Therapeutic Potential of Mesenchymal Stem Cells (MSCs) and MSC-Derived Extracellular Vesicles for the Treatment of Spinal Cord Injury
Abstract
1. Introduction
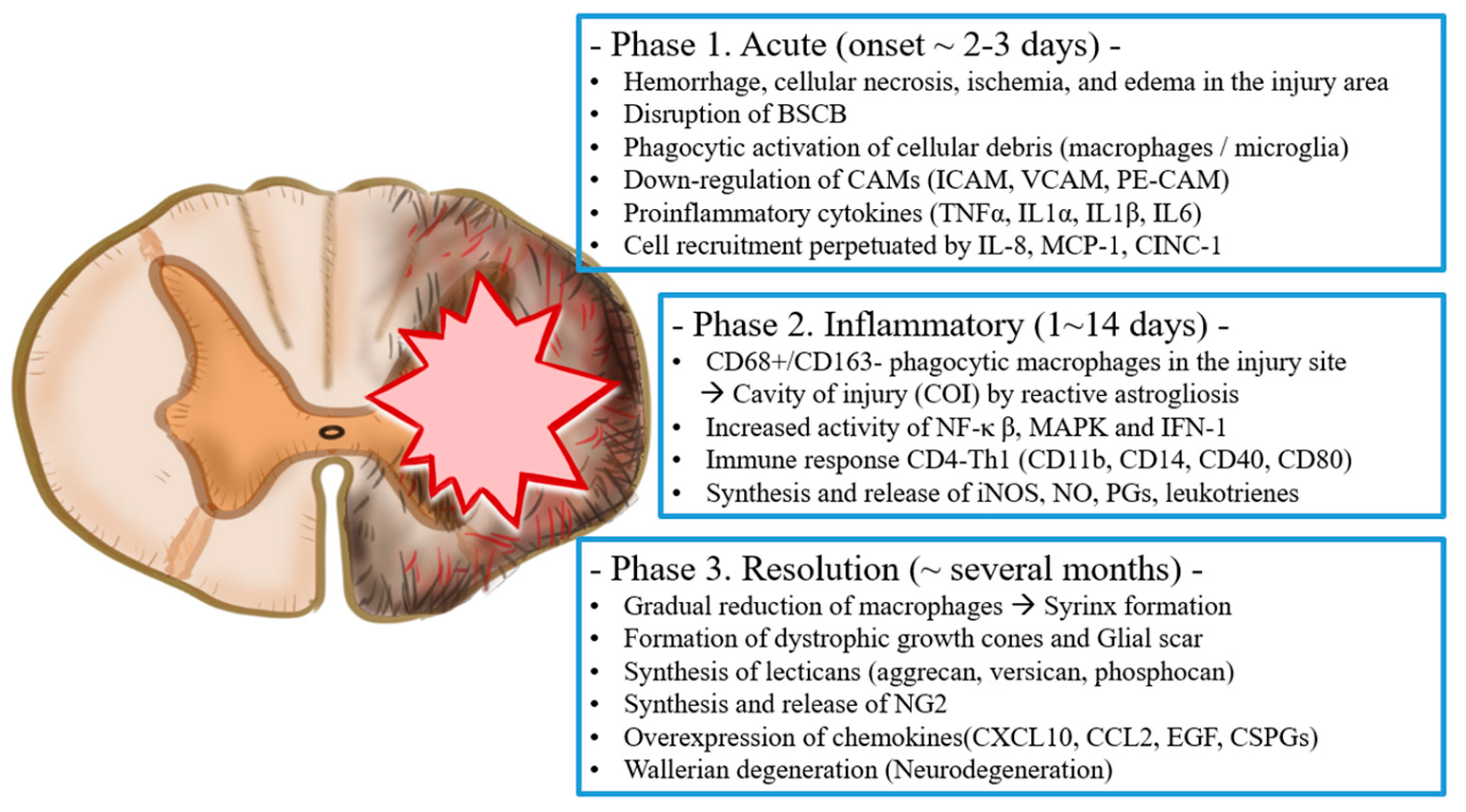
2. Pathophysiology of Spinal Cord Injury
3. Mesenchymal Stem Cells for the Potential Treatment of Spinal Cord Injury
3.1. Bone Marrow Mesenchymal Stem Cells
3.2. Umbilical Cord-Derived Mesenchymal Stem Cells
3.3. Adipose-Derived Mesenchymal Stem Cells
4. Why Should We Pay Attention to Extracellular Vesicles over Mesenchymal Stem Cells as a Therapeutic Source for Spinal Cord Injury?
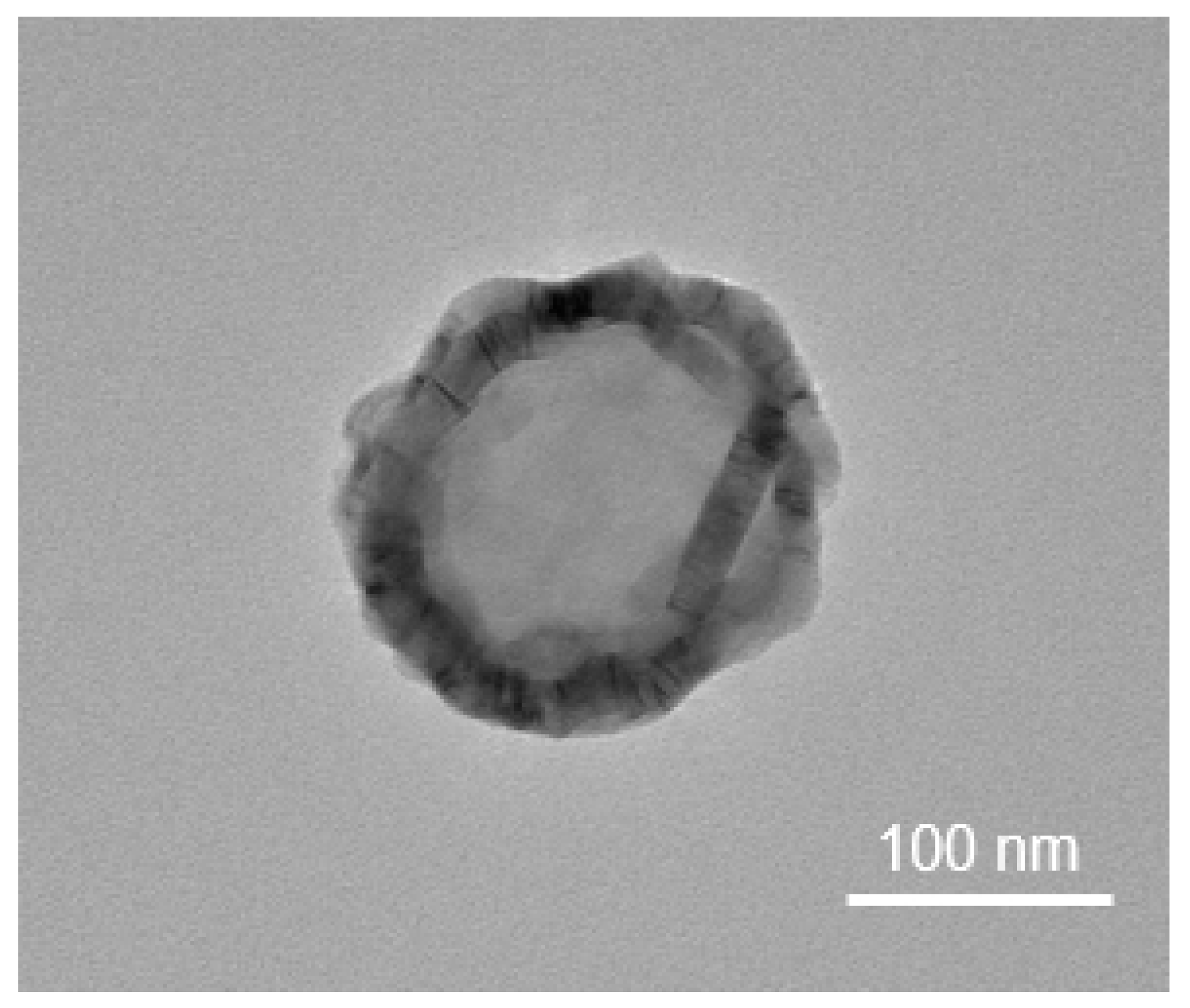
5. Overview and Characteristics of Extracellular Vesicles
6. Research Trials Using Extracellular Vesicles for Spinal Cord Injury
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aziz, I.; Ramli, M.D.C.; Zain, N.S.M.; Sanusi, J. Behavioral and Histopathological Study of Changes in Spinal Cord Injured Rats Supplemented withSpirulina platensis. Evid. Based Complement. Altern. Med. 2014, 2014, 871657. [Google Scholar] [CrossRef] [PubMed]
- Sykova, E.; Cizkova, D.; Kubinova, S. Mesenchymal Stem Cells in Treatment of Spinal Cord Injury and Amyotrophic Lateral Sclerosis. Front. Cell Dev. Biol. 2021, 9, 695900. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Liu, W.-F.; Bai, Y.-Y.; Zhou, Y.-Y.; Zhang, Y.; Wang, C.-M.; Lin, S.; He, H.-F. Transplantation of mesenchymal stem cells for spinal cord injury: A systematic review and network meta-analysis. J. Transl. Med. 2021, 19, 178. [Google Scholar] [CrossRef] [PubMed]
- Bhat, I.A.; Sivanarayanan, T.B.; Somal, A.; Pandey, S.; Bharti, M.K.; Panda, B.S.K.; Indu, B.; Verma, M.; Anand, J.; Sonwane, A.; et al. An allogenic therapeutic strategy for canine spinal cord injury using mesenchymal stem cells. J. Cell. Physiol. 2019, 234, 2705–2718. [Google Scholar] [CrossRef]
- Vismara, I.; Papa, S.; Rossi, F.; Forloni, G.; Veglianese, P. Current Options for Cell Therapy in Spinal Cord Injury. Trends Mol. Med. 2017, 23, 831–849. [Google Scholar] [CrossRef]
- Liau, L.L.; Looi, Q.H.; Chia, W.C.; Subramaniam, T.; Ng, M.H.; Law, J.X. Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar] [CrossRef]
- Kumar, R.; Lim, J.; Mekary, R.A.; Rattani, A.; Dewan, M.C.; Sharif, S.Y.; Osorio-Fonseca, E.; Park, K.B. Traumatic Spinal Injury: Global Epidemiology and Worldwide Volume. World Neurosurg. 2018, 113, e345–e363. [Google Scholar] [CrossRef]
- Witiw, C.D.; Fehlings, M.G. Acute Spinal Cord Injury. J. Spin. Disord. Tech. 2015, 28, 202–210. [Google Scholar] [CrossRef]
- Morales, I.I.; Toscano-Tejeida, D.; Ibarra, A. Non Pharmacological Strategies to Promote Spinal Cord Regeneration: A View on Some Individual or Combined Approaches. Curr. Pharm. Des. 2016, 22, 720–727. [Google Scholar] [CrossRef]
- Tyler, J.Y.; Xu, X.-M.; Cheng, J.-X. Nanomedicine for treating spinal cord injury. Nanoscale 2013, 5, 8821–8836. [Google Scholar] [CrossRef]
- Wang, J.; Pearse, D.D. Therapeutic Hypothermia in Spinal Cord Injury: The Status of Its Use and Open Questions. Int. J. Mol. Sci. 2015, 16, 16848–16879. [Google Scholar] [CrossRef] [PubMed]
- Ramer, L.M.; Ramer, M.S.; Steeves, J.D. Setting the stage for functional repair of spinal cord injuries: A cast of thousands. Spinal Cord 2005, 43, 134–161. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, E.; McDonald, J.W. Rodent models for treatment of spinal cord injury: Research trends and progress toward useful repair. Curr. Opin. Neurol. 2004, 17, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Ropper, A.E.; Lee, S.-H.; Han, I. Propitious Therapeutic Modulators to Prevent Blood-Spinal Cord Barrier Disruption in Spinal Cord Injury. Mol. Neurobiol. 2016, 54, 3578–3590. [Google Scholar] [CrossRef]
- Carlson, G.D.; Gorden, C. Current developments in spinal cord injury research. Spine J. 2002, 2, 116–128. [Google Scholar] [CrossRef]
- Hayta, E.; Elden, H. Acute spinal cord injury: A review of pathophysiology and potential of non-steroidal anti-inflammatory drugs for pharmacological intervention. J. Chem. Neuroanat. 2018, 87, 25–31. [Google Scholar] [CrossRef]
- Tator, C.H.; Fehlings, M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 1991, 75, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Hawryluk, G.W. Scarring after spinal cord injury. J. Neurosurg. Spine 2010, 13, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, J.M.; Dabrowski, W.; Dąbrowska-Bouta, B.; Sulkowski, G.; Oakden, W.; Kwiecien-Delaney, C.J.; Yaron, J.R.; Zhang, L.; Schutz, L.; Marzec-Kotarska, B.; et al. Prolonged inflammation leads to ongoing damage after spinal cord injury. PLoS ONE 2020, 15, e0226584. [Google Scholar] [CrossRef]
- Wilson, J.R.; Forgione, N.; Fehlings, M.G. Emerging therapies for acute traumatic spinal cord injury. Can. Med. Assoc. J. 2013, 185, 485–492. [Google Scholar] [CrossRef]
- Dvorak, M.F.; Noonan, V.K.; Fallah, N.; Fisher, C.G.; Finkelstein, J.; Kwon, B.K.; Rivers, C.S.; Ahn, H.; Paquet, J.; Tsai, E.; et al. The Influence of Time from Injury to Surgery on Motor Recovery and Length of Hospital Stay in Acute Traumatic Spinal Cord Injury: An Observational Canadian Cohort Study. J. Neurotrauma 2015, 32, 645–654. [Google Scholar] [CrossRef]
- Burke, J.F.; Yue, J.K.; Ngwenya, L.B.; Winkler, E.A.; Talbott, J.F.; Pan, J.Z.; Ferguson, A.R.; Beattie, M.S.; Bresnahan, J.C.; Haefeli, J.; et al. Ultra-Early (<12 Hours) Surgery Correlates With Higher Rate of American Spinal Injury Association Impairment Scale Conversion After Cervical Spinal Cord Injury. Neurosurgery 2019, 85, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Vaccaro, A.; Wilson, J.R.; Singh, A.; Cadotte, D.W.; Harrop, J.S.; Aarabi, B.; Shaffrey, C.; Dvorak, M.; Fisher, C.; et al. Early versus Delayed Decompression for Traumatic Cervical Spinal Cord Injury: Results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS ONE 2012, 7, e32037. [Google Scholar] [CrossRef]
- Wutte, C.; Klein, B.; Becker, J.; Mach, O.; Panzer, S.; Strowitzki, M.; Maier, D.; Grassner, L. Earlier Decompression (<8 h) Results in Better Neurological and Functional Outcome after Traumatic Thoracolumbar Spinal Cord Injury. J. Neurotrauma 2019, 36, 2020–2027. [Google Scholar] [CrossRef]
- Rath, N.; Balain, B. Spinal cord injury—The role of surgical treatment for neurological improvement. J. Clin. Orthop. Trauma 2017, 8, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef]
- Streijger, F.; So, K.; Manouchehri, N.; Gheorghe, A.; Okon, E.B.; Chan, R.M.; Ng, B.; Shortt, K.; Sekhon, M.S.; Griesdale, D.E.; et al. A Direct Comparison between Norepinephrine and Phenylephrine for Augmenting Spinal Cord Perfusion in a Porcine Model of Spinal Cord Injury. J. Neurotrauma 2018, 35, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Hawryluk, G.; Whetstone, W.; Saigal, R.; Ferguson, A.; Talbott, J.; Bresnahan, J.; Dhall, S.; Pan, J.; Beattie, M.; Manley, G. Mean Arterial Blood Pressure Correlates with Neurological Recovery after Human Spinal Cord Injury: Analysis of High Frequency Physiologic Data. J. Neurotrauma 2015, 32, 1958–1967. [Google Scholar] [CrossRef]
- Ryken, T.C.; Hurlbert, R.J.; Hadley, M.N.; Aarabi, B.; Dhall, S.S.; Gelb, D.E.; Rozzelle, C.J.; Theodore, N.; Walters, B. The Acute Cardiopulmonary Management of Patients with Cervical Spinal Cord Injuries. Neurosurgery 2013, 72, 84–92. [Google Scholar] [CrossRef]
- Seki, T.; Fehlings, M.G. Mechanistic insights into posttraumatic syringomyelia based on a novel in vivo animal model. J. Neurosurg. Spine 2008, 8, 365–375. [Google Scholar] [CrossRef]
- Kwiecien, J.M.; Jarosz, B.; Oakden, W.; Klapec, M.; Stanisz, G.J.; Delaney, K.H.; Kotlinska-Hasiec, E.; Janik, R.; Rola, R.; Dabrowski, W. An in vivo model of anti-inflammatory activity of subdural dexamethasone following the spinal cord injury. Neurol. Neurochir. Polska 2016, 50, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, J.M.; Jarosz, B.; Urdzikova, L.M.; Rola, R.; Dabrowski, W. Original article Subdural infusion of dexamethasone inhibits leukomyelitis after acute spinal cord injury in a rat model. Folia Neuropathol. 2015, 1, 41–51. [Google Scholar] [CrossRef]
- Hu, R.; Zhou, J.; Luo, C.; Lin, J.; Wang, X.; Li, X.; Bian, X.; Li, Y.; Wan, Q.; Yu, Y.; et al. Glial scar and neuroregeneration: Histological, functional, and magnetic resonance imaging analysis in chronic spinal cord injury. J. Neurosurg. Spine 2010, 13, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, J.M. Original article Cellular mechanisms of white matter regeneration in an adult dysmyelinated rat model. Folia Neuropathol. 2013, 3, 189–202. [Google Scholar] [CrossRef]
- Jain, N.B.; Ayers, G.D.; Peterson, E.N.; Harris, M.B.; Morse, L.R.; O’Connor, K.C.; Garshick, E. Traumatic Spinal Cord Injury in the United States, 1993-2012. JAMA 2015, 313, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Fu, C.; Xiong, F.; He, C.; Wei, Q. Stem Cell Therapy for Spinal Cord Injury. Cell Transplant. 2021, 30, 963689721989266. [Google Scholar] [CrossRef]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef]
- Nori, S.; Ahuja, C.S.; Fehlings, M.G. Translational Advances in the Management of Acute Spinal Cord Injury: What is New? What is Hot? Neurosurgery 2017, 64, 119–128. [Google Scholar] [CrossRef]
- Ruzicka, J.; Urdzikova, L.M.; Gillick, J.; Amemori, T.; Romanyuk, N.; Karova, K.; Zaviskova, K.; Dubisova, J.; Kubinova, S.; Murali, R.; et al. A Comparative Study of Three Different Types of Stem Cells for Treatment of Rat Spinal Cord Injury. Cell Transplant. 2017, 26, 585–603. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedshina, Y.O.; Gracheva, O.A.; Mukhutdinova, D.M.; Chelyshev, Y.A.; Rizvanov, A. Mesenchymal stem cells and the neuronal microenvironment in the area of spinal cord injury. Neural Regen. Res. 2019, 14, 227–237. [Google Scholar] [CrossRef]
- Liu, W.-Z.; Ma, Z.-J.; Li, J.-R.; Kang, X.-W. Mesenchymal stem cell-derived exosomes: Therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res. Ther. 2021, 12, 102. [Google Scholar] [CrossRef]
- McDonald, J.W.; Sadowsky, C. Spinal-cord injury. Lancet 2002, 359, 417–425. [Google Scholar] [CrossRef]
- Tator, C.H. Update on the Pathophysiology and Pathology of Acute Spinal Cord Injury. Brain Pathol. 1995, 5, 407–413. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Snow, D.M.; Beller, J.A. Proteoglycans: Road Signs for Neurite Outgrowth. Neural Regen. Res. 2014, 9, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shibata, A.; Li, C.; Braun, P.E.; McKerracher, L.; Roder, J.; Kater, S.B.; David, S. Myelin-associated glycoprotein inhibits neurite/axon growth and causes growth cone collapse. J. Neurosci. Res. 1996, 46, 404–414. [Google Scholar] [CrossRef]
- Domeniconi, M.; Cao, Z.; Spencer, T.; Sivasankaran, R.; Wang, K.C.; Nikulina, E.; Kimura, N.; Cai, H.; Deng, K.; Gao, Y.; et al. Myelin-Associated Glycoprotein Interacts with the Nogo66 Receptor to Inhibit Neurite Outgrowth. Neuron 2002, 35, 283–290. [Google Scholar] [CrossRef]
- Schwab, M.E. Nogo and axon regeneration. Curr. Opin. Neurobiol. 2004, 14, 118–124. [Google Scholar] [CrossRef]
- Fournier, A.E.; Grandpre, T.; Strittmatter, S. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nat. Cell Biol. 2001, 409, 341–346. [Google Scholar] [CrossRef]
- Moreno-Flores, M.T.; Avila, J. The Quest to Repair the Damaged Spinal Cord. Recent Patents CNS Drug Discov. 2012, 1, 55–63. [Google Scholar] [CrossRef][Green Version]
- Oudega, M. Molecular and cellular mechanisms underlying the role of blood vessels in spinal cord injury and repair. Cell Tissue Res. 2012, 349, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Ulndreaj, A.; Chio, J.C.; Ahuja, C.S.; Fehlings, M.G. Modulating the immune response in spinal cord injury. Expert Rev. Neurother. 2016, 16, 1127–1129. [Google Scholar] [CrossRef]
- Nakamura, M.; Houghtling, R.A.; MacArthur, L.; Bayer, B.M.; Bregman, B.S. Differences in cytokine gene expression profile between acute and secondary injury in adult rat spinal cord. Exp. Neurol. 2003, 184, 313–325. [Google Scholar] [CrossRef]
- Liu, M.; Wu, W.; Li, H.; Li, S.; Huang, L.-T.; Yang, Y.-Q.; Sun, Q.; Wang, C.-X.; Yu, Z.; Hang, C.-H. Necroptosis, a novel type of programmed cell death, contributes to early neural cells damage after spinal cord injury in adult mice. J. Spinal Cord Med. 2014, 38, 745–753. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Tao, Y.; Zhang, S.; Wang, J.; Feng, X. Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience 2014, 266, 91–101. [Google Scholar] [CrossRef]
- Li, S.; Stys, P.K. Mechanisms of Ionotropic Glutamate Receptor-Mediated Excitotoxicity in Isolated Spinal Cord White Matter. J. Neurosci. 2000, 20, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Fazzaro, A.; Xiang, C.; Korsmeyer, S.J.; Jacquin, M.F.; McDonald, J.W. Enhanced Oligodendrocyte Survival after Spinal Cord Injury in Bax-Deficient Mice and Mice with Delayed Wallerian Degeneration. J. Neurosci. 2003, 23, 8682–8691. [Google Scholar] [CrossRef]
- Crowe, M.J.; Bresnahan, J.C.; Shuman, S.L.; Masters, J.N.; Beattie, M.S. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat. Med. 1997, 3, 73–76. [Google Scholar] [CrossRef]
- Beattie, M.S.; Farooqui, A.A.; Bresnahan, J.C. Review of Current Evidence for Apoptosis After Spinal Cord Injury. J. Neurotrauma 2000, 17, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Schanne, F.A.X.; Kane, A.B.; Young, E.E.; Farber, J.L. Calcium Dependence of Toxic Cell Death: A Final Common Pathway. Science 1979, 206, 700–702. [Google Scholar] [CrossRef]
- Garcia, E.; Aguilar-Cevallos, J.; Silva-Garcia, R.; Ibarra, A. Cytokine and Growth Factor Activation In Vivo and In Vitro after Spinal Cord Injury. Mediat. Inflamm. 2016, 2016, 9476020. [Google Scholar] [CrossRef] [PubMed]
- Gazdic, M.; Volarevic, V.; Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Arsenijevic, N.; Stojkovic, M. Stem Cells Therapy for Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 1039. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Nakashima, H.; Nagoshi, N.; Chow, D.S.L.; Grossman, R.G.; Kopjar, B. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): A randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord 2015, 54, 8–15. [Google Scholar] [CrossRef]
- Kwon, B.K.; Streijger, F.; Fallah, N.; Noonan, V.K.; Bélanger, L.M.; Ritchie, L.; Paquette, S.J.; Ailon, T.; Boyd, M.C.; Street, J.; et al. Cerebrospinal Fluid Biomarkers To Stratify Injury Severity and Predict Outcome in Human Traumatic Spinal Cord Injury. J. Neurotrauma 2017, 34, 567–580. [Google Scholar] [CrossRef]
- Biglari, B.; Swing, T.; Child, C.; Büchler, A.; Westhauser, F.; Bruckner, T.; Ferbert, T.; Gerner, H.J.; Moghaddam, A. A pilot study on temporal changes in IL-1β and TNF-α serum levels after spinal cord injury: The serum level of TNF-α in acute SCI patients as a possible marker for neurological remission. Spinal Cord 2015, 53, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Leal-Filho, M.B. Spinal cord injury: From inflammation to glial scar. Surg. Neurol. Int. 2011, 2, 112. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef]
- Orr, M.B.; Gensel, J.C. Spinal Cord Injury Scarring and Inflammation: Therapies Targeting Glial and Inflammatory Responses. Neurother. 2018, 15, 541–553. [Google Scholar] [CrossRef]
- Yuan, Y.-M.; He, C. The glial scar in spinal cord injury and repair. Neurosci. Bull. 2013, 29, 421–435. [Google Scholar] [CrossRef]
- Bartanusz, V.; Jezova, D.; Alajajian, B.; Digicaylioglu, M. The blood-spinal cord barrier: Morphology and Clinical Implications. Ann. Neurol. 2011, 70, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, A.K.; Rittner, H.L. Barrier function in the peripheral and central nervous system—A review. Pflügers Arch. Eur. J. Physiol. 2017, 469, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Figley, S.A.; Khosravi, R.; Legasto, J.M.; Tseng, Y.-F.; Fehlings, M.G. Characterization of Vascular Disruption and Blood–Spinal Cord Barrier Permeability following Traumatic Spinal Cord Injury. J. Neurotrauma 2014, 31, 541–552. [Google Scholar] [CrossRef]
- Hu, J.; Yu, Q.; Xie, L.; Zhu, H. Targeting the blood-spinal cord barrier: A therapeutic approach to spinal cord protection against ischemia-reperfusion injury. Life Sci. 2016, 158, 1–6. [Google Scholar] [CrossRef]
- Jo, D.H.; Kim, J.H.; Heo, J.-I.; Kim, J.H.; Cho, C.-H. Interaction between pericytes and endothelial cells leads to formation of tight junction in hyaloid vessels. Mol. Cells 2013, 36, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Shi, L.; Wang, Y.; Chen, S.; Zhang, J. Recent Advances of the NLRP3 Inflammasome in Central Nervous System Disorders. J. Immunol. Res. 2016, 2016, 9238290. [Google Scholar] [CrossRef]
- Martinon, F.; Mayor, A.; Tschopp, J. The Inflammasomes: Guardians of the Body. Annu. Rev. Immunol. 2009, 27, 229–265. [Google Scholar] [CrossRef]
- Davis, B.K.; Wen, H.; Ting, J.P.-Y. The Inflammasome NLRs in Immunity, Inflammation, and Associated Diseases. Annu. Rev. Immunol. 2011, 29, 707–735. [Google Scholar] [CrossRef]
- Jiang, W.; Li, M.; He, F.; Zhou, S.; Zhu, L. Targeting the NLRP3 inflammasome to attenuate spinal cord injury in mice. J. Neuroinflamm. 2017, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Huang, Y.; Han, N.; He, F.; Li, M.; Bian, Z.; Liu, J.; Sun, T.; Zhu, L. Quercetin suppresses NLRP3 inflammasome activation and attenuates histopathology in a rat model of spinal cord injury. Spinal Cord 2016, 54, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Shi, D.; Zhi, Z.; Ao, R.; Yu, B. Melatonin ameliorates spinal cord injury by suppressing the activation of inflammasomes in rats. J. Cell. Biochem. 2019, 120, 5183–5192. [Google Scholar] [CrossRef]
- Zendedel, A.; Mönnink, F.; Hassanzadeh, G.; Zaminy, A.; Ansar, M.M.; Habib, P.; Slowik, A.D.; Kipp, M.; Beyer, C. Estrogen Attenuates Local Inflammasome Expression and Activation after Spinal Cord Injury. Mol. Neurobiol. 2018, 55, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Huang, Y.; He, F.; Liu, J.; Li, M.; Sun, T.; Ren, W.; Hou, J.; Zhu, L. Dopamine D1 Receptor Agonist A-68930 Inhibits NLRP3 Inflammasome Activation, Controls Inflammation, and Alleviates Histopathology in a Rat Model of Spinal Cord Injury. Spine 2016, 41, E330–E334. [Google Scholar] [CrossRef]
- Anderson, A.J.; Robert, S.; Huang, W.; Young, W.; Cotman, C.W. Activation of complement pathways after contusion-induced spinal cord injury. J. Neurotrauma 2004, 21, 1831–1846. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Lin, H.; Li, W.; Huang, Y.; Dai, H. Complement Component C3 Promotes Cerebral Ischemia/Reperfusion Injury Mediated by TLR2/NFκB Activation in Diabetic Mice. Neurochem. Res. 2018, 43, 1599–1607. [Google Scholar] [CrossRef]
- Fraser, D.A.; Arora, M.; Bohlson, S.S.; Lozano, E.; Tenner, A.J. Generation of inhibitory NFkappaB complexes and phosphorylated cAMP response element-binding protein correlates with the anti-inflammatory activity of complement protein C1q in human monocytes. J. Biol. Chem. 2007, 282, 7360–7367. [Google Scholar] [CrossRef]
- Guerrero, A.R.; Uchida, K.; Nakajima, H.; Watanabe, S.; Nakamura, M.; Johnson, W.E.; Baba, H. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J. Neuroinflamm. 2012, 9, 40. [Google Scholar] [CrossRef]
- Kigerl, K.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of Two Distinct Macrophage Subsets with Divergent Effects Causing either Neurotoxicity or Regeneration in the Injured Mouse Spinal Cord. J. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrogliosis. Cold Spring Harb. Perspect. Biol. 2014, 7, a020420. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Akcakaya, P.; Akçakaya, P.; Ekelund, S.; Kolosenko, I.; Caramuta, S.; Özata, D.M.; Xie, H.; Lindforss, U.; Olivecrona, H.; Lui, W.-O. miR-185 and miR-133b deregulation is associated with overall survival and metastasis in colorectal cancer. Int. J. Oncol. 2011, 39, 311–318. [Google Scholar] [CrossRef]
- Xu, G.; Ao, R.; Zhi, Z.; Jia, J.; Yu, B. miR-21 and miR-19b delivered by hMSC-derived EVs regulate the apoptosis and differentiation of neurons in patients with spinal cord injury. J. Cell Physiol. 2019, 234, 10205–10217. [Google Scholar] [CrossRef]
- Han, Z.; Chen, F.; Ge, X.; Tan, J.; Lei, P.; Zhang, J. miR-21 alleviated apoptosis of cortical neurons through promoting PTEN-Akt signaling pathway in vitro after experimental traumatic brain injury. Brain Res. 2014, 1582, 12–20. [Google Scholar] [CrossRef]
- Liu, N.-K.; Wang, X.-F.; Lu, Q.-B.; Xu, X.-M. Altered microRNA expression following traumatic spinal cord injury. Exp. Neurol. 2009, 219, 424–429. [Google Scholar] [CrossRef]
- Xia, C.; Cai, Y.; Lin, Y.; Guan, R.; Xiao, G.; Yang, J. MiR-133b-5p regulates the expression of the heat shock protein 70 during rat neuronal cell apoptosis induced by the gp120 V3 loop peptide. J. Med. Virol. 2016, 88, 437–447. [Google Scholar] [CrossRef]
- Lu, X.C.; Zheng, J.Y.; Tang, L.J.; Huang, B.S.; Li, K.; Tao, Y.; Yu, Z.J.; Zhu, R.L.; Li, S.; Li, L.X. MiR-133b Promotes Neurite Outgrowth by Targeting RhoA Expression. Cell. Physiol. Biochem. 2015, 35, 246–258. [Google Scholar] [CrossRef]
- Heyer, M.P.; Pani, A.K.; Smeyne, R.J.; Kenny, P.J.; Feng, G. Normal midbrain dopaminergic neuron development and function in miR-133b mutant mice. J. Neurosci. 2012, 32, 10887–10894. [Google Scholar] [CrossRef]
- Yu, Y.-M.; Gibbs, K.M.; Davila, J.; Campbell, N.; Sung, S.; Todorova, T.I.; Otsuka, S.; Sabaawy, H.E.; Hart, R.P.; Schachner, M. MicroRNA miR-133b is essential for functional recovery after spinal cord injury in adult zebrafish. Eur. J. Neurosci. 2011, 33, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zeng, L.; Huang, J.; Wang, G.; Lu, H. miR-126 promotes angiogenesis and attenuates inflammation after contusion spinal cord injury in rats. Brain Res. 2015, 1608, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Groh, M.E.; Maitra, B.; Szekely, E.; Koç, O.N. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp. Hematol. 2005, 33, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, F.; Shi, H.; Tan, R.; Han, S.; Ye, G.; Pan, S.; Sun, F.; Liu, X. Comparisons of Rabbit Bone Marrow Mesenchymal Stem Cell Isolation and Culture Methods In Vitro. PLoS ONE 2014, 9, e88794. [Google Scholar] [CrossRef]
- Stewart, M.C.; Stewart, A.A. Mesenchymal Stem Cells: Characteristics, Sources, and Mechanisms of Action. Veter Clin. N. Am. Equine Pr. 2011, 27, 243–261. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- Ren, Z.; Qi, Y.; Sun, S.; Tao, Y.; Shi, R. Mesenchymal Stem Cell-Derived Exosomes: Hope for Spinal Cord Injury Repair. Stem Cells Dev. 2020, 29, 1467–1478. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, 2. [Google Scholar] [CrossRef]
- Park, H.-W.; Cho, J.-S.; Park, C.-K.; Jung, S.J.; Park, C.-H.; Lee, S.-J.; Oh, S.B.; Park, Y.-S.; Chang, M.-S. Directed Induction of Functional Motor Neuron-Like Cells from Genetically Engineered Human Mesenchymal Stem Cells. PLoS ONE 2012, 7, e35244. [Google Scholar] [CrossRef]
- Tropel, P.; Platet, N.; Platel, J.; Noël, D.; Albrieux, M.; Benabid, A.; Berger, F. Functional Neuronal Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells. Stem. Cells 2006, 24, 2868–2876. [Google Scholar] [CrossRef]
- Mezey, E.; Chandross, K.J.; Harta, G.; Maki, R.A.; McKercher, S.R. Turning Blood into Brain: Cells Bearing Neuronal Antigens Generated in Vivo from Bone Marrow. Science 2000, 290, 1779–1782. [Google Scholar] [CrossRef]
- Qi, X.; Shao, M.; Peng, H.; Bi, Z.; Su, Z.; Li, H. In vitro differentiation of bone marrow stromal cells into neurons and glial cells and differential protein expression in a two-compartment bone marrow stromal cell/neuron co-culture system. J. Clin. Neurosci. 2010, 17, 908–913. [Google Scholar] [CrossRef]
- Sanchez-Ramos, J.; Song, S.; Cardozo-Pelaez, F.; Hazzi, C.; Stedeford, T.; Willing, A.; Freeman, T.; Saporta, S.; Janssen, W.; Patel, N.A.; et al. Adult Bone Marrow Stromal Cells Differentiate into Neural Cells in Vitro. Exp. Neurol. 2000, 164, 247–256. [Google Scholar] [CrossRef]
- Lim, W.L.; Liau, L.L.; Ng, M.H.; Chowdhury, S.R.; Law, J.X. Current Progress in Tendon and Ligament Tissue Engineering. Tissue Eng. Regen. Med. 2019, 16, 549–571. [Google Scholar] [CrossRef]
- Shin, S.; Lee, J.; Kwon, Y.; Park, K.-S.; Jeong, J.-H.; Choi, S.-J.; Bang, S.I.; Chang, J.W.; Lee, C. Comparative Proteomic Analysis of the Mesenchymal Stem Cells Secretome from Adipose, Bone Marrow, Placenta and Wharton’s Jelly. Int. J. Mol. Sci. 2021, 22, 845. [Google Scholar] [CrossRef] [PubMed]
- Marconi, S.; Castiglione, G.; Turano, E.; Bissolotti, G.; Angiari, S.; Farinazzo, A.; Constantin, G.; Bedogni, G.; Bedogni, A.; Bonetti, B. Human Adipose-Derived Mesenchymal Stem Cells Systemically Injected Promote Peripheral Nerve Regeneration in the Mouse Model of Sciatic Crush. Tissue Eng. Part A 2012, 18, 1264–1272. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- Sorrell, J.M.; Baber, M.A.; Caplan, A.I. Influence of Adult Mesenchymal Stem Cells onIn VitroVascular Formation. Tissue Eng. Part A 2009, 15, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131. [Google Scholar] [CrossRef]
- Lim, J.; Razi, Z.R.M.; Law, J.X.; Nawi, A.M.; Idrus, R.B.H.; Chin, T.G.; Mustangin, M.; Ng, M.H. Mesenchymal Stromal Cells from the Maternal Segment of Human Umbilical Cord is Ideal for Bone Regeneration in Allogenic Setting. Tissue Eng. Regen. Med. 2018, 15, 75–87. [Google Scholar] [CrossRef]
- Avanzini, M.A.; Bernardo, M.E.; Cometa, A.M.; Perotti, C.; Zaffaroni, N.; Novara, F.; Visai, L.; Moretta, A.; Del Fante, C.; Villa, R.; et al. Generation of mesenchymal stromal cells in the presence of platelet lysate: A phenotypic and functional comparison of umbilical cord blood- and bone marrow-derived progenitors. Haematologica 2009, 94, 1649–1660. [Google Scholar] [CrossRef]
- Weiss, M.L.; Anderson, C.; Medicetty, S.; Seshareddy, K.B.; Weiss, R.J.; VanderWerff, I.; Troyer, D.; McIntosh, K.R. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells 2008, 26, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, F.; Xiao, Z.; Han, G.; Wang, N.; Yin, N.; Chen, B.; Jiang, X.; Yun, C.; Han, W.; et al. Clinical Study of NeuroRegen Scaffold Combined with Human Mesenchymal Stem Cells for the Repair of Chronic Complete Spinal Cord Injury. Cell Transplant. 2017, 26, 891–900. [Google Scholar] [CrossRef]
- Hafez, P.; Chowdhury, S.R.; Jose, S.; Law, J.X.; Ruszymah, B.H.I.; Ramzisham, A.R.M.; Ng, M.H. Development of an In Vitro Cardiac Ischemic Model Using Primary Human Cardiomyocytes. Cardiovasc. Eng. Technol. 2018, 9, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Gonçalves, F.; Grings, M.; Nunes, N.S.; Pinto, F.O.; Garcez, T.N.A.; Visioli, F.; Leipnitz, G.; Paz, A.H. Antioxidant properties of mesenchymal stem cells against oxidative stress in a murine model of colitis. Biotechnol. Lett. 2017, 39, 613–622. [Google Scholar] [CrossRef]
- Kemp, K.; Mallam, E.; Hares, K.; Witherick, J.; Scolding, N.; Wilkins, A. Mesenchymal Stem Cells Restore Frataxin Expression and Increase Hydrogen Peroxide Scavenging Enzymes in Friedreich Ataxia Fibroblasts. PLoS ONE 2011, 6, e26098. [Google Scholar] [CrossRef]
- Hofstetter, C.P.; Schwarz, E.J.; Hess, D.; Widenfalk, J.; El Manira, A.; Prockop, D.J.; Olson, L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl. Acad. Sci. USA 2002, 99, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Charbord, P. Bone Marrow Mesenchymal Stem Cells: Historical Overview and Concepts. Hum. Gene Ther. 2010, 21, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Matyas, J.J.; Stewart, A.N.; Goldsmith, A.; Nan, Z.; Skeel, R.L.; Rossignol, J.; Dunbar, G.L. Effects of bone-marrow-derived MSC transplantation on functional recovery in a rat model of spinal cord injury: Comparisons of transplant locations and cell concentrations. Cell Transplant. 2017, 26, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wu, C.; Xiong, Q.; Zhou, L.; Tian, Y. Anti-inflammatory Mechanism of Bone Marrow Mesenchymal Stem Cell Transplantation in Rat Model of Spinal Cord Injury. Cell Biophys. 2015, 71, 1341–1347. [Google Scholar] [CrossRef]
- Matsushita, T.; Lankford, K.L.; Arroyo, E.J.; Sasaki, M.; Neyazi, M.; Radtke, C.; Kocsis, J.D. Diffuse and persistent blood–spinal cord barrier disruption after contusive spinal cord injury rapidly recovers following intravenous infusion of bone marrow mesenchymal stem cells. Exp. Neurol. 2015, 267, 152–164. [Google Scholar] [CrossRef]
- Kim, C.; Kim, H.J.; Lee, H.; Lee, H.; Lee, S.J.; Lee, S.T.; Yang, S.R.; Chung, C.K. Mesenchymal Stem Cell Transplantation Promotes Functional Recovery through MMP2/STAT3 Related Astrogliosis after Spinal Cord Injury. Int. J. Stem. Cells 2019, 12, 331–339. [Google Scholar] [CrossRef]
- Nandoe, R.D.S.; Hurtado, A.; Levi, A.D.O.; Grotenhuis, J.A.; Oudega, M. Bone Marrow Stromal Cells for Repair of the Spinal Cord: Towards Clinical Application. Cell Transplant. 2006, 15, 563–577. [Google Scholar] [CrossRef]
- Cížková, D.; Novotna, I.; Slovinska, L.; Vanický, I.; Jergová, S.; Rosocha, J.; Radoňak, J. Repetitive Intrathecal Catheter Delivery of Bone Marrow Mesenchymal Stromal Cells Improves Functional Recovery in a Rat Model of Contusive Spinal Cord Injury. J. Neurotrauma 2011, 28, 1951–1961. [Google Scholar] [CrossRef]
- Osaka, M.; Honmou, O.; Murakami, T.; Nonaka, T.; Houkin, K.; Hamada, H.; Kocsis, J.D. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010, 1343, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Čížková, D.; Rosocha, J.; Vanický, I.; Jergová, S.; Cizek, M. Transplants of Human Mesenchymal Stem Cells Improve Functional Recovery After Spinal Cord Injury in the Rat. Cell. Mol. Neurobiol. 2006, 26, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xuan, A.; Chen, Y.; Zhang, J.; Xu, L.; Yan, Q.; Long, D. Combined effect of nerve growth factor and brain-derived neurotrophic factor on neuronal differentiation of neural stem cells and the potential molecular mechanisms. Mol. Med. Rep. 2014, 10, 1739–1745. [Google Scholar] [CrossRef]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef]
- Jeon, S.R.; Park, J.H.; Lee, J.H.; Kim, D.Y.; Kim, H.S.; Sung, I.Y.; Choi, G.H.; Jeon, M.H.; Kim, G.G. Treatment of Spinal Cord Injury with Bone Marrow-Derived, Cultured Autologous Mesenchymal Stem Cells. Tissue Eng. Regen Med. 2010, 7, 316–322. [Google Scholar]
- Saito, F.; Nakatani, T.; Iwase, M.; Maeda, Y.; Murao, Y.; Suzuki, Y.; Fukushima, M.; Ide, C. Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: A pilot study. Restor. Neurol. Neurosci. 2012, 30, 127–136. [Google Scholar] [CrossRef] [PubMed]
- El-Kheir, W.A.; Gabr, H.; Awad, M.R.; Ghannam, O.; Barakat, Y.; Farghali, H.A.M.A.; El Maadawi, Z.M.; Ewes, I.; Sabaawy, H.E. Autologous Bone Marrow-Derived Cell Therapy Combined with Physical Therapy Induces Functional Improvement in Chronic Spinal Cord Injury Patients. Cell Transplant. 2014, 23, 729–745. [Google Scholar] [CrossRef]
- Karamouzian, S.; Nematollahi-Mahani, S.N.; Nakhaee, N.; Eskandary, H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin. Neurol. Neurosurg. 2012, 114, 935–939. [Google Scholar] [CrossRef]
- Jiang, P.-C.; Xiong, W.-P.; Wang, G.; Ma, C.; Yao, W.-Q.; Kendell, S.F.; Mehling, B.M.; Yuan, X.-H.; Wu, D.-C. A clinical trial report of autologous bone marrow-derived mesenchymal stem cell transplantation in patients with spinal cord injury. Exp. Ther. Med. 2013, 6, 140–146. [Google Scholar] [CrossRef]
- Dai, G.; Liu, X.; Zhang, Z.; Yang, Z.; Dai, Y.; Xu, R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013, 1533, 73–79. [Google Scholar] [CrossRef]
- Mendonça, M.V.P.; Larocca, T.F.; Souza, B.S.D.F.; Villarreal, C.F.; Silva, L.F.M.; Matos, A.C.; Novaes, M.A.; Bahia, C.M.P.; Martinez, A.C.D.O.M.; Kaneto, C.M.; et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 2014, 5, 126. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Venkataramana, N.K.; Bansal, A.; Balaraju, S.; Jan, M.; Chandra, R.; Dixit, A.; Rauthan, A.; Murgod, U.; Totey, S. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: A pilot clinical study. Cytotherapy 2009, 11, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Liau, L.; Ruszymah, B.; Ng, M.; Law, J. Characteristics and clinical applications of Wharton’s jelly-derived mesenchymal stromal cells. Curr. Res. Transl. Med. 2020, 68, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Barry, F.P.; Murphy, J.M.; English, K.; Mahon, B.P. Immunogenicity of Adult Mesenchymal Stem Cells: Lessons from the Fetal Allograft. Stem Cells Dev. 2005, 14, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, H.; Bicknese, A.; Chien, S.-N.; Bogucki, B.; Oliver, D.; Quinn, C.; Wall, D. Multilineage differentiation activity by cells isolated from umbilical cord blood: Expression of bone, fat, and neural markers. Biol. Blood Marrow Transplant. 2001, 7, 581–588. [Google Scholar] [CrossRef]
- Sabapathy, V.; Sundaram, B.; Sreelakshmi, V.M.; Mankuzhy, P.; Kumar, S. Human Wharton’s Jelly Mesenchymal Stem Cells Plasticity Augments Scar-Free Skin Wound Healing with Hair Growth. PLoS ONE 2014, 9, e93726. [Google Scholar] [CrossRef]
- Nagamura-Inoue, T.; He, H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J. Stem. Cells 2014, 6, 195–202. [Google Scholar] [CrossRef]
- Kim, D.-W.; Staples, M.; Shinozuka, K.; Pantcheva, P.; Kang, S.-D.; Borlongan, C.V. Wharton’s Jelly-Derived Mesenchymal Stem Cells: Phenotypic Characterization and Optimizing Their Therapeutic Potential for Clinical Applications. Int. J. Mol. Sci. 2013, 14, 11692–11712. [Google Scholar] [CrossRef]
- De Girolamo, L.; Lucarelli, E.; Alessandri, G.; Avanzini, M.A.; Bernardo, M.E.; Biagi, E.; Brini, A.T.; D’Amico, G.; Fagioli, F.; Ferrero, I.; et al. Mesenchymal stem/stromal cells: A new “cells as drugs” paradigm. Efficacy and critical aspects in cell therapy. Curr. Pharm. Des. 2013, 19, 2459–2473. [Google Scholar] [CrossRef]
- Dasari, V.R.; Veeravalli, K.K.; Tsung, A.J.; Gondi, C.S.; Gujrati, M.; Dinh, D.H.; Rao, J.S. Neuronal apoptosis is inhibited by cord blood stem cells after spinal cord injury. J. Neurotrauma 2009, 26, 2057–2069. [Google Scholar] [CrossRef]
- Veeravalli, K.K.; Dasari, V.R.; Tsung, A.J.; Dinh, D.H.; Gujrati, M.; Fassett, D.; Rao, J.S. Human umbilical cord blood stem cells upregulate matrix metalloproteinase-2 in rats after spinal cord injury. Neurobiol. Dis. 2009, 36, 200–212. [Google Scholar] [CrossRef]
- Judas, G.I.; Ferreira, S.G.; Simas, R.; Sannomiya, P.; Benício, A.; Da Silva, L.F.F.; Moreira, L.F.P. Intrathecal injection of human umbilical cord blood stem cells attenuates spinal cord ischaemic compromise in rats. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 757–762. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaner, T.; Karadag, T.; Cirak, B.; Erken, H.A.; Karabulut, A.; Kiroglu, Y.; Akkaya, S.; Acar, F.; Coskun, E.; Genc, O.; et al. The effects of human umbilical cord blood transplantation in rats with experimentally induced spinal cord injury. J. Neurosurg. Spine 2010, 13, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Dasari, V.R.; Spomar, D.G.; Li, L.; Gujrati, M.; Rao, J.S.; Dinh, D.H. Umbilical Cord Blood Stem Cell Mediated Downregulation of Fas Improves Functional Recovery of Rats after Spinal Cord Injury. Neurochem. Res. 2008, 33, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Li, E.; Yang, B.; Wang, B. Human umbilical cord blood-derived mesenchymal stem cell transplantation for the treatment of spinal cord injury. Exp. Ther. Med. 2014, 7, 1233–1236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, S.-L.; Luo, H.-S.; Li, J.-T.; Xia, Y.-Z.; Li, L.; Zhang, L.-J.; Meng, H.; Cui, G.-Y.; Chen, Z.; Wu, N.; et al. Functional recovery in acute traumatic spinal cord injury after transplantation of human umbilical cord mesenchymal stem cells. Crit. Care Med. 2010, 38, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, D.; Wang, Z.; Xue, M.; Zhu, L.; Yan, H.; Zheng, X.; Guo, Z.; Wang, H. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy 2013, 15, 185–191. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, X.; Hua, R.; Dai, G.; Wang, X.; Gao, J.; An, Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J. Transl. Med. 2014, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-S.; Kim, S.; Oh, Y.; Yu, J.; Kim, K.-Y.; Park, H.; Song, C.-H.; Han, H.J. A 37-year-old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: A case study. Cytotherapy 2005, 7, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of Multi-Lineage Cells from Human Adipose Tissue and Bone Marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Danisovic, L.; Varga, I.; Polák, S.; Ulicná, M.; Hlavacková, L.; Böhmer, D.; Vojtassák, J. Comparison of in vitro chondrogenic potential of human mesenchymal stem cells derived from bone marrow and adipose tissue. Gen. Physiol. Biophys. 2009, 28, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.; Asgari, A.; Lokmic, Z.T.; Sinclair, R.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Comparative Analysis of Paracrine Factor Expression in Human Adult Mesenchymal Stem Cells Derived from Bone Marrow, Adipose, and Dermal Tissue. Stem Cells Dev. 2012, 21, 2189–2203. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian Kia, N.; Bahrami, A.R.; Ebrahimi, M.; Matin, M.M.; Neshati, Z.; Almohaddesin, M.R.; Aghdami, N.; Bidkhori, H.R. Comparative analysis of chemokine receptor’s expression in mesenchymal stem cells derived from human bone marrow and adipose tissue. J. Mol. Neurosci. 2011, 44, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Hamaguchi, A.; Ootaki, M.; Watanabe, M.; Takeba, Y.; Iiri, T.; Matsumoto, N.; Takenaga, M. Intravenous infusion of adipose-derived stem/stromal cells improves functional recovery of rats with spinal cord injury. Cytotherapy 2017, 19, 839–848. [Google Scholar] [CrossRef]
- Aras, Y.; Sabanci, P.A.; Kabatas, S.; Duruksu, G.; Subasi, C.; Erguven, M.; Karaoz, E. The Effects of Adipose Tissue-Derived Mesenchymal Stem Cell Transplantation During the Acute and Subacute Phases Following Spinal Cord Injury. Turk. Neurosurg. 2015, 26, 127–139. [Google Scholar] [CrossRef]
- Zhou, Z.; Tian, X.; Mo, B.; Xu, H.; Zhang, L.; Huang, L.; Yao, S.; Huang, Z.; Wang, Y.; Xie, H.; et al. Adipose mesenchymal stem cell transplantation alleviates spinal cord injury-induced neuroinflammation partly by suppressing the Jagged1/Notch pathway. Stem. Cell Res. Ther. 2020, 11, 212. [Google Scholar] [CrossRef]
- Leu, S.; Lin, Y.-C.; Yuen, C.-M.; Yen, C.-H.; Kao, Y.-H.; Sun, C.-K.; Yip, H.-K. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J. Transl. Med. 2010, 8, 63. [Google Scholar] [CrossRef]
- Gao, S.; Guo, X.; Zhao, S.; Jin, Y.; Zhou, F.; Yuan, P.; Cao, L.; Wang, J.; Qiu, Y.; Sun, C.; et al. Differentiation of human adipose-derived stem cells into neuron/motoneuron-like cells for cell replacement therapy of spinal cord injury. Cell Death Dis. 2019, 10, 597. [Google Scholar] [CrossRef]
- Ra, J.C.; Shin, I.S.; Kim, S.H.; Kang, S.K.; Kang, B.C.; Lee, H.Y.; Kim, Y.J.; Jo, J.Y.; Yoon, E.J.; Choi, H.J.; et al. Safety of Intravenous Infusion of Human Adipose Tissue-Derived Mesenchymal Stem Cells in Animals and Humans. Stem Cells Dev. 2011, 20, 1297–1308. [Google Scholar] [CrossRef]
- Hur, J.W.; Cho, T.-H.; Park, D.-H.; Lee, J.-B.; Park, J.-Y.; Chung, Y.-G. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: A human trial. J. Spinal Cord Med. 2015, 39, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Bydon, M.; Dietz, A.B.; Goncalves, S.; Moinuddin, F.M.; Alvi, M.A.; Goyal, A.; Yolcu, Y.; Hunt, C.L.; Garlanger, K.L.; Del Fabro, A.S.; et al. CELLTOP Clinical Trial: First Report From a Phase 1 Trial of Autologous Adipose Tissue–Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury. Mayo Clin. Proc. 2020, 95, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Heldring, N.; Mäger, I.; Wood, M.J.; Le Blanc, K.; Andaloussi, S.E. Therapeutic Potential of Multipotent Mesenchymal Stromal Cells and Their Extracellular Vesicles. Hum. Gene Ther. 2015, 26, 506–517. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Jin, Y.-Q.; Liu, W.; Zhang, W.J.; Hong, T.-H.; Zhou, G.; Baggett, L.S.; Mikos, A.G.; Cao, Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Shon, O.-J.; Seo, M.-S.; Choi, Y.; Park, W.; Lee, G. Mesenchymal Stem Cell-Derived Exosomes and Their Therapeutic Potential for Osteoarthritis. Biology 2021, 10, 285. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, J.; Kim, M.Y.; Bae, Y.-S.; Ryu, S.H.; Lee, T.G.; Kim, J.H. Proteomic Analysis of Tumor Necrosis Factor-α-Induced Secretome of Human Adipose Tissue-Derived Mesenchymal Stem Cells. J. Proteome Res. 2010, 9, 1754–1762. [Google Scholar] [CrossRef]
- Familtseva, A.; Jeremic, N.; Tyagi, S.C. Exosomes: Cell-created drug delivery systems. Mol. Cell. Biochem. 2019, 459, 1–6. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Sarko, D.K.; McKinney, C.E. Exosomes: Origins and Therapeutic Potential for Neurodegenerative Disease. Front. Neurosci. 2017, 11, 82. [Google Scholar] [CrossRef]
- Van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro-Oncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.W.; Seo, M.-S.; Kang, K.-K.; Oh, S.-K. Epidural fat-derived mesenchymal stem cell: First report of epidural fat-derived mesenchymal stem cell. Asian Spine J. 2019, 13, 361. [Google Scholar] [CrossRef]
- Wahid, F.; Shehzad, A.; Khan, T.; Kim, Y.Y. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1803, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L. Circulating Exosomal miRNA as Diagnostic Biomarkers of Neurodegenerative Diseases. Front. Mol. Neurosci. 2020, 13, 53. [Google Scholar] [CrossRef]
- Chen, J.-J.; Zhao, B.; Zhao, J.; Li, S. Potential Roles of Exosomal MicroRNAs as Diagnostic Biomarkers and Therapeutic Application in Alzheimer’s Disease. Neural Plast. 2017, 2017, 7027380. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.W.; Taylor, K.G.; Peterson, K.E. Location Is Everything: Let-7b microRNA and TLR7 Signaling Results in a Painful TRP. Sci. Signal. 2014, 7, pe14. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.M.; Krüger, C.; Park, B.; Derkow, K.; Rosenberger, K.; Baumgart, J.; Trimbuch, T.; Eom, G.; Hinz, M.; Kaul, D.; et al. An unconventional role for miRNA: Let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 2012, 15, 827–835. [Google Scholar] [CrossRef]
- René, C.; Parks, R. Delivery of Therapeutic Agents to the Central Nervous System and the Promise of Extracellular Vesicles. Pharmaceutics 2021, 13, 492. [Google Scholar] [CrossRef]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome Delivered Anticancer Drugs Across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Didiot, M.-C.; Hall, L.M.; Coles, A.H.; Haraszti, R.A.; Godinho, B.M.; Chase, K.; Sapp, E.; Ly, S.; Alterman, J.F.; Hassler, M.R.; et al. Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Mol. Ther. 2016, 24, 1836–1847. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Wang, F.; Li, Y.; Lu, Q.-E.; Cheung, W.L.; Zhang, Y.; Zhang, Z.G.; Chopp, M. Secondary Release of Exosomes from Astrocytes Contributes to the Increase in Neural Plasticity and Improvement of Functional Recovery after Stroke in Rats Treated with Exosomes Harvested from MicroRNA 133b-Overexpressing Multipotent Mesenchymal Stromal Cells. Cell Transplant. 2017, 26, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Chen, X.; Wang, L.; Yang, G. Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia. Mol. Ther. Nucleic Acids 2017, 7, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.; Sun, H.; Sakai, S.; Mitsutake, S.; Okada, M.; Tahara, H.; Furukawa, J.-I.; Fujitani, N.; Shinohara, Y.; Igarashi, Y. Decreased Amyloid-β Pathologies by Intracerebral Loading of Glycosphingolipid-enriched Exosomes in Alzheimer Model Mice. J. Biol. Chem. 2014, 289, 24488–24498. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Perets, N.; Betzer, O.; Ben-Shaul, S.; Sheinin, A.; Michaelevski, I.; Popovtzer, R.; Offen, D.; Levenberg, S. Intranasal Delivery of Mesenchymal Stem Cell Derived Exosomes Loaded with Phosphatase and Tensin Homolog siRNA Repairs Complete Spinal Cord Injury. ACS Nano 2019, 13, 10015–10028. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kumar, H.; Jo, M.-J.; Kim, J.; Yoon, J.-K.; Lee, J.-R.; Kang, M.; Choo, Y.W.; Song, S.Y.; Kwon, S.P.; et al. Therapeutic Efficacy-Potentiated and Diseased Organ-Targeting Nanovesicles Derived from Mesenchymal Stem Cells for Spinal Cord Injury Treatment. Nano Lett. 2018, 18, 4965–4975. [Google Scholar] [CrossRef]
- Zhong, D.; Cao, Y.; Li, C.-J.; Li, M.; Rong, Z.-J.; Jiang, L.; Guo, Z.; Lu, H.-B.; Hu, J.-Z. Neural stem cell-derived exosomes facilitate spinal cord functional recovery after injury by promoting angiogenesis. Exp. Biol. Med. 2020, 245, 54–65. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.-U.; Sung, S.-E.; Kang, K.-K.; Choi, J.-H.; Lee, S.; Sung, M.; Yang, S.Y.; Kim, S.-K.; Kim, Y.I.; Lim, J.-H.; et al. Therapeutic Potential of Mesenchymal Stem Cells (MSCs) and MSC-Derived Extracellular Vesicles for the Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 13672. https://doi.org/10.3390/ijms222413672
Kim G-U, Sung S-E, Kang K-K, Choi J-H, Lee S, Sung M, Yang SY, Kim S-K, Kim YI, Lim J-H, et al. Therapeutic Potential of Mesenchymal Stem Cells (MSCs) and MSC-Derived Extracellular Vesicles for the Treatment of Spinal Cord Injury. International Journal of Molecular Sciences. 2021; 22(24):13672. https://doi.org/10.3390/ijms222413672
Chicago/Turabian StyleKim, Gang-Un, Soo-Eun Sung, Kyung-Ku Kang, Joo-Hee Choi, Sijoon Lee, Minkyoung Sung, Seung Yun Yang, Seul-Ki Kim, Young In Kim, Ju-Hyeon Lim, and et al. 2021. "Therapeutic Potential of Mesenchymal Stem Cells (MSCs) and MSC-Derived Extracellular Vesicles for the Treatment of Spinal Cord Injury" International Journal of Molecular Sciences 22, no. 24: 13672. https://doi.org/10.3390/ijms222413672
APA StyleKim, G.-U., Sung, S.-E., Kang, K.-K., Choi, J.-H., Lee, S., Sung, M., Yang, S. Y., Kim, S.-K., Kim, Y. I., Lim, J.-H., Seo, M.-S., & Lee, G. W. (2021). Therapeutic Potential of Mesenchymal Stem Cells (MSCs) and MSC-Derived Extracellular Vesicles for the Treatment of Spinal Cord Injury. International Journal of Molecular Sciences, 22(24), 13672. https://doi.org/10.3390/ijms222413672







