The Toxic Mechanism of Gliotoxins and Biosynthetic Strategies for Toxicity Prevention
Abstract
:1. Introduction
2. The Underlying Toxic Mechanism of Gliotoxins
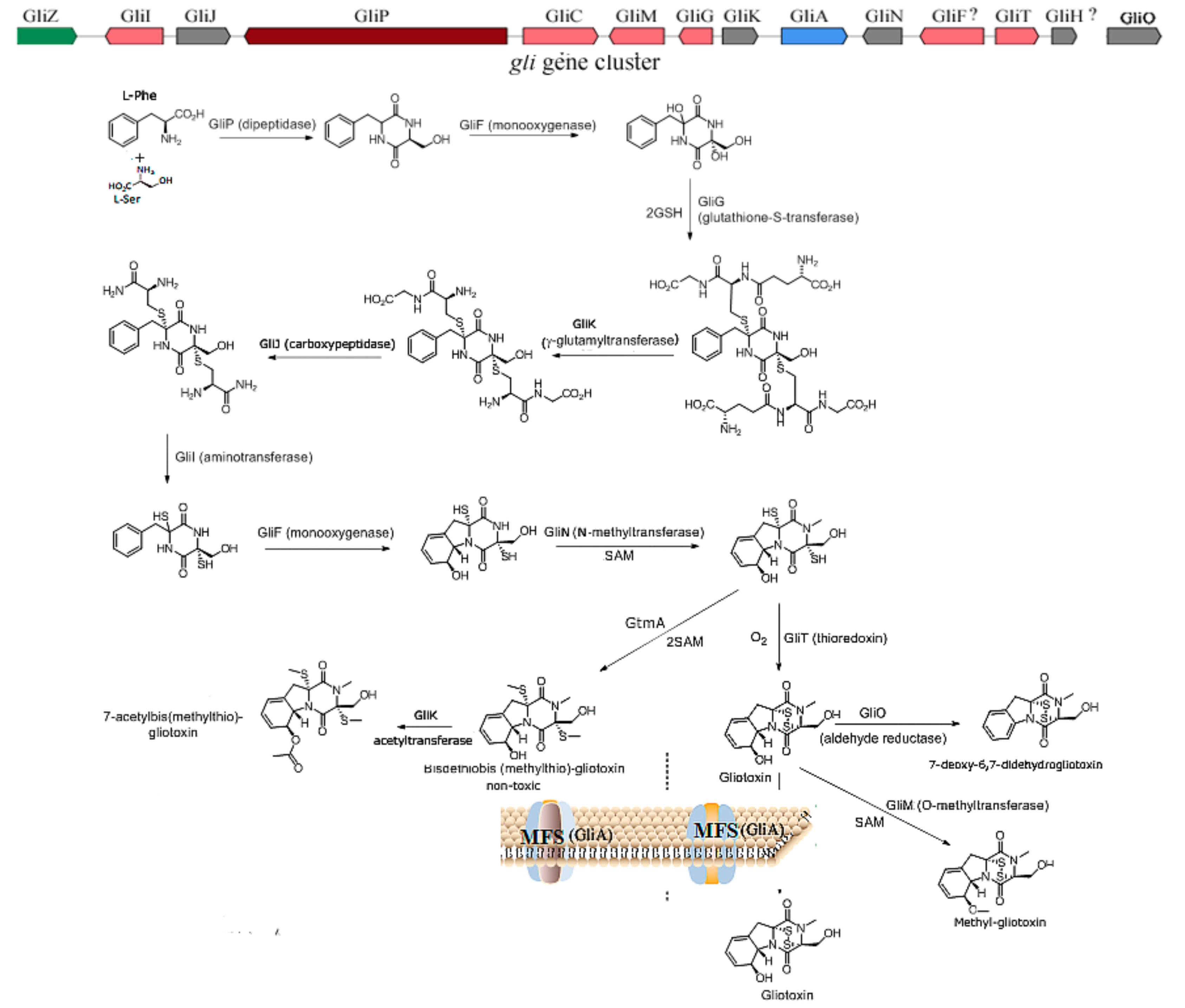
2.1. The Biosynthesis of Gliotoxin
2.2. The Underlying Toxic Mechanism of Gliotoxin
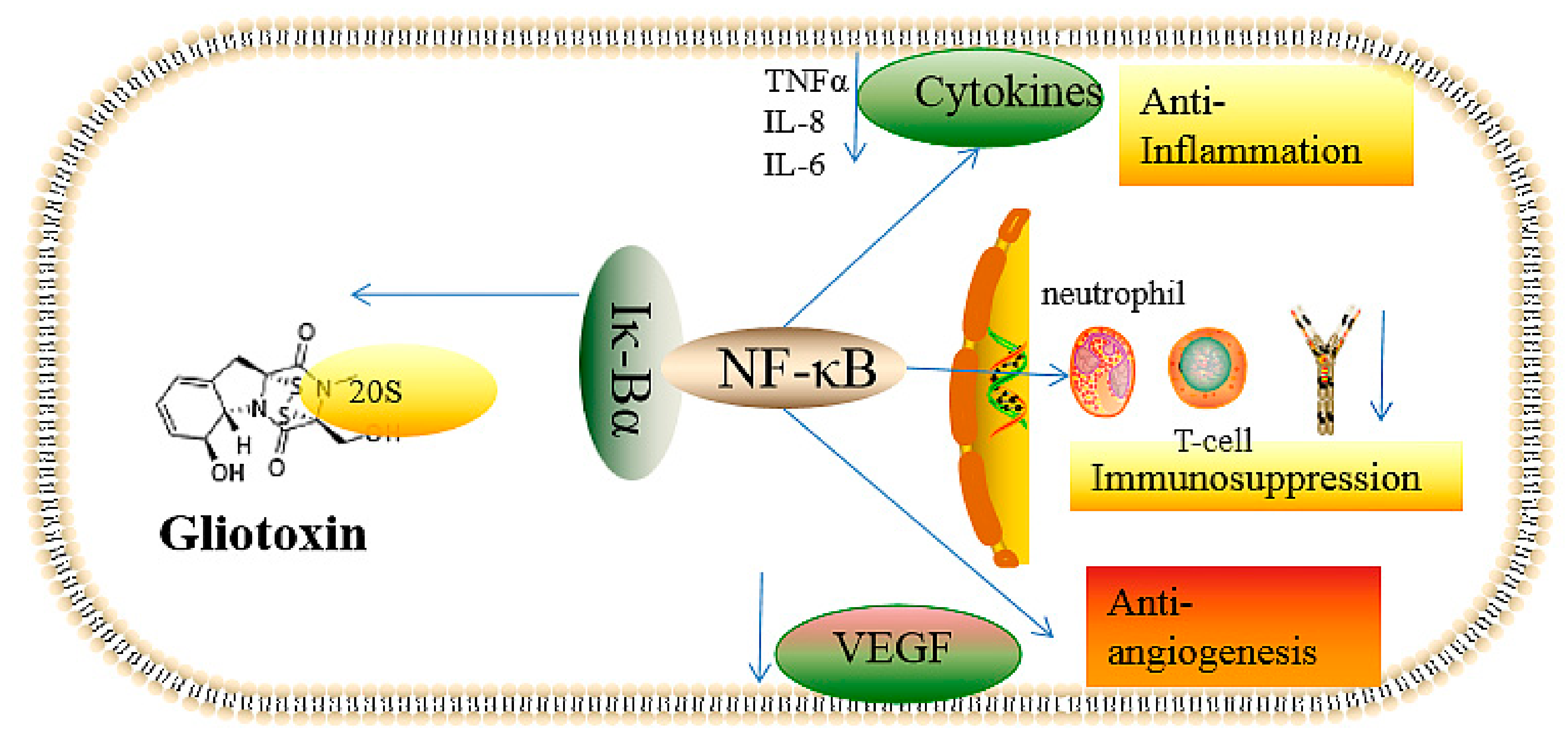
2.2.1. The Inhibition of Angiogenesis
2.2.2. Immunosuppressive Activity
2.2.3. Inflammatory and Anti-Inflammatory Effects
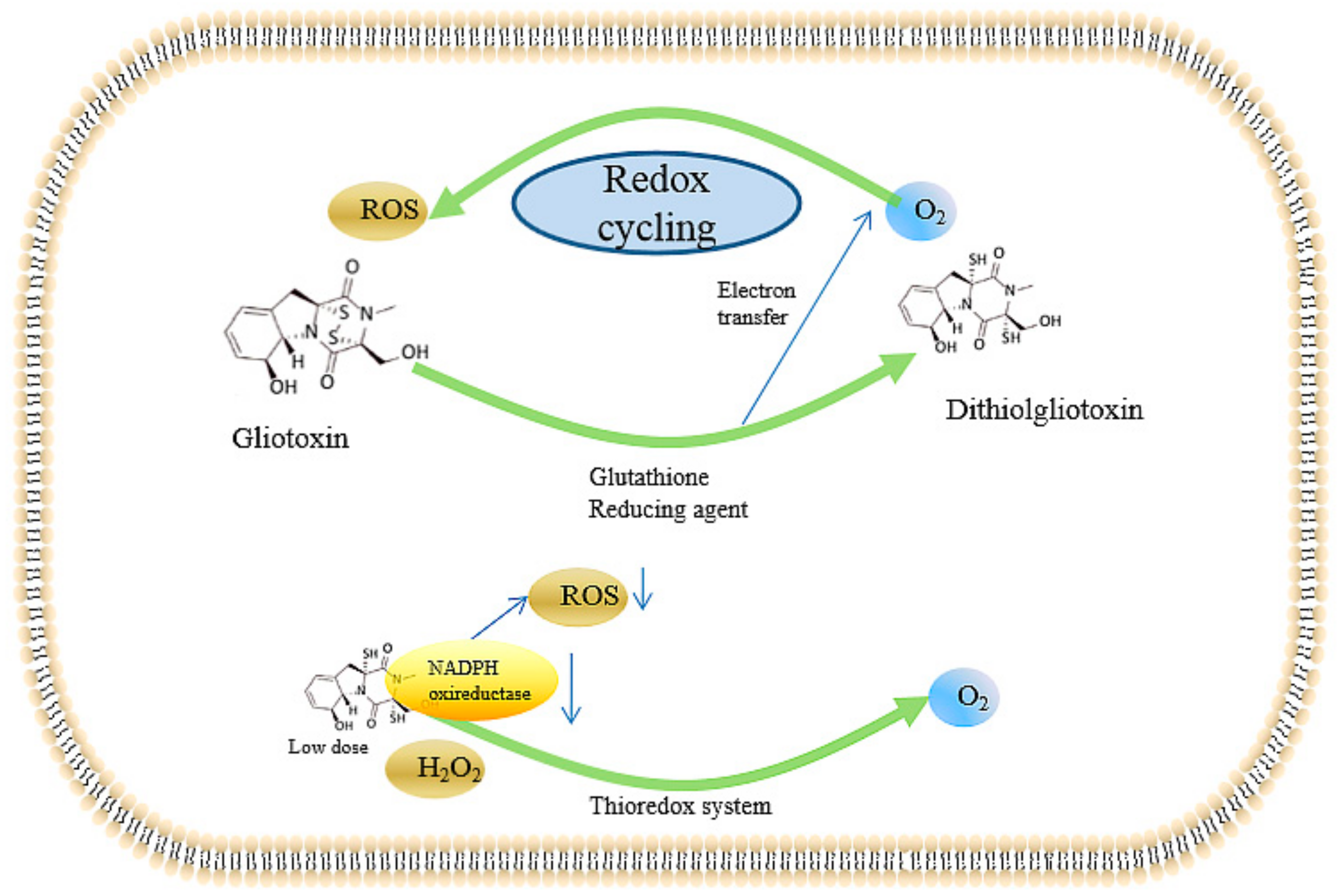
2.2.4. Inducing the Production of ROS and the Inhibition of Peroxidase
2.2.5. Genotoxicity of Gliotoxin
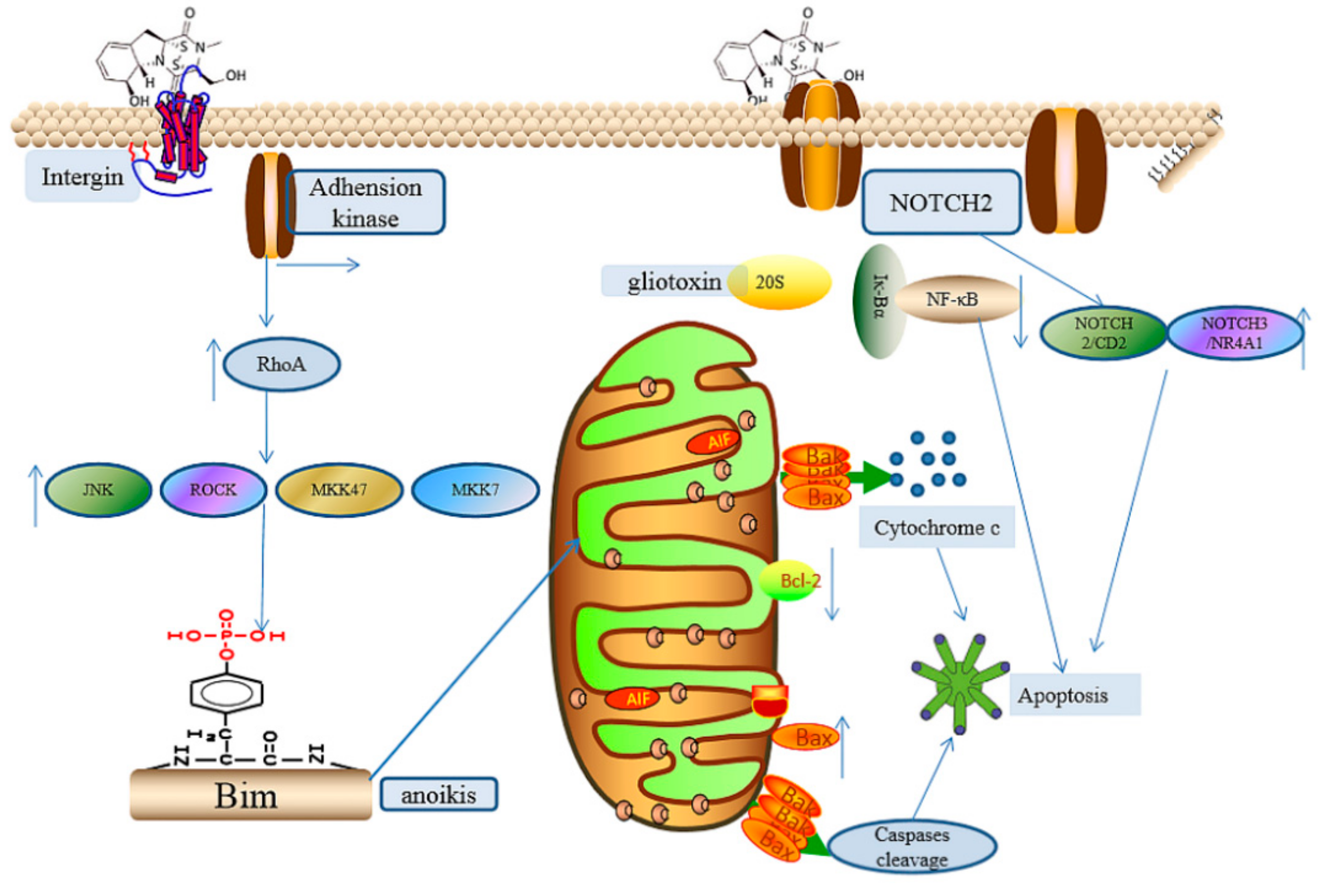
2.2.6. The Induction of Cell Apoptosis
2.2.7. Other Effects Induced by Gliotoxin
3. The Prevention of the Toxicity of Gliotoxin and the Producing Strain A. fumigutas
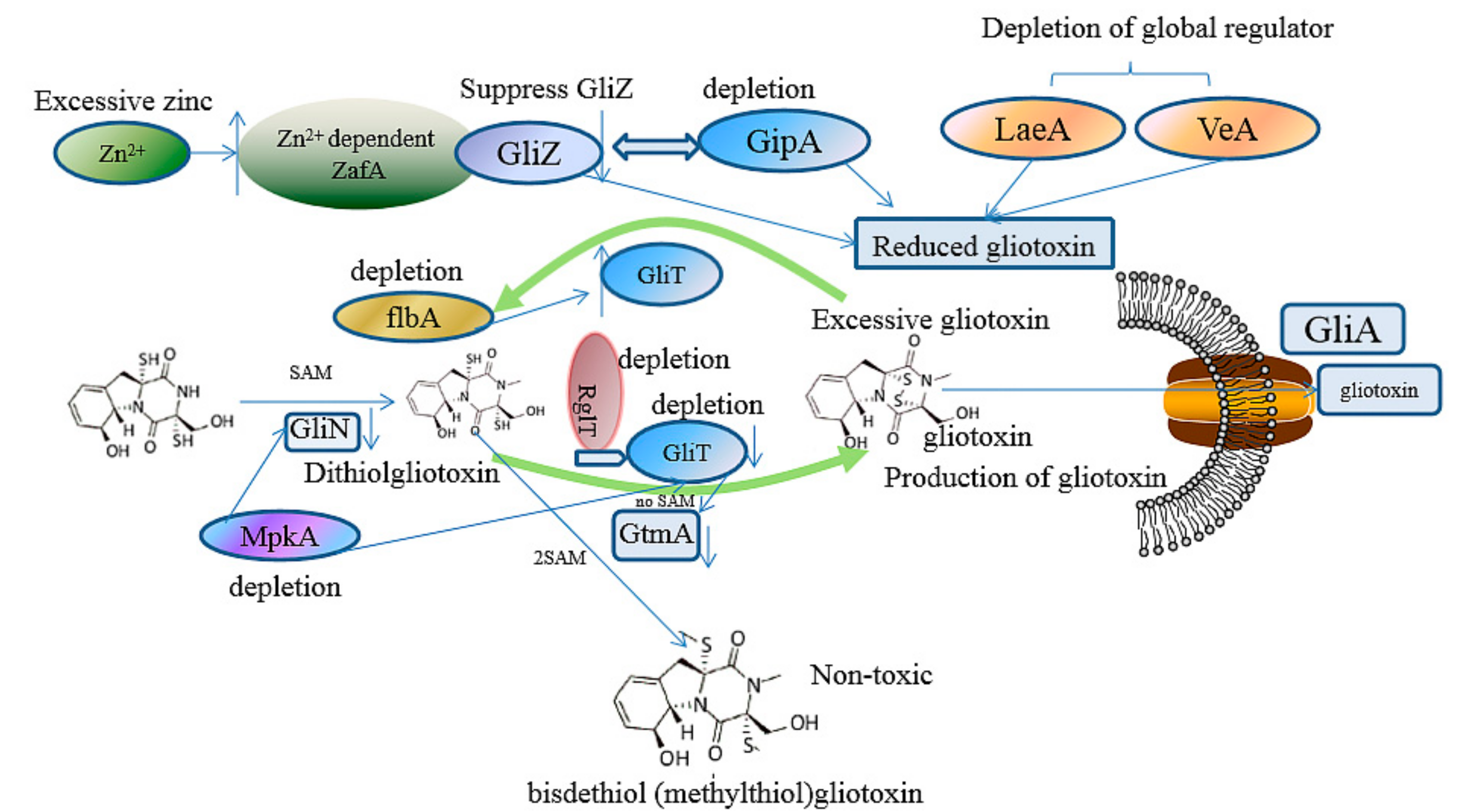
3.1. The Prevention of the Toxicity of Gliotoxin and A. fumigutas via Biosynthetic Approaches
3.2. The Regulation of Gliotoxin by Tailoring Genes
3.3. The Utilization of Toxin Transporter
3.4. The Deletion of Regulator
3.5. Other Microbial or Biosynthetic Strategiesand
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| adriamycin-resistant | ADR |
| adenine nucleotide transporter | ANT |
| arginase 1 | ARG1 |
| arginine-glysine-aspartic acid | RGD |
| Chinese hamster ovary | CHO |
| chronic lymphocytic leukemia cells | CLL |
| colorectal cancer cells | CRC |
| Cyclic Adenosine monophosphate | cAMP |
| C-terminal domain | CTD |
| cytochrome | cyt |
| 2′,7′-dichlorofluorescein diacetate | DCFH-DA |
| dithiothreitol | DTT |
| epipolythiodioxopiperazine | EPT |
| endoplasmic reticulum | ER |
| glutathione-S-transferase | GST |
| granulocyte macrophage-colony stimulating factor | GM-CSF |
| glutathione | GSH |
| glutathione disulfide | GSSG |
| hypoxia-inducible factor 1-alpha | HIF1α |
| human umbilical vein endothelial cells | HUVECs |
| Intercellular adhesion molecule-1 | ICAM-1 |
| interferon-gamma | IFN-γ |
| interleukin | IL |
| inducible nitric oxide synthase | iNOS |
| invasive pulmonary aspergillosis | IPA |
| La-related protein 7 | LARP7 |
| icotinamide adenine dinucleotid phosphate | NADPH |
| non-small-cell lung cancer cell | NSCLC |
| nuclear factor-kappa B | NF-κB |
| oxidant-producing intracellular compartments | OPIC |
| pancreas carcinoma | PANC1 |
| protein kinase A | PKA |
| phorbol myristate acetate | PMA |
| polymorphonuclear leucocytes | PMNLs |
| reactive oxygen species | ROS |
| adenosylmethionine | SAM |
| S-adenosylhomocysteine | SAH |
| superoxide dismutase | SOD |
| T-box transcription factor | TBX21 |
| transforming growth factor | TGF |
| trinitrobenzene sulfonic acid | TNBS |
| tumor necrosis factor alpha | TNFα |
| vascular endothelial growth factor | VGEFs |
References
- Reeves, E.P.; Messina, C.; Doyle, S.; Kavanagh, K. Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella. Mycopathologia 2004, 158, 73–79. [Google Scholar] [CrossRef]
- Kupfahl, C.; Michalka, A.; Lass-Flörl, C.; Fischer, G.; Haase, G.; Ruppert, T.; Geginat, G.; Hof, H. Gliotoxin production by clinical and environmental Aspergillus fumigatus strains. Int. J. Med. Microbiol. Suppl. 2008, 298, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Anitha, R.; Murugesan, K. Production of gliotoxin on natural substrates by Trichoderma virens. J. Basic Microbiol. Int. J. Biochem. Physiol. Genet. Morphol. Ecol. Microorg. 2005, 45, 12–19. [Google Scholar]
- Fan, Z.; Sun, Z.-H.; Liu, Z.; Chen, Y.-C.; Liu, H.-X.; Li, H.-H.; Zhang, W.-M. Dichotocejpins A–C: New Diketopiperazines from a Deep-Sea-Derived Fungus Dichotomomyces cejpii FS110. Mar. Drugs 2016, 14, 164. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Fan, Z.; Sun, Z.; Liu, H.; Zhang, W. Dechdigliotoxins A–C, Three Novel Disulfide-Bridged Gliotoxin Dimers from Deep-Sea Sediment Derived Fungus Dichotomomyces cejpii. Mar. Drugs 2019, 17, 596. [Google Scholar] [CrossRef] [Green Version]
- Scharf, D.H.; Brakhage, A.A.; Mukherjee, P.K. Gliotoxin–bane or boon? Environ. Microbiol. 2016, 18, 1096–1109. [Google Scholar] [CrossRef]
- Singh, S.; Dureja, P.; Tanwar, R.; Singh, A. Production and antifungal activity of secondary metabolites of Trichoderma virens. Pestic. Res. J. 2005, 17, 26–29. [Google Scholar]
- Carberry, S.; Molloy, E.; Hammel, S.; O’Keeffe, G.; Jones, G.W.; Kavanagh, K.; Doyle, S. Gliotoxin effects on fungal growth: Mechanisms and exploitation. Fungal Genet. Biol. 2012, 49, 302–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, J.R.; Benson, D.M. Biocontrol of Damping-off of Catharanthus roseus Caused by Pythium ultimum with Trichoderma virens and Binucleate Rhizoctonia Fungi. Plant Dis. 2000, 84, 644–648. [Google Scholar] [CrossRef] [Green Version]
- Manoharachary, C.; Nagaraju, D. Trichoderma: Boon for Agriculture. In Trichoderma: Agricultural Applications and Beyond; Springer: Berlin/Heidelberg, Germany, 2020; pp. 87–112. [Google Scholar]
- Stanzani, M.; Orciuolo, E.; Lewis, R.; Kontoyiannis, D.P.; Martins, S.L.R.; John, L.S.S.; Komanduri, K. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood 2005, 105, 2258–2265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekel, R.; Zvibel, I.; Brill, S.; Brazovsky, E.; Halpern, Z.; Oren, R. Gliotoxin ameliorates development of fibrosis and cirrhosis in a thioacetamide rat model. Dig. Dis. Sci. 2003, 48, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Hossain, M.A.; German, N.; Al-Ahmad, A.J. Gliotoxin penetrates and impairs the integrity of the human blood-brain barrier in vitro. Mycotoxin Res. 2018, 34, 257–268. [Google Scholar] [CrossRef]
- Lewis, R.E.; Wiederhold, N.P.; Lionakis, M.S.; Prince, R.A.; Kontoyiannis, D.P. Frequency and Species Distribution of Gliotoxin-Producing Aspergillus Isolates Recovered from Patients at a Tertiary-Care Cancer Center. J. Clin. Microbiol. 2005, 43, 6120–6122. [Google Scholar] [CrossRef] [Green Version]
- Scharf, D.H.; Heinekamp, T.; Remme, N.; Hortschansky, P.; Brakhage, A.A.; Hertweck, C. Biosynthesis and function of gliotoxin in Aspergillus fumigatus. Appl. Microbiol. Biotechnol. 2011, 93, 467–472. [Google Scholar] [CrossRef]
- Dolan, S.K.; O’Keeffe, G.; Jones, G.W.; Doyle, S. Resistance is not futile: Gliotoxin biosynthesis, functionality and utility. Trends Microbiol. 2015, 23, 419–428. [Google Scholar] [CrossRef]
- Bok, J.W.; Chung, D.; Balajee, S.A.; Marr, K.A.; Andes, D.; Nielsen, K.F.; Frisvad, J.C.; Kirby, K.A.; Keller, N.P. GliZ, a tran-scriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect. Immun. 2006, 74, 6761–6768. [Google Scholar] [CrossRef] [Green Version]
- Bok, J.W.; Keller, N.P. LaeA, a Regulator of Secondary Metabolism in Aspergillus spp. Eukaryot. Cell 2004, 3, 527–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhingra, S.; Andes, D.; Calvo, A.M. VeA Regulates Conidiation, Gliotoxin Production, and Protease Activity in the Opportunistic Human Pathogen Aspergillus fumigatus. Eukaryot. Cell 2012, 11, 1531–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, W.; Zhang, W.; Liu, T.; Huang, Z.; Zhu, M.; Chen, Y.; Li, H.; Li, S. De Novo Transcriptome Sequencing of the Deep-Sea-Derived Fungus Dichotomomyces cejpii and Analysis of Gliotoxin Biosynthesis Genes. Int. J. Mol. Sci. 2018, 19, 1910. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.; Li, S.; Liu, S.; Kong, Y.; Zhang, W.; Liu, S.; Liu, T.; Zhang, W. Characterization of novel gliotoxin biosynthesis-related genes from deep-sea-derived fungus Geosmithia pallida FS140. Biochimie 2021, 191, 1–10. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Lewis, R.E.; Leventakos, K.; Kontoyiannis, D.P. Aspergillus fumigatus inhibits angiogenesis through the production of gliotoxin and other secondary metabolites. Blood 2009, 114, 5393–5399. [Google Scholar] [CrossRef] [Green Version]
- Erjavec, Z.; Kluin-Nelemans, H.; Verweij, P. Trends in invasive fungal infections, with emphasis on invasive aspergillosis. Clin. Microbiol. Infect. 2009, 15, 625–633. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ami, R.; Lewis, R.E.; Kontoyiannis, D.P. Enemy of the (immunosuppressed) state: An update on the pathogenesis of Aspergillus fumigatus infection. Br. J. Haematol. 2010, 150, 406–417. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.H.; Hwang, B.Y.; Kim, H.S.; Lee, J.J. Anti-angiogenic activities of gliotoxin and its methylthio-derivative, fungal metabolites. Arch. Pharm. Res. 2001, 24, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R. Angiogenesis at the mold–host interface: A potential key to understanding and treating invasive aspergillosis. Future Microbiol. 2013, 8, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Dikmen, M.; Cantürk, Z.; Engur, S.; Kaya Tilki, E. Inhibitory effects of secondary metabolites of halotolerant Pythium ultimum terreus on angiogenesis. Biomed. Res. 2017, 28, 8. [Google Scholar]
- Maulik, N. Redox signaling of angiogenesis. Antioxid. Redox Signal. 2002, 4, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Orciuolo, E.; Stanzani, M.; Canestraro, M.; Galimberti, S.; Carulli, G.; Lewis, R.; Petrini, M.; Komanduri, K.V. Effects of As-pergillus fumigatus gliotoxin and methylprednisolone on human neutrophils: Implications for the pathogenesis of invasive as-pergillosis. J. Leukoc. Biol. 2007, 82, 839–848. [Google Scholar] [CrossRef]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta Gene Regul. Mech. 2010, 1799, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Lignelli, E.; Palumbo, F.; Myti, D.; Morty, R.E. Recent advances in our understanding of the mechanisms of lung alveolarization and bronchopulmonary dysplasia. Am. J. Physiol. Cell. Mol. Physiol. 2019, 317, L832–L887. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Albert, N.D.; Lewis, R.E.; Kontoyiannis, D.P. Proangiogenic Growth Factors Potentiate In Situ Angiogenesis and Enhance Antifungal Drug Activity in Murine Invasive Aspergillosis. J. Infect. Dis. 2013, 207, 1066–1074. [Google Scholar] [CrossRef] [Green Version]
- Bondy, G.S.; Pestka, J.J. Immunomodulation by fungal toxins. J. Toxicol. Environ. Health Part B Crit. Rev. 2000, 3, 109–143. [Google Scholar]
- Higurashi, H.; Arai, M.; Watanabe, A.; Igari, H.; Seki, N.; Kamei, K.; Kuriyama, T. Gene expression profiling of polymorphonuclear leukocytes treated with the culture filtrate of Aspergillus fumigatus and gliotoxin. Microbiol. Immunol. 2007, 51, 407–419. [Google Scholar] [CrossRef] [PubMed]
- König, S.; Pace, S.; Pein, H.; Heinekamp, T.; Kramer, J.; Romp, E.; Straßburger, M.; Troisi, F.; Proschak, A.; Dworschak, J.; et al. Gliotoxin from Aspergillus fumigatus Abrogates Leukotriene B4 Formation through Inhibition of Leukotriene A4 Hydrolase. Cell Chem. Biol. 2019, 26, 524–534. [Google Scholar] [CrossRef]
- Coméra, C.; André, K.; Laffitte, J.; Collet, X.; Galtier, P.; Maridonneau-Parini, I. Gliotoxin from Aspergillus fumigatus affects phagocytosis and the organization of the actin cytoskeleton by distinct signaling pathways in human neutrophils. Microbes Infect. 2007, 9, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speth, C.; Kupfahl, C.; Pfaller, K.; Hagleitner, M.; Deutinger, M.; Würzner, R.; Mohsenipour, I.; Lass-Flörl, C.; Rambach, G. Gliotoxin as putative virulence factor and immunotherapeutic target in a cell culture model of cerebral aspergillosis. Mol. Immunol. 2011, 48, 2122–2129. [Google Scholar] [CrossRef]
- Kroll, M.; Arenzana-Seisdedos, F.; Bachelerie, F.; Thomas, D.; Friguet, B.; Conconi, M. The secondary fungal metabolite gliotoxin targets proteolytic activities of the proteasome. Chem. Biol. 1999, 6, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Gaynor, R.B. Role of the NF-κB pathway in the pathogenesis of human disease states. Curr. Mol. Med. 2001, 1, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Iyer, S.; Razani, B.; Cheng, G. NF-κB and innate immunity. Curr. Top. Microbiol. Immunol. 2011, 349, 115–143. [Google Scholar]
- Bacher, S.; Schmitz, M.L. The NF-κB pathway as a potential target for autoimmune disease therapy. Curr. Pharm. Des. 2004, 10, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Kupfahl, C.; Heinekamp, T.; Geginat, G.; Ruppert, T.; Härtl, A.; Hof, H.; Brakhage, A.A. Deletion of the gliP gene of Aspergillus fumigatus results in loss of gliotoxin production but has no effect on virulence of the fungus in a low-dose mouse infection model. Mol. Microbiol. 2006, 62, 292–302. [Google Scholar] [CrossRef]
- Schlam, D.; Canton, J.; Carreño, M.; Kopinski, H.; Freeman, S.A.; Grinstein, S.; Fairn, G.D. Gliotoxin suppresses macrophage immune function by subverting phosphatidylinositol 3, 4, 5-trisphosphate homeostasis. MBio 2016, 7, e02242–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haun, F.; Neumann, S.; Peintner, L.; Wieland, K.; Habicht, J.; Schwan, C.; Østevold, K.; Koczorowska, M.M.; Biniossek, M.; Kist, M.; et al. Identification of a novel anoikis signalling pathway using the fungal virulence factor gliotoxin. Nat. Commun. 2018, 9, 3524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, X.; Chen, F.; Pan, W.; Yu, R.; Tian, S.; Han, G.; Fang, H.; Wang, S.; Zhao, J.; Li, X.; et al. Gliotoxin promotes Aspergillus fumigatus internalization into type II human pneumocyte A549 cells by inducing host phospholipase D activation. Microbes Infect. 2014, 16, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, M.; Han, X.; Tao, S.; Zheng, D.; Cheng, Y.; Yu, R.; Han, G.; Schmidt, M.; Han, L. Disruption of the Phospholipase D Gene Attenuates the Virulence of Aspergillus fumigatus. Infect. Immun. 2011, 80, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Gayathri, L.; AkbκBarsha, M.A.; Ruckmani, K. In vitro study on aspects of molecular mechanisms underlying invasive asper-gillosis caused by gliotoxin and fumagillin, alone and in combination. Sci. Rep. 2020, 10, 14473. [Google Scholar] [CrossRef] [PubMed]
- Fraga-Silva, T.F.D.C.; Mimura, L.A.N.; Leite, L.D.C.T.; Borim, P.A.; Ishikawa, L.L.W.; Venturini, J.; De Arruda, M.S.P.; Sartori, A. Gliotoxin Aggravates Experimental Autoimmune Encephalomyelitis by Triggering Neuroinflammation. Toxins 2019, 11, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herfarth, H.; Brand, K.; Rath, H.; Rogler, G.; Schölmerich, J.; Falk, W. Nuclear factor-κB activity and intestinal inflammation in dextran sulphate sodium (DSS)-induced colitis in mice is suppressed by gliotoxin. Clin. Exp. Immunol. 2000, 120, 59–65. [Google Scholar] [CrossRef]
- Oh, J.; Hur, J.; Kim, Y.; Kwon, Y.-M.; Kim, K.; Chung, Y.; Choi, M. Gliotoxin Protects Trinitrobenzene Sulfonic Acid-Induced Colonic Damage through Induction of Heme Oxygenase-1. Toxicol. Res. 2004, 20, 293–298. [Google Scholar]
- Araujo, J.A.; Zhang, M.; Yin, F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol. 2012, 3, 119. [Google Scholar] [CrossRef] [Green Version]
- Waring, P.; Sjaarda, A.; Lin, Q.H. Gliotoxin inactivates alcohol dehydrogenase by either covalent modification or free radical damage mediated by redox cycling. Biochem. Pharmacol. 1995, 49, 1195–1201. [Google Scholar] [CrossRef]
- Yoshida, L.S.; Abe, S.; Tsunawaki, S. Fungal Gliotoxin Targets the Onset of Superoxide-Generating NADPH Oxidase of Human Neutrophils. Biochem. Biophys. Res. Commun. 2000, 268, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Konstantinovas, C.; de Oliveira Mendes, T.A.; Vannier-Santos, M.A.; Lima-Santos, J. Modulation of human immune response by fungal biocontrol agents. Front Microbiol. 2017, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardo, P.H.; Brasch, N.; Chai, C.L.; Waring, P. A Novel Redox Mechanism for the Glutathione-dependent Reversible Uptake of a Fungal Toxin in Cells. J. Biol. Chem. 2003, 278, 46549–46555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.S.; Shim, J.S.; Kim, J.-A.; Kang, S.W.; Kwon, H.J. Discovery of gliotoxin as a new small molecule targeting thioredoxin redox system. Biochem. Biophys. Res. Commun. 2007, 359, 523–528. [Google Scholar] [CrossRef]
- Piaz, F.; Braca, A.; Belisario, M.; De Tommasi, N. Thioredoxin System Modulation by Plant and Fungal Secondary Metabolites. Curr. Med. Chem. 2010, 17, 479–494. [Google Scholar] [CrossRef]
- Tsunawaki, S.; Yoshida, L.S.; Nishida, S.; Kobayashi, T.; Shimoyama, T. Fungal Metabolite Gliotoxin Inhibits Assembly of the Human Respiratory Burst NADPH Oxidase. Infect. Immun. 2004, 72, 3373–3382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warris, A.; Ballou, E.R. Oxidative responses and fungal infection biology. Semin. Cell Dev. Biol. 2019, 89, 34–46. [Google Scholar] [CrossRef]
- Nishida, S.; Yoshida, L.S.; Shimoyama, T.; Nunoi, H.; Kobayashi, T.; Tsunawaki, S. Fungal Metabolite Gliotoxin Targets Flavocytochrome b558 in the Activation of the Human Neutrophil NADPH Oxidase. Infect. Immun. 2005, 73, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Kobayashi, T.; Tsunawaki, S.; Ogawa, Y.; Seguchi, H. Gliotoxin Inhibits Superoxide Production and Exocytosis of the Oxidant-producing Intracellular Compartments in Human Neutrophils Stimulated with Phorbol Myristate Acetate. Acta Histochem. Cytochem. 2004, 37, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zhang, J.; Peng, S.; Liu, R.; Li, X.; Hou, Y.; Fang, J. Thioredoxin reductase inhibitors: A patent review. Expert Opin. Ther. Pat. 2017, 27, 547–556. [Google Scholar] [CrossRef]
- Kweon, Y.-O.; Paik, Y.-H.; Schnabl, B.; Qian, T.; Lemasters, J.J.; Brenner, D.A. Gliotoxin-mediated apoptosis of activated human hepatic stellate cells. J. Hepatol. 2003, 39, 38–46. [Google Scholar] [CrossRef]
- Miralles, L.M. Investigating and Exploiting Fungal Natural Products in Aspergillus Spp.; National University of Ireland: Maynooth, Ireland, 2015. [Google Scholar]
- Chai, C.; Waring, P. Redox sensitive epidithiodioxopiperazines in biological mechanisms of toxicity. Redox Rep. 2000, 5, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.A.; Al-Halbosiy, M.M.; Dheeb, B.I.; Hashim, A.J. Cytotoxicity and genotoxicity of gliotoxin on human lymphocytes in vitro. J. King Saud Univ. Sci. 2015, 27, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Nieminen, S.M.; Mäki-Paakkanen, J.; Hirvonen, M.R.; Roponen, M.; von Wright, A. Genotoxicity of gliotoxin, a secondary metabolite of Aspergillus fumigatus, in a battery of short-term test systems. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2002, 520, 161–170. [Google Scholar] [CrossRef]
- Golden, M.C.; Hahm, S.J.; Elessar, R.; Saksonov, S.; Steinberg, J. DNA damage by gliotoxin from Aspergillus fumigatus. An occupational and environmental propagule: Adduct detection as measured by 32P DNA radiolabelling and two-dimensional thin-layer chromatography: DNA-Schädigung durch Gliotoxin von Aspergillus fumigatus. Eine arbeits-und umwelt-medizinische Studie: Addukt-Nachweis mittels 32P-Markierung und zweidimensionaler Dünnschichtchromatographie. Mycoses 1998, 41, 97–104. [Google Scholar] [PubMed]
- Suen, Y.K.; Fung, K.P.; Lee, C.Y.; Kong, S.K. Gliotoxin induces apoptosis in cultured macrophages via production of reactive oxygen species and cytochrome c release without mitochondrial depolarization. Free Radic. Res. 2001, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eichner, R.D.; Waring, P.; Geue, A.M.; Braithwaite, A.W.; Müllbacher, A. Gliotoxin causes oxidative damage to plasmid and cellular DNA. J. Biol. Chem. 1988, 263, 3772–3777. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Q.; Niu, Y.; Gu, L.; Hu, L.; Li, C.; Zhao, G. Antifungal and anti-inflammatory effect of punicalagin on murine Aspergillus fumigatus keratitis. Curr. Eye Res. 2021. [Google Scholar] [CrossRef]
- Ramalingam, S.; Bahuguna, A.; Kim, M. The effects of mycotoxin patulin on cells and cellular components. Trends Food Sci. Technol. 2018, 83, 99–113. [Google Scholar] [CrossRef]
- Ali, I.; Lone, M.N.; Suhail, M.; Mukhtar, S.D.; Asnin, L. Advances in Nanocarriers for Anticancer Drugs Delivery. Curr. Med. Chem. 2016, 23, 2159–2187. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, I.; Venkatesan, J.; Kim, S.-K. Polymer Functionalized Single Walled Carbon Nanotubes Mediated Drug Delivery of Gliotoxin in Cancer Cells. J. Biomed. Nanotechnol. 2014, 10, 120–130. [Google Scholar] [CrossRef]
- Sherpa, A.D.; Van De Nes, P.; Xiao, F.; Weedon, J.; Hrabetova, S. Gliotoxin-induced swelling of astrocytes hinders diffusion in brain extracellular space via formation of dead-space microdomains. Glia 2014, 62, 1053–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moussavou, G.; Kwak, D.H.; Obiang-Obonou, B.W.; Maranguy, C.A.O.; Dinzouna-Boutamba, S.-D.; Lee, D.H.; Pissibanganga, O.G.M.; Ko, K.; Seo, J.I.; Choo, Y.K. Anticancer Effects of Different Seaweeds on Human Colon and Breast Cancers. Mar. Drugs 2014, 12, 4898–4911. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zuo, J.; Han, Y.; Zhang, L.; Yan, L.; Shen, L.; Jiang, Y.; Cao, Y.; Zhao, J. Gliotoxin produced by Aspergillus fumigatus induces apoptosis of human bronchial epithelial cells via the Bcl-2 pathway. Int. J. Clin. Exp. Med. 2017, 10, 8854–8865. [Google Scholar]
- Femenia, F.; Huet, D.; Lair-Fulleringer, S.; Wagner, M.C.; Sarfati, J.; Shingarova, L.; Guillot, J.; Boireau, P.; Chermette, R.; Berkova, N. Effects of Conidia of Various Pythium ultimum Species on Apoptosis of Human Pneumocytes and Bronchial Epithelial Cells. Mycopathologia 2009, 167, 249–262. [Google Scholar] [CrossRef]
- Abad, A.; Fernández-Molina, J.V.; Bikandi, J.; Ramírez, A.; Margareto, J.; Sendino, J.; Hernando, F.L.; Pontón, J.; Garaizar, J.; Rementeria, A. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev. Iberoam. Micol. 2010, 27, 155–182. [Google Scholar] [CrossRef]
- Waring, P.; Khan, T.; Sjaarda, A. Apoptosis Induced by Gliotoxin Is Preceded by Phosphorylation of Histone H3 and Enhanced Sensitivity of Chromatin to Nuclease Digestion. J. Biol. Chem. 1997, 272, 17929–17936. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.-T.; Lee, J.S.; Qian, Z.-J.; Li, Y.-X.; Kim, K.-N.; Heo, S.-J.; Jeon, Y.-J.; Park, W.S.; Choi, I.-W.; Je, J.-Y.; et al. Gliotoxin Isolated from Marine Fungus Pythium ultimum sp. Induces Apoptosis of Human Cervical Cancer and Chondrosarcoma Cells. Mar. Drugs 2013, 12, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Geissler, A.; Haun, F.; Frank, D.O.; Wieland, K.; Simon, M.M.; Idzko, M.; Davis, R.J.; Maurer, U.; Borner, C. Apoptosis induced by the fungal pathogen gliotoxin requires a triple phosphorylation of Bim by JNK. Cell Death Differ. 2013, 20, 1317–1329. [Google Scholar] [CrossRef] [Green Version]
- Putcha, G.V.; Moulder, K.L.; Golden, J.P.; Bouillet, P.; Adams, J.A.; Strasser, A.; Johnson, E.M., Jr. Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron 2001, 29, 615–628. [Google Scholar] [CrossRef] [Green Version]
- Pardo, J.; Urban, C.; Galvez, E.M.; Ekert, P.G.; Müller, U.; Kwon-Chung, J.; Lobigs, M.; Müllbacher, A.; Wallich, R.; Borner, C. The mitochondrial protein Bak is pivotal for gliotoxin-induced apoptosis and a critical host factor of Aspergillus fumigatus vir-ulence in mice. J. Cell Biol. 2006, 174, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.; Wright, M.C.; Marek, C.J.; Haughton, E.L. In The role of the adenine nucleotide translocator (ANT) in apoptosis in response to gliotoxin. In Proceedings of the British Toxicological Society Annual Meeting, Warwick, UK, 16 March 2005. [Google Scholar]
- Chen, J.; Lou, Q.; He, L.; Wen, C.; Lin, M.; Zhu, Z.; Wang, F.; Huang, L.; Lan, W.; Iwamoto, A.; et al. Reduced-gliotoxin induces ROS-mediated anoikis in human colorectal cancer cells. Int. J. Oncol. 2018, 52, 1023–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubmann, R.; Sieghart, W.; Schnabl, S.; Araghi, M.; Hilgarth, M.; Reiter, M.; Demirtas, D.; Valent, P.; Zielinski, C.; Jäger, U.; et al. Gliotoxin Targets Nuclear NOTCH2 in Human Solid Tumor Derived Cell Lines In Vitro and Inhibits Melanoma Growth in Xenograft Mouse Model. Front. Pharmacol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Hubmann, R.; Schnabl, S.; Araghi, M.; Schmidl, C.; Shehata, M. Targeting Nuclear NOTCH2 by Gliotoxin Recovers a Tumor-Suppressor NOTCH3 Activity in CLL. Cells 2020, 9, 1484. [Google Scholar] [CrossRef]
- Park, G.-B.; Jeong, J.-Y.; Kim, D. Gliotoxin Enhances Autophagic Cell Death via the DAPK1-TAp63 Signaling Pathway in Paclitaxel-Resistant Ovarian Cancer Cells. Mar. Drugs 2019, 17, 412. [Google Scholar] [CrossRef] [Green Version]
- Hung, L.V.M.; Song, Y.W.; Cho, S.K. Effects of the Combination of Gliotoxin and Adriamycin on the Adriamycin-Resistant Non-Small-Cell Lung Cancer A549 Cell Line. Mar. Drugs 2018, 16, 105. [Google Scholar] [CrossRef]
- Vigushin, D.M.; Mirsaidi, N.; Brooke, G.; Sun, C.; Pace, P.; Inman, L.; Moody, C.; Coombes, R.C. Gliotoxin Is a Dual Inhibitor of Farnesyltransferase and Geranylgeranyltransferase I with Antitumor Activity Against Breast Cancer In Vivo. Med. Oncol. 2004, 21, 21–30. [Google Scholar] [CrossRef]
- Hussain, A.F.; Sulaiman, G.M.; Dheeb, B.I.; Hashim, A.J.; Abd Alrahman, E.S.; Seddiq, S.H.; Khashman, B.M. Histopathological changes and expression of transforming growth factor beta (TGF-β3) in mice exposed to gliotoxin. J. King Saud Univ. Sci. 2020, 32, 716–725. [Google Scholar] [CrossRef]
- Tang, W.; Liu, Z.-L.; Mai, X.-Y.; Qi, X.; Li, D.-H.; Gu, Q.-Q.; Li, J. Identification of Gliotoxin isolated from marine fungus as a new pyruvate kinase M2 inhibitor. Biochem. Biophys. Res. Commun. 2020, 528, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, F.; Liu, X.; Han, X.; Hu, Y.; Su, X.; Chen, Y.; Sun, Y.; Han, L. Gliotoxin Induces Cofilin Phosphorylation to Promote Actin Cytoskeleton Dynamics and Internalization of Aspergillus fumigatus Into Type II Human Pneumocyte Cells. Front. Microbiol. 2019, 10, 1345. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Chen, F.; Hu, Y.; Li, Z.; Liu, Y.; Han, X.; Sun, Y.; Han, L. Gliotoxin destructs the pulmonary epithelium barrier function by reducing cofilin oligomer formation to promote the dissolution of actin stress fibers. Microb. Pathog. 2018, 123, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Sugui, J.A.; Pardo, J.; Chang, Y.C.; Zarember, K.A.; Nardone, G.; Galvez, E.M.; Mullbacher, A.; Gallin, J.I.; Simon, M.M.; Kwon-Chung, K.J. Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot. Cell 2007, 6, 1562–1569. [Google Scholar] [CrossRef] [Green Version]
- Stoszko, M.; Al-Hatmi, A.M.S.; Skriba, A.; Roling, M.; Ne, E.; Crespo, R.; Mueller, Y.M.; Najafzadeh, M.J.; Kang, J.; Ptackova, R.; et al. Gliotoxin, identified from a screen of fungal metabolites, disrupts 7SK snRNP, releases P-TEFb, and reverses HIV-1 latency. Sci. Adv. 2020, 6, eaba6617. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, D.; Carolyn, C. Rodent Models of Invasive Aspergillosis due to Aspergillus fumigatus: Still a Long Path toward Standardization. Front. Microbiol. 2017, 8, 841. [Google Scholar]
- Lionakis, M.S.; Kontoyiannis, D.P. Drosophila melanogaster as a Model Organism for Invasive Aspergillosis. Front. Cell. Infect. Microbiol. 2012, 845, 455–468. [Google Scholar] [CrossRef] [Green Version]
- Dolan, S.; Bock, T.; Hering, V.; Owens, R.A.; Jones, G.; Blankenfeldt, W.; Doyle, S. Structural, mechanistic and functional insight into gliotoxin bis-thiomethylation in Aspergillus fumigatus. Open Biol. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Davis, C.; Carberry, S.; Schrettl, M.; Singh, I.; Stephens, J.C.; Barry, S.; Kavanagh, K.; Challis, G.; Brougham, D.; Doyle, S. The Role of Glutathione S-Transferase GliG in Gliotoxin Biosynthesis in Aspergillus fumigatus. Chem. Biol. 2011, 18, 542–552. [Google Scholar] [CrossRef] [Green Version]
- Davis, C. The Glutathione S-Transferase GliG Mediates Gliotoxin Biosynthesis, not Self-Protection. In Aspergillus Fumigatus: A Functional Genomic Investigation; RIAN: Ireland, 2011. [Google Scholar]
- Schrettl, M.; Carberry, S.; Kavanagh, K.; Haas, H.; Jones, G.W.; O’Brien, J.; Nolan, A.; Stephens, J.; Fenelon, O.; Doyle, S. Self-protection against gliotoxin—A component of the gliotoxin biosynthetic cluster, GliT, completely protects Aspergillus fumigatus against exogenous gliotoxin. PLoS Pathog. 2010, 6, e1000952. [Google Scholar] [CrossRef]
- O’Keeffe, G.; Hammel, S.; Owens, R.A.; Keane, T.M.; Fitzpatrick, D.A.; Jones, G.W.; Doyle, S. RNA-seq reveals the pan-transcriptomic impact of attenuating the gliotoxin self-protection mechanism in Aspergillus fumigatus. BMC Genom. 2014, 15, 894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzanares-Miralles, L.; Sarikaya-Bayram, Ö.; Smith, E.B.; Dolan, S.; Bayram, O.; Jones, G.; Doyle, S. Quantitative proteomics reveals the mechanism and consequence of gliotoxin-mediated dysregulation of the methionine cycle in Pythium ultimum niger. J. Proteom. 2016, 131, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Dolan, S.; Owens, R.; O’Keeffe, G.; Hammel, S.; Fitzpatrick, D.; Jones, G.; Doyle, S. Regulation of Nonribosomal Peptide Synthesis: Bis-Thiomethylation Attenuates Gliotoxin Biosynthesis in Aspergillus fumigatus. Chem. Biol. 2014, 21, 999–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, R.; O’Keeffe, G.; Smith, E.B.; Dolan, S.; Hammel, S.; Sheridan, K.J.; Fitzpatrick, D.; Keane, T.; Jones, G.W.; Doyle, S. Interplay between Gliotoxin Resistance, Secretion, and the Methyl/Methionine Cycle in Aspergillus fumigatus. Eukaryot. Cell 2015, 14, 941–957. [Google Scholar] [CrossRef] [Green Version]
- Duell, E.; Glaser, M.; Le Chapelain, C.; Antes, I.; Groll, M.; Huber, E.M. Sequential Inactivation of Gliotoxin by the S-Methyltransferase TmtA. ACS Chem. Biol. 2016, 11, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Marion, A.; Groll, M.; Scharf, D.; Scherlach, K.; Glaser, M.; Sievers, H.; Schuster, M.; Hertweck, C.; Brakhage, A.A.; Antes, I.; et al. Gliotoxin Biosynthesis: Structure, Mechanism, and Metal Promiscuity of Carboxypeptidase GliJ. ACS Chem. Biol. 2017, 12, 1874–1882. [Google Scholar] [CrossRef]
- Gallagher, L.; Owens, R.A.; Dolan, S.K.; O’Keeffe, G.; Schrettl, M.; Kavanagh, K.; Jones, G.W.; Doyle, S. The Aspergillus fumigatus protein GliK protects against oxidative stress and is essential for gliotoxin biosynthesis. Eukaryot. Cell 2012, 11, 1226–1238. [Google Scholar] [CrossRef] [Green Version]
- Traynor, A.M.; Owens, R.A.; Coughlin, C.M.; Holton, M.C.; Jones, G.W.; Calera, J.A.; Doyle, S. At the metal–metabolite in-terface in Aspergillus fumigatus: Towards untangling the intersecting roles of zinc and gliotoxin. Microbiology 2021, 167, 001106. [Google Scholar] [CrossRef]
- Scharf, D.; Habel, A.; Heinekamp, T.; Brakhage, A.A.; Hertweck, C. Opposed Effects of Enzymatic Gliotoxin N- and S-Methylations. J. Am. Chem. Soc. 2014, 136, 11674–11679. [Google Scholar] [CrossRef]
- Wang, D.-N.; Toyotome, T.; Muraosa, Y.; Watanabe, A.; Wuren, T.; Bunsupa, S.; Aoyagi, K.; Yamazaki, M.; Takino, M.; Kamei, K. GliA in Aspergillus fumigatus is required for its tolerance to gliotoxin and affects the amount of extracellular and intracellular gliotoxin. Med. Mycol. 2014, 52, 506–518. [Google Scholar] [CrossRef] [Green Version]
- Gardiner, D.M.; Jarvis, R.S.; Howlett, B.J. The ABC transporter gene in the sirodesmin biosynthetic gene cluster of Leptosphaeria maculans is not essential for sirodesmin production but facilitates self-protection. Fungal Genet. Biol. 2005, 42, 257–263. [Google Scholar] [CrossRef]
- Huang, Z.-L.; Ye, W.; Zhu, M.-Z.; Kong, Y.-L.; Li, S.-N.; Liu, S.; Zhang, W.-M. Interaction of a Novel Zn2Cys6 Transcription Factor DcGliZ with Promoters in the Gliotoxin Biosynthetic Gene Cluster of the Deep-Sea-Derived Fungus Dichotomomyces cejpii. Biomolecules 2019, 10, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoberle, T.J.; Nguyen-Coleman, C.K.; Herold, J.; Yang, A.; Weirauch, M.; Hughes, T.R.; McMurray, J.S.; May, G.S. A Novel C2H2 Transcription Factor that Regulates gliA Expression Interdependently with GliZ in Aspergillus fumigatus. PLoS Genet. 2014, 10, e1004336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, T.D.; Calvo, A.M. The mtfA Transcription Factor Gene Controls Morphogenesis, Gliotoxin Production, and Virulence in the Opportunistic Human Pathogen Aspergillus fumigatus. Eukaryot. Cell 2014, 13, 766–775. [Google Scholar] [CrossRef] [Green Version]
- Palmer, J.M.; Bok, J.W.; Lee, S.; Dagenais, T.R.; Andes, D.R.; Kontoyiannis, D.P.; Keller, N.P. Loss of CclA, required for histone 3 lysine 4 methylation, decreases growth but increases secondary metabolite production in Aspergillus fumigatus. PeerJ 2013, 1, e4. [Google Scholar] [CrossRef] [Green Version]
- Ries, L.N.A.; Pardeshi, L.; Dong, Z.; Tan, K.; Steenwyk, J.L.; Colabardini, A.C.; Filho, J.A.F.; De Castro, P.A.; Silva, L.P.; Preite, N.W.; et al. The Aspergillus fumigatus transcription factor RglT is important for gliotoxin biosynthesis and self-protection, and virulence. PLOS Pathog. 2020, 16, e1008645. [Google Scholar] [CrossRef]
- Shin, K.-S.; Park, H.-S.; Kim, Y.-H.; Yu, J.-H. Comparative proteomic analyses reveal that FlbA down-regulates gliT expression and SOD activity in Aspergillus fumigatus. J. Proteom. 2013, 87, 40–52. [Google Scholar] [CrossRef]
- Park, H.-S.; Kim, M.-J.; Yu, J.-H.; Shin, K.-S. Heterotrimeric G-protein signalers and RGSs in Aspergillus fumigatus. Pathogens 2020, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Valiante, V.; Remme, N.; Docimo, T.; Heinekamp, T.; Hertweck, C.; Gershenzon, J.; Haas, H.; Brakhage, A.A. The MAP kinase MpkA controls cell wall integrity, oxidative stress response, gliotoxin production and iron adaptation in Aspergillus fumigatus. Mol. Microbiol. 2011, 82, 39–53. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.A.; Jones, G.W.; Tinley, F.C.; Delaney, S.F.; Alabbadi, S.H.; Fenlon, K.; Doyle, S.; Owens, R.A. Systems impact of zinc chelation by the epipolythiodioxopiperazine dithiol gliotoxin in Aspergillus fumigatus: A new direction in natural product functionality. Metallomics 2018, 10, 854–866. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.; Kang, S.; Park, Y.-S.; Yun, C.-W. The Role of Zinc in Gliotoxin Biosynthesis of Aspergillus fumigatus. Int. J. Mol. Sci. 2019, 20, 6192. [Google Scholar] [CrossRef] [Green Version]
- Svahn, K.S.; Göransson, U.; Chryssanthou, E.; Olsen, B.; Sjölin, J.; Strömstedt, A.A. Induction of Gliotoxin Secretion in Aspergillus fumigatus by Bacteria-Associated Molecules. PLoS ONE 2014, 9, e93685. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, W.; Liu, T.; Zhang, W.; Zhang, W. The Toxic Mechanism of Gliotoxins and Biosynthetic Strategies for Toxicity Prevention. Int. J. Mol. Sci. 2021, 22, 13510. https://doi.org/10.3390/ijms222413510
Ye W, Liu T, Zhang W, Zhang W. The Toxic Mechanism of Gliotoxins and Biosynthetic Strategies for Toxicity Prevention. International Journal of Molecular Sciences. 2021; 22(24):13510. https://doi.org/10.3390/ijms222413510
Chicago/Turabian StyleYe, Wei, Taomei Liu, Weiyang Zhang, and Weimin Zhang. 2021. "The Toxic Mechanism of Gliotoxins and Biosynthetic Strategies for Toxicity Prevention" International Journal of Molecular Sciences 22, no. 24: 13510. https://doi.org/10.3390/ijms222413510





