Glial-Neuronal Interactions in Pathogenesis and Treatment of Spinal Cord Injury
Abstract
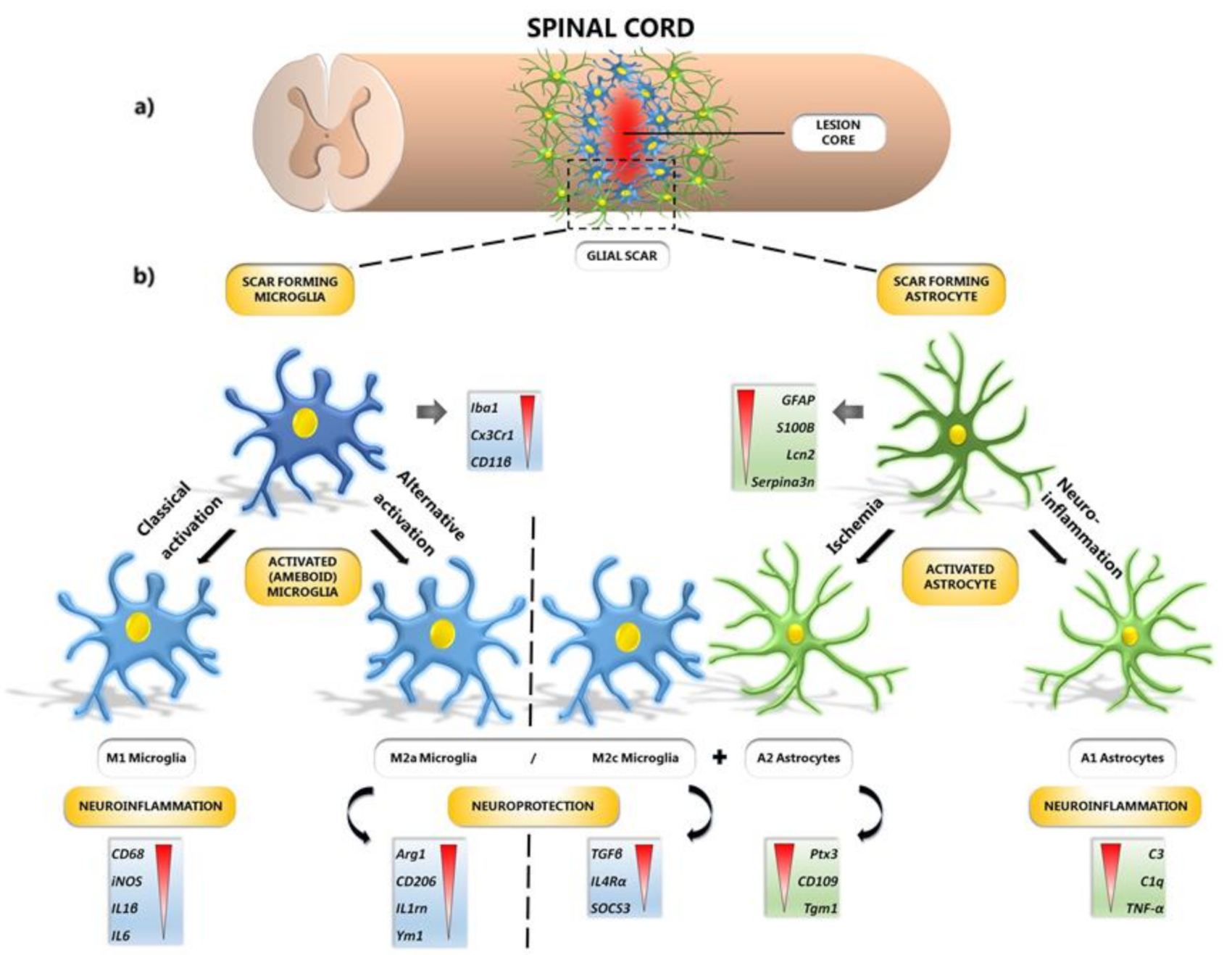
:1. Introduction
2. Neutrophils and Monocytes Respond Early after SCI
3. Microglia Rapidly Accumulate around the Lesion Site and Influences Neurons and Astrocytes in the Subacute Phase after SCI
4. Protection of Microglial Phenotypes after SCI
5. Early Modulation of Inflammatory Response after SCI
6. Changes in Gut Microbiota Resulting from SCI as a Trigger of Inflammatory Response
7. Promising Modulation Strategies of SCI-Induced Microglial Plasticity and Gut Dysbiosis
8. Interactions in Pathogenesis, Maintenance and Escalation of SCI-Induced Neuropathic Pain, and Development of Therapy
9. Astrocytes Response to SCI
10. Microglial and Astrocyte Polarization after SCI
11. In Vivo Conversion of Astrocytes to Neurons
12. The Effect of Weak Long-Term Electrostimulation on Spinal Cord Functional Recovery
13. Rehabilitation-Comprehensive and Effective Therapeutic Strategy after SCI
14. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blesch, A.; Tuszynski, M.H. Spinal cord injury: Plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009, 32, 41–47. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Nguyen, D.H. Immunoglobulin G: A potential treatment to attenuate neuroinflammation following spinal cord injury. J. Clin. Immunol. 2010, 30 (Suppl. 1), 109–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tator, C.H.; Fehlings, M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 1991, 75, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, J.; Kellerova, E.; Bimbova, K.; Pavel, J. The Histopathology of Severe Graded Compression in Lower Thoracic Spinal Cord Segment of Rat, Evaluated at Late Post-injury Phase. Cell. Mol. Neurobiol. 2021. [CrossRef]
- Tator, C.H. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995, 5, 407–413. [Google Scholar] [CrossRef]
- Min, K.J.; Jeong, H.K.; Kim, B.; Hwang, D.H.; Shin, H.Y.; Nguyen, A.T.; Kim, J.H.; Jou, I.; Kim, B.G.; Joe, E.H. Spatial and temporal correlation in progressive degeneration of neurons and astrocytes in contusion-induced spinal cord injury. J. Neuroinflamm. 2012, 9, 100. [Google Scholar] [CrossRef] [Green Version]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef]
- Saxena, T.; Deng, B.; Stelzner, D.; Hasenwinkel, J.; Chaiken, J. Raman spectroscopic investigation of spinal cord injury in a rat model. J. Biomed. Opt. 2011, 16, 027003. [Google Scholar] [CrossRef]
- Kisucka, A.; Bimbova, K.; Bacova, M.; Galik, J.; Lukacova, N. Activation of Neuroprotective Microglia and Astrocytes at the Lesion Site and in the Adjacent Segments Is Crucial for Spontaneous Locomotor Recovery after Spinal Cord Injury. Cells 2021, 10, 1943. [Google Scholar] [CrossRef] [PubMed]
- Teo, L.; Bourne, J.A. Current opinion on a role of the astrocytes in neuroprotection. Neural Regen. Res. 2018, 13, 797–798. [Google Scholar]
- Boghdadi, A.G.; Teo, L.; Bourne, J.A. The Neuroprotective Role of Reactive Astrocytes after Central Nervous System Injury. J. Neurotrauma 2020, 37, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Ao, Y.; Sofroniew, M.V. Heterogeneity of reactive astrocytes. Neurosci. Lett. 2014, 565, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukácová, N.; Kolesárová, M.; Kuchárová, K.; Pavel, J.; Kolesár, D.; Radonák, J.; Marsala, M.; Chalimoniuk, M.; Langfort, J.; Marsala, J. The effect of a spinal cord hemisection on changes in nitric oxide synthase pools in the site of injury and in regions located far away from the injured site. Cell. Mol. Neurobiol. 2006, 26, 1367–1385. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.A.; Yang, M.S.; Jeong, H.K.; Min, K.J.; Kang, S.H.; Jou, I.; Joe, E.H. Resident microglia die and infiltrated neutrophils and monocytes become major inflammatory cells in lipopolysaccharide-injected brain. Glia 2007, 55, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; John, S.M.; Chen, Y.; Mathison, R.D.; Weaver, L.C. The tripeptide phenylalanine-(D) glutamate-(D) glycine modulates leukocyte infiltration and oxidative damage in rat injured spinal cord. Neuroscience 2006, 140, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.J.; Rothwell, N.J. Cytokines and nervous system. I: Expression and recognition. Trends Neurosci. 1995, 18, 83–88. [Google Scholar] [CrossRef]
- Donnelly, D.J.; Popovich, P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef] [Green Version]
- Bimbova, K.; Bacova, M.; Kisucka, A.; Pavel, J.; Galik, J.; Zavacky, P.; Marsala, M.; Stropkovska, A.; Fedorova, J.; Papcunova, S.; et al. A Single Dose of Atorvastatin Applied Acutely after Spinal Cord Injury Suppresses Inflammation, Apoptosis, and Promotes Axon Outgrowth, Which Might Be Essential for Favorable Functional Outcome. Int. J. Mol. Sci. 2018, 19, 1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Xu, C.; Lin, J.; Hu, H.; Zhang, C.; Mei, X. Regulation of inflammatory cytokines for spinal cord injury recovery. Histol. Histopathol. 2021, 36, 137–142. [Google Scholar]
- Garcia, E.; Aguilar-Cevallos, J.; Silva-Garcia, R.; Ibarra, A. Cytokine and Growth Factor Activation In Vivo and In Vitro after Spinal Cord Injury. Mediat. Inflamm. 2016, 2016, 9476020. [Google Scholar] [CrossRef] [Green Version]
- Newman, S.L.; Henson, J.E.; Henson, P.M. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J. Exp. Med. 1982, 156, 430–442. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.K.; Jou, I.; Joe, E.H. Systemic LPS administration induces brain inflammation but not dopaminergic neuronal death in the substantia nigra. Exp. Mol. Med. 2010, 42, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.E.; Vernoux, N.; Tremblay, M.È.; Fuehrmann, T.; Shoichet, M.S. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef] [Green Version]
- Filous, A.R.; Silver, J. Targeting astrocytes in CNS injury and disease: A translational research approach. Prog. Neurobiol. 2016, 144, 173–187. [Google Scholar] [CrossRef] [Green Version]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Chen, Z.; Trapp, B.D. Microglia and neuroprotection. J. Neurochem. 2016, 136, 10–17. [Google Scholar] [CrossRef]
- Tremblay, M.È.; Majewska, A.K. A role for microglia in synaptic plasticity? Commun. Integr. Biol. 2011, 2, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Zheng, S.; Eacker, S.M.; Hong, S.J.; Gronostajski, R.M.; Dawson, T.M.; Dawson, V.L. NMDA-induced neuronal survival is mediated through nuclear factor I-A in mice. J. Clin. Investig. 2010, 120, 2446–2456. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Jalabi, W.; Hu, W.; Park, H.J.; Gale, J.T.; Kidd, G.J.; Bernatowicz, R.; Gossman, Z.C.; Chen, J.T.; Dutta, R.; et al. Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain. Nat. Commun. 2014, 5, 4486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thored, P.; Heldmann, U.; Gomes-Leal, W.; Gisler, R.; Darsalia, V.; Taneera, J.; Nygren, J.M.; Jacobsen, S.E.; Ekdahl, C.T.; Kokaia, Z.; et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia 2009, 8, 835–849. [Google Scholar] [CrossRef]
- Nikolakopoulou, A.M.; Dutta, R.; Chen, Z.; Miller, R.H.; Trapp, B.D. Activated microglia enhance neurogenesis via trypsinogen secretion. Proc. Natl. Acad. Sci. USA 2013, 110, 8714–8719. [Google Scholar] [CrossRef] [Green Version]
- Schmid, C.D.; Sautkulis, L.N.; Danielson, P.E.; Cooper, J.; Hasel, K.W.; Hilbush, B.S.; Sutcliffe, J.G.; Carson, M.J. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J. Neurochem. 2002, 83, 1309–1320. [Google Scholar] [CrossRef] [Green Version]
- Derecki, N.C.; Cronk, J.C.; Lu, Z.; Xu, E.; Abbott, S.B.; Guyenet, P.G.; Kipnis, J. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature 2012, 484, 105–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.; Younkin, S.; et al. Alzheimer Genetic Analysis Group. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar]
- Colton, C.A. Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 2009, 4, 399–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramaniam, S.R.; Federoff, H.J. Targeting Microglial Activation States as a Therapeutic Avenue in Parkinson’s Disease. Front. Aging Neurosci. 2017, 9, 176. [Google Scholar] [CrossRef]
- Ferreira, S.A.; Romero-Ramos, M. Microglia Response during Parkinson’s Disease: Alpha-Synuclein Intervention. Front. Cell. Neurosci. 2018, 12, 247. [Google Scholar] [CrossRef] [Green Version]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- Ferrante, C.J.; Pinhal-Enfield, G.; Elson, G.; Cronstein, B.N.; Hasko, G.; Outram, S.; Leibovich, S.J. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Rα) signaling. Inflammation 2013, 36, 921–931. [Google Scholar] [CrossRef]
- Kroner, A.; Greenhalgh, A.D.; Zarruk, J.G.; Passos Dos Santos, R.; Gaestel, M.; David, S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 2014, 83, 1098–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Lu, F.; Su, Q.; Liu, Z.; Xia, X.; Yan, Z.; Zhou, F.; Qin, R. Knockdown of long noncoding RNA XIST mitigates the apoptosis and inflammatory injury of microglia cells after spinal cord injury through miR-27a/Smurf1 axis. J. Neurosci. Lett. 2020, 715, 134649. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Caron, I.; Erba, E.; Panini, N.; De Paola, M.; Mariani, A.; Colombo, C.; Ferrari, R.; Pozzer, D.; Zanier, E.R.; et al. Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials 2016, 75, 13–24. [Google Scholar] [CrossRef]
- Jorge, A.; Taylor, T.; Agarwal, N.; Hamilton, D.K. Current Agents and Related Therapeutic Targets for Inflammation After Acute Traumatic Spinal Cord Injury. World Neurosurg. 2019, 132, 138–147. [Google Scholar] [CrossRef]
- Pannu, R.; Barbosa, E.; Singh, A.K.; Singh, I. Attenuation of acute inflammatory response by atorvastatin after spinal cord injury in rats. J. Neurosci. Res. 2005, 79, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Pannu, R.; Christie, D.K.; Barbosa, E.; Singh, I.; Singh, A.K. Post-trauma Lipitor treatment prevents endothelial dysfunction, facilitates neuroprotection, and promotes locomotor recovery following spinal cord injury. J. Neurochem. 2007, 101, 182–200. [Google Scholar] [CrossRef]
- Déry, M.A.; Rousseau, G.; Benderdour, M.; Beaumont, E. Atorvastatin prevents early apoptosis after thoracic spinal cord contusion injury and promotes locomotion recovery. Neurosci. Lett. 2009, 453, 73–76. [Google Scholar] [CrossRef]
- Mann, C.; Lee, J.H.T.; Hillyer, J.; Stammers, A.T.; Tetzlaff, W.; Kwon, B.K. Lack of robust neurologic benefits with simvastatin or atorvastatin treatment after acute thoracic spinal cord contusion injury. Exp. Neurology. 2010, 221, 285–295. [Google Scholar] [CrossRef]
- Nacar, O.A.; Eroglu, H.; Cetinalp, N.E.; Menekse, G.; Yildirim, A.E.; Uckun, O.M.; Daglioglu, E.; Turkoglu, O.F.; Belen, A.D. Systemic administration of atorvastatin improves locomotor functions and hyperacute-acute response after experimental spinal cord injury: An ultrastructural and biochemical analysis. Turk. Neurosurg. 2014, 24, 337–343. [Google Scholar]
- Gao, S.; Zhang, Z.; Shen, Z.; Gao, K.; Chang, L.; Guo, Y.; Li, Z.; Wang, W.; Wang, A. Atorvastatin activates autophagy and promotes neurological function recovery after spinal cord injury. Neural Regen. Res. 2016, 11, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Astaneh, M.E.; Goodarzi, A.; Khanmohammadi, M.; Shokati, A.; Mohandesnezhad, S.; Ataollahi, M.R.; Najafipour, S.; Farahani, M.S.; Ai, J. Chitosan/gelatin hydrogel and endometrial stem cells with subsequent atorvastatin injection impact in regenerating spinal cord tissue. J. Drug Deliv. Sci. Technol. 2020, 58, 101831. [Google Scholar] [CrossRef]
- Nakajima, H.; Honjoh, K.; Watanabe, S.; Kubota, A.; Matsumine, A. Distribution and polarization of microglia and macrophages at injured sites and the lumbar enlargement after spinal cord injury. Neurosci. Lett. 2020, 737, 135152. [Google Scholar] [CrossRef]
- Sohn, H.M.; Hwang, J.Y.; Ryu, J.H.; Kim, J.; Park, S.; Park, J.W.; Han, S.H. Simvastatin protects ischemic spinal cord injury from cell death and cytotoxicity through decreasing oxidative stress: In vitro primary cultured rat spinal cord model under oxygen and glucose deprivation-reoxygenation conditions. J. Orthop. Surg. Res. 2017, 12, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.L.; Chen, H.J.; Liliang, P.C.; Wang, H.K.; Tsai, Y.D.; Cho, C.L.; Lu, K.; Wang, K.W. Simvastatin and Simvastatin-Ezetimibe Improve the Neurological Function and Attenuate the Endothelial Inflammatory Response after Spinal Cord Injury in Rat. Ann. Clin. Lab. Sci. 2019, 49, 105–111. [Google Scholar]
- Wan, G.; An, Y.; Tao, J.; Wang, Y.; Zhou, Q.; Yang, R.; Liang, Q. MicroRNA-129-5p alleviates spinal cord injury in mice via suppressing the apoptosis and inflammatory response through HMGB1/TLR4/NF-κB pathway. Biosci. Rep. 2020, 40, BSR20193315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kigerl, K.A.; Zane, K.; Adams, K.; Sullivan, M.B.; Popovich, P.G. The spinal cord-gut-immune axis as a master regulator of health and neurological function after spinal cord injury. Exp. Neurol. 2020, 323, 113085. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [Green Version]
- Krych, L.; Hansen, C.H.; Hansen, A.K.; van den Berg, F.W.; Nielsen, D.S. Quantitatively different, yet qualitatively alike: A meta-analysis of the mouse core gut microbiome with a view towards the human gut microbiome. PLoS ONE 2013, 8, e62578. [Google Scholar] [CrossRef] [Green Version]
- Tate, D.G.; Forchheimer, M.; Rodriguez, G.; Chiodo, A.; Cameron, A.P.; Meade, M.; Krassioukov, A. Risk Factors Associated With Neurogenic Bowel Complications and Dysfunction in Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2016, 97, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; Hall, J.C.; Wang, L.; Mo, X.; Yu, Z.; Popovich, P.G. Gut dysbiosis impairs recovery after spinal cord injury. J. Exp. Med. 2016, 213, 2603–2620. [Google Scholar] [CrossRef]
- O’Connor, G.; Jeffrey, E.; Madorma, D.; Marcillo, A.; Abreu, M.T.; Deo, S.K.; Dietrich, W.D.; Daunert, S. Investigation of Microbiota Alterations and Intestinal Inflammation Post-Spinal Cord Injury in Rat Model. J. Neurotrauma 2018, 35, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.A.; Gobejishvili, L.; Saraswat Ohri, S.; Garrett Wilson, C.; Andres, K.R.; Riegler, A.S.; Donde, H.; Joshi-Barve, S.; Barve, S.; Whittemore, S.R. Following spinal cord injury, PDE4B drives an acute, local inflammatory response and a chronic, systemic response exacerbated by gut dysbiosis and endotoxemia. Neurobiol. Dis. 2019, 124, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.P.; Ricciardi-Castagnoli, P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immun. 2001, 2, 361–367. [Google Scholar]
- Rachmilewitz, D.; Katakura, K.; Karmeli, F.; Hayashi, T.; Reinus, C.; Rudensky, B.; Akira, S.; Takeda, K.; Lee, J.; Takabayashi, K.; et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 2004, 126, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kim, H.G.; Kim, J.Y.; Kim, N.R.; Jung, B.J.; Jeong, J.H.; Chung, D.K. Probiotic genomic DNA reduces the production of pro-inflammatory cytokine tumor necrosis factor-alpha. FEMS Microbiol. Lett. 2012, 328, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Chytilova, M.; Mudronova, D.; Nemcova, R.; Gancarcikova, S.; Buleca, V.; Koscova, J.; Tkacikova, L. Anti-inflammatory and immunoregulatory effects of flax-seed oil and Lactobacillus plantarum—Biocenol™ LP96 in gnotobiotic pigs challenged with enterotoxigenic Escherichia coli. Res. Vet. Sci. 2013, 95, 103–109. [Google Scholar] [CrossRef]
- Chytilova, M.; Nemcova, R.; Gancarcikova, S.; Mudronova, D.; Tkacikova, L. Flax-seed oil and Lactobacillus plantarum supplementation modulate TLR and NF-κB gene expression in enterotoxigenic Escherichia coli challenged gnotobiotic pigs. Acta Vet. Hung. 2014, 62, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Ringø, E.; Bendiksen, H.R.; Gausen, S.J.; Sundsfjord, A.; Olsen, R.E. The effect of dietary fatty acids on lactic acid bacteria associated with the epithelial mucosa and from faecalia of Arctic charr, Salvelinus alpinus (L.). J. Appl. Microbiol. 1998, 85, 855–864. [Google Scholar]
- Nemcová, R.; Borovská, D.; Koščová, J.; Gancarčíková, S.; Mudroňová, D.; Buleca, V.; Pistl, J. The effect of supplementation of flax-seed oil on interaction of Lactobacillus plantarum—Biocenol™ LP96 and Escherichia coli O8:K88ab:H9 in the gut of germ-free piglets. Res. Vet. Sci. 2012, 93, 39–41. [Google Scholar] [CrossRef]
- Kankaanpää, P.E.; Salminen, S.J.; Isolauri, E.; Lee, Y.K. The influence of polyunsaturated fatty acids on probiotic growth and adhesion. FEMS Microbiol. Lett. 2001, 194, 149–153. [Google Scholar] [CrossRef]
- Bi, J.; Chen, C.; Sun, P.; Tan, H.; Feng, F.; Shen, J. Neuroprotective effect of omega-3 fatty acids on spinal cord injury induced rats. Brain Behav. 2019, 9, e01339. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, S.; Vacca, V.; De Angelis, F.; Pieroni, L.; Orsini, T.; Parisi, C.; Soligo, M.; Protto, V.; Manni, L.; Guerrieri, R.; et al. Innovative mouse model mimicking human-like features of spinal cord injury: Efficacy of Docosahexaenoic acid on acute and chronic phases. Sci. Rep. 2019, 9, 8883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Turczyn, P.; Frasuńska, J.; Paradowska-Gorycka, A.; Tarnacka, B. Significance of Omega-3 Fatty Acids in the Prophylaxis and Treatment after Spinal Cord Injury in Rodent Models. Mediat. Inflamm. 2020, 29, 3164260. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, Y.Y.; Zhou, M.W.; Liu, N.; Xing, H.Y.; Liu, X.X.; Li, F. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci. Lett. 2018, 683, 13–18. [Google Scholar] [CrossRef]
- Jing, Y.; Yang, D.; Bai, F.; Zhang, C.; Qin, C.; Li, D.; Wang, L.; Yang, M.; Chen, Z.; Li, J. Melatonin Treatment Alleviates Spinal Cord Injury-Induced Gut Dysbiosis in Mice. J. Neurotrauma 2019, 36, 2646–2664. [Google Scholar] [CrossRef]
- Schmidt, E.K.A.; Torres-Espin, A.; Raposo, P.J.F.; Madsen, K.L.; Kigerl, K.A.; Popovich, P.G.; Fenrich, K.K.; Fouad, K. Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. PLoS ONE 2020, 15, e0226128. [Google Scholar] [CrossRef]
- Cheng, J.; Li, W.; Wang, Y.; Cao, Q.; Ni, Y.; Zhang, W.; Guo, J.; Chen, B.; Zang, Y.; Zhu, Y. Electroacupuncture modulates the intestinal microecology to improve intestinal motility in spinal cord injury rats. Microb. Biotechnol. 2021, 1–12. [Google Scholar] [CrossRef]
- Schmidt, E.K.A.; Raposo, P.J.F.; Torres-Espin, A.; Fenrich, K.K.; Fouad, K. Beyond the lesion site: Minocycline augments inflammation and anxiety-like behavior following SCI in rats through action on the gut microbiota. J. Neuroinflamm. 2021, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Hulsebosch, C.E.; Hains, B.C.; Crown, E.D.; Carlton, S.M. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res. Rev. 2009, 60, 202–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naser, P.V.; Kuner, R. Molecular, Cellular and Circuit Basis of Cholinergic Modulation of Pain. Neuroscience 2018, 387, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Jergova, S.; Gordon, C.E.; Gajavelli, S.; Sagen, J. Experimental Gene Therapy with Serine-Histogranin and Endomorphin 1 for the Treatment of Chronic Neuropathic Pain. Front. Mol. Neurosci. 2017, 10, 406. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Baastrup, C. Spinal cord injury pain: Mechanisms and management. Curr. Pain Headache Rep. 2012, 16, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Yaksh, T.L.; Fisher, C.J.; Hockman, T.M.; Wiese, A.J. Current and Future Issues in the Development of Spinal Agents for the Management of Pain. Curr. Neuropharmacol. 2017, 15, 232–259. [Google Scholar] [CrossRef] [Green Version]
- Sotgiu, M.L.; Biella, G. Differential effects of MK-801, a N-methyl-D-aspartate non-competitive antagonist, on the dorsal horn neuron hyperactivity and hyperexcitability in neuropathic rats. Neurosci. Lett. 2000, 283, 153–156. [Google Scholar] [CrossRef]
- Suzuki, R.; Matthews, E.A.; Dickenson, A.H. Comparison of the effects of MK-801, ketamine and memantine on responses of spinal dorsal horn neurones in a rat model of mononeuropathy. Pain 2001, 91, 101–109. [Google Scholar] [CrossRef]
- Hawksworth, C.; Serpell, M. Intrathecal anesthesia with ketamine. Reg. Anesth. Pain Med. 1998, 23, 283–288. [Google Scholar]
- Galluzzi, K.E. Management of neuropathic pain. J. Am. Osteopath. Assoc. 2005, 105, S12–19. [Google Scholar] [PubMed]
- Lemaire, S.; Shukla, V.K.; Rogers, C.; Ibrahim, I.H.; Lapierre, C.; Parent, P.; Dumont, M. Isolation and characterization of histogranin, a natural peptide with NMDA receptor antagonist activity. Eur. J. Pharmacol. 1993, 245, 247–256. [Google Scholar] [CrossRef]
- Lemaire, S.; Rogers, C.; Dumont, M.; Shukla, V.K.; Lapierre, C.; Prasad, J.; Lemaire, I. Histogranin, a modified histone H4 fragment endowed with N-methyl-D-aspartate antagonist and immunostimulatory activities. Life Sci. 1995, 56, 1233–1241. [Google Scholar] [CrossRef]
- Prasad, J.A.; Shukla, V.K.; Lemaire, S. Synthesis and biological activity of histogranin and related peptides. Can. J. Physiol. Pharmacol. 1995, 73, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Hama, A.T.; Siegan, J.B.; Herzberg, U.; Sagen, J. NMDA-induced spinal hypersensitivity is reduced by naturally derived peptide analog [Ser1] histogranin. Pharmacol. Biochem. Behav. 1999, 62, 67–74. [Google Scholar] [CrossRef]
- Hama, A.; Sagen, J. Selective antihyperalgesic effect of [Ser1] histogranin on complete Freund’s adjuvant-induced hyperalgesia in rats. Pain 2002, 95, 15–21. [Google Scholar] [CrossRef]
- Hentall, I.D.; Hargraves, W.A.; Sagen, J. Inhibition by the chromaffin cell-derived peptide serine-histogranin in the rat’s dorsal horn. Neurosci. Lett. 2007, 419, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Thakor, D.K.; Han, I.; Ropper, A.E.; Haragopal, H.; Sidman, R.L.; Zafonte, R.; Schachter, S.C.; Teng, Y.D. Alleviation of chronic pain following rat spinal cord compression injury with multimodal actions of huperzine A. Proc. Natl. Acad. Sci. USA 2013, 110, 746–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trujillo, K.A.; Akil, H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science 1991, 251, 85–87. [Google Scholar] [CrossRef]
- Trujillo, K.A.; Akil, H. Inhibition of opiate tolerance by non-competitive N-methyl-D-aspartate receptor antagonists. Brain Res. 1994, 633, 178–188. [Google Scholar] [CrossRef] [Green Version]
- Sallerin-Caute, B.; Lazorthes, Y.; Deguine, O.; Francés, B.; Verdié, J.C.; Charlet, J.P.; Bastide, R. Does intrathecal morphine in the treatment of cancer pain induce the development of tolerance? Neurosurgery 1998, 42, 44–49. [Google Scholar] [CrossRef]
- Przewłocki, R.; Przewłocka, B. Opioids in chronic pain. Eur. J. Pharmacol. 2001, 429, 79–91. [Google Scholar] [CrossRef]
- Clayton, B.A.; Hayashida, K.; Childers, S.R.; Xiao, R.; Eisenach, J.C. Oral donepezil reduces hypersensitivity after nerve injury by a spinal muscarinic receptor mechanism. Anesthesiology 2007, 106, 1019–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hama, A.; Sagen, J. Selective antinociceptive effects of a combination of the N-methyl-D-aspartate receptor peptide antagonist [Ser(1)]histogranin and morphine in rat models of pain. Pharmacol. Res. Perspect. 2014, 2, e00032. [Google Scholar] [CrossRef]
- Nasirinezhad, F.; Gajavelli, S.; Priddy, B.; Jergova, S.; Zadina, J.; Sagen, J. Viral vectors encoding endomorphins and serine histogranin attenuate neuropathic pain symptoms after spinal cord injury in rats. Mol. Pain 2015, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J. Huperzine A-an interesting anticholinesterase compound from the Chinese herbal medicine. Acta Med. 1998, 41, 155–157. [Google Scholar] [CrossRef]
- Jones, P.G.; Dunlop, J. Targeting the cholinergic system as a therapeutic strategy for the treatment of pain. Neuropharmacology 2007, 53, 197–206. [Google Scholar] [CrossRef]
- Cai, Y.Q.; Chen, S.R.; Han, H.D.; Sood, A.K.; Lopez-Berestein, G.; Pan, H.L. Role of M2, M3, and M4 muscarinic receptor subtypes in the spinal cholinergic control of nociception revealed using siRNA in rats. J. Neurochem. 2009, 111, 1000–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.J.; Eisenach, J.C. Intrathecal clonidine reduces hypersensitivity after nerve injury by a mechanism involving spinal m4 muscarinic receptors. Anesth. Analg. 2003, 96, 1403–1408. [Google Scholar] [CrossRef]
- Lee, J.W.; Jergova, S.; Furmanski, O.; Gajavelli, S.; Sagen, J. Predifferentiated GABAergic neural precursor transplants for alleviation of dysesthetic central pain following excitotoxic spinal cord injury. Front. Physiol. 2012, 3, 167. [Google Scholar] [CrossRef] [Green Version]
- Rashid, M.H.; Ueda, H. Neuropathy-specific analgesic action of intrathecal nicotinic agonists and its spinal GABA-mediated mechanism. Brain Res. 2002, 953, 53–62. [Google Scholar] [CrossRef]
- Harte, S.E.; Hoot, M.R.; Borszcz, G.S. Involvement of the intralaminar parafascicular nucleus in muscarinic-induced antinociception in rats. Brain Res. 2004, 1019, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Detloff, M.R.; Fisher, L.C.; McGaughy, V.; Longbrake, E.E.; Popovich, P.G.; Basso, D.M. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp. Neurol. 2008, 212, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Paixão, S.; Klein, R. Neuron-astrocyte communication and synaptic plasticity. Curr. Opin. Neurobiol. 2010, 20, 466–473. [Google Scholar] [CrossRef]
- Blanco-Suárez, E.; Caldwell, A.L.; Allen, N.J. Role of astrocyte-synapse interactions in CNS disorders. J. Physiol. 2017, 595, 1903–1916. [Google Scholar] [CrossRef] [Green Version]
- Takano, T.; Tian, G.F.; Peng, W.; Lou, N.; Libionka, W.; Han, X.; Nedergaard, M. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006, 9, 260–267. [Google Scholar] [CrossRef]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; Macvicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldskogius, H. Repairing CNS myelin--astrocytes have to do their jobs. Exp. Neurol. 2005, 19, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, E.N.; Lukanidin, E. Metastasis-associated mts1 (S100A4) protein is selectively expressed in white mater astrocytes and is up-regulated after peripheral nerve or dosal root injury. Glia 1999, 27, 249–258. [Google Scholar] [CrossRef]
- Kozlova, E.N.; Lukanidin, E. Mts1 protein expression in the central nervous system after injury. Glia 2002, 37, 337–348. [Google Scholar] [CrossRef]
- Pedersen, M.V.; Kohler, L.B.; Grigorian, M.; Novitskaya, V.; Bock, E.; Lukanidin, E.; Berezin, V. The Mts1/S100A4 protein is a neuroprotectant. J. Neurosci. Res. 2004, 77, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Rusnakova, V.; Honsa, P.; Dzamba, D.; Ståhlberg, A.; Kubista, M.; Anderova, M. Heterogeneity of astrocytes: From development to injury—Single cell gene expression. PLoS ONE 2013, 8, e69734. [Google Scholar] [CrossRef]
- Ben Haim, L.; Rowitch, D.H. Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci. 2017, 18, 31–41. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Multiple Roles for Astrocytes as Effectors of Cytokines and Inflammatory Mediators. Neuroscientist 2014, 20, 160–172. [Google Scholar] [CrossRef]
- Auguste, K.I.; Jin, S.; Uchida, K.; Yan, D.; Manley, G.T.; Papadopoulos, M.C.; Verkman, A.S. Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J. 2007, 21, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Goldshmit, Y.; Sztal, T.E.; Jusuf, P.R.; Hall, T.E.; Nguyen, C.; Currie, P.D. Fgf-dependent glial cell bridges facilitate spinal cord regeneration in zebrafish. J. Neurosci. 2012, 32, 7477–7492. [Google Scholar] [CrossRef] [Green Version]
- Dawley, E.M.; Samson, O.S.; Woodard, K.T.; Matthias, K.A. Spinal cord regeneration in a tail autotomizing urodele. J. Morphol. 2012, 273, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef] [Green Version]
- Myer, D.J.; Gurkoff, G.G.; Lee, S.M.; Hovda, D.A.; Sofroniew, M.V. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 2006, 129, 2761–2772. [Google Scholar] [CrossRef]
- Okada, S.; Nakamura, M.; Katoh, H.; Miyao, T.; Shimazaki, T.; Ishii, K.; Yamane, J.; Yoshimura, A.; Iwamoto, Y.; Toyama, Y.; et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 2006, 12, 829–834. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilhelmsson, U.; Bushong, E.A.; Price, D.L.; Smarr, B.L.; Phung, V.; Terada, M.; Ellisman, M.H.; Pekny, M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc. Natl. Acad. Sci. USA 2006, 103, 17513–17518. [Google Scholar] [CrossRef] [Green Version]
- Andrews, E.M.; Richards, R.J.; Yin, F.Q.; Viapiano, M.S.; Jakeman, L.B. Alterations in chondroitin sulfate proteoglycan expression occur both at and far from the site of spinal contusion injury. Exp. Neurol. 2012, 235, 174–187. [Google Scholar] [CrossRef] [Green Version]
- Galtrey, C.M.; Fawcett, J.W. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res. Rev. 2007, 54, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Massey, J.M.; Hubscher, C.H.; Wagoner, M.R.; Decker, J.A.; Amps, J.; Silver, J.; Onfer, S.M. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J. Neurosci. 2006, 26, 4406–4414. [Google Scholar] [CrossRef]
- Massey, J.M.; Amps, J.; Viapiano, M.S.; Matthews, R.T.; Wagoner, M.R.; Whitaker, C.M.; Alilain, W.; Yonkof, A.L.; Khalyfa, A.; Cooper, N.G.F.; et al. Increased chondroitin sulfate proteoglycan expression in denervated brainstem targets following spinal cord injury creates a barrier to axonal regeneration overcome by chondroitinase ABC and neurotrophin-3. Exp. Neurol. 2008, 209, 426–445. [Google Scholar] [CrossRef] [Green Version]
- Hara, M.; Kobayakawa, K.; Ohkawa, Y.; Kumamaru, H.; Yokota, K.; Saito, T.; Kijima, K.; Yoshizaki, S.; Harimaya, K.; Nakashima, Y.; et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat. Med. 2017, 23, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Cheng, X.; Huang, X.; Yuan, Y.; Qin, S.; Tan, Z.; Wang, D.; Hu, X.; He, C.; Su, Z. Conditional ablation of reactive astrocytes to dissect their roles in spinal cord injury and repair. Brain Behav. Immun. 2019, 80, 394–405. [Google Scholar] [CrossRef]
- Dias, D.O.; Kim, H.; Holl, D.; Solnestam, B.W.; Lundeberg, J.; Carlen, M.; Goritz, C.; Frisen, J. Reducing Pericyte-Derived Scarring Promotes Recovery after Spinal Cord Injury. Cell 2018, 173, 153–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Kong, X.; Gao, J. Macrophage polarization: A key event in the secondary phase of acute spinal cord injury. J. Cell. Mol. Med. 2017, 21, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Niu, W.; Liu, M.L.; Zou, Y.; Zhang, C.L. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 2014, 5, 3338. [Google Scholar] [CrossRef]
- Mattugini, N.; Bocchi, R.; Scheuss, V.; Russo, G.L.; Torper, O.; Lao, C.L.; Götz, M. Inducing Different Neuronal Subtypes from Astrocytes in the Injured Mouse Cerebral Cortex. Neuron 2019, 103, 1086–1095. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Qin, S.; Huang, X.; Yuan, Y.; Tan, Z.; Gu, Y.; Cheng, X.; Wang, D.; Lian, X.F.; He, C.; et al. Region-Restrict Astrocytes Exhibit Heterogeneous Susceptibility to Neuronal Reprogramming. Stem Cell Rep. 2019, 12, 290–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buffo, A.; Vosko, M.R.; Ertürk, D.; Hamann, G.F.; Jucker, M.; Rowitch, D.; Götz, M. Expression pattern of the transcription factor Olig2 in response to brain injuries: Implications for neuronal repair. Proc. Natl. Acad. Sci. USA 2005, 102, 18183–18188. [Google Scholar] [CrossRef] [Green Version]
- Berninger, B.; Costa, M.R.; Koch, U.; Schroeder, T.; Sutor, B.; Grothe, B.; Götz, M. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J. Neurosci. 2007, 27, 8654–8664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinrich, C.; Blum, R.; Gascón, S.; Masserdotti, G.; Tripathi, P.; Sánchez, R.; Tiedt, S.; Schroeder, T.; Götz, M.; Berninger, B. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010, 8, e1000373. [Google Scholar] [CrossRef] [Green Version]
- Graham, V.; Khudyakov, J.; Ellis, P.; Pevny, L. SOX2 functions to maintain neural progenitor identity. Neuron 2003, 39, 749–765. [Google Scholar] [CrossRef] [Green Version]
- Chew, L.J.; Gallo, V. The Yin and Yang of Sox proteins: Activation and repression in development and disease. J. Neurosci. Res. 2009, 87, 3277–3287. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Engler, A.; Taylor, V. Notch: An interactive player in neurogenesis and disease. Cell Tissue Res. 2018, 371, 73–89. [Google Scholar] [CrossRef]
- Gascón, S.; Masserdotti, G.; Russo, G.L.; Götz, M. Direct Neuronal Reprogramming: Achievements, Hurdles, and New Roads to Success. Cell Stem Cell 2017, 21, 18–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.L.; Zhang, C.L. Engineering new neurons: In vivo reprogramming in mammalian brain and spinal cord. Cell Tissue Res. 2018, 371, 201–212. [Google Scholar] [CrossRef]
- Miyanohara, A.; Kamizato, K.; Juhas, S.; Juhasova, J.; Navarro, M.; Marsala, S.; Lukacova, N.; Hruska-Plochan, M.; Curtis, E.; Gabel, B.; et al. Potent spinal parenchymal AAV9-mediated gene delivery by subpial injection in adult rats and pigs. Mol. Ther. Methods Clin. Dev. 2016, 3, 16046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadokoro, T.; Miyanohara, A.; Navarro, M.; Kamizato, K.; Juhas, S.; Juhasova, J.; Marsala, S.; Platoshyn, O.; Curtis, E.; Gabel, B.; et al. Subpial Adeno-associated Virus 9 (AAV9) Vector Delivery in Adult Mice. J. Vis. Exp. 2017, 13, 55770. [Google Scholar] [CrossRef]
- Bravo-Hernández, M.; Tadokoro, T.; Marsala, M. Subpial AAV Delivery for Spinal Parenchymal Gene Regulation in Adult Mammals. Methods Mol. Biol. 2019, 1950, 209–233. [Google Scholar]
- Bravo-Hernandez, M.; Tadokoro, T.; Navarro, M.R.; Platoshyn, O.; Kobayashi, Y.; Marsala, S.; Miyanohara, A.; Juhas, S.; Juhasova, J.; Skalnikova, H. Spinal subpial delivery of AAV9 enables widespread gene silencing and blocks motoneuron degeneration in ALS. Nat. Med. 2020, 26, 118–130. [Google Scholar] [CrossRef]
- Marsala, M.; Kamizato, K.; Tadokoro, T.; Navarro, M.; Juhas, S.; Juhasova, J.; Marsala, S.; Studenovska, H.; Proks, V.; Hazel, T.; et al. Spinal parenchymal occupation by neural stem cells after subpial delivery in adult immunodeficient rats. Stem Cells Transl. Med. 2020, 9, 177–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Fan, Z.K.; Gu, G.F.; Jia, Z.Q.; Zhang, Q.Q.; Dai, J.Y.; He, S.S. Epidural Spinal Cord Stimulation Promotes Motor Functional Recovery by Enhancing Oligodendrocyte Survival and Differentiation and by Protecting Myelin after Spinal Cord Injury in Rats. Neurosci. Bull. 2020, 36, 372–384. [Google Scholar] [CrossRef]
- McKasson, M.J.; Huang, L.; Robinson, K.R. Chick embryonic Schwann cells migrate anodally in small electrical fields. Exp. Neurol. 2008, 211, 585–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Song, B.; Pu, J.; Wada, T.; Reid, B.; Tai, G.; Wang, F.; Guo, A.; Walczysko, P.; Gu, Y.; et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 2006, 442, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Blackiston, D.J.; McLaughlin, K.A.; Levin, M. Bioelectric controls of cell proliferation: Ion channels, membrane voltage and the cell cycle. Cell Cycle 2009, 8, 3527–3536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mie, M.; Endoh, T.; Yanagida, Y.; Kobatake, E.; Aizawa, M. Induction of neural differentiation by electrically stimulated gene expression of NeuroD2. J. Biotechnol. 2003, 100, 231–238. [Google Scholar] [CrossRef]
- Li, Y.; Weiss, M.; Yao, L. Directed migration of embryonic stem cell-derived neural cells in an applied electric field. Stem Cell Rev. 2014, 10, 653–662. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Agius-Fernandez, A.; Forrester, J.V.; McCaig, C.D. Orientation and directed migration of cultured corneal epithelial cells in small electric fields are serum dependent. J. Cell Sci. 1996, 109, 1405–1414. [Google Scholar] [CrossRef]
- Rajnicek, A.M.; Robinson, K.R.; McCaig, C.D. The direction of neurite growth in a weak DC electric field depends on the substratum: Contributions of adhesivity and net surface charge. Dev. Biol. 1998, 203, 412–423. [Google Scholar] [CrossRef] [Green Version]
- McCaig, C.D.; Rajnicek, A.M.; Song, B.; Zhao, M. Controlling cell behavior electrically: Current views and future potential. Physiol. Rev. 2005, 85, 943–978. [Google Scholar] [CrossRef] [Green Version]
- McCaig, C.D.; Song, B.; Rajnicek, A.M. Electrical dimensions in cell science. J. Cell Sci. 2009, 122, 4267–4276. [Google Scholar] [CrossRef] [Green Version]
- Borgens, R.B.; Shi, R.; Mohr, T.J.; Jaeger, C.B. Mammalian Cortical Astrocytes Align Themselves in a Physiological Voltage Gradient. Exp. Neurol. 1994, 128, 41–49. [Google Scholar] [CrossRef]
- Moriarty, L.J.; Borgens, R.B. An oscillating extracellular voltage gradient reduces the density and infuences the orientation of astrocytes in injured mammalian spinal cord. J. Neurocytol. 2001, 30, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, L.J.; Borgens, R.B. The effect of an applied electric field on macrophage accumulation within the subacute spinal injury. Restor. Neurol. Neurosci. 1999, 14, 53–64. [Google Scholar] [PubMed]
- Borgens, R.B.; Toombs, J.P.; Breur, G.; Widmer, W.R.; Waters, D.; Harbath, A.M.; March, P.; Adams, L.G. An imposed oscillating electrical field improves the recovery of function in neurologically complete paraplegic dogs. J. Neurotrauma 1999, 16, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Bacova, M.; Bimbova, K.; Fedorova, J.; Lukacova, N.; Galik, J. Epidural oscillating field stimulation as an effective therapeutic approach in combination therapy for spinal cord injury. J. Neurosci. Methods 2019, 311, 102–110. [Google Scholar] [CrossRef]
- Bacova, M.; Bimbova, K.; Kisucka., A.; Lukacova, N.; Galik, J. Epidural oscillating field stimulation as a trigger to increase axonal regenerative capacity and myelination after spinal cord trauma. J. Neural Regen. Res. 2021. [CrossRef]
- Zhang, C.; Zhang, G.; Rong, W.; Wang, A.; Wu, C.; Huo, X. Oscillating field stimulation promotesspinal cord remyelination by inducing differentiation of oligodendrocyte precursor cells after spinal cord injury. Biomed. Mater. Eng. 2014, 24, 3629–3636. [Google Scholar] [PubMed] [Green Version]
- Jing, J.H.; Qian, J.; Zhu, N.; Chou, W.B.; Huang, X.J. Improved differentiation of oligodendrocyte precursor cells and neurological function after spinal cord injury in rats by oscillating field stimulation. Neuroscience 2015, 303, 346–351. [Google Scholar] [CrossRef]
- Keough, M.B.; Rogers, J.A.; Zhang, P.; Jensen, S.K.; Stephenson, E.L.; Chen, T.; Hurlbert, M.G.; Lau, L.W.; Rawji, K.S.; Plemel, J.R.; et al. An inhibitor of chondroitin sulfate proteoglycan synthesis promotes central nervous system remyelination. Nat. Commun. 2016, 7, 11312. [Google Scholar] [CrossRef]
- McCaig, C.D.; Erskine, L. Nerve growth and nerve guidance in a physiological electrical field. In Nerve Growth and Guidance (Frontiers in Neurobiology); McCaig, C.D., Ed.; Portland Press Ltd.: London, UK, 1996; Volume 2, pp. 151–170. [Google Scholar]
- McCaig, C.D.; Sangster, L.; Stewart, R. Neurotrophins enhance electric field-directed growth cone guidance and directed nerve branching. Dev. Dyn. 2000, 217, 299–308. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Brushart, T.M.; Gordon, T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regeneratingrat femoral motoneurons. Eur. J. Neurosci. 2000, 12, 4381–4390. [Google Scholar]
- Al-Majed, A.A.; Tam, S.L.; Gordon, T. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cell. Mol. Neurobiol. 2004, 24, 379–402. [Google Scholar] [CrossRef]
- Puttagunta, R.; Tedeschi, A.; Sória, M.G.; Hervera, A.; Lindner, R.; Rathore, K.I.; Gaub, P.; Joshi, Y.; Nguyen, T.; Schmadke, A.; et al. PCAF-dependent epigenetic changes promote axonal regeneration in the central nervous system. Nat. Commun. 2014, 5, 3527. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.; Cavalli, V. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J. 2012, 31, 3063–3078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekki, M.; Delgado, A.D.; Fry, A.; Putrino, D.; Huang, V. Robotic Rehabilitation and Spinal Cord Injury: A Narrative Review. Neurotherapeutics 2018, 15, 604–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detloff, M.R.; Smith, E.J.; Molina, D.Q.; Ganzer, P.D.; Houlé, J.D. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp. Neurol. 2014, 255, 38–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalki, L.; Sadlaoud, K.; Lerond, J.; Coq, J.O.; Brezun, J.M.; Vinay, L.; Coulon, P.; Bras, H. Changes in innervation of lumbar motoneurons and organization of premotor network following training of transected adult rats. Exp. Neurol. 2018, 299, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sandrow-Feinberg, H.R.; Houlé, J.D. Exercise after spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res. 2015, 1619, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, Q.; Xie, C.; Wang, C.; Wang, Q.; Dong, C.; Fang, L.; Ding, J.; Wang, T. Blocking of BDNF-TrkB signaling inhibits the promotion effect of neurological function recovery after treadmill training in rats with spinal cord injury. Spinal Cord 2018, 57, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Kiss Bimbova, K.; Bacova, M.; Kisucka, A.; Galik, J.; Zavacky, P.; Lukacova, N. Activation of three major signaling pathways after endurance training and spinal cord injury. Mol. Neurobiol. 2021. [CrossRef]
- Zhang, Q.; Bian, G.; Chen, P.; Liu, L.; Yu, C.; Liu, F.F.; Xue, Q.; Chung, S.K.; Song, B.; Ju, G.; et al. Aldose Reductase Regulates Microglia/Macrophages Polarization Through the cAMP Response Element-Binding Protein After Spinal Cord Injury in Mice. Mol. Neurobiol. 2016, 53, 662–676. [Google Scholar] [CrossRef] [Green Version]
- Mohanraj, M.; Sekar, P.; Liou, H.H.; Chang, S.F.; Lin, W.W. The Mycobacterial Adjuvant Analogue TDB Attenuates Neuroinflammation via Mincle-Independent PLC-γ1/PKC/ERK Signaling and Microglial Polarization. Mol. Neurobiol. 2018, 56, 1167–1187. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukacova, N.; Kisucka, A.; Kiss Bimbova, K.; Bacova, M.; Ileninova, M.; Kuruc, T.; Galik, J. Glial-Neuronal Interactions in Pathogenesis and Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 13577. https://doi.org/10.3390/ijms222413577
Lukacova N, Kisucka A, Kiss Bimbova K, Bacova M, Ileninova M, Kuruc T, Galik J. Glial-Neuronal Interactions in Pathogenesis and Treatment of Spinal Cord Injury. International Journal of Molecular Sciences. 2021; 22(24):13577. https://doi.org/10.3390/ijms222413577
Chicago/Turabian StyleLukacova, Nadezda, Alexandra Kisucka, Katarina Kiss Bimbova, Maria Bacova, Maria Ileninova, Tomas Kuruc, and Jan Galik. 2021. "Glial-Neuronal Interactions in Pathogenesis and Treatment of Spinal Cord Injury" International Journal of Molecular Sciences 22, no. 24: 13577. https://doi.org/10.3390/ijms222413577





