A Comprehensive Review on the Interaction of Milk Protein Concentrates with Plant-Based Polyphenolics
Abstract
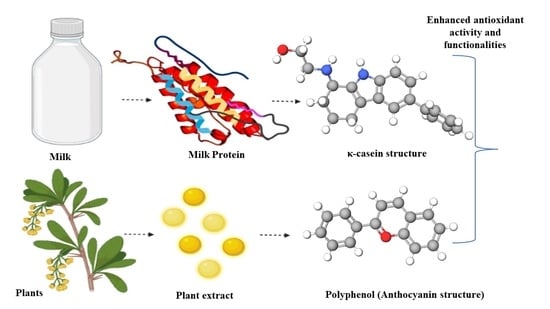
:1. Introduction
2. (Poly)phenolic Compounds
2.1. Types of (Poly)phenols
2.1.1. Flavonoids
2.1.2. Phenolic Acids
2.1.3. Non-Flavonoids
2.2. Extraction Process
2.3. Factors Causing Detrimental Effects on the Bioactivity of (Poly)phenols
2.3.1. Effect of Temperature on (Poly)phenols
2.3.2. Effect of pH on (Poly)phenols
2.3.3. Effect of Oxygen on (Poly)phenols
2.3.4. Effect of Light on (Poly)phenols
3. Milk Proteins
3.1. Interaction of Milk Proteins with (Poly)phenols
3.1.1. Non-Covalent Interaction between Milk Proteins and (Poly)phenols
3.1.2. Covalent Interaction between Milk Proteins and (Poly)phenol
4. Functional Properties of (Poly)phenol–Milk Protein Complexes
4.1. Solubility
4.2. Thermal Stability
4.3. Gelation
5. Factors Affecting Binding Interactions between (Poly)phenols and Protein Complexes
5.1. Ionic Strength and pH
5.2. Temperature
5.3. Type of Protein Complex
5.4. Structure of (Poly)phenolic Compound
6. Significances of Binding Reactions of Protein and (Poly)phenolic Complexes
Structural Changes
7. Analysis of Protein and (Poly)phenolic Binding Interactions
7.1. Ultrafiltration
- Km is the total initial ligand.
- Kn is the bound ligand.
- R is the amount of total protein.
7.2. Isothermal Titration Calorimetry
7.3. Molecular Docking
7.4. Thermodynamic Methods
8. Biological Activity of (Poly)phenol-Milk Protein Complexes
8.1. Antioxidant Activity
8.2. Anti-Proliferative Activity
8.3. Anti-Carcinogenic Properties
9. Impacts of (Poly)phenol and Protein Complexes on Food Quality
10. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, C.; Li, C.; Chen, B.; Shen, Y. Composition and antioxidant, antibacterial, and anti-HepG2 cell activities of polyphenols from seed coat of Amygdalus pedunculata Pall. Food Chem. 2018, 265, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Sadh, P.K.; Kumar, S.; Chawla, P.; Duhan, J.S. Fermentation: A boon for production of bioactive compounds by processing of food industries wastes (byproducts). Molecules 2018, 23, 2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucarini, M.; Durazzo, A.; Romani, A.; Campo, M.; Lombardi-Boccia, G.; Cecchini, F. Bio-based compounds from grape seeds: A biorefinery approach. Molecules 2018, 23, 1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Bao, J. Polyphenols in whole rice grain: Genetic diversity and health benefits. Food Chem. 2015, 180, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Moga, M.A.; Dimienescu, O.G.; Arvatescu, C.A.; Mironescu, A.; Dracea, L.; Ples, L. The role of natural polyphenols in the prevention and treatment of cervical cancer—An overview. Molecules 2016, 21, 1055. [Google Scholar] [CrossRef] [Green Version]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. In Phenolic Compounds—Biological Activity; Soto-Hernandez, M., Palma-Tenango, M., del Rosario Garcia Mateos, M., Eds.; InTech: Nappanee, IN, USA, 2017; pp. 1–24. [Google Scholar]
- Turturică, M.; Oancea, A.M.; Râpeanu, G.; Bahrim, G. Anthocyanins: Naturally occuring fruit pigments with functional properties. Ann. Univ. Dunarea De Jos Galati Fascicle VI-Food Technol. 2015, 39, 9–24. [Google Scholar]
- Broyard, C.; Gaucheron, F. Modifications of structures and functions of caseins: A scientific and technological challenge. Dairy Sci. Technol. 2015, 95, 831–862. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein-polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Chawla, P.; Kumar, N.; Kaushik, R.; Dhull, S.B. Synthesis, characterization and cellular mineral absorption of nanoemulsions of Rhododendron arboreum flower extracts stabilized with gum arabic. J. Food Sci. Technol. 2019, 56, 5194–5203. [Google Scholar] [CrossRef]
- Shilpashree, B.G.; Arora, S.; Chawla, P.; Tomar, S.K. Effect of succinylation on physicochemical and functional properties of milk protein concentrate. Food Res. Int. 2015, 72, 223–230. [Google Scholar] [CrossRef]
- Chen, X.X.; Leung, G.P.H.; Zhang, Z.J.; Xiao, J.B.; Lao, L.X.; Feng, F.; Zhang, K.Y.B. Proanthocyanidins from Uncaria rhynchophylla induced apoptosis in MDA-MB-231 breast cancer cells while enhancing cytotoxic effects of 5-fluorouracil. Food Chem. Toxicol. 2017, 107, 248–260. [Google Scholar] [CrossRef]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef]
- Gorusupudi, A.; Liu, A.; Hageman, G.S.; Bernstein, P.S. Associations of human retinal very long-chain polyunsaturated fatty acids with dietary lipid biomarkers. J. Lipid Res. 2016, 57, 499–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuper-Szablewska, K.; Perkowski, J. Phenolic acids in cereal grain: Occurrence, biosynthesis, metabolism and role in living organisms. Crit. Rev. Food Sci. Nutr. 2019, 59, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Adams, Z.P.; Ehlting, J.; Edwards, R. The regulatory role of shikimate in plant phenylalanine metabolism. J. Theor. Biol. 2019, 462, 158–170. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Tomás-Barberán, F.A.; García-Villalba, R. Structural diversity of polyphenols and distribution in foods. Diet. Polyphen. Metab. Health Eff. 2020, 15, 1–29. [Google Scholar] [CrossRef]
- Dwivedi, S.; Malik, C.; Chhokar, V. Molecular structure, biological functions, and metabolic regulation of flavonoids. In Plant Biotechnology: Recent Advancements and Developments; Springer: Singapore, 2017; pp. 171–188. [Google Scholar] [CrossRef]
- Abenavoli, L.; Izzo, A.A.; Milić, N.; Cicala, C.; Santini, A.; Capasso, R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- D’Evoli, L.; Lucarini, M.; del Pulgar, J.S.; Aguzzi, A.; Gabrielli, P.; Gambelli, L.; Lombardi-Boccia, G. Phenolic acids content and nutritional quality of conventional, organic and biodynamic cultivations of the tomato CXD271BIO breeding line (Solanum lycopersicum L.). Food Nutr. Sci. 2016, 7, 1112. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef]
- Reis, J.; Gaspar, A.; Milhazes, N.; Borges, F. Chromone as a Privileged Scaffold in Drug Discovery: Recent Advances: Miniperspective. J. Med. Chem. 2017, 60, 7941–7957. [Google Scholar] [CrossRef] [PubMed]
- Roche, A.; Ross, E.; Walsh, N.; O’Donnell, K.; Williams, A.; Klapp, M.; Edelstein, S. Representative literature on the phytonutrients category: Phenolic acids. Crit. Rev. Food Sci. Nutr. 2017, 57, 1089–1096. [Google Scholar] [CrossRef]
- Arraki, K.; Renouf, É.; Waffo-Téguo, P.; Mérillon, J.M.; Richard, T.; Decendit, A. Identification and quantification of stilbenes in some Tunisian red wines using UPLC-MS and HPLC-DAD. OENO One 2017, 51, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Theppakorn, T. Stability and chemical changes of phenolic compounds during Oolong tea processing. Int. Food Res. J. 2016, 23, 564–574. [Google Scholar]
- Ianni, A.; Martino, G. Dietary Grape Pomace Supplementation in Dairy Cows: Effect on Nutritional Quality of Milk and Its Derived Dairy Products. Foods 2020, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Sarker, S.A.; Sultana, S.; Pietroni, M.; Dover, A. Safety of a Bioactive Polyphenol Dietary Supplement in Pediatric Subjects with Acute Diarrhoea. Int. J. Pediatr. 2015, 2015, 387159. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Xu, Q.; Li, X.; Wang, Y.; Zhu, J.; Ning, C.; Meng, X. Effects of high hydrostatic pressure on physicochemical properties, enzymes activity, and antioxidant capacities of anthocyanins extracts of wild Lonicera caerulea berry. Innov. Food Sci. Emerg. Technol. 2016, 36, 48–58. [Google Scholar] [CrossRef]
- Deng, J.; Yang, H.; Capanoglu, E.; Cao, H.; Xiao, J. Technological aspects and stability of polyphenols. In Polyphenols: Properties, Recovery, and Applications; Woodhead Publishing: Sawston, UK, 2018; pp. 295–323. [Google Scholar] [CrossRef]
- Reis, A.; Perez-Gregorio, R.; Mateus, N.; de Freitas, V. Interactions of dietary polyphenols with epithelial lipids: Advances from membrane and cell models in the study of polyphenol absorption, transport and delivery to the epithelium. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–24. [Google Scholar] [CrossRef]
- Cao, H.; Högger, P.; Arroo, R.; Xiao, J. Flavonols with a catechol or pyrogallol substitution pattern on ring B readily form stable dimers in phosphate buffered saline at four degrees celsius. Food Chem. 2020, 311, 125902. [Google Scholar] [CrossRef]
- Sun, R.Z.; Cheng, G.; Li, Q.; He, Y.N.; Wang, Y.; Lan, Y.B.; Cui, X.D. Light-induced variation in phenolic compounds in Cabernet Sauvignon grapes (Vitis vinifera L.) involves extensive transcriptome reprogramming of biosynthetic enzymes, transcription factors, and phytohormonal regulators. Front. Plant Sci. 2017, 8, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Woo, M.W.; Patel, H.; Metzger, L.; Selomulya, C. Improvement of rheological and functional properties of milk protein concentrate by hydrodynamic cavitation. J. Food Eng. 2018, 221, 106–113. [Google Scholar] [CrossRef]
- Yildirim-Elikoglu, S.; Erdem, Y.K. Interactions between milk proteins and polyphenols: Binding mechanisms, related changes, and the future trends in the dairy industry. Food Rev. Int. 2018, 34, 665–697. [Google Scholar] [CrossRef]
- Ni, H.; Hayes, H.; Stead, D.; Liu, G.; Yang, H.; Li, H.; Raikos, V. Interaction of whey protein with polyphenols from salal fruits (Gaultheria shallon) and the effects on protein structure and hydrolysis pattern by Flavourzyme®. Int. J. Food Sci. Technol. 2019, 55, 1281–1288. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Z.; Zhao, J.; Liu, Y. The effect of non-covalent interaction of chlorogenic acid with whey protein and casein on physicochemical and radical-scavenging activity of in vitro protein digests. Food Chem. 2018, 268, 334–341. [Google Scholar] [CrossRef]
- Lila, M.A.; Schneider, M.; Devlin, A.; Plundrich, N.; Laster, S.; Foegeding, E.A. Polyphenol-enriched berry extracts naturally modulate reactive proteins in model foods. Food Funct. 2017, 8, 4760–4767. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Pathera, A.K.; Kaushik, R.; Kumar, N.; Dhull, S.B.; Arora, S.; Chawla, P. Succinylation of milk proteins: Influence on micronutrient binding and functional indices. Trends Food Sci. Technol. 2020, 97, 254264. [Google Scholar] [CrossRef]
- Yuksel, Z.; Avci, E.; Erdem, Y.K. Characterization of binding interactions between green tea flavanoids and milk proteins. Food Chem. 2010, 121, 450–456. [Google Scholar] [CrossRef]
- Gupta, C.; Arora, S.; Syama, M.A.; Sharma, A. Preparation of milk protein-vitamin A complexes and their evaluation for vitamin A binding ability. Food Chem. 2017, 237, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Arora, S.; Gupta, C.; Kapila, S. Effect of sodium caseinate and vitamin A complexation on bioaccessibility and bioavailability of vitamin A in Caco-2 cells. Food Res. Int. 2019, 121, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, C.D.; Hasni, I.; Bourassa, P.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.A. Milk β-lactoglobulin complexes with tea polyphenols. Food Chem. 2011, 123, 1046–1055. [Google Scholar] [CrossRef]
- Gallo, M.; Vinci, G.; Graziani, G.; De Simone, C.; Ferranti, P. The interaction of cocoa polyphenols with milk proteins studied by proteomic techniques. Food Res. Int. 2013, 54, 406–415. [Google Scholar] [CrossRef]
- Ye, J.; Fan, F.; Xu, X.; Liang, Y. Interactions of black and green tea polyphenols with whole milk. Food Res. Int. 2013, 53, 449–455. [Google Scholar] [CrossRef]
- El-Messery, T.M.; Mwafy, E.A.; Mostafa, A.M.; El-Din, H.M.F.; Mwafy, A.; Amarowicz, R.; Ozçelik, B. Spectroscopic studies of the interaction between isolated polyphenols from coffee and the milk proteins. Surf. Interfaces 2020, 20, 100558. [Google Scholar] [CrossRef]
- Bourassa, P.; Côté, R.; Hutchandani, S.; Samson, G.; Tajmir-Riahi, H.A. The effect of milk alpha-casein on the antioxidant activity of tea polyphenols. J. Photochem. Photobiol. B Biol. 2013, 128, 43–49. [Google Scholar] [CrossRef]
- Han, J.; Chang, Y.; Britten, M.; St-Gelais, D.; Champagne, C.P.; Fustier, P.; Lacroix, M. Interactions of phenolic compounds with milk proteins. Eur. Food Res. Technol. 2019, 245, 1881–1888. [Google Scholar] [CrossRef]
- Ojha, H.; Mishra, K.; Hassan, M.I.; Chaudhury, N.K. Spectroscopic and isothermal titration calorimetry studies of binding interaction of ferulic acid with bovine serum albumin. Thermochim. Acta 2012, 548, 56–64. [Google Scholar] [CrossRef]
- Liu, F.; Wang, D.; Ma, C.; Gao, Y. Conjugation of polyphenols prevents lactoferrin from thermal aggregation at neutral pH. Food Hydrocoll. 2016, 58, 49–59. [Google Scholar] [CrossRef]
- Staszewski, M.V.; Jara, F.L.; Ruiz, A.L.T.G.; Jagus, R.J.; Carvalho, J.E.; Pilosof, A.M.R. Nanocomplex formation between β-lactoglobulin or caseinomacropeptide and green tea polyphenols: Impact on protein gelation and polyphenols antiproliferative activity. J. Funct. Foods 2012, 4, 800–809. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Q.; Li, G.; Chen, F.; Qian, Y.; Wang, R. In vitro antioxidant, anti-mutagenic, anti-cancer and anti-angiogenic effects of Chinese Bowl tea. J. Funct. Foods 2014, 7, 590–598. [Google Scholar] [CrossRef]
- Hasni, I.; Bourassa, P.; Hamdani, S.; Samson, G.; Carpentier, R.; Tajmir-Riahi, H.A. Interaction of milk α-and β-caseins with tea polyphenols. Food Chem. 2011, 126, 630–639. [Google Scholar] [CrossRef]
- Dalgleish, D.G. On the structural models of bovine casein micelles—Review and possible improvements. Soft Matter 2011, 7, 2265–2272. [Google Scholar] [CrossRef]
- Rosa Perez-Gregorio, M.; Simal-Gandara, J. A critical review of the characterization of polyphenol-protein interactions and of their potential use for improving food quality. Curr. Pharm. Des. 2017, 23, 2742–2753. [Google Scholar] [CrossRef]
- Mehranfar, F.; Bordbar, A.K.; Parastar, H. A combined spectroscopic, molecular docking and molecular dynamic simulation study on the interaction of quercetin with β-casein nanoparticles. J. Photochem. Photobiol. B Biol. 2013, 127, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Dey, R.; Wu, H.U.I.; Liu, Z.; He, Q.; Zeng, X. Studies on the interaction of epigallocatechin 3 gallate from green tea with bovine β lactoglobulin by spectroscopic methods and docking. Int. J. Dairy Technol. 2013, 66, 7–13. [Google Scholar] [CrossRef]
- Skrt, M.; Benedik, E.; Podlipnik, Č.; Ulrih, N.P. Interactions of different polyphenols with bovine serum albumin using fluorescence quenching and molecular docking. Food Chem. 2012, 135, 2418–2424. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, M.; Radosavljevic, J.; Ognjenovic, J.; Vesic, J.; Prodic, I.; StanicVucinic, D.; Velickovic, T.C. Binding affinity between dietary polyphenols and β-lactoglobulin negatively correlates with the protein susceptibility to digestion and total antioxidant activity of complexes formed. Food Chem. 2013, 136, 1263–1271. [Google Scholar] [CrossRef]
- Wu, X.; Wu, H.; Liu, M.; Liu, Z.; Xu, H.; Lai, F. Analysis of binding interaction between (−)-epigallocatechin (EGC) and β-lactoglobulin by multispectroscopic method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 82, 164–168. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.A. Probing the binding sites of resveratrol, genistein, and curcumin with milk βlactoglobulin. J. Biomol. Struct. Dyn. 2013, 31, 14551466. [Google Scholar] [CrossRef]
- Bourassa, P.; Bariyanga, J.; Tajmir-Riahi, H.A. Binding sites of resveratrol, genistein, and curcumin with milk α-and β-caseins. J. Phys. Chem. B 2013, 117, 1287–1295. [Google Scholar] [CrossRef]
- Roy, D.; Dutta, S.; Maity, S.S.; Ghosh, S.; Roy, A.S.; Ghosh, K.S.; Dasgupta, S. Spectroscopic and docking studies of the binding of two stereoisomeric antioxidant catechins to serum albumins. J. Lumin. 2012, 132, 13641375. [Google Scholar] [CrossRef]
- Bohin, M.C.; Vincken, J.P.; van der Hijden, H.T.; Gruppen, H. Efficacy of food proteins as carriers for flavonoids. J. Agric. Food Chem. 2012, 60, 4136–4143. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Han, X.; Li, J.; Wang, Y.; Yu, W.; Wang, R.; Chang, J. Comparative study of the bindings between 3-phenyl-1H-indazole and five proteins by isothermal titration calorimetry, spectroscopy and docking methods. J. Biomol. Struct. Dyn. 2019, 37, 4580–4589. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Sun, Y.; Ding, L.; Wang, Y.; Gao, Z.; Wu, Z.; Bi, Y. Mechanism evaluation of the interactions between flavonoids and bovine serum albumin based on multi-spectroscopy, molecular docking and Q-TOF HR-MS analyses. Food Chem. 2016, 203, 150–157. [Google Scholar] [CrossRef]
- Ren, C.; Xiong, W.; Li, J.; Li, B. Comparison of binding interactions of cyanidin-3-O-glucoside to β-conglycinin and glycinin using multi-spectroscopic and thermodynamic methods. Food Hydrocoll. 2019, 92, 155–162. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Gidley, M.J.; Guo, Y.; Miao, M. Mechanism of binding interactions between young apple polyphenols and porcine pancreatic αamylase. Food Chem. 2019, 283, 468–474. [Google Scholar] [CrossRef]
- Pelitli, E.P.; Janiak, M.A.; Amarowicz, R.; Alasalvar, C. Protein precipitating capacity and antioxidant activity of Turkish Tombul hazelnut phenolic extract and its fractions. Food Chem. 2017, 218, 584–590. [Google Scholar] [CrossRef]
- Oliveira, A.; Amaro, A.L.; Pintado, M. Impact of food matrix components on nutritional and functional properties of fruit-based products. Curr. Opin. Food Sci. 2018, 22, 153–159. [Google Scholar] [CrossRef]
- Tao, J.; Li, Y.; Li, S.; Li, H.B. Plant foods for the prevention and management of colon cancer. J. Funct. Foods 2018, 42, 95–110. [Google Scholar] [CrossRef]
- Chanphai, P.; Bourassa, P.; Kanakis, C.D.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.A. Review on the loading efficacy of dietary tea polyphenols with milk proteins. Food Hydrocoll. 2018, 77, 322–332. [Google Scholar] [CrossRef]
- Oancea, A.M.; Aprodu, I.; Ghinea, I.O.; Barbu, V.; Ioniţă, E.; Bahrim, G.; Stănciuc, N. A bottom-up approach for encapsulation of sour cherries anthocyanins by using β-lactoglobulin as matrices. J. Food Eng. 2017, 210, 83–90. [Google Scholar] [CrossRef]
- Stanisavljević, N.; Samardžić, J.; Janković, T.; Šavikin, K.; Mojsin, M.; Topalović, V.; Stevanović, M. Antioxidant and antiproliferative activity of chokeberry juice phenolics during in vitro simulated digestion in the presence of food matrix. Food Chem. 2015, 175, 516–522. [Google Scholar] [CrossRef]
- Mehanna, N.S.; Hassan, Z.M.R.; El-Din, H.M.F.; Ali, A.A.E.; Amarowicz, R.; El-Messery, T.M. Effect of interaction phenolic compounds with milk proteins on cell line. Food Nutr. Sci. 2014, 5, 2130. [Google Scholar] [CrossRef] [Green Version]
- Rai, S.; Kureel, A.K.; Dutta, P.; Mehrotra, G. Phenolic compounds based conjugates from dextran aldehyde and BSA: Preparation, characterization and evaluation of their anti-cancer efficacy for therapeutic applications. Int. J. Biol. Macromol. 2018, 110, 425–436. [Google Scholar] [CrossRef]
- Chuang, C.Y.; Liu, H.C.; Wu, L.C.; Chen, C.Y.; Chang, J.T.; Hsu, S.L. Gallic acid induces apoptosis of lung fibroblasts via a reactive oxygen species-dependent ataxia telangiectasia mutated-p53 activation pathway. J. Agric. Food Chem. 2010, 58, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xu, M.; Zeng, M.; Qin, F.; Chen, J. Interactions of milk α-and β-casein with malvidin-3-O-glucoside and their effects on the stability of grape skin anthocyanin extracts. Food Chem. 2016, 199, 314–322. [Google Scholar] [CrossRef] [PubMed]







| Milk Protein Concentrates | (Poly)phenols | Type of Interaction | References |
|---|---|---|---|
| β-lactoglobulin | Tea (poly)phenols (catechin, epicatechin, epigallocatechin and epigallocatechin gallate) | Hydrophobic and Hydrophilic | [46] |
| Casein, whey proteins and β-lactoglobulin | Cocoa (poly)phenols (catechin and epicatechin) | Non-covalent bonding | [47] |
| Casien micelles and whey proteins | Black tea and green the (poly)phenols (catechin) | Hydrophobic | [48] |
| β-casein, α-casein, κ-casein, and whey protein | Coffee (poly)phenols (tannins) | Hydrogen bonding | [49] |
| α-caseins and β-caseins | Antioxidant (poly)phenols (resveratrol, genistein, and curcumin) | Hydrophilic and Hydrophobic | [50] |
| β-casein | Green tea (poly)phenols (catechin) | Hydrophobic, and non-covalent bonding | [43] |
| Casein and whey proteins | Green tea, grapes, and cranberry (poly)phenols (catechin, tannic acid, homovanillic acid, and hesperetin) | Hydrophobic | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tosif, M.M.; Najda, A.; Bains, A.; Krishna, T.C.; Chawla, P.; Dyduch-Siemińska, M.; Klepacka, J.; Kaushik, R. A Comprehensive Review on the Interaction of Milk Protein Concentrates with Plant-Based Polyphenolics. Int. J. Mol. Sci. 2021, 22, 13548. https://doi.org/10.3390/ijms222413548
Tosif MM, Najda A, Bains A, Krishna TC, Chawla P, Dyduch-Siemińska M, Klepacka J, Kaushik R. A Comprehensive Review on the Interaction of Milk Protein Concentrates with Plant-Based Polyphenolics. International Journal of Molecular Sciences. 2021; 22(24):13548. https://doi.org/10.3390/ijms222413548
Chicago/Turabian StyleTosif, Mansuri M., Agnieszka Najda, Aarti Bains, Thummalacharla Chaitanya Krishna, Prince Chawla, Magdalena Dyduch-Siemińska, Joanna Klepacka, and Ravinder Kaushik. 2021. "A Comprehensive Review on the Interaction of Milk Protein Concentrates with Plant-Based Polyphenolics" International Journal of Molecular Sciences 22, no. 24: 13548. https://doi.org/10.3390/ijms222413548
APA StyleTosif, M. M., Najda, A., Bains, A., Krishna, T. C., Chawla, P., Dyduch-Siemińska, M., Klepacka, J., & Kaushik, R. (2021). A Comprehensive Review on the Interaction of Milk Protein Concentrates with Plant-Based Polyphenolics. International Journal of Molecular Sciences, 22(24), 13548. https://doi.org/10.3390/ijms222413548