 307, 479–485. [Google Scholar] [CrossRef]
- Leonard, W.J. Role of JAK kinases and stats in cytokine signal transduction. Int. J. Hematol. 2001, 73, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Nishio, H.; Matsui, K.; Tsuji, H.; Tamura, A.; Suzuki, K. Immunolocalisation of the janus kinases (JAK)-signal transducers and activators of transcription (STAT) pathway in human epidermis. J. Anat. 2001, 198, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.J.; Whittaker, S.J.; John, S. Dysregulated Expression of COOH-Terminally Truncated Stat5 and Loss of IL2-Inducible Stat5-Dependent Gene Expression in Sezary Syndrome. Cancer Res. 2003, 63, 9048–9054. [Google Scholar] [PubMed]
- Bladon, J.; Taylor, P. Extracorporeal photopheresis differentially regulates the expression of phosphorylated STAT-1 and STAT-5 in treated monocytes and T cells, respectively. J. Cutan. Med. Surg. 2004, 8, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Sommer, V.H.; Clemmensen, O.J.; Nielsen, O.; Wasik, M.; Lovato, P.; Brender, C.; Eriksen, K.W.; Woetmann, A.; Kaestel, C.G.; Nissen, M.H.; et al. In vivo activation of STAT3 in cutaneous T-cell lymphoma. Evidence for an antiapoptotic function of STAT3. Leukemia 2004, 18, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Brender, C.; Lovato, P.; Sommer, V.H.; Woetmann, A.; Mathiesen, A.M.; Geisler, C.; Wasik, M.; Ødum, N. Constitutive SOCS-3 expression protects T-cell lymphoma against growth inhibition by IFNα. Leukemia 2005, 19, 209–213. [Google Scholar] [CrossRef]
- Zhang, C.; Li, B.; Gaikwad, A.S.; Haridas, V.; Xu, Z.; Gutterman, J.U.; Duvic, M. Avicin D selectively induces apoptosis and downregulates p-STAT-3, bcl-2, and survivin in cutaneous T-cell lymphoma cells. J. Investig. Dermatol. 2008, 128, 2728–2735. [Google Scholar] [CrossRef]
- Fantin, V.R.; Loboda, A.; Paweletz, C.P.; Hendrickson, R.C.; Pierce, J.W.; Roth, J.A.; Li, L.; Gooden, F.; Korenchuk, S.; Hou, X.S.; et al. Constitutive activation of signal transducers and activators of transcription predicts vorinostat resistance in cutaneous T-cell lymphoma. Cancer Res. 2008, 68, 3785–3794. [Google Scholar] [CrossRef] [PubMed]
- Van Kester, M.S.; Out-Luiting, J.J.; Von Dem Borne, P.A.; Willemze, R.; Tensen, C.P.; Vermeer, M.H. Cucurbitacin I inhibits Stat3 and induces apoptosis in Sézary cells. J. Investig. Dermatol. 2008, 128, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, B.; Zhang, X.; Hazarika, P.; Aggarwal, B.B.; Duvic, M. Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients PBMCs: Potential role for STAT-3 and NF-B signaling. J. Investig. Dermatol. 2010, 130, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.K.; Davies, A.M.; Long, A.; Kelleher, D.; Volkov, Y. STAT3 knockdown by siRNA induces apoptosis in human cutaneous T-cell lymphoma line Hut78 via downregulation of Bcl-xL. Cell. Mol. Biol. Lett. 2010, 15, 342–355. [Google Scholar] [CrossRef]
- Kameda, T.; Shide, K.; Shimoda, H.K.; Hidaka, T.; Kubuki, Y.; Katayose, K.; Taniguchi, Y.; Sekine, M.; Kamiunntenn, A.; Maeda, K.; et al. Absence of gain-of-function JAK1 and JAK3 mutations in adult T cell leukemia/lymphoma. Int. J. Hematol. 2010, 92, 320–325. [Google Scholar] [CrossRef]
- Wu, J.; Siddiqui, J.; Nihal, M.; Vonderheid, E.C.; Wood, G.S. Structural alterations of the FAS gene in cutaneous T-cell lymphoma (CTCL). Arch. Biochem. Biophys. 2011, 508, 185–191. [Google Scholar] [CrossRef]
- Persson, J.L. MiR-155 meets the JAK/STAT pathway. Cell Cycle 2013, 12, 2170. [Google Scholar] [CrossRef]
- Kopp, K.L.; Ralfkiaer, U.; Gjerdrum, L.M.R.; Helvad, R.; Pedersen, I.H.; Litman, T.; Jønson, L.; Hagedorn, P.H.; Krejsgaard, T.; Gniadecki, R.; et al. STAT5-mediated expression of oncogenic miR-155 in cutaneous T-cell lymphoma. Cell Cycle 2013, 12, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Ehrentraut, S.; Nagel, S.; Scherr, M.E.; Schneider, B.; Quentmeier, H.; Geffers, R.; Kaufmann, M.; Meyer, C.; Prochorec-Sobieszek, M.; Ketterling, R.P.; et al. t(8;9)(p22;p24)/PCM1-JAK2 Activates SOCS2 and SOCS3 via STAT5. PLoS ONE 2013, 8, e53767. [Google Scholar] [CrossRef] [PubMed]
- Döbbeling, U.; Waeckerle-Men, Y.; Zabel, F.; Graf, N.; Kündig, T.M.; Johansen, P. The antihistamines clemastine and desloratadine inhibit STAT3 and c-Myc activities and induce apoptosis in cutaneous T-cell lymphoma cell lines. Exp. Dermatol. 2013, 22, 119–124. [Google Scholar] [CrossRef]
- Litvinov, I.V.; Cordeiro, B.; Fredholm, S.; Ødum, N.; Zargham, H.; Huang, Y.; Zhou, Y.; Pehr, K.; Kupper, T.S.; Woetmann, A.; et al. Analysis of STAT4 expression in cutaneous T-cell lymphoma (CTCL) patients and patient-derived cell lines. Cell Cycle 2014, 13, 2975–2982. [Google Scholar] [CrossRef]
- Van Der Fits, L.; Out-Luiting, J.J.; Tensen, C.P.; Zoutman, W.H.; Vermeer, M.H. Exploring the IL-21-STAT3 axis as therapeutic target for Sézary syndrome. J. Investig. Dermatol. 2014, 134, 2639–2647. [Google Scholar] [CrossRef] [PubMed]
- Willerslev-Olsen, A.; Litvinov, I.V.; Fredholm, S.M.; Petersen, D.L.; Sibbesen, N.A.; Gniadecki, R.; Zhang, Q.; Bonefeld, C.M.; Wasik, M.A.; Geisler, C.; et al. IL-15 and IL-17F are differentially regulated and expressed in mycosis fungoides (MF). Cell Cycle 2014, 13, 1306–1312. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.Y.; Wei, F.; Liu, X.; Paterson, J.C.; Roy, D.; Mihova, D.; Woetmann, A.; Ptasznik, A.; Odum, N.; et al. Cutaneous T Cell Lymphoma Expresses Immunosuppressive CD80 (B7-1) Cell Surface Protein in a STAT5-Dependent Manner. J. Immunol. 2014, 192, 2913–2919. [Google Scholar] [CrossRef] [PubMed]
- Netchiporouk, E.; Litvinov, I.V.; Moreau, L.; Gilbert, M.; Sasseville, D.; Duvic, M. Deregulation in STAT signaling is important for cutaneous T-cell lymphoma (CTCL) pathogenesis and cancer progression. Cell Cycle 2014, 13, 3331–3335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Nowak, I.; Vonderheid, E.C.; Rook, A.H.; Kadin, M.E.; Nowell, P.C.; Shaw, L.M.; Wasik, M.A. Activation of Jak/STAT proteins involved in signal transduction pathway mediated by receptor for interleukin 2 in malignant T lymphocytes derived from cutaneous anaplastic large T-cell lymphoma and Sezary syndrome. Proc. Natl. Acad. Sci. USA 1996, 93, 9148–9153. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.; González-Rincón, J.; Onaindia, A.; Almaráz, C.; García-Díaz, N.; Pisonero, H.; Curiel-Olmo, S.; Gómez, S.; Cereceda, L.; Madureira, R.; et al. Mutated JAK kinases and deregulated STAT activity are potential therapeutic targets in cutaneous T-cell lymphoma. Haematologica 2015, 100, e450–e453. [Google Scholar] [CrossRef]
- Sibbesen, N.A.; Kopp, K.L.; Litvinov, I.V.; Jønson, L.; Willerslev-Olsen, A.; Fredholm, S.; Petersen, D.L.; Nastasi, C.; Krejsgaard, T.; Lindahl, L.M.; et al. Jak3, STAT3, and STAT5 inhibit expression of miR-22, a novel tumor suppressor microRNA, in cutaneous T-Cell lymphoma. Oncotarget 2015, 6, 20555–20569. [Google Scholar] [CrossRef]
- McGirt, L.Y.; Jia, P.; Baerenwald, D.A.; Duszynski, R.J.; Dahlman, K.B.; Zic, J.A.; Zwerner, J.P.; Hucks, D.; Dave, U.; Zhao, Z.; et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood 2015, 126, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, I.V.; Shtreis, A.; Kobayashi, K.; Glassman, S.; Tsang, M.; Woetmann, A.; Sasseville, D.; Ødum, N.; Duvic, M. Investigating potential exogenous tumor initiating and promoting factors for Cutaneous T-Cell Lymphomas (CTCL), a rare skin malignancy. Oncoimmunology 2016, 5, 1175799. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chevret, E.; Merlio, J.P. Sézary Syndrome: Translating Genetic Diversity into Personalized Medicine. J. Investig. Dermatol. 2016, 136, 1319–1324. [Google Scholar] [CrossRef][Green Version]
- Woollard, W.J.; Pullabhatla, V.; Lorenc, A.; Patel, V.M.; Butler, R.M.; Bayega, A.; Begum, N.; Bakr, F.; Dedhia, K.; Fisher, J.; et al. Candidate driver genes involved in genome maintenance and DNA repair in Sézary syndrome. Blood 2016, 127, 3387–3397. [Google Scholar] [CrossRef]
- Ehrentraut, S.; Schneider, B.; Nagel, S.; Pommerenke, C.; Quentmeier, H.; Geffers, R.; Feist, M.; Kaufmann, M.; Meyer, C.; Kadin, M.E.; et al. Th17 cytokine differentiation and loss of plasticity after SOCS1 inactivation in a cutaneous T-cell lymphoma. Oncotarget 2016, 7, 34201–34216. [Google Scholar] [CrossRef]
- Benoit, B.M.; Jariwala, N.; O’Connor, G.; Oetjen, L.K.; Whelan, T.M.; Werth, A.; Troxel, A.B.; Sicard, H.; Zhu, L.; Miller, C.; et al. CD164 identifies CD4+ T cells highly expressing genes associated with malignancy in Sézary syndrome: The Sézary signature genes, FCRL3, Tox, and miR-214. Arch. Dermatol. Res. 2017, 309, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Liszewski, W.; Naym, D.G.; Biskup, E.; Gniadecki, R. Psoralen with ultraviolet A-induced apoptosis of cutaneous lymphoma cell lines is augmented by type I interferons via the JAK1–STAT1 pathway. Photodermatol. Photoimmunol. Photomed. 2017, 33, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Zhang, X.; Hu, C.H.; Langridge, T.; Tarapore, R.S.; Allen, J.E.; Oster, W.; Duvic, M. ONC201 selectively induces apoptosis in cutaneous T-cell lymphoma cells via activating pro-apoptotic integrated stress response and inactivating JAK/STAT and NF-κB pathways. Oncotarget 2017, 8, 61761–61776. [Google Scholar] [CrossRef]
- Nielsen, M.; Kaltoft, K.; Nordahl, M.; Röpke, C.; Geisler, C.; Mustelin, T.; Dobson, P.; Svejgaard, A.; Ødum, N. Constitutive activation of a slowly migrating isoform of Stat3 in mycosis fungoides: Tyrphostin AG490 inhibits Stat3 activation and growth of mycosis fungoides tumor cell lines. Proc. Natl. Acad. Sci. USA 1997, 94, 6764–6769. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A.; Chen, J. Disorders of the JAK/STAT pathway in T cell lymphoma pathogenesis: Implications for immunotherapy. Annu. Rev. Immunol. 2017, 35, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, I.; Gorunova, L.; Spetalen, S.; Bassarova, A.; Beiske, K.; Micci, F.; Heim, S. Fusion of the genes ataxin 2 like, ATXN2L, and Janus kinase 2, JAK2, in cutaneous CD4 positive T-cell lymphoma. Oncotarget 2017, 8, 103775–103784. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.N.B.; Najidh, S.; Tensen, C.P.; Vermeer, M.H. Molecular advances in cutaneous T-cell lymphoma. Semin. Cutan. Med. Surg. 2018, 37, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Seto, A.G.; Beatty, X.; Lynch, J.M.; Hermreck, M.; Tetzlaff, M.; Duvic, M.; Jackson, A.L. Cobomarsen, an oligonucleotide inhibitor of miR-155, co-ordinately regulates multiple survival pathways to reduce cellular proliferation and survival in cutaneous T-cell lymphoma. Br. J. Haematol. 2018, 183, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, E.D.; Weinstock, D.M. Challenges and implications of genomics for T-cell lymphomas. Hematology 2018, 2018, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Bastidas Torres, A.N.; Cats, D.; Mei, H.; Szuhai, K.; Willemze, R.; Vermeer, M.H.; Tensen, C.P. Genomic analysis reveals recurrent deletion of JAK-STAT signaling inhibitors HNRNPK and SOCS1 in mycosis fungoides. Genes Chromosom. Cancer 2018, 57, 653–664. [Google Scholar] [CrossRef]
- Lindahl, L.M.; Willerslev-Olsen, A.; Gjerdrum, L.M.R.; Nielsen, P.R.; Blümel, E.; Rittig, A.H.; Celis, P.; Herpers, B.; Becker, J.C.; Stausbøl-Grøn, B.; et al. Antibiotics inhibit tumor and disease activity in cutaneous T-cell lymphoma. Blood 2019, 134, 1072–1083. [Google Scholar] [CrossRef]
- Yang, H.; Tao, Y.; Zhang, M.; Ma, P.; Li, L.; Diao, Q. Effects of 9-cis-retinoic acid on the proliferation and apoptosis of cutaneous T-cell lymphoma cells. Anticancer Drugs 2019, 30, 56–64. [Google Scholar] [CrossRef]
- Moosic, K.B.; Paila, U.; Olson, K.C.; Dziewulska, K.; Wang, T.T.; Xing, J.C.; Ratan, A.; Feith, D.J.; Loughran, T.P.; Olson, T.L. Genomics of LGL leukemia and select other rare leukemia/lymphomas. Best Pract. Res. Clin. Haematol. 2019, 32, 196–206. [Google Scholar] [CrossRef]
- Seffens, A.; Herrera, A.; Tegla, C.; Buus, T.B.; Hymes, K.B.; Ødum, N.; Geskin, L.J.; Koralov, S.B. STAT3 dysregulation in mature T and NK cell lymphomas. Cancers 2019, 11, 1711. [Google Scholar] [CrossRef]
- Sun, W.H.; Pabon, C.; Alsayed, Y.; Huang, P.P.; Jandeska, S.; Uddin, S.; Platanias, L.C.; Rosen, S.T. Interferon-α resistance in a cutaneous T-cell lymphoma cell line is associated with lack of STAT1 expression. Blood 1998, 91, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Trochopoulos, A.G.X.; Zaharieva, M.M.; Marinova, M.H.; Yoncheva, K.; Tibi, P.E.; Berger, M.R.; Konstantinov, S.M. Antineoplastic effect of a novel nanosized curcumin on cutaneous T cell lymphoma. Oncol. Lett. 2020, 20, 304. [Google Scholar] [CrossRef]
- Pérez, C.; Mondéjar, R.; García-Díaz, N.; Cereceda, L.; León, A.; Montes, S.; Durán Vian, C.; Pérez Paredes, M.G.; González-Morán, A.; Alegre de Miguel, V.; et al. Advanced-stage mycosis fungoides: Role of the signal transducer and activator of transcription 3, nuclear factor-κB and nuclear factor of activated T cells pathways. Br. J. Dermatol. 2020, 182, 147–155. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, L.; Shi, X.; Gong, Z.; Yu, R.; Zhang, D.; Zhang, Y.; Ma, W. TPD7 inhibits the growth of cutaneous T cell lymphoma H9 cell through regulating IL-2R signalling pathway. J. Cell. Mol. Med. 2020, 24, 984–995. [Google Scholar] [CrossRef]
- Maurus, K.; Appenzeller, S.; Roth, S.; Brändlein, S.; Kneitz, H.; Goebeler, M.; Rosenwald, A.; Geissinger, E.; Wobser, M. Recurrent Oncogenic JAK and STAT Alterations in Cutaneous CD30-Positive Lymphoproliferative Disorders. J. Investig. Dermatol. 2020, 140, 2023–2031.e1. [Google Scholar] [CrossRef] [PubMed]
- García-Colmenero, L.; González, J.; Sandoval, J.; Guillén, Y.; Diaz-Lagares, A.; Andrades, E.; Iglesias, A.; Nonell, L.; Pujol, R.M.; Bigas, A.; et al. Epigenetic Silencing of Tumor Suppressor miR-124 Directly Supports STAT3 Activation in Cutaneous T-Cell Lymphoma. Cells 2020, 9, 2692. [Google Scholar] [CrossRef]
- Brouwer, I.J.; Out-Luiting, J.J.; Vermeer, M.H.; Tensen, C.P. Cucurbitacin E and I target the JAK/STAT pathway and induce apoptosis in Sézary cells. Biochem. Biophys. Rep. 2020, 24, 100832. [Google Scholar] [CrossRef] [PubMed]
- Yumeen, S.; Mirza, F.N.; Lewis, J.M.; King, A.L.O.; Kim, S.R.; Carlson, K.R.; Umlauf, S.R.; Surovtseva, Y.V.; Foss, F.M.; Girardi, M. JAK inhibition synergistically potentiates BCL2, BET, HDAC, and proteasome inhibition in advanced CTCL. Blood Adv. 2020, 4, 2213–2226. [Google Scholar] [CrossRef]
- Le, M.; Ghazawi, F.M.; Netchiporouk, E.; Litvinov, I.V. The Novel Role of Antibiotic Treatment in the Management of Cutaneous T-Cell Lymphoma (CTCL) Patients. J. Cutan. Med. Surg. 2020, 24, 410–411. [Google Scholar] [CrossRef]
- Vadivel, C.K.; Gluud, M.; Torres-Rusillo, S.; Boding, L.; Willerslev-Olsen, A.; Buus, T.B.; Nielsen, T.K.; Persson, J.L.; Bonefeld, C.M.; Geisler, C.; et al. JAK3 is expressed in the nucleus of malignant T cells in cutaneous T cell lymphoma (CTCL). Cancers 2021, 13, 280. [Google Scholar] [CrossRef]
- Karagianni, F.; Piperi, C.; Mpakou, V.; Spathis, A.; Foukas, P.G.; Dalamaga, M.; Pappa, V.; Papadavid, E. Ruxolitinib with resminostat exert synergistic antitumor effects in Cutaneous T-cell Lymphoma. PLoS ONE 2021, 16, e0248298. [Google Scholar] [CrossRef] [PubMed]
- Woetmann, A.; Nielsen, M.; Christensen, S.T.; Brockdorff, J.; Kaltoft, K.; Engel, A.M.; Skov, S.; Brender, C.; Geisler, C.; Svejgaard, A.; et al. Inhibition of protein phosphatase 2A induces serine/threonine phosphorylation, subcellular redistribution, and functional inhibition of STAT3. Proc. Natl. Acad. Sci. USA 1999, 96, 10620–10625. [Google Scholar] [CrossRef] [PubMed]
- Bastidas Torres, A.N.; Cats, D.; Out-Luiting, J.J.; Fanoni, D.; Mei, H.; Venegoni, L.; Willemze, R.; Vermeer, M.H.; Berti, E.; Tensen, C.P. Deregulation of JAK2 signaling underlies primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma. Haematologica 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Willerslev-Olsen, A.; Gjerdrum, L.M.R.; Lindahl, L.M.; Buus, T.B.; Pallesen, E.M.H.; Gluud, M.; Bzorek, M.; Nielsen, B.S.; Kamstrup, M.R.; Rittig, A.H.; et al. Staphylococcus aureus Induces Signal Transducer and Activator of Transcription 5–Dependent miR-155 Expression in Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2021, 141, 2449–2458. [Google Scholar] [CrossRef]
- Pavlidis, A.; Piperi, C.; Papadavid, E. Novel therapeutic approaches for cutaneous T cell lymphomas. Expert Rev. Clin. Immunol. 2021, 17, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.R.; Patrone, C.C.; Quinn, S.A.; Gu, Y.; Sanchez-Martin, M.; Mackey, A.; Cooke, A.J.; Shih, B.B.; Laurent, A.P.; Trager, M.H.; et al. Jak-STAT Inhibition Mediates Romidepsin and Mechlorethamine Synergism in Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2021, 141, 2908–2920.e7. [Google Scholar] [CrossRef] [PubMed]
- Nihal, M.; Wu, J.; Stonesifer, C.J.; Daniels, J.; Choi, J.; Geskin, L.; Rook, A.H.; Wood, G.S. Epigenetic Regulation of Apoptosis in Cutaneous T-Cell Lymphoma: Implications for Therapy with Methotrexate, Jak Inhibitors, and Resveratrol. J. Investig. Dermatol. 2021, in press. [Google Scholar] [CrossRef]
- García-Díaz, N.; Piris, M.Á.; Ortiz-Romero, P.L.; Vaqué, J.P. Mycosis fungoides and sézary syndrome: An integrative review of the pathophysiology, molecular drivers, and targeted therapy. Cancers 2021, 13, 1391. [Google Scholar] [CrossRef]
- McKenzie, R.C.T.; Jones, C.L.; Tosi, I.; Caesar, J.A.; Whittaker, S.J.; Mitchell, T.J. Constitutive activation of STAT3 in Sézary syndrome is independent of SHP-1. Leukemia 2012, 26, 323–331. [Google Scholar] [CrossRef]
- Willerslev-Olsen, A.; Krejsgaard, T.; Lindahl, L.M.; Litvinov, I.V.; Fredholm, S.; Petersen, D.L.; Nastasi, C.; Gniadecki, R.; Mongan, N.P.; Sasseville, D.; et al. Staphylococcal enterotoxin A (SEA) stimulates STAT3 activation and IL-17 expression in cutaneous T-cell lymphoma. Blood 2016, 127, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Shreberk-Hassidim, R.; Ramot, Y.; Zlotogorski, A. Janus kinase inhibitors in dermatology: A systematic review. J. Am. Acad. Dermatol. 2017, 76, 745–753.e19. [Google Scholar] [CrossRef]
- Qin, J.Z.; Kamarashev, J.; Zhang, C.L.; Dummer, R.; Burg, G.; Döbbeling, U. Constitutive and interleukin-7- and interleukin-15-stimulated DNA binding of STAT and novel factors in cutaneous T cell lymphoma cells. J. Investig. Dermatol. 2001, 117, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, K.W.; Kaltoft, K.; Mikkelsen, G.; Nielsen, M.; Zhang, Q.; Geisler, C.; Nissen, M.H.; Röpke, C.; Wasik, M.A.; Ødum, N. Constitutive STAT3-activation in Sezary syndrome: Tyrphostin AG490 inhibits STAT3-activation, interleukin-2 receptor expression and growth of leukemic Sezary cells. Leukemia 2001, 15, 787–793. [Google Scholar] [CrossRef]
- Nielsen, M.; Nissen, M.H.; Gerwien, J.; Zocca, M.B.; Rasmussen, H.M.; Nakajima, K.; Röpke, C.; Geisler, C.; Kaltoft, K.; Ødum, N. Spontaneous interleukin-5 production in cutaneous T-cell lymphoma lines is mediated by constitutively activated Stat3. Blood 2002, 99, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Dequidt, L.; Franck, N.; Sanchez-Pena, P.; Dalle, S.; Adamski, H.; Boulinguez, S.; Ingen-Housz-Oro, S.; Ram-Wolff, C.; Boccara, O.; Bonnet, N.; et al. Cutaneous lymphomas appearing during treatment with biologics: 44 cases from the French Study Group on Cutaneous Lymphomas and French Pharmacovigilance Database. Br. J. Dermatol. 2019, 181, 616–618. [Google Scholar] [CrossRef]
- Kołkowski, K.; Sokołowska-Wojdyło, M. Safety and danger of biologic treatments in psoriasis in context of cutaneous T-cell lymphoma (CTCL). Adv. Dermatol. Allergol. 2021, in press. [Google Scholar] [CrossRef]
- Elston, D.M. Dupilumab and cutaneous T-cell lymphoma. J. Am. Acad. Dermatol. 2020, 83, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Drug Safety-Related Labeling Changes (SrLC). Available online: https://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges/index.cfm?event=searchResult.page (accessed on 16 October 2021).
- European Medicines Agency. Adtralza. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/adtralza#authorisation-details-section (accessed on 21 October 2021).
- European Medicines Agency. EMEA-002536-PIP01-18. Available online: https://www.ema.europa.eu/en/medicines/human/paediatric-investigation-plans/emea-002536-pip01-18 (accessed on 21 October 2021).
- Klein, K.; Stoiber, D.; Sexl, V.; Witalisz-Siepracka, A. Untwining anti-tumor and immunosuppressive effects of JAK inhibitors—A strategy for hematological malignancies? Cancers 2021, 13, 2611. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Octagam. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Octagam/human_referral_000265.jsp&mid=WC0b01ac0580024e99 (accessed on 20 October 2021).
- European Medicines Agency. Xeljanz. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xeljanz#patient-safety-section (accessed on 20 October 2021).
- European Medicines Agency. Beromun. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/beromun (accessed on 20 October 2021).
- European Medicines Agency. Olumiant. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant#product-information-section (accessed on 20 October 2021).
- European Medicines Agency. EMA Tagrisso: Pending EC Decision. Available online: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/tagrisso (accessed on 20 October 2021).
- Drug Safety-Related Labeling Changes (SrLC). Available online: https://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges/index.cfm?event=searchdetail.page&DrugNameID=2084 (accessed on 16 October 2021).
- Food and Drug Administration. FDA Requires Warnings about Increased Risk of Serious Heart-Related Events, Cancer, Blood Clots, and Death for JAK Inhibitors That Treat Certain Chronic Inflammatory Conditions. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death (accessed on 22 October 2021).
- Drug Safety-Related Labeling Changes (SrLC). Available online: https://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges/index.cfm?event=searchdetail.page&DrugNameID=2285 (accessed on 16 October 2021).
- Drug Safety-Related Labeling Changes (SrLC). Available online: https://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges/index.cfm?event=searchdetail.page&DrugNameID=87 (accessed on 16 October 2021).
- Murphrey, M.; Waldman, R.A.; Druso, T.; Grant-Kels, J.M. Special Editorial: When Prescribing Janus Kinase Inhibitors for Dermatologic Conditions, Be Mindful of the FDA’s 9/1/2021 Data Safety Communication. J. Am. Acad. Dermatol. 2021, in press. [Google Scholar] [CrossRef]
- Chiba, T.; Nagai, T.; Osada, S.I.; Manabe, M. Diagnosis of mycosis fungoides following administration of dupilumab for misdiagnosed atopic dermatitis. Acta Derm.-Venereol. 2019, 99, 818–819. [Google Scholar] [CrossRef]
- Tran, J.; Morris, L.; Vu, A.; Duvic, M. Development of Sézary syndrome following the administration of dupilumab. Dermatol. Online J. 2020, 26, 17. [Google Scholar] [CrossRef]
- Miyashiro, D.; Vivarelli, A.G.; Gonçalves, F.; Cury-Martins, J.; Sanches, J.A. Progression of mycosis fungoides after treatment with dupilumab: A case report. Dermatol. Ther. 2020, 33, e13880. [Google Scholar] [CrossRef]
- Espinosa, M.L.; Nguyen, M.T.; Aguirre, A.S.; Martinez-Escala, M.E.; Kim, J.; Walker, C.J.; Pontes, D.S.; Silverberg, J.I.; Choi, J.; Pro, B.; et al. Progression of cutaneous T-cell lymphoma after dupilumab: Case review of 7 patients. J. Am. Acad. Dermatol. 2020, 83, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Claire Hollins, L.; Wirth, P.; Fulchiero, G.J.; Foulke, G.T. Long-standing dermatitis treated with dupilumab with subsequent progression to cutaneous T-cell lymphoma. Cutis 2020, 106, E8–E11. [Google Scholar] [CrossRef]
- Mollanazar, N.K.; Savage, K.T.; Pousti, B.T.; Jariwala, N.; Del Guzzo, C.; Haun, P.; Vittorio, C.C.; Rook, A.H.; Kim, E.J. Cutaneous T-cell lymphoma and concomitant atopic dermatitis responding to dupilumab. Cutis 2020, 106, 131–132. [Google Scholar] [CrossRef]
- Lazaridou, I.; Ram-Wolff, C.; Bouaziz, J.D.; Begon, E.; Battistella, M.; Rivet, J.; Jachiet, M.; Bagot, M.; De Masson, A. Dupilumab treatment in two patients with cutaneous T-cell lymphomas. Acta Derm.-Venereol. 2020, 100, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Steck, O.; Bertschi, N.L.; Luther, F.; van den Berg, J.; Winkel, D.J.; Holbro, A.; Schlapbach, C. Rapid and sustained control of itch and reduction in Th2 bias by dupilumab in a patient with Sézary syndrome. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, N.; Demitsu, T.; Otaki, K.; Matsumoto, T.; Takazawa, M.; Yamada, A.; Kimura, S.-i.; Kakurai, M. Dupilumab therapy in Sézary syndrome misdiagnosed as atopic dermatitis: A case report. J. Dermatol. 2020, 47, e356–e357. [Google Scholar] [CrossRef] [PubMed]
- Ayasse, M.; Kamaria, N.; Glass, F.; Silverberg, J.I. Mycosis Fungoides Unmasked by Dupilumab Treatment in a Patient With a History of Atopic Dermatitis. Dermatitis 2020, 32, e88–e89. [Google Scholar] [CrossRef] [PubMed]
- Russomanno, K.; Carver DeKlotz, C.M. Acceleration of cutaneous T-cell lymphoma following dupilumab administration. JAAD Case Rep. 2021, 8, 83–85. [Google Scholar] [CrossRef]
- Newsom, M.; Hrin, M.L.; Hamid, R.N.; Strowd, L.C.; Ahn, C.; Jorizzo, J.L.; Feldman, S.R. Two cases of mycosis fungoides diagnosed after treatment non-response to dupilumab. Dermatol. Online J. 2021, 27, 18. [Google Scholar] [CrossRef]
- Trum, N.A.; Zain, J.; Abad, C.; Rosen, S.T.; Querfeld, C. Dupilumab as a therapy option for treatment refractory mogamulizumab-associated rash. JAAD Case Rep. 2021, 14, 37–42. [Google Scholar] [CrossRef]
- Lévy, R.; Fusaro, M.; Guerin, F.; Chetouani, A.; Moshous, D.; Fischer, A.; de Saint Basile, G.; Sepulveda, F.E.; Neven, B. Efficacy of ruxolitinib in subcutaneous panniculitis-like T-cell lymphoma and hemophagocytic lymphohistiocytosis. Blood Adv. 2020, 4, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, A.J.; Ghione, P.; Jacobsen, E.D.; Ruan, J.; Schatz, J.H.; Noor, S.J.; Myskowski, P.L.; Vardhana, S.A.; Ganesan, N.; Hancock, H.; et al. Phase II biomarker-driven study of ruxolitinib demonstrates effectiveness of JAK/STAT targeting in T-cell lymphomas. Blood 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Francuzik, W.; Alexiou, A.; Worm, M. Safety of dupilumab in patients with atopic dermatitis: Expert opinion. Expert Opin. Drug Saf. 2021, 9, 997–1004. [Google Scholar] [CrossRef]
- Sugaya, M. Clinical Guidelines and New Molecular Targets for Cutaneous Lymphomas. Int. J. Mol. Sci. 2021, 22, 11079. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacol. Res. 2016, 111, 784–803. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Guan, P.; Sprague, L.; Verbist, K.; Tedrick, P.; An, Q.A.; Cheng, C.; Kurachi, M.; Levine, R.; Wherry, E.J.; et al. Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis. Blood 2016, 127, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Maschalidi, S.; Sepulveda, F.E.; Garrigue, A.; Fischer, A.; De Saint Basile, G. Therapeutic effect of JAK1/2 blockade on the manifestations of hemophagocytic lymphohistiocytosis in mice. Blood 2016, 128, 60–71. [Google Scholar] [CrossRef] [PubMed]
| Similarities | Atopic Dermatitis | Cutaneous T-Cell Lymphoma |
|---|---|---|
| Eosinophilia | Often present | May be present in the advanced stage |
| Immunoglobulin E (IgE) | Often elevated | May be elevated in the advanced stage |
| Lactate dehydrogenase (LDH) | May be elevated | Severity marker of MF/SS |
| Soluble interleukin receptor 2 (sIL-2R) | May be elevated | Severity marker of MF/SS |
| Th-2 microenvironment activation | Always present | Present in the advanced stage |
| Levels of filaggrin | Significantly lowered | May be significantly lowered |
| Transepidermal water loss (TEWL) | Significantly lowered | May be significantly lowered |
| Levels of antimicrobial peptides (AMPs) | Significantly lowered | Significantly lowered |
| Colonization of S. aureus | 80% of patients | 50–60% of patients |
| Drug | Age (Years) | Sex | Pre-Diagnosis | Final Diagnosis | Response to Treatment | Death | Reference |
|---|---|---|---|---|---|---|---|
| Dupilumab | 58 | M | AD | MF | Progression of MF | No | [199] |
| Dupilumab | 64 | M | AD | SS | Progression of SS | No | [200] |
| Dupilumab | 51 | F | AD | MF | Progression of MF | No | [201] |
| Dupilumab | 64 | M | AD | CTCL-NOS | Progression of erythroderma | No | [202] |
| Dupilumab | 72 | M | AD | MF | Progression of MF | No | [202] |
| Dupilumab | 59 | F | AD | MF and AD | Progression of MF | No | [202] |
| Dupilumab | 40 | F | AD | MF | Progression of MF | No | [202] |
| Dupilumab | 67 | M | MF | SS | Progression of SS | Yes | [202] |
| Dupilumab | 58 | M | MF | SS | Progression of SS | Yes | [202] |
| Dupilumab | 77 | F | MF | SS | Progression of SS | No | [202] |
| Dupilumab | 61 | M | Eczema | MF | Progression of MF | No | [203] |
| Dupilumab | 52 | M | Eczema | MF | No clinical improvement | No | [203] |
| Dupilumab | 60 | F | Eczema | MF | No clinical improvement | No | [203] |
| Dupilumab | 68 | M | SS and AD | SS and AD | Improvement in SS and AD | No | [204] |
| Dupilumab | 37 | F | Eczema | SS | Progression of SS | No | [205] |
| Dupilumab | 55 | M | MF and AD | MF and AD | Improvement of MF and AD | No | [205] |
| Dupilumab | 74 | F | SS | SS | Improvement of SS | No | [206] |
| Dupilumab | 48 | F | AD | SS and AD | No clinical improvement | No | [207] |
| Dupilumab | 40 | F | AD | MF | Progression of MF | No | [208] |
| Dupilumab | 43 | M | AD | MF and AD | Progression of MF | No | [209] |
| Dupilumab | 48 | F | AD | MF | Progression of MF | No | [210] |
| Dupilumab | 55 | M | AD | MF | Progression of MF | No | [210] |
| Dupilumab | 26 | M | MF | MF | No clinical improvement | No | [211] |
| Ruxolitinib | 13 | M | HLH | HLH and SPTCL | Improvement of SPTCL and HLH | No | [212] |
| Ruxolitinib | NS | NS | MF | MF | Progression of MF | No | [213] |
| Ruxolitinib | NS | NS | CTCL | CTCL | No clinical improvement/Stable disease | No | [213] |
| Ruxolitinib | NS | NS | CTCL | CTCL | Progression of CTCL | No | [213] |
| Ruxolitinib | NS | NS | CTCL | CTCL | Progression of CTCL | No | [213] |
| Ruxolitinib | NS | NS | MF | MF | Progression of MF | No | [213] |
| Ruxolitinib | NS | NS | MF | MF | No clinical improvement/Stable disease | No | [213] |
| Ruxolitinib | NS | NS | MF | MF | Improvement of MF/Partial remission | No | [213] |
| Ruxolitinib | NS | NS | pcALCL | pcALCL | Improvement of MF/Complete response | No | [213] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kołkowski, K.; Trzeciak, M.; Sokołowska-Wojdyło, M. Safety and Danger Considerations of Novel Treatments for Atopic Dermatitis in Context of Primary Cutaneous Lymphomas. Int. J. Mol. Sci. 2021, 22, 13388. https://doi.org/10.3390/ijms222413388
Kołkowski K, Trzeciak M, Sokołowska-Wojdyło M. Safety and Danger Considerations of Novel Treatments for Atopic Dermatitis in Context of Primary Cutaneous Lymphomas. International Journal of Molecular Sciences. 2021; 22(24):13388. https://doi.org/10.3390/ijms222413388
Chicago/Turabian StyleKołkowski, Karol, Magdalena Trzeciak, and Małgorzata Sokołowska-Wojdyło. 2021. "Safety and Danger Considerations of Novel Treatments for Atopic Dermatitis in Context of Primary Cutaneous Lymphomas" International Journal of Molecular Sciences 22, no. 24: 13388. https://doi.org/10.3390/ijms222413388
APA StyleKołkowski, K., Trzeciak, M., & Sokołowska-Wojdyło, M. (2021). Safety and Danger Considerations of Novel Treatments for Atopic Dermatitis in Context of Primary Cutaneous Lymphomas. International Journal of Molecular Sciences, 22(24), 13388. https://doi.org/10.3390/ijms222413388






