Protein-Based Nanoparticle Vaccines for SARS-CoV-2
Abstract
:1. Introduction
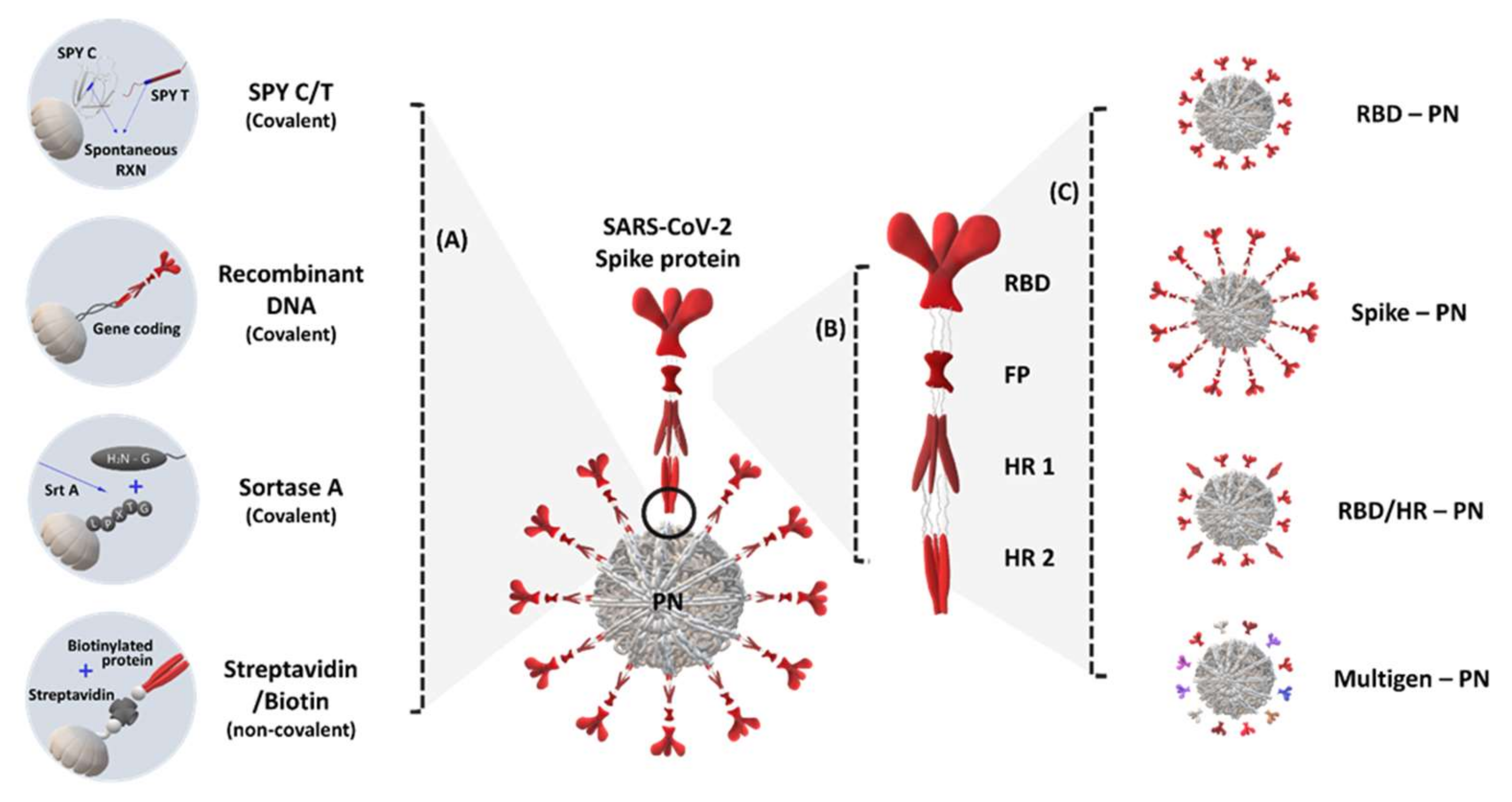
2. S Protein Domain-Conjugated (Presenting) Protein Nanoparticles
3. RBD and Other Domain-Conjugated (Presenting) Protein Nanoparticles
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—7 September 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---7-september-2020 (accessed on 30 September 2021).
- Coronavirus Resource Center. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available online: https://coronavirus.jhu.edu/map.html (accessed on 30 September 2021).
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 30 September 2021).
- Wrapp, D.; Wang, N.; Corbett Kizzmekia, S.; Goldsmith Jory, A.; Hsieh, C.-L.; Abiona, O.; Graham Barney, S.; McLellan Jason, S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.-Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e9. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Kim, D.; Yu, K.-M.; Seo, H.D.; Lee, S.-A.; Casel, M.A.B.; Jang, S.-G.; Kim, S.; Jung, W.; Lai, C.-J.; et al. Development of Spike Receptor-Binding Domain Nanoparticles as a Vaccine Candidate against SARS-CoV-2 Infection in Ferrets. mBio 2021, 12, e00230-21. [Google Scholar] [CrossRef]
- Ingale, J.; Stano, A.; Guenaga, J.; Sharma, S.K.; Nemazee, D.; Zwick, M.B.; Wyatt, R.T. High-Density Array of Well-Ordered HIV-1 Spikes on Synthetic Liposomal Nanoparticles Efficiently Activate B Cells. Cell Rep. 2016, 15, 1986–1999. [Google Scholar] [CrossRef] [Green Version]
- Thompson, E.A.; Ols, S.; Miura, K.; Rausch, K.; Narum, D.L.; Spångberg, M.; Juraska, M.; Wille-Reece, U.; Weiner, A.; Howard, R.F.; et al. TLR-adjuvanted nanoparticle vaccines differentially influence the quality and longevity of responses to malaria antigen Pfs25. JCI Insight 2018, 3, e120692. [Google Scholar] [CrossRef] [PubMed]
- Marcandalli, J.; Fiala, B.; Ols, S.; Perotti, M.; de van der Schueren, W.; Snijder, J.; Hodge, E.; Benhaim, M.; Ravichandran, R.; Carter, L.; et al. Induction of Potent Neutralizing Antibody Responses by a Designed Protein Nanoparticle Vaccine for Respiratory Syncytial Virus. Cell 2019, 176, 1420–1431.e17. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Wang, J.; Dou, J.; Yang, H.; He, X.; Xu, W.; Zhang, Y.; Hu, K.; Gu, N. Nanoparticle-based adjuvant for enhanced protective efficacy of DNA vaccine Ag85A-ESAT-6-IL-21 against Mycobacterium tuberculosis infection. Nanomedicine 2012, 8, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, M.; Wei, C.-J.; Yassine, H.M.; McTamney, P.M.; Boyington, J.C.; Whittle, J.R.R.; Rao, S.S.; Kong, W.-P.; Wang, L.; Nabel, G.J. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 2013, 499, 102–106. [Google Scholar] [CrossRef]
- Joyce, M.G.; Chen, W.-H.; Sankhala, R.S.; Hajduczki, A.; Thomas, P.V.; Choe, M.; Chang, W.; Peterson, C.E.; Martinez, E.; Morrison, E.B.; et al. SARS-CoV-2 ferritin nanoparticle vaccines elicit broad SARS coronavirus immunogenicity. bioRxiv 2021. [Google Scholar] [CrossRef]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines Against Infectious Diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef] [Green Version]
- Bangaru, S.; Ozorowski, G.; Turner, H.L.; Antanasijevic, A.; Huang, D.; Wang, X.; Torres, J.L.; Diedrich, J.K.; Tian, J.H.; Portnoff, A.D.; et al. Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. Science 2020, 370, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Gause, K.T.; Wheatley, A.K.; Cui, J.; Yan, Y.; Kent, S.J.; Caruso, F. Immunological Principles Guiding the Rational Design of Particles for Vaccine Delivery. ACS Nano 2017, 11, 54–68. [Google Scholar] [CrossRef]
- Link, A.; Zabel, F.; Schnetzler, Y.; Titz, A.; Brombacher, F.; Bachmann, M.F. Innate immunity mediates follicular transport of particulate but not soluble protein antigen. J. Immunol. 2012, 188, 3724–3733. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Shang, J.; Jiang, S.; Du, L. Subunit Vaccines against Emerging Pathogenic Human Coronaviruses. Front. Microbio. 2020, 11, 298. [Google Scholar] [CrossRef]
- Kang, Y.-F.; Sun, C.; Zhuang, Z.; Yuan, R.-Y.; Zheng, Q.; Li, J.-P.; Zhou, P.-P.; Chen, X.-C.; Liu, Z.; Zhang, X.; et al. Rapid Development of SARS-CoV-2 Spike Protein Receptor-Binding Domain Self-Assembled Nanoparticle Vaccine Candidates. ACS Nano 2021, 15, 2738–2752. [Google Scholar] [CrossRef]
- Kih, M.; Lee, E.J.; Lee, N.K.; Kim, Y.K.; Lee, K.E.; Jeong, C.; Yang, Y.; Kim, D.-H.; Kim, I.-S. Designed trimer-mimetic TNF superfamily ligands on self-assembling nanocages. Biomaterials 2018, 180, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Je, H.; Nam, G.-H.; Kim, G.B.; Kim, W.; Kim, S.R.; Kim, I.-S.; Lee, E.J. Overcoming therapeutic efficiency limitations against TRAIL-resistant tumors using re-sensitizing agent-loaded trimeric TRAIL-presenting nanocages. J. Control. Release 2021, 331, 7–18. [Google Scholar] [CrossRef]
- Choi, Y.; Nam, G.-H.; Kim, G.B.; Kim, S.; Kim, Y.K.; Kim, S.A.; Kim, H.-J.; Lee, E.J.; Kim, I.-S. Nanocages displaying SIRP gamma clusters combined with prophagocytic stimulus of phagocytes potentiate anti-tumor immunity. Cancer Gene Ther. 2021, 28, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Deng, Y.; Chen, X.; Zhou, Y.; Zhang, H.; Wu, H.; Yang, S.; Chen, F.; Zhou, Z.; Wang, M.; et al. Immune Response of A Novel ATR-AP205-001 Conjugate Anti-hypertensive Vaccine. Sci. Rep. 2017, 7, 12580. [Google Scholar] [CrossRef] [Green Version]
- Pardi, N.; Hogan, M.J.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E.; et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018, 215, 1571–1588. [Google Scholar] [CrossRef] [PubMed]
- López-Sagaseta, J.; Malito, E.; Rappuoli, R.; Bottomley, M.J. Self-assembling protein nanoparticles in the design of vaccines. Comput. Struct. Biotechnol. J. 2016, 14, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Slifka, M.K.; Amanna, I.J. Role of Multivalency and Antigenic Threshold in Generating Protective Antibody Responses. Front. Immunol. 2019, 10, 956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, H.G.; Kent, S.J.; Wheatley, A.K. Immunological basis for enhanced immunity of nanoparticle vaccines. Expert Rev. Vaccines 2019, 18, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Tokatlian, T.; Read, B.J.; Jones, C.A.; Kulp, D.W.; Menis, S.; Chang, J.Y.H.; Steichen, J.M.; Kumari, S.; Allen, J.D.; Dane, E.L.; et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science 2019, 363, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, X.; Bian, Y.; Wang, S.; Chai, Q.; Guo, Z.; Wang, Z.; Zhu, P.; Peng, H.; Yan, X.; et al. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat. Nanotechnol. 2020, 15, 406–416. [Google Scholar] [CrossRef]
- Kato, Y.; Abbott, R.K.; Freeman, B.L.; Haupt, S.; Groschel, B.; Silva, M.; Menis, S.; Irvine, D.J.; Schief, W.R.; Crotty, S. Multifaceted Effects of Antigen Valency on B Cell Response Composition and Differentiation In Vivo. Immunity 2020, 53, 548–563.e8. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Lazarovits, J.; Poon, W.; Ouyang, B.; Nguyen, L.N.M.; Kingston, B.R.; Chan, W.C.W. Nanoparticle Size Influences Antigen Retention and Presentation in Lymph Node Follicles for Humoral Immunity. Nano Lett. 2019, 19, 7226–7235. [Google Scholar] [CrossRef]
- Kim, G.B.; Sung, H.-D.; Nam, G.-H.; Kim, W.; Kim, S.; Kang, D.; Lee, E.J.; Kim, I.-S. Design of PD-1-decorated nanocages targeting tumor-draining lymph node for promoting T cell activation. J. Control. Release 2021, 333, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Nam, G.H.; Lee, N.K.; Kih, M.; Koh, E.; Kim, Y.K.; Hong, Y.; Kim, S.; Park, S.Y.; Jeong, C.; et al. Nanocage-Therapeutics Prevailing Phagocytosis and Immunogenic Cell Death Awakens Immunity against Cancer. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Ma, X.; Zou, F.; Yu, F.; Li, R.; Yuan, Y.; Zhang, Y.; Zhang, X.; Deng, J.; Chen, T.; Song, Z.; et al. Nanoparticle Vaccines Based on the Receptor Binding Domain (RBD) and Heptad Repeat (HR) of SARS-CoV-2 Elicit Robust Protective Immune Responses. Immunity 2020, 53, 1315–1330.e9. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, M.; Bu, W.; Joyce, M.G.; Meng, G.; Whittle, J.R.; Baxa, U.; Yamamoto, T.; Narpala, S.; Todd, J.P.; Rao, S.S.; et al. Rational Design of an Epstein-Barr Virus Vaccine Targeting the Receptor-Binding Site. Cell 2015, 162, 1090–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, H.G.; Tan, H.-X.; Juno, J.A.; Esterbauer, R.; Ju, Y.; Jiang, W.; Wimmer, V.C.; Duckworth, B.C.; Groom, J.R.; Caruso, F.; et al. Self-assembling influenza nanoparticle vaccines drive extended germinal center activity and memory B cell maturation. JCI Insight 2020, 5, e136653. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Marles-Wright, J. Ferritin family proteins and their use in bionanotechnology. New Biotechnol. 2015, 32, 651–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.K.; Lee, E.J.; Kim, S.; Nam, G.H.; Kih, M.; Hong, Y.; Jeong, C.; Yang, Y.; Byun, Y.; Kim, I.S. Ferritin nanocage with intrinsically disordered proteins and affibody: A platform for tumor targeting with extended pharmacokinetics. J. Control. Release 2017, 267, 172–180. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, X.; Liu, Y.; Zhang, T.; Chen, H.; Zang, J.; Zheng, B.; Zhao, G. Redesign of protein nanocages: The way from 0D, 1D, 2D to 3D assembly. Chem. Soc. Rev. 2021, 50, 3957–3989. [Google Scholar] [CrossRef]
- Ren, H.; Zhu, S.; Zheng, G. Nanoreactor Design Based on Self-Assembling Protein Nanocages. Int. J. Mol. Sci. 2019, 20, 592. [Google Scholar] [CrossRef] [Green Version]
- Diaz, D.; Care, A.; Sunna, A. Bioengineering Strategies for Protein-Based Nanoparticles. Genes 2018, 9, 370. [Google Scholar] [CrossRef] [Green Version]
- Bhaskar, S.; Lim, S. Engineering protein nanocages as carriers for biomedical applications. NPG Asia Mater. 2017, 9, e371. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, N.K.; Kim, I.-S. Bioengineered protein-based nanocage for drug delivery. Adv. Drug Del. Rev. 2016, 106, 157–171. [Google Scholar] [CrossRef]
- Demchuk, A.M.; Patel, T.R. The biomedical and bioengineering potential of protein nanocompartments. Biotechnol. Adv. 2020, 41, 107547. [Google Scholar] [CrossRef]
- Nguyen, B.; Tolia, N.H. Protein-based antigen presentation platforms for nanoparticle vaccines. NPJ Vaccines 2021, 6, 70. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.-F.; Xu, W.; Liu, S.-W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacolol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Powell, A.E.; Zhang, K.; Sanyal, M.; Tang, S.; Weidenbacher, P.A.; Li, S.; Pham, T.D.; Pak, J.E.; Chiu, W.; Kim, P.S. A Single Immunization with Spike-Functionalized Ferritin Vaccines Elicits Neutralizing Antibody Responses against SARS-CoV-2 in Mice. ACS Cent. Sci. 2021, 7, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.J.; Shin, H.J.; Lee, J.-H.; Kim, K.-J.; Park, S.S.; Lee, Y.; Lee, C.; Park, S.S.; Kim, K.H. The Crystal Structure of Ferritin from Helicobacter pylori Reveals Unusual Conformational Changes for Iron Uptake. J. Mol. Biol. 2009, 390, 83–98. [Google Scholar] [CrossRef]
- Carmen, J.M.; Shrivastava, S.; Lu, Z.; Anderson, A.; Morrison, E.B.; Sankhala, R.S.; Chen, W.-H.; Chang, W.C.; Bolton, J.S.; Matyas, G.R.; et al. A spike-ferritin nanoparticle vaccine induces robust innate immune activity and drives polyfunctional SARS-CoV-2-specific T cells. bioRxiv 2021. [Google Scholar] [CrossRef]
- Joyce, M.G.; King, H.A.D.; Naouar, I.E.; Ahmed, A.; Peachman, K.K.; Cincotta, C.M.; Subra, C.; Chen, R.E.; Thomas, P.V.; Chen, W.-H.; et al. Efficacy of a Broadly Neutralizing SARS-CoV-2 Ferritin Nanoparticle Vaccine in Nonhuman Primates. bioRxiv 2021. [Google Scholar] [CrossRef]
- Saunders, K.O.; Lee, E.; Parks, R.; Martinez, D.R.; Li, D.; Chen, H.; Edwards, R.J.; Gobeil, S.; Barr, M.; Mansouri, K.; et al. Neutralizing antibody vaccine for pandemic and pre-emergent coronaviruses. Nature 2021, 594, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Torres, J.L.; Greenhouse, J.; Wallace, S.; Chiang, C.-I.; Jackson, A.M.; Porto, M.; Kar, S.; Li, Y.; Ward, A.B.; et al. One dose of COVID-19 nanoparticle vaccine REVC-128 protects against SARS-CoV-2 challenge at two weeks post-immunization. Emerg Microbes Infect. 2021, 10, 2016–2029. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, B.; Zhu, Y.; Tan, W.; Zhu, M. Ferritin nanoparticle-based SARS-CoV-2 RBD vaccine induces a persistent antibody response and long-term memory in mice. Cell. Mol. Immunol. 2021, 18, 749–751. [Google Scholar] [CrossRef]
- Brouwer, P.J.M.; Brinkkemper, M.; Maisonnasse, P.; Dereuddre-Bosquet, N.; Grobben, M.; Claireaux, M.; de Gast, M.; Marlin, R.; Chesnais, V.; Diry, S.; et al. Two-component spike nanoparticle vaccine protects macaques from SARS-CoV-2 infection. Cell 2021, 184, 1188–1200.e19. [Google Scholar] [CrossRef]
- Walls, A.C.; Fiala, B.; Schäfer, A.; Wrenn, S.; Pham, M.N.; Murphy, M.; Tse, L.V.; Shehata, L.; O’Connor, M.A.; Chen, C.; et al. Elicitation of Potent Neutralizing Antibody Responses by Designed Protein Nanoparticle Vaccines for SARS-CoV-2. Cell 2020, 183, 1367–1382.e17. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.A.; Gnanapragasam, P.N.P.; Lee, Y.E.; Hoffman, P.R.; Ou, S.; Kakutani, L.M.; Keeffe, J.R.; Wu, H.-J.; Howarth, M.; West, A.P.; et al. Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice. Science 2021, 371, 735. [Google Scholar] [CrossRef]
- Chiba, S.; Frey, S.J.; Halfmann, P.J.; Kuroda, M.; Maemura, T.; Yang, J.E.; Wright, E.R.; Kawaoka, Y.; Kane, R.S. Multivalent nanoparticle-based vaccines protect hamsters against SARS-CoV-2 after a single immunization. Commun. Bio. 2021, 4, 597. [Google Scholar] [CrossRef] [PubMed]
- Salzer, R.; Clark, J.J.; Vaysburd, M.; Chang, V.T.; Albecka-Moreau, A.; Kiss, L.; Sharma, P.; Llamazares, A.G.; Kipar, A.; Hiscox, J.A.; et al. Single-dose immunisation with a multimerised SARS-CoV-2 receptor binding domain (RBD) induces an enhanced and protective response in mice. FEBS Lett. 2021, 595, 2323–2340. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lin, X.; Wang, Y.; Abraham, C.; Sou, C.; Ngo, T.; Zhang, Y.; Wilson, I.A.; Zhu, J. Single-component, self-assembling, protein nanoparticles presenting the receptor binding domain and stabilized spike as SARS-CoV-2 vaccine candidates. Sci. Adv. 2021, 7, eabf1591. [Google Scholar] [CrossRef] [PubMed]
- Lainšček, D.; Fink, T.; Forstnerič, V.; Hafner-Bratkovič, I.; Orehek, S.; Strmšek, Ž.; Manček-Keber, M.; Pečan, P.; Esih, H.; Malenšek, Š.; et al. A Nanoscaffolded Spike-RBD Vaccine Provides Protection against SARS-CoV-2 with Minimal Anti-Scaffold Response. Vaccines 2021, 9, 431. [Google Scholar] [CrossRef]
- Boyoglu-Barnum, S.; Ellis, D.; Gillespie, R.A.; Hutchinson, G.B.; Park, Y.-J.; Moin, S.M.; Acton, O.; Ravichandran, R.; Murphy, M.; Pettie, D.; et al. Elicitation of broadly protective immunity to influenza by multivalent hemagglutinin nanoparticle vaccines. bioRxiv 2020. [Google Scholar] [CrossRef]
- Brouwer, P.J.M.; Antanasijevic, A.; Berndsen, Z.; Yasmeen, A.; Fiala, B.; Bijl, T.P.L.; Bontjer, I.; Bale, J.B.; Sheffler, W.; Allen, J.D.; et al. Enhancing and shaping the immunogenicity of native-like HIV-1 envelope trimers with a two-component protein nanoparticle. Nat. Commun. 2019, 10, 4272. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Li, W.; Farzan, M.; Harrison, S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 2005, 309, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Escolano, A.; Gristick, H.B.; Abernathy, M.E.; Merkenschlager, J.; Gautam, R.; Oliveira, T.Y.; Pai, J.; West, A.P.; Barnes, C.O.; Cohen, A.A.; et al. Immunization expands B cells specific to HIV-1 V3 glycan in mice and macaques. Nature 2019, 570, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, P.J.; Castro, A.; Loeffler, K.; Frey, S.J.; Chiba, S.; Kawaoka, Y.; Kane, R.S. Potent neutralization of SARS-CoV-2 including variants of concern by vaccines presenting the receptor-binding domain multivalently from nanoscaffolds. Bioeng. Transl. Med. 2021, 6, e10253. [Google Scholar] [CrossRef] [PubMed]
| Nanoparticle | Immunogen | In Vivo Model | Adjuvant | Route | Dose (μg) | Infection | Ref. |
|---|---|---|---|---|---|---|---|
| Ferritin (Bullfrog and Helicobacter pylori) | S protein RBD protein RBD protein and N-terminal domain | BALB/c mouse C57BL/6 mouse K18-hACE2 mouse | ALFQ or Alhydrogel | Intramuscular | 0.08–10 | Intranasal (1.25 × 104 PFU) | [13] |
| S protein | C57BL/6 mouse | ALFQ or Alhydrogel | Intramuscular | 10 | N/A | [50] | |
| S protein | Rhesus macaque | ALFQ | Intramuscular | 5–50 | Intranasal and Intratracheal (1.00 × 106 TCID50) | [51] | |
| RBD protein | Ferret | AddaVax | Intramuscular | 15 | Intranasal (1.00 × 105–1.00 × 106 TCID50) | [7] | |
| Intramuscular and Intranasal | |||||||
| Ferritin (Helicobacter pylori) | RBD protein | Cynomol gusmacaque | 3M-052 and Alhydrogel | Intramuscular | 100 | Intranasal and Intratracheal (1.00 × 105 PFU) | [52] |
| Rhesus macaque | |||||||
| RBD protein and HR domain | BALB/c mouse | Sigma Adjuvant System | Subcutaneous | 10 | Intranasal (4.00 × 104 FFU) | [35] | |
| C57BL/6 mouse(hACE2 transgenic) | |||||||
| Rhesus macaque | Intramuscular | 50 | |||||
| S protein | C57BL/6 mouse | Sigma Adjuvant System | Subcutaneous | 20 | N/A | [53] | |
| Syrian golden hamster | Intramuscular | 100 | Intranasal (1.99 × 104 TCID50) | ||||
| S protein | BALB/c mouse | Quil-A and MPLA | Subcutaneous | 0.1–20 | N/A | [48] | |
| Ferritin (Pyrococcus furiosus) | RBD protein | C57BL/6 mouse | CpG 1826 | Subcutaneous | 12.3–30.7 | N/A | [54] |
| I53-50 (Artificial) | S protein | BALB/c mouse | Poly(I:C) | Subcutaneous | 13 | N/A | [55] |
| New Zealand white rabbit | Squalene emulsion | Intramuscular | 39 | ||||
| Cynomolgus macaque | MPLA liposome | Intramuscular | 50 | Intranasal and Intratracheal (1.00 × 106 PFU) | |||
| RBD protein | BALB/c mouse | AddaVax | Intramuscular | 0.9–5 | Intranasal (1.00 × 105 PFU) | [56] | |
| Kymab Darwin mouse | N/A | ||||||
| Mi3 (Artificial) | RBD protein (4a, 4b, or 8) | BALB/c mouse | AddaVax | Intraperitoneal | 5 | N/A | [57] |
| MS2 (Emesvirus zinderi) | S protein | Syrian golden hamster | Alhydrogel | Subcutaneous | 60 | Intranasal (1.00 × 103 PFU) | [58] |
| Dps (Sulfolobus islandicus) | RBD protein | C57BL/6J mouse | CpG 1668 | Subcutaneous | 25–50 | N/A | [59] |
| K18 mouse (hACE2 transgenic) | 25 | Intranasal (1.00 × 104 PFU) | |||||
| S protein | C57BL/6J mouse | 25–50 | N/A | ||||
| Nucleocapsid protein | C57BL/6J mouse | 25–50 | N/A | ||||
| I3-01v9 (Artificial) | S protein | BALB/c mouse | AddaVax or Adju-Phos | Intraperitoneal | 50 | N/A | [60] |
| E2p (Geobacillus sterothermophilus) | S protein | ||||||
| Ferritin (Helicobacter pylori) | S protein | ||||||
| RBD protein | |||||||
| Bann (Tomato bushystunt virus) | RBD protein | BALB/c mouse | N/A | Intramuscular, Intranasal, or Sublingual | 20 (Plasmid) | Intranasal (70 μL of VSV-S pseudovirus) | [61] |
| AddaVax | Intramuscular | 100 (protein) | |||||
| Foldon (T4 bacteriophage fibritin) | N/A | Intramuscular | 20 (Plasmid) | ||||
| Ferritin (Bullfrog and Helicobacter pylori) | N/A | Intramuscular | 20 (Plasmid) | ||||
| AaLS (Aquifex aeolicus) | N/A | Intramuscular | 20 (Plasmid) | ||||
| I53-50 (Artificial) | RBD protein | BALB/c mouse | AddaVax or Sigma Adjuvant System | Subcutaneous | 11.91 | N/A | [20] |
| Mi3 (Artificial) | 9.51 | ||||||
| Ferritin (Helicobacter pylori) | 9.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, H.-D.; Kim, N.; Lee, Y.; Lee, E.J. Protein-Based Nanoparticle Vaccines for SARS-CoV-2. Int. J. Mol. Sci. 2021, 22, 13445. https://doi.org/10.3390/ijms222413445
Sung H-D, Kim N, Lee Y, Lee EJ. Protein-Based Nanoparticle Vaccines for SARS-CoV-2. International Journal of Molecular Sciences. 2021; 22(24):13445. https://doi.org/10.3390/ijms222413445
Chicago/Turabian StyleSung, Hyo-Dong, Nayeon Kim, Yeram Lee, and Eun Jung Lee. 2021. "Protein-Based Nanoparticle Vaccines for SARS-CoV-2" International Journal of Molecular Sciences 22, no. 24: 13445. https://doi.org/10.3390/ijms222413445






