The Role of Prostaglandin E1 as a Pain Mediator through Facilitation of Hyperpolarization-Activated Cyclic Nucleotide-Gated Channel 2 via the EP2 Receptor in Trigeminal Ganglion Neurons of Mice
Abstract
1. Introduction
2. Results
2.1. PGE1 Induces Mechanical Allodynia and Enhances the Excitability of TG Neurons
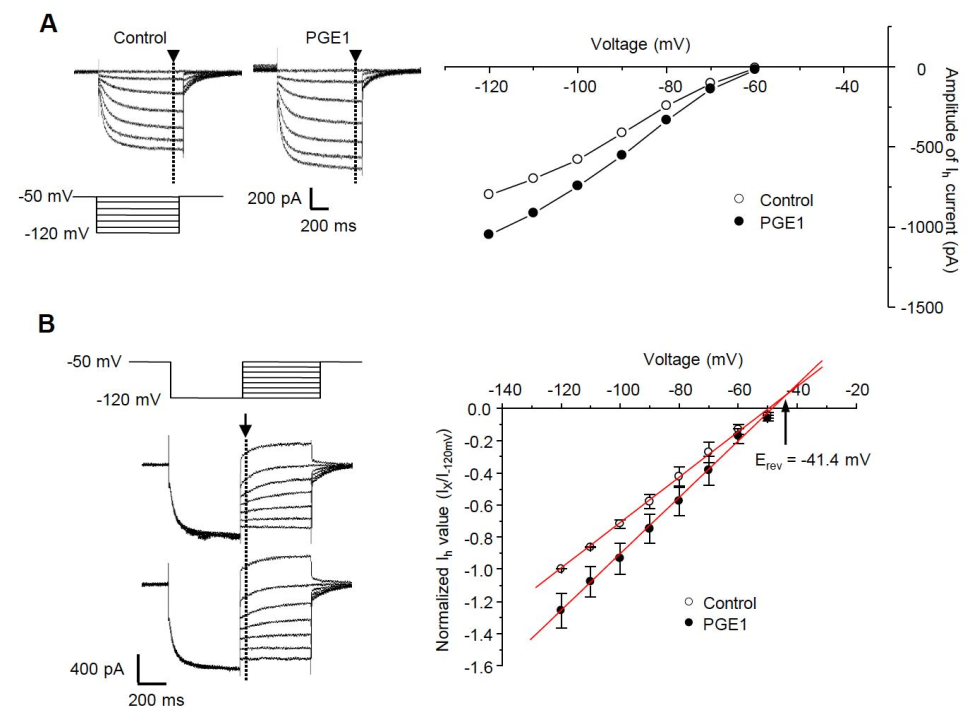
2.2. PGE1 Increases Ih Current in TG Neurons in a Dose-Dependent Manner
2.3. I-V Relationship of PGE1-Induced Ih Facilitation
2.4. PGE1-Induced Orofacial Pain Is Associated with the HCN Channel
2.5. PGE1-Induced Ih Facilitation Is Mediated by Adenylyl Cyclase and cAMP via the EP2 Receptor
2.6. PGE1-Induced Facilitation of Ih Is Mediated via Activation of the EP2 Receptor, Not the EP4 Receptor
3. Discussion
3.1. Hyperalgesic Action of PGE1
3.2. The Role of the HCN2 Channel in PGE1-Induced Mechanical Allydonia
3.3. The Identification of E-Type Prostaglandin (EP)-Receptor Involved in PGE1-Induced Pain
4. Materials and Methods
4.1. Animals
4.2. Behavioral Study—Orofacial Pain
4.3. Preparation of Trigeminal Ganglia (TG) Neurons
4.4. Electrophysiological Recordings
4.5. Immunofluorescent Staining
4.6. Drugs
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wang, X.; Lin, H.; Gu, Y. Multiple roles of dihomo-gamma-linolenic acid against proliferation diseases. Lipids Health Dis. 2012, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Raz, A.; Minkes, M.S.; Needleman, P. Endoperoxides and thromboxanes. Structural determinants for platelet aggregation and vasoconstriction. Biochim. Biophys. Acta 1977, 488, 305–311. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Chapkin, R.S.; Ramos, K.S. Dietary lipid source alters murine macrophage/vascular smooth muscle cell interactions in vitro. J. Nutr. 1996, 126, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Negishi, M.; Sugimoto, Y.; Ichikawa, A. Prostanoid Receptors and Their Biological Actions. Prog. Lipid Res. 1993, 32, 417–434. [Google Scholar] [CrossRef]
- Jain, A.; Iqbal, O.A. Alprostadil. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Zurier, R.B.; Quagliata, F. Effect of prostaglandin E 1 on adjuvant arthritis. Nature 1971, 234, 304–305. [Google Scholar] [CrossRef]
- Zurier, R.B. Prostaglandins, immune responses, and murine lupus. Arthritis Rheum. 1982, 25, 804–809. [Google Scholar] [CrossRef]
- Greenberg, S.; Kadowitz, P.J.; Long, J.P.; Wilson, W.R. Studies on the nature of a prostaglandin receptor in canine and rabbit vascular smooth muscle. Circ. Res. 1976, 39, 66–76. [Google Scholar] [CrossRef]
- Fang, W.; Li, H.; Zhou, L.; Su, L.; Liang, Y.; Mu, Y. Effect of prostaglandin E1 on TNF-induced vascular inflammation in human umbilical vein endothelial cells. Can. J. Physiol. Pharm. 2010, 88, 576–583. [Google Scholar] [CrossRef]
- Kida, T.; Sawada, K.; Kobayashi, K.; Hori, M.; Ozaki, H.; Murata, T. Diverse effects of prostaglandin E₂ on vascular contractility. Heart Vessel. 2014, 29, 390–395. [Google Scholar] [CrossRef]
- Lopshire, J.C.; Nicol, G.D. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: Whole-cell and single-channel studies. J. Neurosci. 1998, 18, 6081–6092. [Google Scholar] [CrossRef]
- Hingtgen, C.M.; Waite, K.J.; Vasko, M.R. Prostaglandins facilitate peptide release from rat sensory neurons by activating the adenosine 3’,5’-cyclic monophosphate transduction cascade. J. Neurosci. 1995, 15, 5411–5419. [Google Scholar] [CrossRef]
- Yeon, K.Y.; Chung, G.; Kim, Y.H.; Hwang, J.H.; Davies, A.J.; Park, M.K.; Ahn, D.K.; Kim, J.S.; Jung, S.J.; Oh, S.B. Eugenol reverses mechanical allodynia after peripheral nerve injury by inhibiting hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. Pain 2011, 152, 2108–2116. [Google Scholar] [CrossRef]
- Emery, E.C.; Young, G.T.; Berrocoso, E.M.; Chen, L.; McNaughton, P.A. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science 2011, 333, 1462–1466. [Google Scholar] [CrossRef]
- Lee, D.H.; Chang, L.; Sorkin, L.S.; Chaplan, S.R. Hyperpolarization-activated, cation-nonselective, cyclic nucleotide-modulated channel blockade alleviates mechanical allodynia and suppresses ectopic discharge in spinal nerve ligated rats. J. Pain 2005, 6, 417–424. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Chapkin, R.S. Importance of dietary gamma-linolenic acid in human health and nutrition. J. Nutr. 1998, 128, 1411–1414. [Google Scholar] [CrossRef]
- Sato, Y.; Suzuki, N.; Adachi, H.; Hisasue, S.; Horita, H.; Tsukamoto, T. Evaluation of the alleviative action of neurotropin for penile pain associated with intracavernous injection of prostaglandin E1 assessed using the visual analogue scale. Int. J. Impot. Res. 1998, 10, 1–3. [Google Scholar] [CrossRef][Green Version]
- Kaupp, U.B.; Seifert, R. Molecular diversity of pacemaker ion channels. Annu. Rev. Physiol. 2001, 63, 235–257. [Google Scholar] [CrossRef]
- Moosmang, S.; Stieber, J.; Zong, X.; Biel, M.; Hofmann, F.; Ludwig, A. Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur. J. Biochem. 2001, 268, 1646–1652. [Google Scholar] [CrossRef]
- Yoshihara, H. Prostaglandin E1 Treatment for Lumbar Spinal Canal Stenosis: Review of the Literature. Pain Pr. 2016, 16, 245–256. [Google Scholar] [CrossRef]
- Polito, M.; d’Anzeo, G.; Conti, A.; Muzzonigro, G. Erectile rehabilitation with intracavernous alprostadil after radical prostatectomy: Refusal and dropout rates. BJU Int. 2012, 110, E954–E957. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, P.B.; Choe, G.Y.; Moon, J.Y.; Nahm, F.S.; Kim, Y.C. Therapeutic effect of epidurally administered lipo-prostaglandin e1 agonist in a rat spinal stenosis model. Korean J. Pain 2014, 27, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Staikopoulos, V.; Ivanusic, J.J.; Jennings, E.A. Hyperpolarization-activated cyclic-nucleotide gated 4 (HCN4) protein is expressed in a subset of rat dorsal root and trigeminal ganglion neurons. Cell Tissue Res. 2009, 338, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, P.J.; Dinarello, C.A.; Strom, T.B. Prostaglandins posttranscriptionally inhibit monocyte expression of interleukin 1 activity by increasing intracellular cyclic adenosine monophosphate. J. Immunol. 1986, 137, 3189–3194. [Google Scholar] [PubMed]
- Horrobin, D.F. The regulation of prostaglandin biosynthesis: Negative feedback mechanisms and the selective control of formation of I and 2 series prostaglandins: Relevance to inflammation and immunity. Med. Hypotheses 1980, 6, 687–709. [Google Scholar] [CrossRef]
- Cho, H.J.; Staikopoulos, V.; Furness, J.B.; Jennings, E.A. Inflammation-induced increase in hyperpolarization-activated, cyclic nucleotide-gated channel protein in trigeminal ganglion neurons and the effect of buprenorphine. Neuroscience 2009, 162, 453–461. [Google Scholar] [CrossRef]
- Wells, J.E.; Rowland, K.C.; Proctor, E.K. Hyperpolarization-activated channels in trigeminal ganglia innervating healthy and pulp-exposed teeth. Int. Endod. J. 2007, 40, 715–721. [Google Scholar] [CrossRef]
- Yamamoto, T.; Habuchi, Y.; Tanaka, H.; Suto, F.; Morikawa, J.; Kashima, K.; Yoshimura, M. EP receptor-mediated inhibition by prostaglandin E(1) of cardiac L-type Ca(2+) current of rabbits. Am. J. Physiol. 1999, 277, H1369–H1374. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Guo, H.Q.; Lee, D.H.; Luo, L.; Liu, C.; Kuei, C.; Velumian, A.A.; Butler, M.P.; Brown, S.M.; Dubin, A.E. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J. Neurosci. 2003, 23, 1169–1178. [Google Scholar] [CrossRef]
- Papp, I.; Szucs, P.; Hollo, K.; Erdelyi, F.; Szabo, G.; Antal, M. Hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 ion channels modulate synaptic transmission from nociceptive primary afferents containing substance P to secondary sensory neurons in laminae I-IIo of the rodent spinal dorsal horn. Eur. J. Neurosci. 2006, 24, 1341–1352. [Google Scholar] [CrossRef]
- Antal, M.; Papp, I.; Bahaerguli, N.; Veress, G.; Vereb, G. Expression of hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 in axon terminals of peptidergic nociceptive primary sensory neurons in the superficial spinal dorsal horn of rats. Eur. J. Neurosci. 2004, 19, 1336–1342. [Google Scholar] [CrossRef]
- Kadowitz, P.J.; Chapnick, B.M.; Joiner, P.D.; Hyman, A.L. Influence of inhibitors of prostaglandin synthesis on the canine pulmonary vascular bed. Am. J. Physiol. 1975, 229, 941–946. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, M.; Yang, X.; Wu, F.; Wang, G.; Feng, X.; Ombati, R.; Zuo, R.; Yang, C.; Liu, J.; et al. Prostaglandin E1 Is an Efficient Molecular Tool for Forest Leech Blood Sucking. Front. Vet. Sci. 2020, 7, 615915. [Google Scholar] [CrossRef]
- Kreutz, R.P.; Nystrom, P.; Kreutz, Y.; Miao, J.; Kovacs, R.; Desta, Z.; Flockhart, D.A.; Jin, Y. Inhibition of platelet aggregation by prostaglandin E1 (PGE1) in diabetic patients during therapy with clopidogrel and aspirin. Platelets 2013, 24, 145–150. [Google Scholar] [CrossRef]
- Levin, G.; Duffin, K.L.; Obukowicz, M.G.; Hummert, S.L.; Fujiwara, H.; Needleman, P.; Raz, A. Differential metabolism of dihomo-gamma-linolenic acid and arachidonic acid by cyclo-oxygenase-1 and cyclo-oxygenase-2: Implications for cellular synthesis of prostaglandin E1 and prostaglandin E2. Biochem. J. 2002, 365, 489–496. [Google Scholar] [CrossRef]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid receptors: Structures, properties, and functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef]
- Namba, T.; Sugimoto, Y.; Negishi, M.; Irie, A.; Ushikubi, F.; Kakizuka, A.; Ito, S.; Ichikawa, A.; Narumiya, S. Alternative splicing of C-terminal tail of prostaglandin E receptor subtype EP3 determines G-protein specificity. Nature 1993, 365, 166–170. [Google Scholar] [CrossRef]
- Patwardhan, A.M.; Vela, J.; Farugia, J.; Vela, K.; Hargreaves, K.M. Trigeminal nociceptors express prostaglandin receptors. J. Dent. Res. 2008, 87, 262–266. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Krzyzanowska, A.; Pittolo, S.; Cabrerizo, M.; Sánchez-López, J.; Krishnasamy, S.; Venero, C.; Avendaño, C. Assessing nociceptive sensitivity in mouse models of inflammatory and neuropathic trigeminal pain. J. Neurosci. Methods 2011, 201, 46–54. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.; Choi, Y.I.; Jo, H.J.; Lee, S.H.; Lee, H.K.; Kim, H.; Moon, J.Y.; Jung, S.J. The Role of Prostaglandin E1 as a Pain Mediator through Facilitation of Hyperpolarization-Activated Cyclic Nucleotide-Gated Channel 2 via the EP2 Receptor in Trigeminal Ganglion Neurons of Mice. Int. J. Mol. Sci. 2021, 22, 13534. https://doi.org/10.3390/ijms222413534
Kwon J, Choi YI, Jo HJ, Lee SH, Lee HK, Kim H, Moon JY, Jung SJ. The Role of Prostaglandin E1 as a Pain Mediator through Facilitation of Hyperpolarization-Activated Cyclic Nucleotide-Gated Channel 2 via the EP2 Receptor in Trigeminal Ganglion Neurons of Mice. International Journal of Molecular Sciences. 2021; 22(24):13534. https://doi.org/10.3390/ijms222413534
Chicago/Turabian StyleKwon, Jean, Young In Choi, Hang Joon Jo, Sang Hoon Lee, Han Kyu Lee, Heesoo Kim, Jee Youn Moon, and Sung Jun Jung. 2021. "The Role of Prostaglandin E1 as a Pain Mediator through Facilitation of Hyperpolarization-Activated Cyclic Nucleotide-Gated Channel 2 via the EP2 Receptor in Trigeminal Ganglion Neurons of Mice" International Journal of Molecular Sciences 22, no. 24: 13534. https://doi.org/10.3390/ijms222413534
APA StyleKwon, J., Choi, Y. I., Jo, H. J., Lee, S. H., Lee, H. K., Kim, H., Moon, J. Y., & Jung, S. J. (2021). The Role of Prostaglandin E1 as a Pain Mediator through Facilitation of Hyperpolarization-Activated Cyclic Nucleotide-Gated Channel 2 via the EP2 Receptor in Trigeminal Ganglion Neurons of Mice. International Journal of Molecular Sciences, 22(24), 13534. https://doi.org/10.3390/ijms222413534





