Abstract
The capacity of T cells to identify and kill cancer cells has become a central pillar of immune-based cancer therapies. However, T cells are characterized by a dysfunctional state in most tumours. A major obstacle for proper T-cell function is the metabolic constraints posed by the tumour microenvironment (TME). In the TME, T cells compete with cancer cells for macronutrients (sugar, proteins, and lipid) and micronutrients (vitamins and minerals/ions). While the role of macronutrients in T-cell activation and function is well characterized, the contribution of micronutrients and especially ions in anti-tumour T-cell activities is still under investigation. Notably, ions are important for most of the signalling pathways regulating T-cell anti-tumour function. In this review, we discuss the role of six biologically relevant ions in T-cell function and in anti-tumour immunity, elucidating potential strategies to adopt to improve immunotherapy via modulation of ion metabolism.
1. Introduction
T lymphocytes undergo a metabolic reprogramming upon TCR-stimulation, which sustains the biosynthetic requirements of clonal expansion and differentiation. Indeed, the engagement of specific metabolic pathways requires the presence of particular metabolites that are not only necessary to promote the synthesis of ATP and macromolecules but also to mediate signalling regulation of T-cell function and fate [1,2,3]. The role of metabolism in modulating T-cell responses becomes evident in the context of anti-tumour immunity, where cancer cells acquire suppressive mechanisms to evade the immune system [4]. Nutrient competition between cancer and immune cells in the tumour microenvironment (TME) or the secretion of cancer-cell suppressive metabolic waste products (e.g., adenosine or kynurenine) have been deeply studied during the last decade [5,6], and a myriad of promising interventions has been developed to overcome these metabolic barriers and to boost anti-tumour T-cell responses [7,8].
The role of glucose, amino acids, and lipids in the regulation of T-cell responses against cancer has been studied and reviewed extensively elsewhere [9,10,11,12,13,14]. Here, we focus on the underrepresented function of ions. T cells require an appropriate balance of extracellular and intracellular ion levels to maintain cell and mitochondrial membrane potential (∆Ψm). Furthermore, ions operate as second messengers for TCR signalling, act as cofactors for a multitude of enzymes, and interact with DNA to stabilise its structure. Disturbances in ionic concentrations or in the expression of ionic channels are detrimental for T-cell performance and lead to the appearance of immune-related diseases. Although it is well-known that ionic homeostasis is essential for T-cell survival and activity, the functional relevance of ions within tumours remains poorly understood. Recent reports have shown that tumour necrotic cells release ions within the TME and that several cancer types modify ion-channel expression to adapt to the ionic conditions of the TME [15,16]. In this review, we will discuss how ions shape T-cell immunity and describe the latest advances in the context of anti-tumour immunity. Specifically, we will focus on six ions with potential translational application: potassium, manganese, zinc, selenium, iron, and magnesium.
2. Potassium
Potassium (K+) is the most abundant ion in mammalian cells, with intracellular K+ levels ([K+]i) reaching ~130 mM, while extracellular levels [K+]e are ~3–5 mM [17,18]. In T lymphocytes, K+ gradient is balanced through the action of two ion channels mediating K+ efflux: the voltage-gated Kv1.3 and the Ca2+-activated KCa3.1 channels (Figure 1) [19]. Alterations in the expression of these channels and, subsequently, in [K+]i, lead to aberrant T-cell functionality.
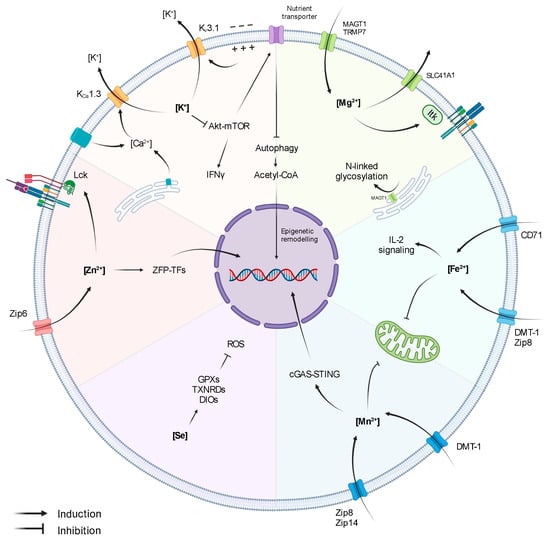
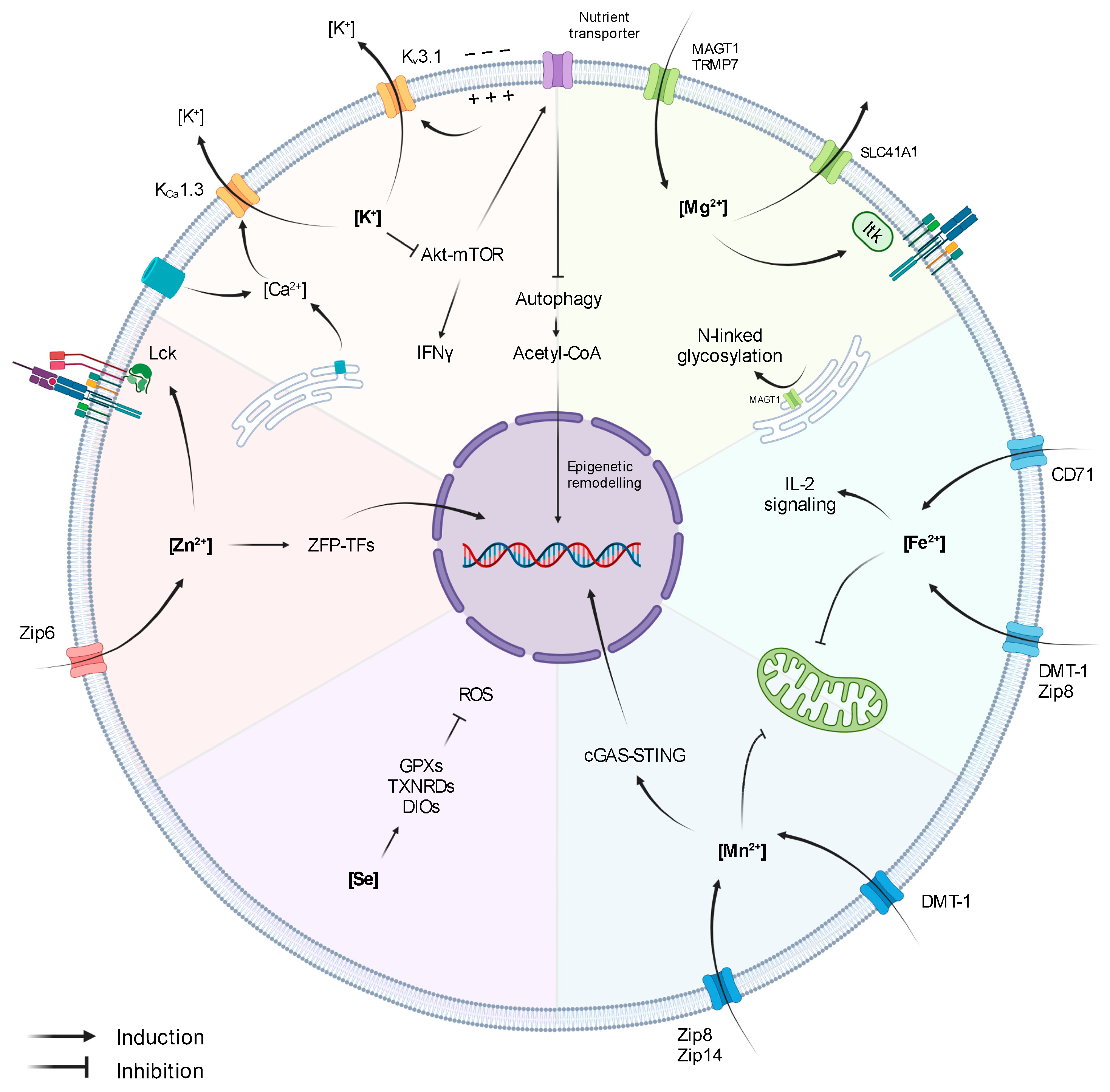
Figure 1.
Influence of Ions on T-cell activity.
The role of K+ in T cells is tightly linked to Ca2+ signalling. Upon antigen recognition, the activation of TCR signalling triggers the opening of Ca2+ channels (Orai1 in the ER and CRAC channels in the cell membrane), leading to increased intracellular Ca2+ levels. Importantly, Ca2+ induces NFAT expression and subsequent IL-2 production and T-cell activation [20]. However, the first wave of Ca2+ release generates an electrochemical imbalance that depolarises the membrane and hampers further Ca2+ influx. Membrane depolarisation and the elevated Ca2+ levels activate Kv1.3 and KCa3.1, respectively, promoting K+ efflux, thus restoring membrane potential and enabling continuous Ca2+ entry and signalling amplification (Figure 1) [19,21]. Indeed, a blockade of Kv1.3 and KCa3.1 reduces Ca2+ signalling, demonstrating the key role of K+ gradient in preserving the equilibrium of the membrane potential upon TCR stimulation and ensuring efficient T-cell activation [22]. In accordance, Kv1.3 and KCa3.1 are highly expressed upon T-cell activation, and they co-localize at the immunological synapse, together with CRAC channels [15,21,23]. Moreover, Kv1.3 and KCa3.1 have also been shown to influence T-cell migratory capacity [24,25].
Importantly, Kv1.3 and KCa3.1 expression vary between T-cell subsets. It has been described that Th1 and Th2 cells predominantly express KCa3.1, whilst Th17 and Tregs express Kv1.3. In fact, KCa3.1−/− mice are resistant to the induction of autoimmune colitis, characterised by the presence of autoreactive Th1 cells. In this model, depletion of KCa3.1 disrupted Th1 activity without affecting the functionality of Tregs and Th17 cells [26]. Similarly in humans, effector-memory T cells (CD45RA−CCR7+; Tem) are highly dependent on Kv1.3 for Ca2+ signalling, whereas central-memory T cells (CD45RA−CCR7−; Tcm) are mostly dependent on KCa3.1, and Kv1.3 inhibition only mildly affects their functionality [27,28]. The differences in expression levels are interesting from an immunotherapeutic perspective, as the application of K+ channel blockers could be used to target specific T-cell populations.
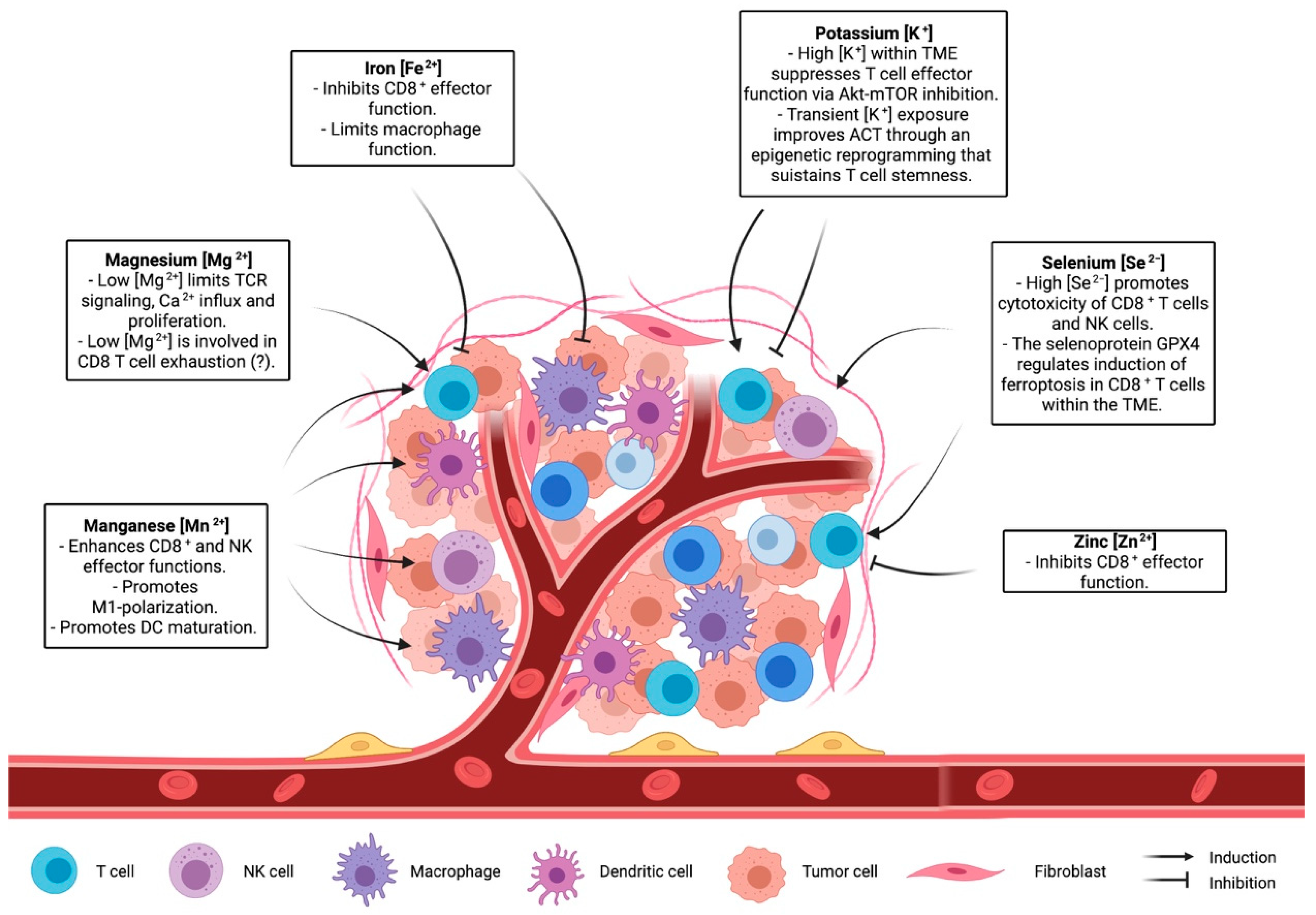
In the context of anti-tumour T-cell responses, it has been shown that necrotic cancer cells within hypoxic areas release large amounts of K+, which directly inhibit effector functions of murine and human CD8+ T cells [15,18]. Mechanistically, T-cell suppression derived from exposure to high extracellular [K+] ([K+]e) is not directly caused by membrane-potential variations or Ca2+ signalling alterations but is rather due to an increase in intracellular [K+]i, which affects the Akt-mTOR pathway (Figure 1) [15,18]. In addition, it has been described that hypoxia downregulates Kv1.3 and KCa3.1 [29], suggesting that intracellular [K+]I could be further augmented in the TME through other synergistic mechanisms. Importantly, overexpression of KCa3.1 decreases intracellular [K+]I and restores T-cell Akt-mTOR signalling and IFNγ secretion, resulting in improved tumour growth control and survival [15]. These reports indicate that levels of [K+]I and K+ channels in T cells might be used as markers of T-cell fitness within tumours. Accordingly, Kv1.3 and KCa3.1 activity in CD8+ T cells derived from head- and neck-cancer patients correlate with increased T-cell infiltration and functionality [25,30,31]. Moreover, K+ is also an important cofactor for the glycolytic enzyme hexokinase-II (HK-II), suggesting that K+ might not only be involved in the regulation of anti-tumour immunity but also in the adaptation of cancer-cell metabolism in the TME. Altogether, these studies support the concept that K+ acts as a suppressive element of anti-tumour immunity. However, a more recent report by Vodnala et al. (2019) showed that despite dampening T-cell effector functions, mTOR inactivation derived from high [K+]e is accompanied by a decreased nutrient uptake, which initiates a starvation response. The authors define this state as ‘functional caloric restriction’, characterised by autophagy induction and acetyl-CoA-dependent epigenetic remodelling (Figure 1). Specifically, exposure to [K+]e reduced the acetylation of effector/exhaustion-associated loci of genes such as Pdcd1 (PD1), Cd244 (2B4), and Havcr2 (Tim-3) while preserving T-cell stemness through the induction of TCF1 expression. Consequently, T cells exposed to high [K+]e during in vitro expansion enhanced T-cell persistence and anti-tumour response upon adoptive cell transfer in a B16 melanoma mouse model [32]. On the contrary, CD19-directed human CAR-T cells cultured for 48 h in cerebrospinal fluid (CSF), which contains low concentrations of glucose and K+, expressed elevated levels of genes encoding for survival and memory markers (e.g., BCL2, IL7R) and lower levels of effector genes (e.g., IFNγ, GrB, Tbet) [33]. Although plenty of evidence points at K+ as an interesting target for immunotherapy, the dual roles of K+ in anti-tumour T cells, the discrepancies observed in murine and human settings, and the direct effect of K+ on cancer cells indicate that further investigations are required to unveil the best strategy to exploit K+ in cancer therapy.
3. Manganese
Manganese (Mn2+) is one of the most abundant metals found in the tissues of mammals, and it is crucial for intracellular processes regulating energy production, development, antioxidant defence and immune response [34]. Indeed, uptake, retention, and excretion of Mn2+ are tightly regulated due to its key role as cofactor of a variety of enzymes, such as Mn2+ superoxide dismutase (SOD), glutamine synthetase (GS), arginase, and pyruvate carboxylase. Intracellular Mn2+ homeostasis is regulated through non-exclusive metal-ion transporters, including divalent metal transporter A (DMT1), calcium channel-dependent protein, and metal-transporter-family proteins like Zip8 and Zip14 (Figure 1) [35,36,37].
Mn2+ is present in all compartments. However, most intracellular Mn2+ is stored in the Golgi apparatus and in the mitochondria [38]. When supplemented at high concentrations in culture media, Mn2+ accumulates in the mitochondria and in the nucleus, impairing mitochondrial activity and inducing DNA damage (Figure 1) [39]. In HeLa and in THP1 cells, Mn2+ release from the mitochondria and Golgi to the cytosol increases the sensitivity of the DNA sensor cGAS and the downstream adaptor protein STING, which, in turn, induces type I IFNs and cytokine production [40]. However, its function in both adaptive and innate immunity has been poorly investigated. A recent study has shown that Mn2+ supplementation improved tumour-specific antigen presentation acting on macrophages and dendritic cell maturation [41]. As a consequence, both dendritic cells and macrophage maturation contribute to CD8+ T-cell activation and better tumour control in a B16 melanoma model. Congruently, as first reported in the 1980s, Mn2+ supplementation leads to a significant increase in the number of TILs [41,42,43]. In addition, Mn2+ treatment increases cytokine production capacity in both CD8+ T and NK infiltrating tumours, while depletion of Mn2+ from the diet results in a reduced T cells differentiation and increased tumour size. Mn2+ anti-tumoural activities, such as increased TIL number, function, or shifting macrophage polarization to a more anti-tumoural phenotype, has been exploited in combination with conventional chemotherapy and immune checkpoint blockade therapy to boost anti-tumour response [44,45]. Indeed, Mn2+ can induce type I IFN production and dendritic cell maturation, similarly to STING agonist, making Mn2+ a potential novel adjuvant for cancer vaccines (Figure 1) [41,46]. Taken together, due to its promiscuous effect in stimulating both myeloid (dendritic cells) and lymphoid (CD8+ T cells and NK) compartments, Mn2+ metabolism emerges as a potential novel target for anti-tumour therapies.
4. Zinc
Zinc (Zn2+) is the second most abundant trace metal in the human body after iron. It is an essential component of several proteins [47] and participates in a variety of cellular processes, including cell proliferation, differentiation, redox regulation, and apoptosis. [48,49,50]. Zn2+ is mostly intracellular and conjugated to zinc-binding proteins [51]. Zn2+ homeostasis is tightly controlled by a variety of transporters and chaperone proteins called metallothioneins [52]. Importantly, Zn2+ regulates both innate and adaptive immunity [53,54]. Chronic Zn2+ deficiency impairs proper T-cell development, differentiation, and function [55]. Indeed, Zn2+ deficiency reduces expression of the cytotoxic T lymphocyte marker CD73 in patients with sickle cell anaemia [56] and leads to a significant reduction of thymus-derived hormone thymulin, regulating T-cell differentiation and maturation [57]. Zn2+ is also involved in T-cell activation and differentiation, being involved in the interaction between the short cytoplasmatic domain of CD4 or CD8α with p56lck (Figure 1) [58]. Upon TCR signalling, cytoplasmatic Zn2+ concentration increases within 1 min due to the rapid upregulation of the zinc transporter Zip6 (Figure 1) [59], leading to Zap70 phosphorylation and sustained calcium influx, which supports T-cell proliferation in suboptimal conditions [59]. Moreover, inhibition of Zn2+ influx through Zip6 silencing impairs T-cell activation, resulting in reduced expression of activation markers, such as CD25 and CD69, and reduced production of cytokines, such as IL-2 [60]. Similarly, Zn2+ depletion blocks the ERK1/2 and PI3K/Akt pathways, inhibiting T-cell activation [61,62]. While the direct effect of Zn2+ on tumour growth has not been elucidated yet, few studies have indicated a potential immunosuppressive role of Zn2+ both in vitro [63] and in the tumour microenvironment [64]. Notably, it has been shown that a Zn2+-rich diet can promote prostate carcinogenesis and increase the risk of prostate cancer progression [65].
Finally, in the B16F10 murine melanoma model, it has been observed that TILs upregulate metallothieins and zinc-finger transcription factors, such as GATA-3 and IKZF2 (Figure 1) [66], indicating a possible role of Zn2+ homeostasis in T-cell differentiation and exhaustion within the TME. The evidence gathered so far places Zn2+ metabolism as a potential target to dampen the immunosuppressive mechanism adopted by cancer cells. However, how Zn2+ acts as an immunosuppressive factor and which zinc-dependent proteins are involved in the process has yet to be defined.
5. Selenium
Selenium (Se2−) is taken up through the diet in either organic forms, seleno-L-methionine (SeMet) and seleno-L-cysteine (SeCys), or as inorganic forms, selenide and selenite, which are all ultimately metabolized within mammalian cells into SeCys. Indeed, SeCys, also known as the 21st amino acid, is an essential element of selenoprotein catalytic sites [67,68]. In humans, 25 genes encoding for selenoproteins have been identified, with most of them involved in the regulation of redox balance and protection against oxidative stress. Enzymatic glutathione peroxidases (GPXs), thioredoxin reductases (TXNRDs), or iodothyronine deiodinases (DIOs) (Figure 1), as well as the non-enzymatic selenoprotein P (SELENOP) and selenoprotein K (SELENOK), are amongst the most important selenoproteins [67,68]. SELENOP is known to be one of the most important Se2− carriers in circulation. On the other hand, the molecular mechanisms involved in Se2− cellular uptake have not yet been completely elucidated [67,68].
In an immunological context, Se2− supplementation boosts immune function via regulation of selenoprotein levels. Shrimali et al. (2008) generated mice with T-cell-specific ablation of the SeCys tRNA[Ser]Sec and described that loss of selenoprotein synthesis in T cells leads to ROS hyperproduction and suppression of T-cell expansion after TCR stimulation [69]. Furthermore, another report by Verma et al. (2011) showed that T cells lacking SELENOK, an ER transmembrane protein that regulates Ca2+ flux, display reduced Ca2+ signalling during T-cell activation and, subsequently, defective immune responses during viral infection [70]. These investigations, together with epidemiological studies showing that Se-deficient diets are associated with a loss of immunocompetence [71], indicate that Se2− levels and selenoproteins are essential for appropriate regulation of T-cell-mediated immunity.
Even though the role of Se2− in T-cell anti-tumour responses has been poorly elucidated, a combination of preclinical and clinical studies indicate that increased Se2− serum levels are associated with overall improved survival in patients [72]. In particular, sodium-selenite-enriched diets have shown to reduce tumour size in mice by enhancing the cytotoxicity of both CD8+ T cells and NK cells, suggesting a direct effect on anti-tumour immunity [73]. Importantly, selenoprotein GPX4 has been described as a fate and functional determinant of TILs. Specifically, decreased GPX4 expression in TILs is associated with an accumulation of oxidized lipids that induces T-cell death via ferroptosis [74]. GPX4-mediated regulation of ferroptosis is also a survival mechanisms of cancer cells, which can increase GPX4 levels through the induction of selenophosphate synthetase 2 (SEPHS2), an enzyme involved in SeCys biosynthesis [75]. Altogether, these reports indicate that cancer progression is influenced by Se2− levels, by both affecting cancer-cell survival and immune-cell function, opening the way to Se2− modulation as a possible future strategy to boost cancer immunotherapy.
6. Magnesium
Magnesium (Mg2+) is the most abundant divalent cation in eukaryotic cells (~10–30 mM). While only ~5% of intracellular Mg2+ is found free ([Mg2+]i), most of it is complexed to ATP or bonded to other molecules functioning as a cofactor. In T cells, [Mg2+]i levels are finely regulated by the ion channels MAGT1, TRPM7, mediating Mg2+ influx, and SLC41A1, mediating Mg2+ efflux through Na+ exchange (Figure 1) [76].
T-cell antigen recognition is followed by a rapid transient Mg2+ influx, which acts as second messenger in TCR signalling [77,78]. Specifically, Mg2+ directly interacts with IL-2-inducible T-cell kinase (ITK) promoting its activation (Figure 1) [79]. On the contrary, lymphocyte activation in low [Mg2+] conditions limits CD69 and CD25 upregulation, Ca2+ influx, and cell proliferation [79]. Indeed, mice fed Mg2+-restricted diets and infected with influenza A virus have reduced numbers of virus-specific T cells [79]. Furthermore, patients carrying loss-of-function mutations in MAGT1 gene develop a rare primary immunodeficiency known as XMEN disease (‘X-linked immunodeficiency with Mg2+ defect, Epstein-Barr virus (EBV) infection, and neoplasia’). T cells from patients with XMEN disease exhibit limited Mg2+ influx and recapitulate most of the features observed in low [Mg2+] conditions (i.e., deficient TCR signalling, Ca2+ influx, T-cell activation and proliferation) [77,78,80]. Interestingly, MAGT1 localizes in the ER, where it mediates N-linked glycosylation, a post-translational modification influencing protein half-life (Figure 1) [81,82]. In XMEN patients, CD8+ T cells lose CD70 and NKG2D expression due to its diminished glycosylation, which has been linked to an increased susceptibility to EBV infection [81,82,83].
Mg2+ is important for the stabilisation of DNA structure and operates as a cofactor for enzymes involved in DNA repair, suggesting that Mg2+ deprivation might lead to accumulation of DNA damage and carcinogenesis. Accordingly, low Mg2+ intake is associated with higher risk of pancreatic, lung, and breast cancer [84,85,86], while alterations in MAGT1 and SLC41A1 expression have been associated with aggressive colorectal cancers and pancreatic ductal adenocarcinomas (PDAC) [87,88]. Interestingly, Diao et al. (2017) described that chronically activated CD8+ T cells in hepatitis B virus (HBV)-infected patients show a decline in [Mg2+]i and MAGT1 expression associated with PD-1 upregulation and loss of NKG2D [89]. To date, this phenotype has not been identified in the exhausted TILs. However, the necrosis-derived release of ions [15] added to the alterations in the expression of Mg2+ transporters in cancer cells suggests that Mg2+ levels might vary in the TME and thus have an immunomodulatory role within the TME.
7. Iron
Iron (Fe2+) is an essential element involved in several enzymatic reactions and cellular processes, such as proliferation, DNA synthesis, metabolism [90], and immune function [91,92]. For this reason, Fe2+ levels are tightly regulated. Most of the Fe2+ delivered to the cells is bound to transferrin protein (Tf). The Tf-iron complex is taken up by the cells through transferrin receptor (CD71) endocytosis. Notably, T cells can also take up Fe2+ via non-specific metal-ion transporters, like DMT-1 and ZIP-8 (Figure 1) [93,94]. During activation, T cells increase expression of CD71 (Figure 1) [95]. On the contrary, anergic T cells have reduced expression of CD71 [96]. Reduced Fe2+ uptake due to defective Tf-receptor endocytosis impairs T-cell function and results in severe immunodeficiency [97]. Furthermore, reduced intracellular Fe2+ levels impaired CD25 expression and IL-2R signalling and compromised mitochondrial function in T cells (Figure 1). Notably, Fe2+ supplementation in an iron-deficiency culture system restore proper mitochondrial potential and biogenesis [98].
A recent report revealed a role of Fe2+ in an inflammatory context. In autoinflammatory diseases, iron deposition is frequently observed. According to Wang et al., Fe2+ promotes proinflammatory cytokine production in immune cells, including T cells [99]. On the other hand, it has been reported that Fe2+ is released by tumour-associated macrophages (TAMs) and tumour-associated neutrophils (TANs) in the TME. In this scenario, Fe2+ might sustain TAMs and TANs in supporting cancer progression and impairing T and B cell activity by inducing cell death. Fe2+ has been involved in the induction of ferroptosis by mechanisms that are still poorly understood [100]. Although the impact of Fe2+ secretion by TAMs and TANs is not directly proven, it is likely that high Fe2+ levels may contribute to the induction of ferroptosis in T cells and cancer cells. Furthermore, TAMs and TANs can also impair proper APC maturation and antigen presentation [101,102]. In light of the cited reports, altering Fe2+ concentration in the tumour microenvironment could be a promising approach to improve current therapies [102,103].
8. Conclusions and Perspectives
Many clinical trials have demonstrated therapeutic efficacy of T-cell based immunotherapy, which exploits the capacity of T cells to recognize and kill a specific target, including cancer cells [104]. While it is well established that metal ions can regulate immune-system and T-cell function and metabolism, it is not clear how the manipulation of ion concentrations in the TME can improve T-cell activity and possibly T-cell-based immunotherapy. Recent studies cited in this review underline the role of ions in shaping T-cell capacity controlling tumour growth (Figure 2). Although there is an increased interest in understanding the role of ions in the context of the tumour microenvironment [15,105], the complex interplay between ion concentration, immune cells, and cancer cells has not been sufficiently investigated. Recently, cutting-edge gene-targeting technologies, like CRISPR, have been adopted to reveal processes involved in nutrient sensing and consumption in T cells in vivo [106]. Implementing these approaches to ion channels and ion-dependent enzymes would provide a deeper view on the molecular processes orchestrated by specific ions and on how these processes influence T-cell activity. Another challenging aspect is the development of strategies capable of locally altering ionic concentration in the TME. While adequate diet and nutrient supplementation can modulate ion blood levels, it is not known whether a systemic change in ion intake might lead to a local effect. Further studies are needed to elucidate whether a tailored supplementation of a given ion would be adequate to optimize immune function in the TME. Another possibility would be to design methods to locally deliver or deplete a specific ion. Canale et al. used engineered bacteria to locally deliver arginine in the TME, enabling metabolic modulation of the tumour microenvironment and improving adaptive immune responses against cancer cells [107]. A similar approach suited for ions would provide a tool to alter ion concentration only in the TME. Indeed, a large body of evidence has shown how metabolism and nutrient consumption are key factors for a proper and robust anti-tumour immune response [12,108]. In this context, elucidating the role of ions in both homeostatic and anti-tumour T-cell activity might help in the development of novel strategies aimed to improve T-cell-based therapies.
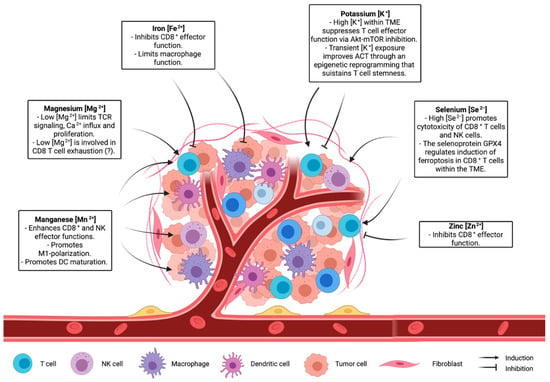
Figure 2.
The role of ions in shaping the immune landscape of the microenvironment.
Author Contributions
P.G. and H.C.H. performed the primary literature search and wrote part of the manuscript. A.Z., M.S. and N.V. wrote part of the manuscript. P.G., H.C.H. and N.V. defined the topic and guided during the writing. All authors have read and agreed to the published version of the manuscript.
Funding
The N.V.’s laboratory is supported by the Swiss Cancer League Grant (KFS-4993-02-2020-R).
Acknowledgments
All the figures were created with BioRender.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buck, M.D.; O’Sullivan, D.; Pearce, E.L. T cell metabolism drives immunity. J. Exp. Med. 2015, 212, 1345–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buck, M.D.; Sowell, R.T.; Kaech, S.M.; Pearce, E.L. Metabolic Instruction of Immunity. Cell 2017, 169, 570–586. [Google Scholar] [CrossRef] [PubMed]
- Geltink, R.I.K.; Kyle, R.L.; Pearce, E.L. Unraveling the Complex Interplay between T Cell Metabolism and Function. Annu. Rev. Immunol. 2018, 36, 461–488. [Google Scholar] [CrossRef]
- Anderson, K.G.; Stromnes, I.M.; Greenberg, P.D. Obstacles Posed by the Tumor Microenvironment to T cell Activity: A Case for Synergistic Therapies. Cancer Cell 2017, 31, 311–325. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, A.; Rathmell, J.C. Metabolic Barriers to T-cell function in Tumors. J. Immunol. 2018, 200, 400. [Google Scholar] [CrossRef]
- DePeaux, K.; Delgoffe, G.M. Metabolic barriers to cancer immunotherapy. Nat. Rev. Immunol. 2021, 21, 785–797. [Google Scholar] [CrossRef]
- Hope, H.C.; Salmond, R.J. Targeting the tumor microenvironment and T cell metabolism for effective cancer immunotherapy. Eur. J. Immunol. 2019, 49, 1147–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Sullivan, D.; Sanin, D.E.; Pearce, E.J.; Pearce, E.L. Metabolic interventions in the immune response to cancer. Nat. Rev. Immunol. 2019, 19, 324–335. [Google Scholar] [CrossRef]
- Hope, H.C.; Salmond, R.J. The Role of Non-essential Amino Acids in T Cell Function and Anti-tumour Immunity. Arch. Immunol. Ther. Exp. 2021, 69, 29. [Google Scholar] [CrossRef]
- Reinfeld, B.I.; Rathmell, W.K.; Kim, T.K.; Rathmell, J.C. The therapeutic implications of immunosuppressive tumor aerobic glycolysis. Cell Mol. Immunol. 2021. [Google Scholar] [CrossRef]
- Kelly, B.; Pearce, E.L. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wenes, M.; Romero, P.; Huang, S.C.; Fendt, S.M.; Ho, P.C. Navigating metabolic pathways to enhance antitumour immunity and immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Lukey, M.J.; Katt, W.P.; Cerione, R.A. Targeting amino acid metabolism for cancer therapy. Drug Discov. Today 2017, 22, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Bai, L.; Li, W.; Zeng, T.; Tian, H.; Cui, J. Targeting T cell metabolism in the tumor microenvironment: An anti-cancer therapeutic strategy. J. Exp. Clin. Cancer Res. 2019, 38, 403. [Google Scholar] [CrossRef] [PubMed]
- Eil, R.; Vodnala, S.K.; Clever, D.; Klebanoff, C.A.; Sukumar, M.; Pan, J.H.; Palmer, D.C.; Gros, A.; Yamamoto, T.N.; Patel, S.J.; et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 2016, 537, 539–543. [Google Scholar] [CrossRef] [Green Version]
- Litan, A.; Langhans, S.A. Cancer as a channelopathy: Ion channels and pumps in tumor development and progression. Front. Cell. Neurosci. 2015, 9, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurusamy, D.; Clever, D.; Eil, R.; Restifo, N.P. Novel “Elements” of Immune Suppression within the Tumor Microenvironment. Cancer Immunol. Res. 2017, 5, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Ong, S.T.; Ng, A.S.; Ng, X.R.; Zhuang, Z.; Wong, B.H.S.; Prasannan, P.; Kok, Y.J.; Bi, X.; Shim, H.; Wulff, H.; et al. Extracellular K(+) Dampens T Cell Functions: Implications for Immune Suppression in the Tumor Microenvironment. Bioelectricity 2019, 1, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Feske, S.; Wulff, H.; Skolnik, E.Y. Ion channels in innate and adaptive immunity. Annu. Rev. Immunol. 2015, 33, 291–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.-R.; Byeon, Y.; Kim, D.; Park, S.-G. Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Exp. Mol. Med. 2020, 52, 750–761. [Google Scholar] [CrossRef]
- Cahalan, M.D.; Chandy, K.G. The functional network of ion channels in T lymphocytes. Immunol. Rev. 2009, 231, 59–87. [Google Scholar] [CrossRef] [Green Version]
- Beeton, C.; Wulff, H.; Standifer, N.E.; Azam, P.; Mullen, K.M.; Pennington, M.W.; Kolski-Andreaco, A.; Wei, E.; Grino, A.; Counts, D.R.; et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. USA 2006, 103, 17414–17419. [Google Scholar] [CrossRef] [Green Version]
- Panyi, G.; Vámosi, G.; Bacsó, Z.; Bagdány, M.; Bodnár, A.; Varga, Z.; Gáspár, R.; Mátyus, L.; Damjanovich, S. Kv1.3 potassium channels are localized in the immunological synapse formed between cytotoxic and target cells. Proc. Natl. Acad. Sci. USA 2004, 101, 1285–1290. [Google Scholar] [CrossRef] [Green Version]
- Sim, J.H.; Kim, K.S.; Park, H.; Kim, K.J.; Lin, H.; Kim, T.J.; Shin, H.M.; Kim, G.; Lee, D.S.; Park, C.W.; et al. Differentially Expressed Potassium Channels Are Associated with Function of Human Effector Memory CD8(+) T Cells. Front. Immunol. 2017, 8, 859. [Google Scholar] [CrossRef] [Green Version]
- Chimote, A.A.; Balajthy, A.; Arnold, M.J.; Newton, H.S.; Hajdu, P.; Qualtieri, J.; Wise-Draper, T.; Conforti, L. A defect in KCa3.1 channel activity limits the ability of CD8(+) T cells from cancer patients to infiltrate an adenosine-rich microenvironment. Sci. Signal. 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Di, L.; Srivastava, S.; Zhdanova, O.; Ding, Y.; Li, Z.; Wulff, H.; Lafaille, M.; Skolnik, E.Y. Inhibition of the K+ channel KCa3.1 ameliorates T cell-mediated colitis. Proc. Natl. Acad. Sci. USA 2010, 107, 1541–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Wang, T.; Gocke, A.R.; Nath, A.; Zhang, H.; Margolick, J.B.; Whartenby, K.A.; Calabresi, P.A. Blockade of Kv1.3 potassium channels inhibits differentiation and granzyme B secretion of human CD8+ T effector memory lymphocytes. PLoS ONE 2013, 8, e54267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panyi, G.; Beeton, C.; Felipe, A. Ion channels and anti-cancer immunity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130106. [Google Scholar] [CrossRef] [Green Version]
- Conforti, L.; Petrovic, M.; Mohammad, D.; Lee, S.; Ma, Q.; Barone, S.; Filipovich, A.H. Hypoxia regulates expression and activity of Kv1.3 channels in T lymphocytes: A possible role in T cell proliferation. J. Immunol. 2003, 170, 695–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimote, A.A.; Hajdu, P.; Sfyris, A.M.; Gleich, B.N.; Wise-Draper, T.; Casper, K.A.; Conforti, L. Kv1.3 Channels Mark Functionally Competent CD8+ Tumor-Infiltrating Lymphocytes in Head and Neck Cancer. Cancer Res. 2017, 77, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, H.S.; Gawali, V.S.; Chimote, A.A.; Lehn, M.A.; Palackdharry, S.M.; Hinrichs, B.H.; Jandarov, R.; Hildeman, D.; Janssen, E.M.; Wise-Draper, T.M.; et al. PD1 blockade enhances K(+) channel activity, Ca(2+) signaling, and migratory ability in cytotoxic T lymphocytes of patients with head and neck cancer. J. Immunother. Cancer 2020, 8, e000844. [Google Scholar] [CrossRef] [PubMed]
- Vodnala, S.K.; Eil, R.; Kishton, R.J.; Sukumar, M.; Yamamoto, T.N.; Ha, N.H.; Lee, P.H.; Shin, M.; Patel, S.J.; Yu, Z.; et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 2019, 363. [Google Scholar] [CrossRef]
- Wang, X.; Huynh, C.; Urak, R.; Weng, L.; Walter, M.; Lim, L.; Vyas, V.; Chang, W.C.; Aguilar, B.; Brito, A.; et al. The Cerebroventricular Environment Modifies CAR T Cells for Potent Activity against Both Central Nervous System and Systemic Lymphoma. Cancer Immunol. Res. 2021, 9, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Bornhorst, J.; Aschner, M. Manganese metabolism in humans. Front. Biosci. 2018, 23, 1655–1679. [Google Scholar] [CrossRef] [Green Version]
- Crossgrove, J.S.; Yokel, R.A. Manganese distribution across the blood-brain barrier III. The divalent metal transporter-1 is not the major mechanism mediating brain manganese uptake. Neurotoxicology 2004, 25, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Aydemir, F.; Nam, H.; Knutson, M.D.; Cousins, R.J. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13612–13617. [Google Scholar] [CrossRef] [Green Version]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese Is Essential for Neuronal Health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef]
- Carmona, A.; Roudeau, S.; Perrin, L.; Veronesi, G.; Ortega, R. Environmental manganese compounds accumulate as Mn(II) within the Golgi apparatus of dopamine cells: Relationship between speciation, subcellular distribution, and cytotoxicity. Metallomics 2014, 6, 822–832. [Google Scholar] [CrossRef]
- Morello, M.; Canini, A.; Mattioli, P.; Sorge, R.P.; Alimonti, A.; Bocca, B.; Forte, G.; Martorana, A.; Bernardi, G.; Sancesario, G. Sub-cellular localization of manganese in the basal ganglia of normal and manganese-treated rats An electron spectroscopy imaging and electron energy-loss spectroscopy study. Neurotoxicology 2008, 29, 60–72. [Google Scholar] [CrossRef]
- Wang, C.; Guan, Y.; Lv, M.; Zhang, R.; Guo, Z.; Wei, X.; Du, X.; Yang, J.; Li, T.; Wan, Y.; et al. Manganese Increases the Sensitivity of the cGAS-STING Pathway for Double-Stranded DNA and Is Required for the Host Defense against DNA Viruses. Immunity 2018, 48, 675–687.e7. [Google Scholar] [CrossRef] [Green Version]
- Lv, M.; Chen, M.; Zhang, R.; Zhang, W.; Wang, C.; Zhang, Y.; Wei, X.; Guan, Y.; Liu, J.; Feng, K.; et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020, 30, 966–979. [Google Scholar] [CrossRef]
- Rogers, R.R.; Garner, R.J.; Riddle, M.M.; Luebke, R.W.; Smialowicz, R.J. Augmentation of murine natural killer cell activity by manganese chloride. Toxicol. Appl. Pharmacol. 1983, 70, 7–17. [Google Scholar] [CrossRef]
- Smialowicz, R.J.; Rogers, R.R.; Riddle, M.M.; Luebke, R.W.; Rowe, D.G.; Garner, R.J. Manganese chloride enhances murine cell-mediated cytotoxicity: Effects on natural killer cells. J. Immunopharmacol. 1984, 6, 1–23. [Google Scholar] [CrossRef]
- Yang, G.; Xu, L.; Chao, Y.; Xu, J.; Sun, X.; Wu, Y.; Peng, R.; Liu, Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017, 8, 902. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Liu, T.; Shi, C.; Zhang, X.; Chen, X. Bioconjugated Manganese Dioxide Nanoparticles Enhance Chemotherapy Response by Priming Tumor-Associated Macrophages toward M1-like Phenotype and Attenuating Tumor Hypoxia. ACS Nano 2016, 10, 633–647. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Liu, Y.; Teo, H.Y.; Hanafi, Z.B.; Mei, Y.; Zhu, Y.; Chua, Y.L.; Lv, M.; Jiang, Z.; Liu, H. Manganese enhances the antitumor function of CD8(+) T cells by inducing type I interferon production. Cell Mol. Immunol. 2021, 18, 1571–1574. [Google Scholar] [CrossRef]
- Krezel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haase, H.; Rink, L. Multiple impacts of zinc on immune function. Metallomics 2014, 6, 1175–1180. [Google Scholar] [CrossRef]
- Bellomo, E.; Hogstrand, C.; Maret, W. Redox and zinc signalling pathways converging on protein tyrosine phosphatases. Free Radic. Biol. Med. 2014, 75 (Suppl. S1), S9. [Google Scholar] [CrossRef] [PubMed]
- Truong-Tran, A.Q.; Carter, J.; Ruffin, R.E.; Zalewski, P.D. The role of zinc in caspase activation and apoptotic cell death. Biometals 2001, 14, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Rink, L.; Gabriel, P. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Haase, H.; Rink, L. Zinc signals and immune function. Biofactors 2014, 40, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Lee, W.W. Regulatory Role of Zinc in Immune Cell Signaling. Mol. Cells 2021, 44, 335–341. [Google Scholar] [CrossRef]
- King, L.E.; Frentzel, J.W.; Mann, J.J.; Fraker, P.J. Chronic zinc deficiency in mice disrupted T cell lymphopoiesis and erythropoiesis while B cell lymphopoiesis and myelopoiesis were maintained. J. Am. Coll. Nutr. 2005, 24, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Beck, F.W.; Kaplan, J.; Fine, N.; Handschu, W.; Prasad, A.S. Decreased expression of CD73 (ecto-5’-nucleotidase) in the CD8+ subset is associated with zinc deficiency in human patients. J. Lab. Clin. Med. 1997, 130, 147–156. [Google Scholar] [CrossRef]
- Saha, A.R.; Hadden, E.M.; Hadden, J.W. Zinc induces thymulin secretion from human thymic epithelial cells in vitro and augments splenocyte and thymocyte responses in vivo. Int. J. Immunopharmacol. 1995, 17, 729–733. [Google Scholar] [CrossRef]
- Lin, R.S.; Rodriguez, C.; Veillette, A.; Lodish, H.F. Zinc is essential for binding of p56(lck) to CD4 and CD8alpha. J. Biol. Chem. 1998, 273, 32878–32882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Lee, W.W.; Tomar, D.; Pryshchep, S.; Czesnikiewicz-Guzik, M.; Lamar, D.L.; Li, G.; Singh, K.; Tian, L.; Weyand, C.M.; et al. Regulation of T cell receptor signaling by activation-induced zinc influx. J. Exp. Med. 2011, 208, 775–785. [Google Scholar] [CrossRef] [Green Version]
- Colomar-Carando, N.; Meseguer, A.; Company-Garrido, I.; Jutz, S.; Herrera-Fernandez, V.; Olvera, A.; Kiefer, K.; Brander, C.; Steinberger, P.; Vicente, R. Zip6 Transporter Is an Essential Component of the Lymphocyte Activation Machinery. J. Immunol. 2019, 202, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Kaltenberg, J.; Plum, L.M.; Ober-Blobaum, J.L.; Honscheid, A.; Rink, L.; Haase, H. Zinc signals promote IL-2-dependent proliferation of T cells. Eur. J. Immunol. 2010, 40, 1496–1503. [Google Scholar] [CrossRef]
- Plum, L.M.; Brieger, A.; Engelhardt, G.; Hebel, S.; Nessel, A.; Arlt, M.; Kaltenberg, J.; Schwaneberg, U.; Huber, M.; Rink, L.; et al. PTEN-inhibition by zinc ions augments interleukin-2-mediated Akt phosphorylation. Metallomics 2014, 6, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Wellinghausen, N.; Driessen, C.; Rink, L. Stimulation of human peripheral blood mononuclear cells by zinc and related cations. Cytokine 1996, 8, 767–771. [Google Scholar] [CrossRef] [Green Version]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitzmann, M.F.; Stampfer, M.J.; Wu, K.; Colditz, G.A.; Willett, W.C.; Giovannucci, E.L. Zinc supplement use and risk of prostate cancer. J. Natl. Cancer Inst. 2003, 95, 1004–1007. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Wang, C.; Cong, L.; Marjanovic, N.D.; Kowalczyk, M.S.; Zhang, H.; Nyman, J.; Sakuishi, K.; Kurtulus, S.; Gennert, D.; et al. A Distinct Gene Module for Dysfunction Uncoupled from Activation in Tumor-Infiltrating T Cells. Cell 2017, 171, 1221–1223. [Google Scholar] [CrossRef] [Green Version]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Hoffmann, P.R. Selenoproteins as regulators of T cell proliferation, differentiation, and metabolism. Semin. Cell Dev. Biol. 2021, 115, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Shrimali, R.K.; Irons, R.D.; Carlson, B.A.; Sano, Y.; Gladyshev, V.N.; Park, J.M.; Hatfield, D.L. Selenoproteins mediate T cell immunity through an antioxidant mechanism. J. Biol. Chem. 2008, 283, 20181–20185. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Hoffmann, F.W.; Kumar, M.; Huang, Z.; Roe, K.; Nguyen-Wu, E.; Hashimoto, A.S.; Hoffmann, P.R. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J. Immunol. 2011, 186, 2127–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Bjornstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef]
- Petrie, H.T.; Klassen, L.W.; Klassen, P.S.; O’Dell, J.R.; Kay, H.D. Selenium and the immune response: 2. Enhancement of murine cytotoxic T-lymphocyte and natural killer cell cytotoxicity in vivo. J. Leukoc. Biol. 1989, 45, 215–220. [Google Scholar] [CrossRef]
- Xu, S.; Chaudhary, O.; Rodriguez-Morales, P.; Sun, X.; Chen, D.; Zappasodi, R.; Xu, Z.; Pinto, A.F.M.; Williams, A.; Schulze, I.; et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8(+) T cells in tumors. Immunity 2021, 54, 1561–1577.e7. [Google Scholar] [CrossRef]
- Carlisle, A.E.; Lee, N.; Matthew-Onabanjo, A.N.; Spears, M.E.; Park, S.J.; Youkana, D.; Doshi, M.B.; Peppers, A.; Li, R.; Joseph, A.B.; et al. Selenium detoxification is required for cancer-cell survival. Nat. Metab. 2020, 2, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Chaigne-Delalande, B.; Lenardo, M.J. Divalent cation signaling in immune cells. Trends Immunol. 2014, 35, 332–344. [Google Scholar] [CrossRef] [Green Version]
- Li, F.Y.; Chaigne-Delalande, B.; Kanellopoulou, C.; Davis, J.C.; Matthews, H.F.; Douek, D.C.; Cohen, J.I.; Uzel, G.; Su, H.C.; Lenardo, M.J. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 2011, 475, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Y.; Lenardo, M.J.; Chaigne-Delalande, B. Loss of MAGT1 abrogates the Mg2+ flux required for T cell signaling and leads to a novel human primary immunodeficiency. Magnes. Res. 2011, 24, S109–S114. [Google Scholar] [CrossRef]
- Kanellopoulou, C.; George, A.B.; Masutani, E.; Cannons, J.L.; Ravell, J.C.; Yamamoto, T.N.; Smelkinson, M.G.; Jiang, P.D.; Matsuda-Lennikov, M.; Reilley, J.; et al. Mg(2+) regulation of kinase signaling and immune function. J. Exp. Med. 2019, 216, 1828–1842. [Google Scholar] [CrossRef] [PubMed]
- Ravell, J.C.; Chauvin, S.D.; He, T.; Lenardo, M. An Update on XMEN Disease. J. Clin. Immunol. 2020, 40, 671–681. [Google Scholar] [CrossRef]
- Matsuda-Lennikov, M.; Biancalana, M.; Zou, J.; Ravell, J.C.; Zheng, L.; Kanellopoulou, C.; Jiang, P.; Notarangelo, G.; Jing, H.; Masutani, E.; et al. Magnesium transporter 1 (MAGT1) deficiency causes selective defects in N-linked glycosylation and expression of immune-response genes. J. Biol. Chem. 2019, 294, 13638–13656. [Google Scholar] [CrossRef]
- Ravell, J.C.; Matsuda-Lennikov, M.; Chauvin, S.D.; Zou, J.; Biancalana, M.; Deeb, S.J.; Price, S.; Su, H.C.; Notarangelo, G.; Jiang, P.; et al. Defective glycosylation and multisystem abnormalities characterize the primary immunodeficiency XMEN disease. J. Clin. Investig. 2020, 130, 507–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaigne-Delalande, B.; Li, F.Y.; O’Connor, G.M.; Lukacs, M.J.; Jiang, P.; Zheng, L.; Shatzer, A.; Biancalana, M.; Pittaluga, S.; Matthews, H.F.; et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science 2013, 341, 186–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.Q.; Long, W.Q.; Mo, X.F.; Zhang, N.Q.; Luo, H.; Lin, F.Y.; Huang, J.; Zhang, C.X. Direct and indirect associations between dietary magnesium intake and breast cancer risk. Sci. Rep. 2019, 9, 5764. [Google Scholar] [CrossRef] [PubMed]
- Dibaba, D.; Xun, P.; Yokota, K.; White, E.; He, K. Magnesium intake and incidence of pancreatic cancer: The VITamins and Lifestyle study. Br. J. Cancer 2015, 113, 1615–1621. [Google Scholar] [CrossRef]
- Mahabir, S.; Wei, Q.; Barrera, S.L.; Dong, Y.Q.; Etzel, C.J.; Spitz, M.R.; Forman, M.R. Dietary magnesium and DNA repair capacity as risk factors for lung cancer. Carcinogenesis 2008, 29, 949–956. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Yang, Q.; Xie, L.; Qiu, Z.; Huang, Y.; Lin, Y.; Tu, L.; Cui, C. Overexpression of MAGT1 is associated with aggressiveness and poor prognosis of colorectal cancer. Oncol. Lett. 2019, 18, 3857–3862. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Cheng, C.S.; Zhu, X.Y.; Shen, Y.H.; Song, L.B.; Chen, H.; Chen, Z.; Liu, L.M.; Meng, Z.Q. Magnesium transporter protein solute carrier family 41 member 1 suppresses human pancreatic ductal adenocarcinoma through magnesium-dependent Akt/mTOR inhibition and bax-associated mitochondrial apoptosis. Aging 2019, 11, 2681–2698. [Google Scholar] [CrossRef]
- Diao, B.; Huang, X.; Guo, S.; Yang, C.; Liu, G.; Chen, Y.; Wu, Y. MAGT1-mediated disturbance of Mg(2+) homeostasis lead to exhausted of HBV-infected NK and CD8(+) T cells. Sci. Rep. 2017, 7, 13594. [Google Scholar] [CrossRef]
- Raza, M.; Chakraborty, S.; Choudhury, M.; Ghosh, P.C.; Nag, A. Cellular iron homeostasis and therapeutic implications of iron chelators in cancer. Curr. Pharm. Biotechnol. 2014, 15, 1125–1140. [Google Scholar] [CrossRef]
- Doherty, C.P. Host-pathogen interactions: The role of iron. J. Nutr. 2007, 137, 1341–1344. [Google Scholar] [CrossRef] [Green Version]
- Walker, E.M., Jr.; Walker, S.M. Effects of iron overload on the immune system. Ann. Clin. Lab. Sci. 2000, 30, 354–365. [Google Scholar] [PubMed]
- Mims, M.P.; Prchal, J.T. Divalent metal transporter 1. Hematology 2005, 10, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Jenkitkasemwong, S.; Duarte, S.; Sparkman, B.K.; Shawki, A.; Mackenzie, B.; Knutson, M.D. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J. Biol. Chem. 2012, 287, 34032–34043. [Google Scholar] [CrossRef] [Green Version]
- Motamedi, M.; Xu, L.; Elahi, S. Correlation of transferrin receptor (CD71) with Ki67 expression on stimulated human and mouse T cells: The kinetics of expression of T cell activation markers. J. Immunol. Methods 2016, 437, 43–52. [Google Scholar] [CrossRef]
- Zheng, Y.; Collins, S.L.; Lutz, M.A.; Allen, A.N.; Kole, T.P.; Zarek, P.E.; Powell, J.D. A Role for Mammalian Target of Rapamycin in Regulating T Cell Activation versus Anergy. J. Immunol. 2007, 178, 2163–2170. [Google Scholar] [CrossRef] [Green Version]
- Jabara, H.H.; Boyden, S.E.; Chou, J.; Ramesh, N.; Massaad, M.J.; Benson, H.; Bainter, W.; Fraulino, D.; Rahimov, F.; Sieff, C.; et al. A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nat. Genet. 2016, 48, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Yarosz, E.L.; Ye, C.; Kumar, A.; Black, C.; Choi, E.-K.; Seo, Y.-A.; Chang, C.-H. Cutting Edge: Activation-Induced Iron Flux Controls CD4 T Cell Proliferation by Promoting Proper IL-2R Signaling and Mitochondrial Function. J. Immunol. 2020, 204, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yin, W.; Zhu, L.; Li, J.; Yao, Y.; Chen, F.; Sun, M.; Zhang, J.; Shen, N.; Song, Y.; et al. Iron Drives T Helper Cell Pathogenicity by Promoting RNA-Binding Protein PCBP1-Mediated Proinflammatory Cytokine Production. Immunity 2018, 49, 80–92.e7. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Duan, C.; Dai, R.; Zeng, Y. Ferroptosis-mediated Crosstalk in the Tumor Microenvironment Implicated in Cancer Progression and Therapy. Front. Cell Dev. Biol. 2021, 9, 739392. [Google Scholar] [CrossRef]
- Shaw, J.; Chakraborty, A.; Nag, A.; Chattopadyay, A.; Dasgupta, A.K.; Bhattacharyya, M. Intracellular iron overload leading to DNA damage of lymphocytes and immune dysfunction in thalassemia major patients. Eur. J. Haematol. 2017, 99, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.M.; Richardson, K.L.; Kabir, T.D.; Trinder, D.; Ganss, R.; Leedman, P.J. Altered Iron Metabolism and Impact in Cancer Biology, Metastasis, and Immunology. Front. Oncol. 2020, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xie, Y.; Cao, L.; Yang, L.; Yang, M.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol. Cell Oncol. 2015, 2, e1054549. [Google Scholar] [CrossRef] [Green Version]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Feske, S.; Skolnik, E.Y.; Prakriya, M. Ion channels and transporters in lymphocyte function and immunity. Nat. Rev. Immunol. 2012, 12, 532–547. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Zhou, P.; Wei, J.; Long, L.; Shi, H.; Dhungana, Y.; Chapman, N.M.; Fu, G.; Saravia, J.; Raynor, J.L.; et al. In vivo CRISPR screening reveals nutrient signaling processes underpinning CD8(+) T cell fate decisions. Cell 2021, 184, 1245–1261.e21. [Google Scholar] [CrossRef] [PubMed]
- Canale, F.P.; Basso, C.; Antonini, G.; Perotti, M.; Li, N.; Sokolovska, A.; Neumann, J.; James, M.J.; Geiger, S.; Jin, W.; et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 2021. [Google Scholar] [CrossRef]
- Reinfeld, B.I.; Madden, M.Z.; Wolf, M.M.; Chytil, A.; Bader, J.E.; Patterson, A.R.; Sugiura, A.; Cohen, A.S.; Ali, A.; Do, B.T.; et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 2021, 593, 282–288. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).