Verteporfin-Loaded Mesoporous Silica Nanoparticles’ Topical Applications Inhibit Mouse Melanoma Lymphangiogenesis and Micrometastasis In Vivo
Abstract
:1. Introduction
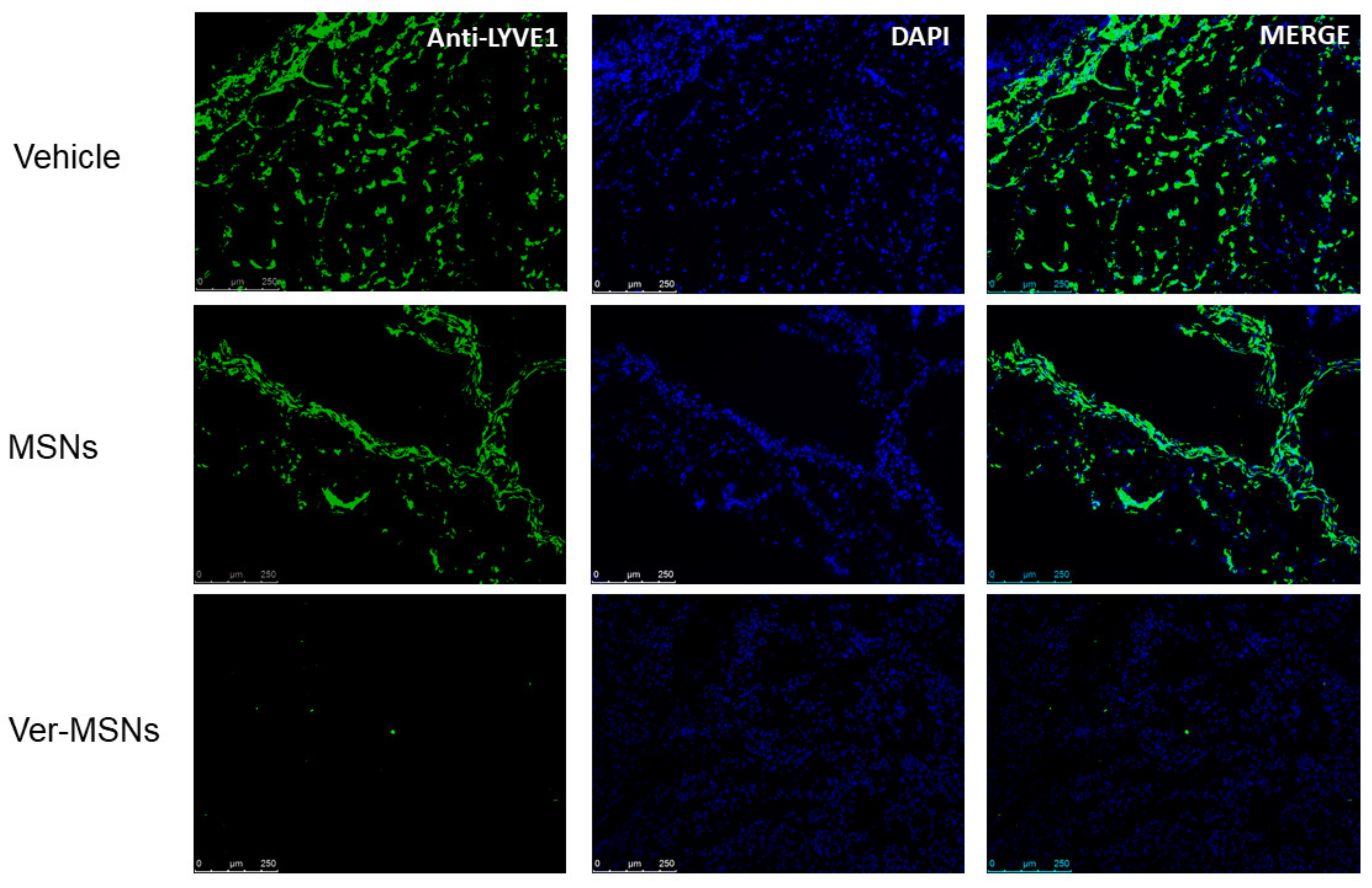
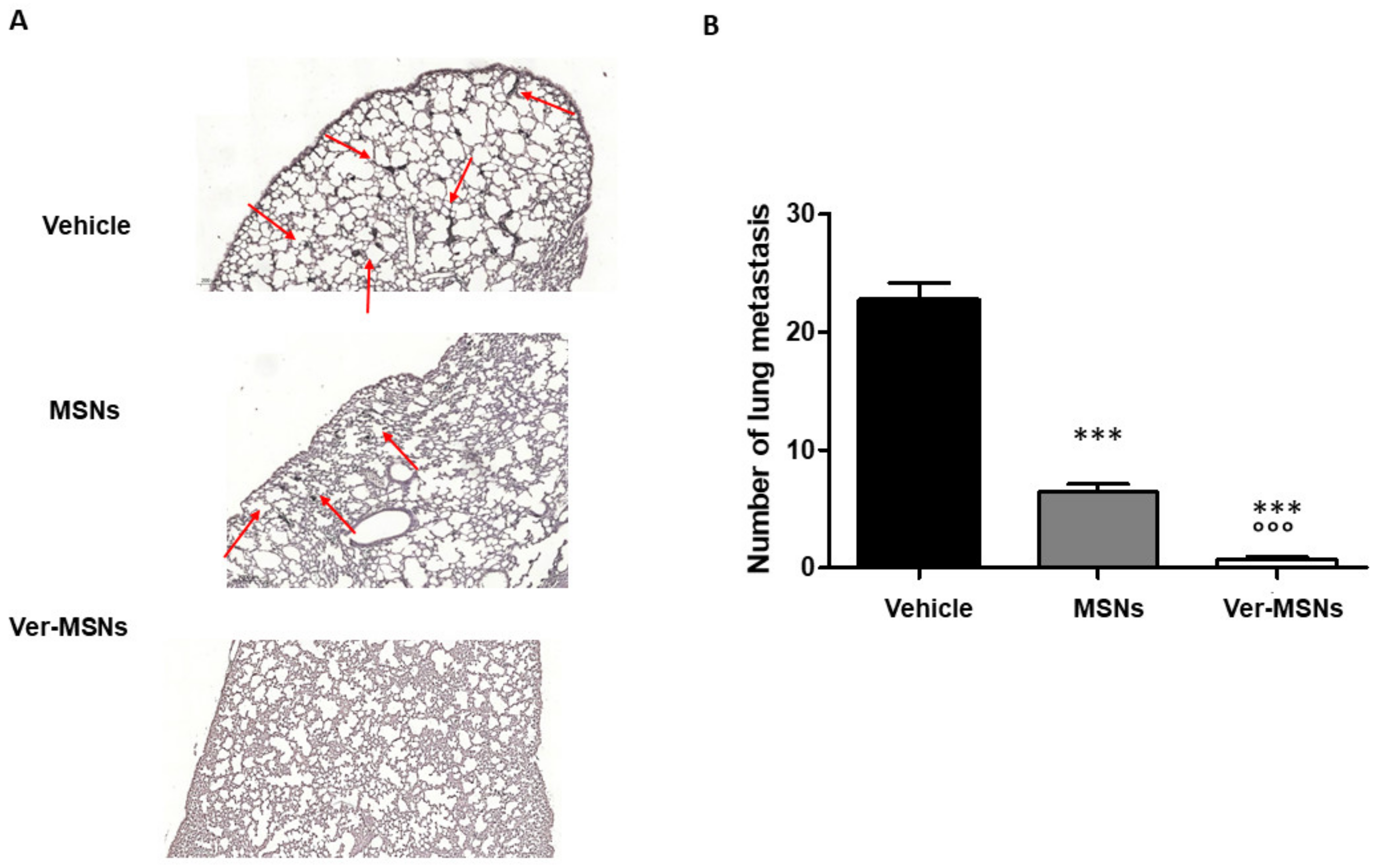
2. Results
3. Discussion
4. Materials and Methods
4.1. Synthesis of Verteporfin-Loaded Mesoporous Silica Nanoparticles
4.2. In Vivo Melanoma Topical Treatment
4.3. Immunofluorescence and Immunohistochemistry of Tumors and Animal Specimens
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suhail, Y.; Cain, M.P.; Gireesan, K.V.; Kurywchak, P.A.; Levchenko, A.; Kalluri, R.; Kshitiz, D.A. Systems biology of cancer metastasis. Cell Syst. 2019, 9, 109–127. [Google Scholar] [CrossRef] [Green Version]
- Rinderknecht, M.; Detmar, M. Tumor lymphangiogenesis and tumor metastasis. J. Cell. Physiol. 2008, 16, 347–354. [Google Scholar] [CrossRef]
- Van Deurzen, C.H.M.; de Boer, M.; Monninkhof, E.M.; Bult, P.; Van Der Wall, E.; Tjan-Heijnen, V.C.G.; Van Diest, P.J. Non-sentinel lymph node metastases associated with isolated breast cancer cells in the sentinel node. J. Natl. Cancer Inst. 2008, 100, 1574–1580. [Google Scholar] [CrossRef] [Green Version]
- Cserni, G. Commentary on in-transit lymph node metastases in breast cancer: A possible source of local recurrence after sentinel node procedure. J. Clin. Pathol. 2008, 61, 1233–1235. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Tammela, T.; Saaristo, A.; Holopainen, T.; Ylä-Herttuala, S.; Andersson, L.C.; Virolainen, S.; Immonen, I.; Alitalo, K. Photodynamic ablation of lymphatic vessels and intralymphatic cancer cells prevents metastasis. Sci. Transl. Med. 2011, 3, 69ra11. [Google Scholar] [CrossRef]
- Duong, T.; Koopman, P.; Francois, M. Tumor Lymphoangiogenesis as a potential therapeutic target. J. Oncol. 2012, 2012, 204946. [Google Scholar] [CrossRef] [Green Version]
- Lund, A.W.; Wagner, M.; Fankhauser, M.; Steinskog, E.S.; Broggi, M.A.; Spranger, S.; Gajewski, T.F.; Alitalo, K.; Eikesdal, H.P.; Wiig, H.; et al. Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J. Clin. Investig. 2016, 126, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Tas, F. Metastatic behavior in melanoma: Timing, pattern, survival and influencing factors. J. Oncol. 2012, 2012, 647684. [Google Scholar] [CrossRef] [Green Version]
- Mallidi, S.; Anbil, S.; Bulin, A.-L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Pautu, V.; Leonetti, D.; Lepeltier, E.; Clere, N.; Passirani, C. Nanomedicine as a potent strategy in melanoma tumor microenvironment. Pharmacol. Res. 2017, 126, 31–53. [Google Scholar] [CrossRef] [Green Version]
- Fioramonti Calixto, G.M.F.; Bernegossi, J.; de Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Battaglia, L.; Scomparin, A.; Dianzani, C.; Milla, P.; Muntoni, E.; Arpicco, S.; Cavalli, R. Nanotechnology addressing cutaneous melanoma: The Italian landscape. Pharmaceutics 2021, 13, 1617. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gao, L.; Chen, K.; Zhang, W.; Zhang, Q.; Li, Q.; Hu, K. Nanoparticles: A new approach to upgrade cancer diagnosis and treatment. Nanoscale Res. Lett. 2021, 16, 88. [Google Scholar] [CrossRef]
- Konstantinou, E.K.; Notomi, S.; Koemidou, C.; Brodowska, K.; Al-Moujahed, A.; Nicolaou, F.; Tsoka, P.; Gragoudas, E.; Miller, J.W.; Young, L.H.; et al. Verteporfin-induced formation of protein cross-linked oligomers and high molecular weight complexes is mediated by light and leads to cell toxicity. Sci. Rep. 2017, 7, 46581. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, E.; Martins Estevão, B.; Miletto, I.; Tonello, S.; Renò, F.; Marchese, L. Verteporfin based silica nanoplatform for photodynamic therapy. Chem. Sel. 2016, 1, 127–131. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. Multifunctional mesoporous silica nanoplatform based on silicon nanoparticles for target two-photon-excited fluorescence imaging-guided chemo/photodynamic synergetic therapy in vitro. Talanta 2020, 209, 120552. [Google Scholar] [CrossRef]
- Rizzi, M.; Tonello, S.; Estevão, B.M.; Gianotti, E.; Marchese, L.; Renò, F. Verteporfin based silica nanoparticle for in vitro selective inhibition of human highly invasive melanoma cell proliferation. J. Photochem. Photobiol. B 2017, 167, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Clemente, N.; Miletto, I.; Gianotti, E.; Invernizzi, M.; Marchese, L.; Dianzani, U.; Renò, F. Verteporfin-loaded mesoporous silica nanoparticles inhibit mouse melanoma proliferation in vitro and in vivo. J. Photochem. Photobiol. B 2019, 197, 111533. [Google Scholar] [CrossRef]
- Chriestiansen, A.; Detmar, M. Lymphoangiogenesis and cancer. Genes Cancer 2011, 2, 1146–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobler, N.E.; Detmar, M. Tumor and lymph node lymphangiogenesis-impact on cancer metastasis. J. Leukoc. Biol. 2006, 80, 691–696. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zarkada, G.; Yi, S.; Eichmann, A. Lymphatic endothelial cell junctions: Molecular regulation in physiology and disease. Front. Physiol. 2020, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Padera, T.P.; Kadambi, A.; Di Tomaso, E.; Carreira, C.M.; Brown, E.B.; Boucher, Y.; Choi, N.C.; Mathisen, D.; Wain, J.; Mark, E.J.; et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 2002, 296, 1883–1886. [Google Scholar] [CrossRef]
- Kim, L.-Y.; Joachim, E.; Choi, H.; Kim, K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomedicine 2015, 11, 1407–1416. [Google Scholar] [CrossRef]
- Wachowska, M.; Osiak, A.; Muchowicz, A.; Gabrysiak, M.; Domagala, A.; Kilarski, W.W.; Golab, J. Investigation of cell death mechanisms in human lymphatic endothelial cells undergoing photodynamic therapy. Photodiagnosis Photodyn. Ther. 2016, 14, 57–65. [Google Scholar] [CrossRef]
- Zhu, D.; Cheng, C.F.; Pauli, B.U. Blocking of lung endothelial cell adhesion molecule-1 (Lu-ECAM-1) inhibits murine melanoma lung metastasis. J. Clin. Investig. 1992, 89, 1718–1724. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente, N.; Miletto, I.; Gianotti, E.; Sabbatini, M.; Invernizzi, M.; Marchese, L.; Dianzani, U.; Renò, F. Verteporfin-Loaded Mesoporous Silica Nanoparticles’ Topical Applications Inhibit Mouse Melanoma Lymphangiogenesis and Micrometastasis In Vivo. Int. J. Mol. Sci. 2021, 22, 13443. https://doi.org/10.3390/ijms222413443
Clemente N, Miletto I, Gianotti E, Sabbatini M, Invernizzi M, Marchese L, Dianzani U, Renò F. Verteporfin-Loaded Mesoporous Silica Nanoparticles’ Topical Applications Inhibit Mouse Melanoma Lymphangiogenesis and Micrometastasis In Vivo. International Journal of Molecular Sciences. 2021; 22(24):13443. https://doi.org/10.3390/ijms222413443
Chicago/Turabian StyleClemente, Nausicaa, Ivana Miletto, Enrica Gianotti, Maurizio Sabbatini, Marco Invernizzi, Leonardo Marchese, Umberto Dianzani, and Filippo Renò. 2021. "Verteporfin-Loaded Mesoporous Silica Nanoparticles’ Topical Applications Inhibit Mouse Melanoma Lymphangiogenesis and Micrometastasis In Vivo" International Journal of Molecular Sciences 22, no. 24: 13443. https://doi.org/10.3390/ijms222413443







