The Role of Panx3 in Age-Associated and Injury-Induced Intervertebral Disc Degeneration
Abstract
:1. Introduction
2. Results
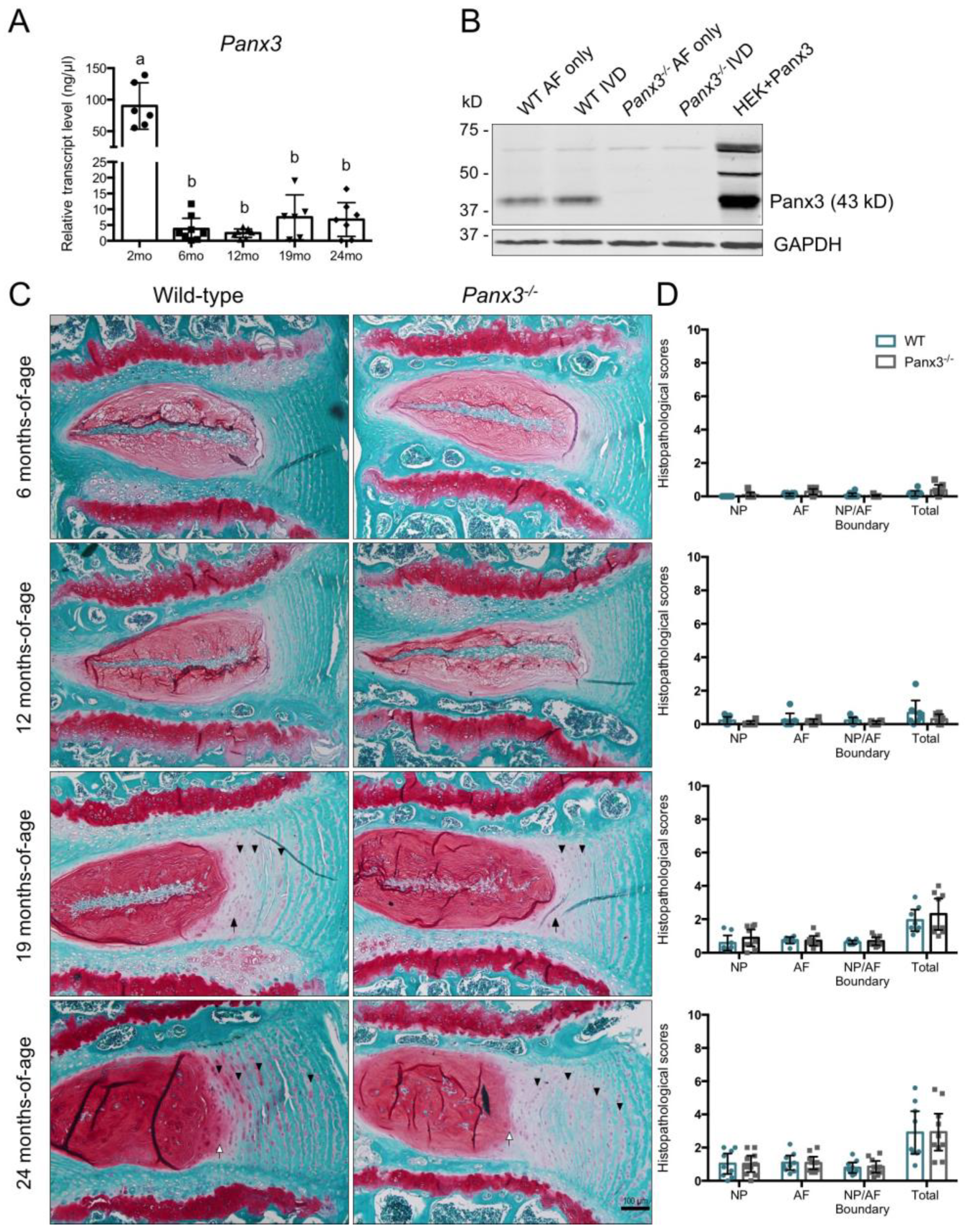
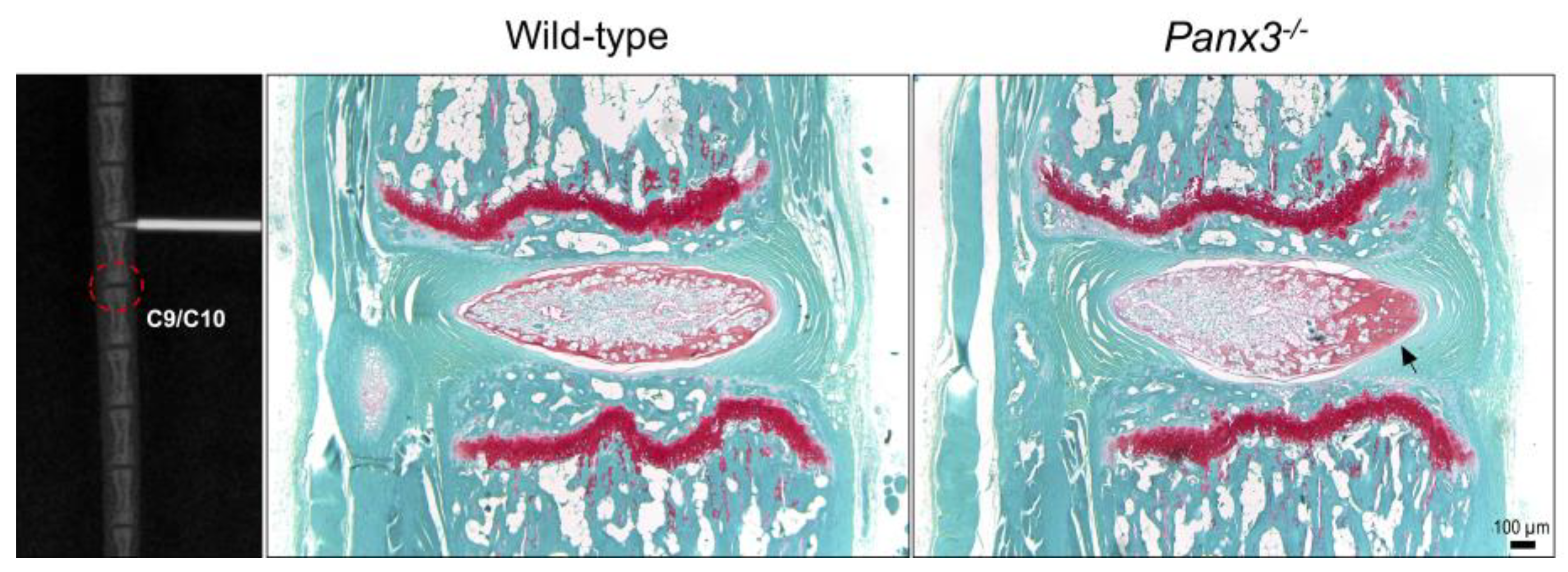
2.1. Loss of Panx3 Does Not Alter Age-Associated IVD Degeneration
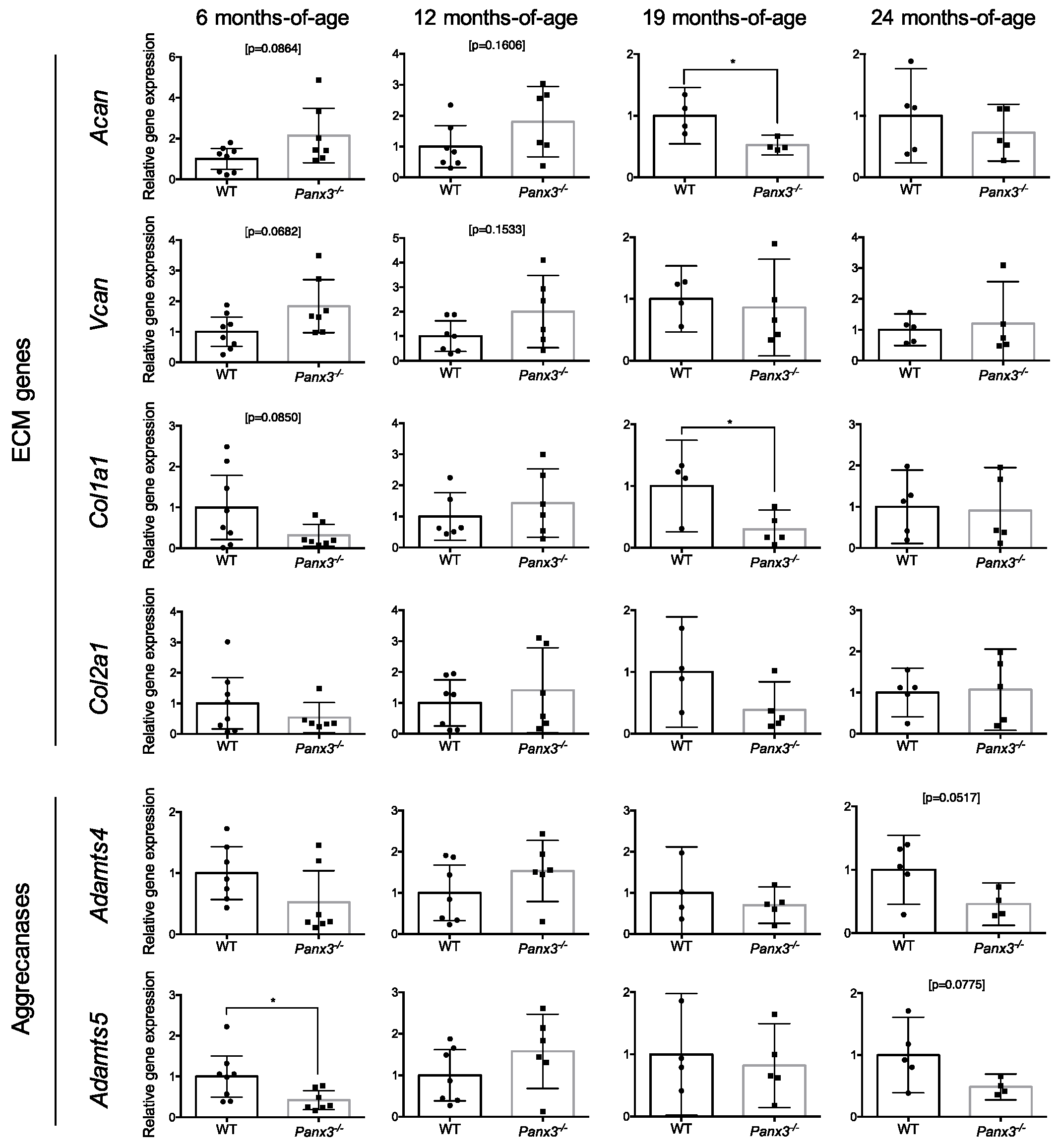
2.2. Loss of Panx3 Is Associated with Altered Gene Expression in the IVD
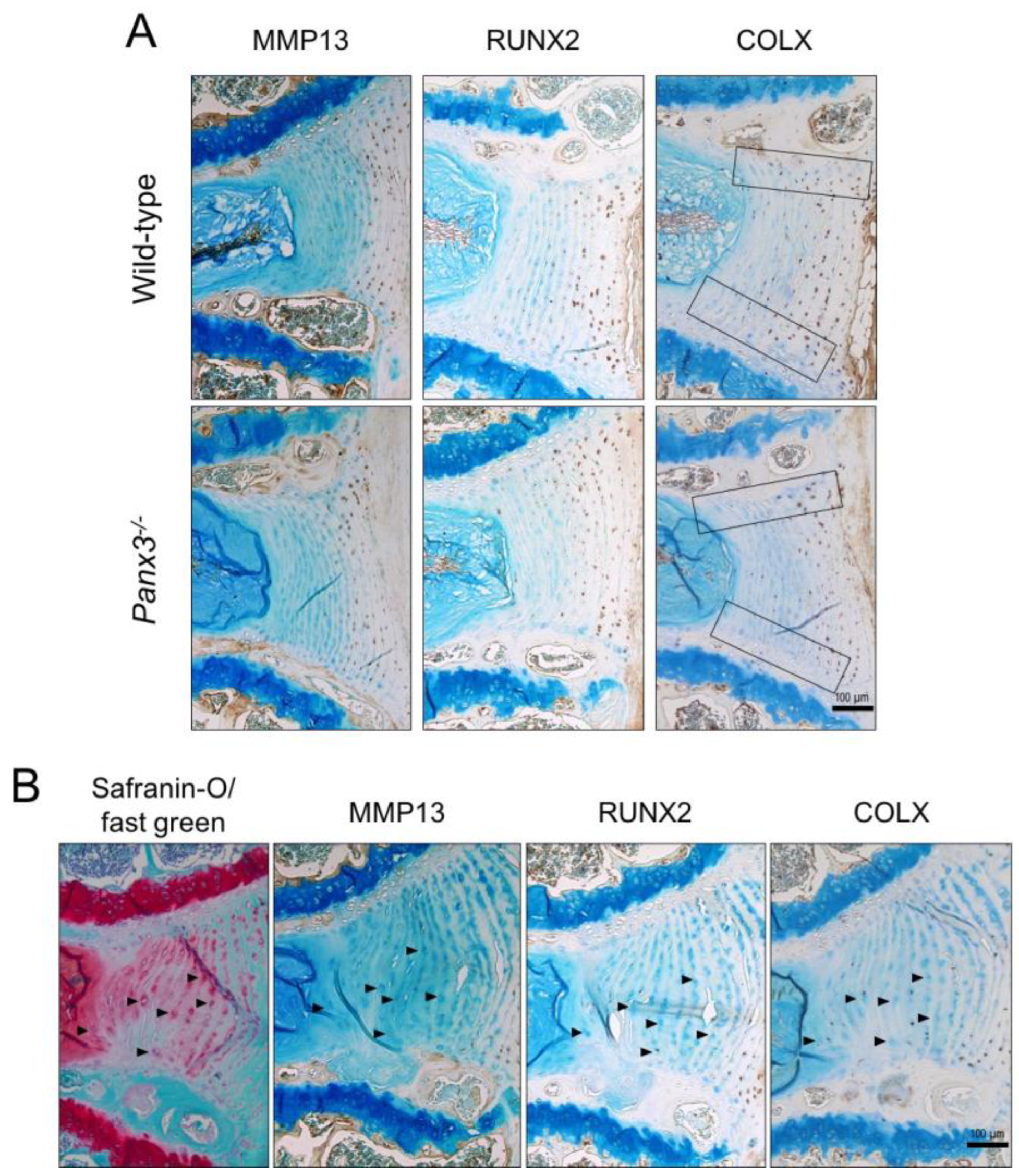
2.3. Loss of Panx3 Alters the Localization of COLX in the IVD
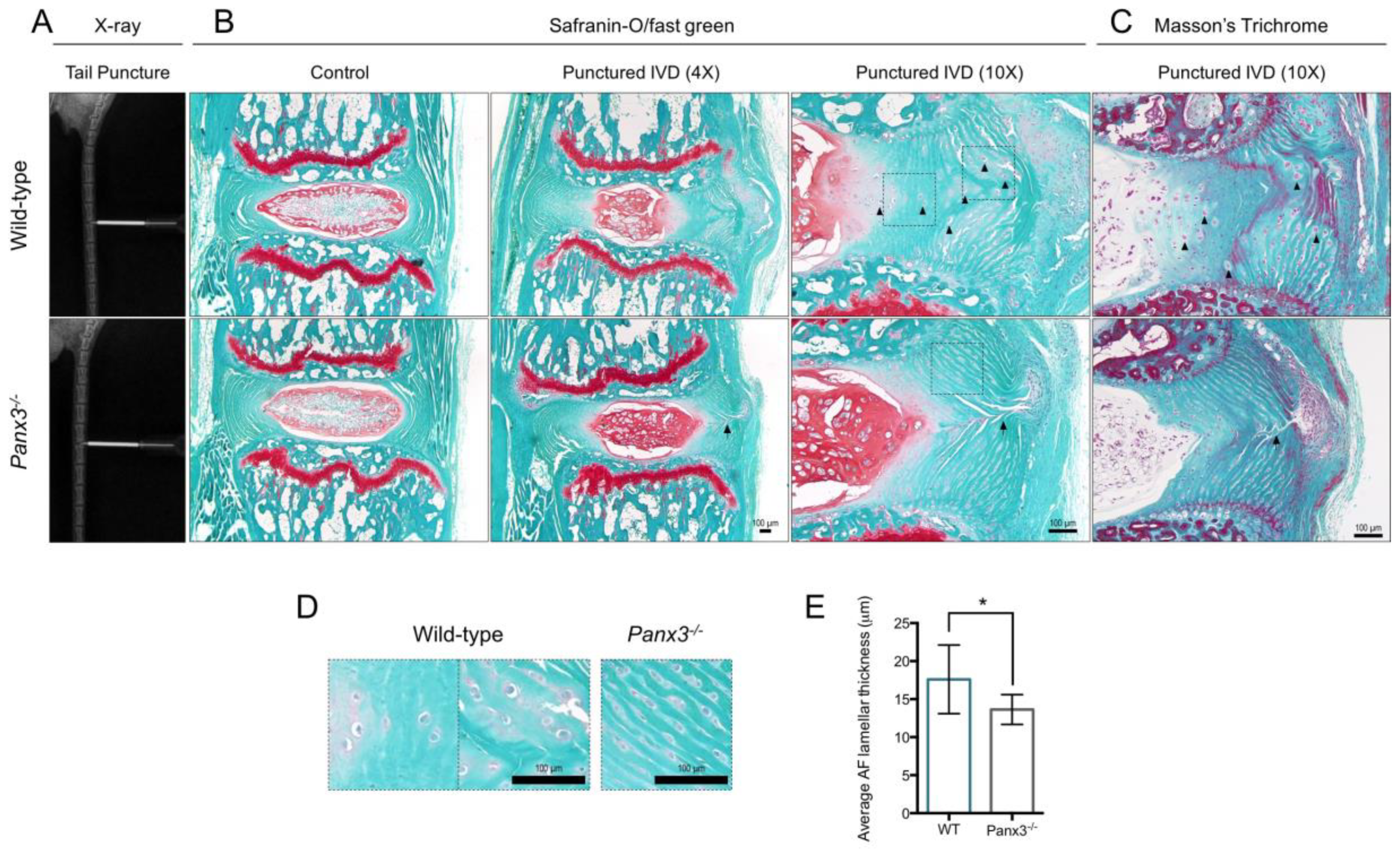
2.4. Loss of Panx3 Is Associated with Maintenance of AF Tissue Integrity Following IVD Injury
2.5. Loss of Panx3 Accelerates NP Degeneration in IVDs Adjacent to Site of Puncture
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Gene Expression Analysis
4.3. Western Blot Analysis
4.4. Histology
4.5. Immunohistochemical Analysis
4.6. Percutaneous IVD Needle Puncture
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| Acan | Aggrecan |
| ADAMTS-4 | A disintegrin and metalloproteinase with thrombospondin motifs 4 |
| ADAMTS-5 | A disintegrin and metalloproteinase with thrombospondin motifs 5 |
| AF | Annulus fibrosus |
| CEP | Cartilaginous endplates |
| CI | Confidence interval |
| Col1a1 | Collagen, type I, alpha 1 |
| Col10a1 | Collagen, type X, alpha1 |
| COLX | Collagen type X |
| Col2a1 | Collagen, type II, alpha 1 |
| ECM | Extracellular matrix |
| GAG | Glycosaminoglycan |
| IVD | Intervertebral disc |
| MMP-13 | Matrix metalloproteinase-13 |
| NP | Nucleus pulposus |
| OA | Osteoarthritis |
| Panx3 | Pannexin 3 |
| Panx3-/- | Whole-body Panx3 knockout |
| PBS | Phosphate buffer saline |
| qPCR | quantitative polymerase chain reaction |
| Rps29 | Ribosomal protein S29 |
| Runx2 | Runt-related transcription factor 2 |
| Vcan | Versican |
| WT | Wild-type |
References
- Vos, T.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; Aboyans, V.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [Green Version]
- Asche, C.V.; Kirkness, C.S.; McAdam-Marx, C.; Fritz, J.M. The societal costs of low back pain: Data published between 2001 and 2007. J. Pain Palliat. Care Pharmacother. 2007, 21, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.M.C.; Karppinen, J.; Chan, D.; Ho, D.W.H.; Song, Y.-Q.; Sham, P.; Cheah, K.S.E.; Leong, J.C.Y.; Luk, K.D.K. Prevalence and Pattern of Lumbar Magnetic Resonance Imaging Changes in a Population Study of One Thousand Forty-Three Individuals. Spine 2009, 34, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Popham, M.; Malkin, I.; Sambrook, P.N.; MacGregor, A.J.; Spector, T.; Williams, F.M.K. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: The UK Twin Spine Study. Ann. Rheum. Dis. 2011, 70, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, D.; Karppinen, J.; Mok, F.; Fong, D.Y.T.; Luk, K.D.K.; Cheung, K.M.C. A population-based study of juvenile disc degeneration and its association with overweight and obesity, low back pain, and diminished functional status. J. Bone Jt. Surg. Ser. A 2011, 93, 662–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, M.A.; Roughley, P.J. What is intervertebral disc degeneration, and what causes it? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef] [Green Version]
- Jensen, G.M. Biomechanics of the lumbar intervertebral disc: A review. Phys. Ther. 1980, 60, 765–773. [Google Scholar] [CrossRef]
- Urban, J.P.G.; Smith, S.; Fairbank, J.C.T. Nutrition of the intervertebral disc. Spine 2004, 29, 2700–2709. [Google Scholar] [CrossRef]
- Battie, M.C.; Videman, T.; Gibbons, L.E.; Fisher, L.; Manninen, H.; Gill, K. Determinants of lumbar disc degeneration: A study relating lifetime exposure and magnetic resonance imaging findings in identical twins. Spine 1995, 20, 2601–2612. [Google Scholar] [CrossRef]
- Buckwalter, J.A. Aging and degeneration of the human intervertebral disc. Spine 1995, 20, 1307–1314. [Google Scholar] [CrossRef]
- Smith, L.J.; Nerurkar, N.L.; Choi, K.-S.; Harfe, B.D.; Elliott, D.M. Degeneration and regeneration of the intervertebral disc: Lessons from development. Dis. Model. Mech. 2011, 4, 31–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boxberger, J.I.; Sen, S.; Yerramalli, C.S.; Elliott, D.M. Nucleus pulposus glycosaminoglycan content is correlated with axial mechanics in rat lumbar motion segments. J. Orthop. Res. 2006, 24, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Le Maitre, C.L.; Pockert, A.P.; Buttle, D.J.; Freemont, A.J.; Hoyland, J.A. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem. Soc. Trans. 2007, 35, 652–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, M.A.; McNally, D.S.; Dolan, P. “Stress” distributions inside intervertebral discs. J. Bone Jt. Surg. 1996, 78, 965–972. [Google Scholar] [CrossRef]
- Tam, V.; Chan, W.C.W.; Leung, V.Y.L.; Cheah, K.S.E.; Cheung, K.M.C.; Sakai, D.; Mccann, M.R.; Bedore, J.; Séguin, C.A.; Chan, D. Histological and reference system for the analysis of mouse intervertebral disc. J. Orthop. Res. 2018, 36, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Nakamura, T.; Doyle, A.; Ishikawa, M.; De Vega, S.; Fukumoto, S.; Yamada, Y. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J. Biol. Chem. 2010, 285, 18948–18958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, M.; Iwamoto, T.; Nakamura, T.; Doyle, A.; Fukumoto, S.; Yamada, Y. Pannexin 3 functions as an ER Ca 2+ channel, hemichannel, and gap junction to promote osteoblast differentiation. J. Cell Biol. 2011, 193, 1257–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, P.M.; Penuela, S.; Barr, K.; Khan, S.; Pin, C.L.; Welch, I.; Attur, M.; Abramson, S.B.; Laird, D.W.; Beier, F. Deletion of Panx3 prevents the development of surgically induced osteoarthritis. J. Mol. Med. 2015, 93, 845–856. [Google Scholar] [CrossRef] [Green Version]
- Bond, S.R.; Lau, A.; Penuela, S.; Sampaio, A.V.; Underhill, T.M.; Laird, D.W.; Naus, C.C. Pannexin 3 is a novel target for Runx2, expressed by osteoblasts and mature growth plate chondrocytes. J. Bone Miner. Res. 2011, 26, 2911–2922. [Google Scholar] [CrossRef]
- Penuela, S.; Bhalla, R.; Gong, X.-Q.; Cowan, K.N.; Celetti, S.J.; Cowan, B.J.; Bai, D.; Shao, Q.; Laird, D.W. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J. Cell Sci. 2007, 120, 3772–3783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.K.; Shin, J.O.; Baek, J.I.; Lee, J.; Bae, J.W.; Ankamerddy, H.; Kim, M.J.; Huh, T.L.; Ryoo, Z.Y.; Kim, U.K.; et al. Pannexin 3 is required for normal progression of skeletal development in vertebrates. FASEB J. 2015, 29, 4473–4484. [Google Scholar] [CrossRef] [PubMed]
- Pitsillides, A.A.; Beier, F. Cartilage biology in osteoarthritis—Lessons from developmental biology. Nat. Rev. Rheumatol. 2011, 7, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Veras, M.A.; McCann, M.R.; Tenn, N.A.; Séguin, C.A. Transcriptional profiling of the murine intervertebral disc and age-associated changes in the nucleus pulposus. Connect. Tissue Res. 2020, 61, 63–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Maitre, C.L.; Freemont, A.J.; Hoyland, J.A. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J. Pathol. 2004, 204, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Pockert, A.J.; Richardson, S.M.; Le Maitre, C.L.; Lyon, M.; Deakin, J.A.; Buttle, D.J.; Freemont, A.J.; Hoyland, J.A. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009, 60, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Tessier, S.; Tran, V.A.; Ottone, O.K.; Novais, E.J.; Doolittle, A.; DiMuzio, M.J.; Shapiro, I.M.; Risbud, M.V. TonEBP-deficiency accelerates intervertebral disc degeneration underscored by matrix remodeling, cytoskeletal rearrangements, and changes in proinflammatory gene expression. Matrix Biol. 2020, 87, 94–111. [Google Scholar] [CrossRef] [PubMed]
- Tolonen, J.; Gronblad, M.; Vanharanta, H.; Virri, J.; Guyer, R.; Rytomaa, T.; Karaharju, E. Growth factor expression in degenerated intervertebral disc tissue—An immunohistochemical analysis of transforming growth factor beta, fibroblast growth factor and platelet-derived growth factor. Eur. Spine J. 2006, 15, 588–596. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Leung, V.Y.; Luk, K.D.; Chan, D.; Cheung, K.M. Injury-induced sequential transformation of notochordal nucleus pulposus to chondrogenic and fibrocartilaginous phenotype in the mouse. J. Pathol. 2009, 218, 113–121. [Google Scholar] [CrossRef]
- Martin, J.T.; Gorth, D.J.; Beattie, E.E.; Harfe, B.D.; Smith, L.J.; Elliott, D.M. Needle puncture injury causes acute and long-term mechanical deficiency in a mouse model of intervertebral disc degeneration. J. Orthop. Res. 2013, 31, 1276–1282. [Google Scholar] [CrossRef]
- Postacchini, F.; Bellocci, M.; Massobrio, M. Morphologic changes in annulus fibrosus during aging. An ultrastructural study in rats. Spine 1984, 9, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Marchand, F.; Ahmed, A.M. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine 1990, 15, 402–410. [Google Scholar] [CrossRef]
- Cs-Szabo, G.; Juan, D.R.; Turumella, V.; Masuda, K.; Thonar, E.J.A.; An, H.S. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine 2002, 27, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, J.; Steffen, T.; Nelson, F.; Winterbottom, N.; Hollander, A.P.; Poole, R.A.; Aebi, M.; Alini, M. The human lumbar intervertebral disc: Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Investig. 1996, 98, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.T.; Lai, P.L.; Liao, J.C.; Fu, T.S.; Niu, C.C.; Chen, L.H.; Lee, M.S.; Chen, W.J.; Fang, H.C.; Ho, N.Y.J.; et al. Increased periostin gene expression in degenerative intervertebral disc cells. Spine J. 2013, 13, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Eyre, B.D.R.; Muir, H. Types I and II collagens in intervertebral disc. Biochem. J. 1976, 157, 267–270. [Google Scholar] [CrossRef]
- Sato, S.; Kimura, A.; Ozdemir, J.; Asou, Y.; Miyazaki, M.; Jinno, T.; Ae, K.; Liu, X.; Osaki, M.; Takeuchi, Y.; et al. The distinct role of the runx proteins in chondrocyte differentiation and intervertebral disc degeneration: Findings in murine models and in human disease. Arthritis Rheum. 2008, 58, 2764–2775. [Google Scholar] [CrossRef] [PubMed]
- Rutges, J.P.H.J.; Duit, R.A.; Kummer, J.A.; Oner, F.C.; van Rijen, M.H.; Verbout, A.J.; Castelein, R.M.; Dhert, W.J.A.; Creemers, L.B. Hypertrophic differentiation and calcification during intervertebral disc degeneration. Osteoarthr. Cartil. 2010, 18, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Bond, S.R.; Naus, C.C. The pannexins: Past and present. Front. Physiol. 2014, 5, 58. [Google Scholar] [CrossRef] [Green Version]
- Penuela, S.; Gehi, R.; Laird, D.W. The biochemistry and function of pannexin channels. Biochim. Biophys. Acta 2013, 1828, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Yorgan, T.A.; Peters, S.; Amling, M.; Schinke, T. Osteoblast-specific expression of Panx3 is dispensable for postnatal bone remodeling. Bone 2019, 127, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Caskenette, D.; Penuela, S.; Lee, V.; Barr, K.; Beier, F.; Laird, D.W.; Willmore, K.E. Global deletion of Panx3 produces multiple phenotypic effects in mouse humeri and femora. J. Anat. 2016, 228, 746–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, M.; Williams, G.L.; Ikeuchi, T.; Sakai, K.; Fukumoto, S.; Yamada, Y. Pannexin 3 and connexin 43 modulate skeletal development through their distinct functions and expression patterns. J. Cell Sci. 2016, 129, 1018–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, M.; Yamada, Y. The role of Pannexin 3 in bone biology. J. Dent. Res. 2017, 96, 372–379. [Google Scholar] [CrossRef]
- Abitbol, J.M.; O’Donnell, B.L.; Wakefield, C.B.; Jewlal, E.; Kelly, J.J.; Barr, K.; Willmore, K.E.; Allman, B.L.; Penuela, S. Double deletion of Panx1 and Panx3 affects skin and bone but not hearing. J. Mol. Med. 2019, 97, 723–736. [Google Scholar] [CrossRef]
- Penuela, S.; Kelly, J.J.; Churko, J.M.; Barr, K.J.; Berger, A.C.; Laird, D.W. Panx1 regulates cellular properties of keratinocytes and dermal fibroblasts in skin development and wound healing. J. Investig. Dermatol. 2014, 134, 2026–2035. [Google Scholar] [CrossRef] [Green Version]
- Abitbol, J.M.; Kelly, J.J.; Barr, K.; Schormans, A.L.; Laird, D.W.; Allman, B.L. Differential effects of pannexins on noise-induced hearing loss. Biochem. J. 2016, 473, 4665–4680. [Google Scholar] [CrossRef]
- Park, P.; Garton, H.J.; Gala, V.C.; Hoff, J.T.; McGillicuddy, J.E. Adjacent segment disease after lumbar or lumbosacral fusion: Review of the literature. Spine 2004, 29, 1938–1944. [Google Scholar] [CrossRef]
- Ii, H.; Warraich, S.; Tenn, N.; Quinonez, D.; Holdsworth, D.W.; Hammond, J.R.; Dixon, S.J.; Séguin, C.A. Disruption of biomineralization pathways in spinal tissues of a mouse model of diffuse idiopathic skeletal hyperostosis. Bone 2016, 90, 37–49. [Google Scholar] [CrossRef]
- McCann, M.R.; Patel, P.; Beaucage, K.L.; Xiao, Y.; Bacher, C.; Siqueira, W.L.; Holdsworth, D.W.; Dixon, S.J.; Séguin, C.A. Acute vibration induces transient expression of anabolic genes in the Murine intervertebral disc. Arthritis Rheum. 2013, 65, 1853–1864. [Google Scholar] [CrossRef] [Green Version]
- Kerr, G.J.; McCann, M.R.; Branch, J.K.; Ratneswaran, A.; Pest, M.A.; Holdsworth, D.W.; Beier, F.; Dixon, S.J.; Séguin, C.A. C57BL/6 mice are resistant to joint degeneration induced by whole-body vibration. Osteoarthr. Cartil. 2017, 25, 421–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veras, M.A.; Tenn, N.A.; Kuljanin, M.; Lajoie, G.A.; Hammond, J.R.; Dixon, S.J.; Séguin, C.A. Loss of ENT1 increases cell proliferation in the annulus fibrosus of the intervertebral disc. J. Cell. Physiol. 2019, 234, 13705–13719. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Ma, X.; Yasen, M.; Mauck, R.L.; Qin, L.; Shofer, F.S.; Smith, L.J.; Pacifici, M.; Enomoto-Iwamoto, M.; Zhang, Y. Intervertebral disc degeneration in a percutaneous mouse tail injury model. Am. J. Phys. Med. Rehabil. 2018, 97, 170–177. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serjeant, M.; Moon, P.M.; Quinonez, D.; Penuela, S.; Beier, F.; Séguin, C.A. The Role of Panx3 in Age-Associated and Injury-Induced Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2021, 22, 1080. https://doi.org/10.3390/ijms22031080
Serjeant M, Moon PM, Quinonez D, Penuela S, Beier F, Séguin CA. The Role of Panx3 in Age-Associated and Injury-Induced Intervertebral Disc Degeneration. International Journal of Molecular Sciences. 2021; 22(3):1080. https://doi.org/10.3390/ijms22031080
Chicago/Turabian StyleSerjeant, Meaghan, Paxton M. Moon, Diana Quinonez, Silvia Penuela, Frank Beier, and Cheryle A. Séguin. 2021. "The Role of Panx3 in Age-Associated and Injury-Induced Intervertebral Disc Degeneration" International Journal of Molecular Sciences 22, no. 3: 1080. https://doi.org/10.3390/ijms22031080




