A New Insight into the Potential Role of Tryptophan-Derived AhR Ligands in Skin Physiological and Pathological Processes
Abstract
1. Introduction
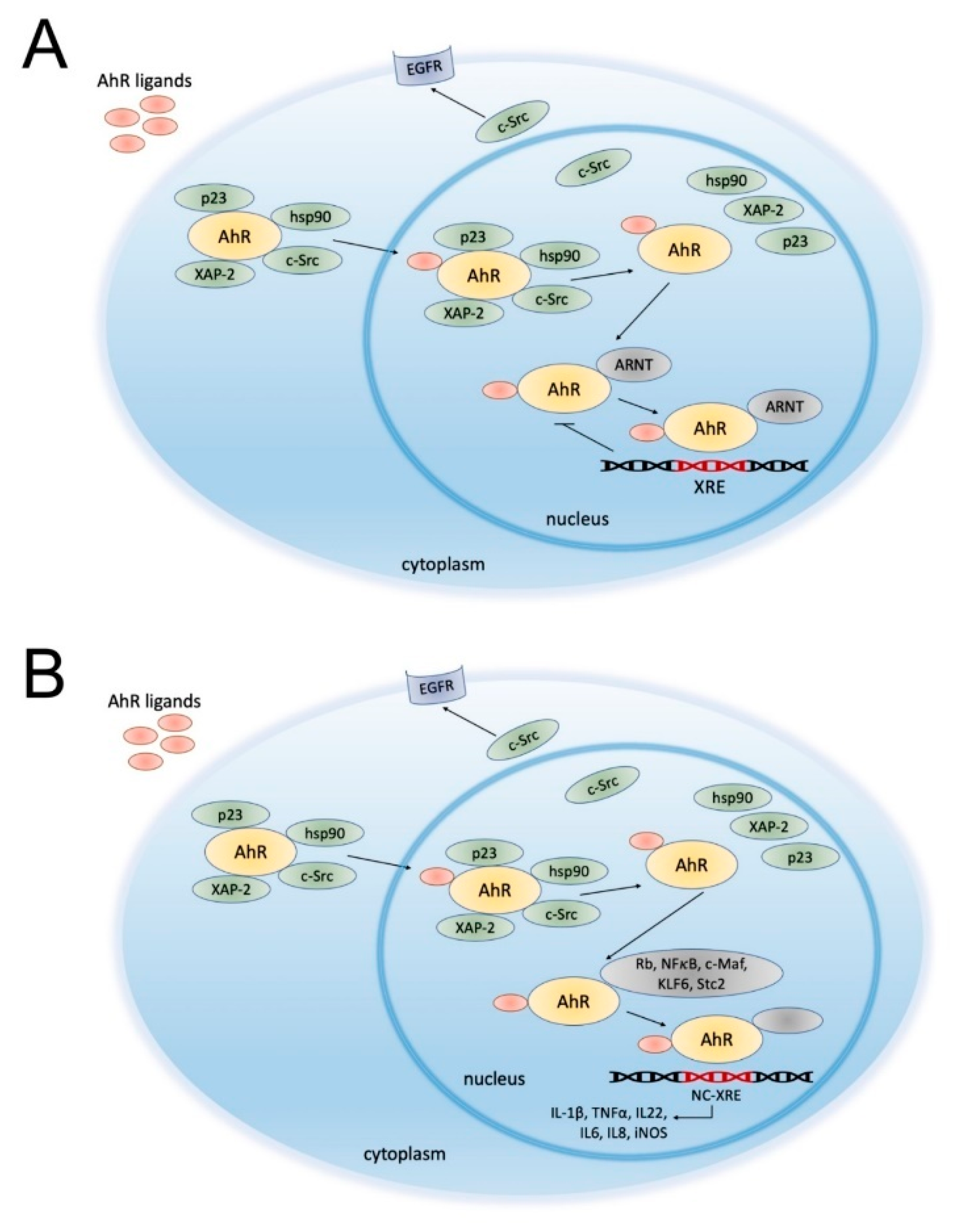
2. Aryl Hydrocarbon Receptor (AhR)
3. The Role of AhR in Skin Physiology
4. AhR and Skin Pathological Processes
A Double Agent: The Role of AhR in Oxidative Stress
5. Role of AhR in Inflammatory Skin Diseases
5.1. Atopic Dermatitis
5.2. Psoriasis
6. Skin Pigmentation Disorders
6.1. Hyperpigmentation
6.2. Vitiligo
7. Skin Appendage Disorder: Chloracne
8. Skin Cancer
8.1. Squamous Cell Carcinoma
8.2. Melanoma
9. The Role of Tryptophan-Derived AhR Ligands in Skin Homeostasis
- Exogenous/synthetic ligands (i.e., TCDD, biphenyls, DMBA, methylcholanthrene, and BaP);
- Exogenous/natural compounds, found in or metabolized from dietary plants (i.e., resveratrol and other glucosinolates, flavonoids, indolcarbinols, and kynurenic acid);
- Endogenous ligands formed in the body (i.e., kynurenine, kynurenic acid, ITE, a tryptophan–cysteine dimer, and FICZ).
9.1. FICZ
9.2. Kynurenine
9.3. Kynurenic Acid
9.4. Skin Microbiome Metabolites
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | atopic dermatitis |
| AhR | aryl hydrocarbon receptor |
| AhRR | aryl hydrocarbon receptor repressor |
| Akt | protein kinase B |
| AMPK | AMP-activated protein kinase |
| ARNT | aryl hydrocarbon receptor nuclear translocator |
| ATP | adenosine triphosphate |
| BaP | benzo[a]pyrene |
| cAMP | 3′5′-cyclic adenosine monophosphate |
| CCL17 | chemokine (C-C motif) ligand 17 |
| CCL22 | chemokine (C-C motif) ligand 22 |
| CDK | cyclin-dependent kinase |
| DC | dendritic cell |
| DMBA | 7,12-dimethylbenz[a]anthracene |
| EDC | epidermal differentiation complex |
| EGFR | epidermal growth factor receptor |
| ER | estrogen receptor |
| ERK | extracellular signal-regulated kinase |
| FICZ | 6-formylindolo[3,2-b]carbazole |
| FLG | Filaggrin |
| HIF | hypoxia-induced factor |
| HNSC | head and neck squamous cell carcinoma |
| HMOX1 | heme oxygenase 1 |
| HSP | heat shock protein |
| IaId | indole-3-aldehyde |
| IDO | indoleamine 2,3-dioxygenase |
| IFN-γ | interferon gamma |
| ITE | 2-(1H-Indol-3-ylcarbonyl)-4-thiazolecarboxylic acid methyl ester |
| KAT | kynurenine aminotransferases |
| KLF6 | Kruppel-like factor 6 |
| KYNA | kynurenic acid |
| KYNU | kynureninase |
| LC | Langerhans cell |
| LOR | Loricrin |
| LPR6 | LDL receptor related protein 6 |
| MAPK | mitogen-activated protein kinase |
| MITF | microphtalmia-associated transcription factor |
| Msrebp-1 | mature sterol-binding protein |
| NAD | nicotinamide adenine dinucleotide |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| Nrf2 | nuclear factor-erythroid 2-related factor-2 |
| PAH | polycyclic aromatic hydrocarbons |
| PBMC | peripheral blood mononuclear cell |
| PCB | polychlorinated biphenyls |
| PCDD | polychlorinated dibenzo-p-dioxins |
| PCDF | polychlorinated dibenzofurans |
| PI3K | phosphoinositide 3-kinase |
| PKA | protein kinase A |
| PKC | protein kinase C |
| PPAR-δ | peroxisome proliferator-activated receptor-δ |
| PTD | photodynamic therapy |
| Rb | retinoblastoma protein |
| ROS | reactive oxygen species |
| SCC | squamous cell carcinoma |
| siRNA | small interfering RNA |
| SKCN | skin cutaneous melanoma |
| SOS1 | son of sevenless 1 |
| STAT | signal transducer and activator of transcription |
| TCDD | 2,3,7,8-tetrachlorodibenzo-p-dioxin |
| TDO | tryptophan 2,3-dioxygenase |
| TGF-β | transforming growth factor beta |
| TNF-α | tumor necrosis factor alpha |
| Treg | T regulatory cell |
| TRP | tryptophan |
| TSLP | thymic stromal lymphopoietin |
| TYR | tyrosinase |
| TYRP | tyrosinase-related protein |
| VEGF | vascular endothelial growth factor |
| XAP2 | the hepatitis B virus X-associated protein 2 |
| XRE | xenobiotic-responsive element |
References
- Abel, J.; Haarmann-Stemmann, T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol. Chem. 2010, 391, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Esser, C.; Rannug, A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev. 2015, 67, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Denison, M.S.; Nagy, S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 309–334. [Google Scholar] [CrossRef]
- Sheipouri, D.; Braidy, N.; Guillemin, G.J. Kynurenine pathway in skin cells: Implications for UV-induced skin damage. Int. J. Tryptophan. Res. 2012, 5, 15–25. [Google Scholar] [CrossRef]
- Mimura, J.; Fujii-Kuriyama, Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim. et Biophys. Acta Gen. Subj. 2003. [Google Scholar] [CrossRef]
- Kawasaki, H.; Chang, H.W.; Tseng, H.C.; Hsu, S.C.; Yang, S.J.; Hung, C.H.; Zhou, Y.; Huang, S.K. A tryptophan metabolite, kynurenine, promotes mast cell activation through aryl hydrocarbon receptor. Allergy 2014, 69, 445–452. [Google Scholar] [CrossRef]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front. Immunol. 2018, 8, 1957. [Google Scholar] [CrossRef]
- Tanaka, Y.; Uchi, H.; Hashimoto-Hachiya, A.; Furue, M. Tryptophan Photoproduct FICZ Upregulates IL1A, IL1B, and IL6 Expression via Oxidative Stress in Keratinocytes. Oxid. Med. Cell Longev. 2018, 2018, 9298052. [Google Scholar] [CrossRef]
- Nakahara, T.; Mitoma, C.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Uchi, H.; Yan, X.; Hachisuka, J.; Chiba, T.; Esaki, H.; et al. Antioxidant opuntia ficus-indica extract activates AHR-NRF2 signaling and upregulates filaggrin and loricrin expression in human keratinocytes. J. Med. Food 2015, 18, 1143–1149. [Google Scholar] [CrossRef]
- Takei, K.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Nakahara, T.; Furue, M. Cynaropicrin attenuates UVB-induced oxidative stress via the AhR-Nrf2-Nqo1 pathway. Toxicol. Lett. 2015, 234, 74–80. [Google Scholar] [CrossRef]
- Uchi, H.; Yasumatsu, M.; Morino-Koga, S.; Mitoma, C.; Furue, M. Inhibition of aryl hydrocarbon receptor signaling and induction of NRF2-mediated antioxidant activity by cinnamaldehyde in human keratinocytes. J. Dermatol. Sci. 2017, 85, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Krämer, H.J.; Podobinska, M.; Bartsch, A.; Battmann, A.; Thoma, W.; Bernd, A.; Kummer, W.; Irlinger, B.; Steglich, W.; Mayser, P. Malassezin, a novel agonist of the aryl hydrocarbon receptor from the yeast Malassezia furfur, induces apoptosis in primary human melanocytes. Chembiochem 2005, 6, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Wincent, E.; Bengtsson, J.; Bardbori, A.M.; Alsberg, T.; Luecke, S.; Rannug, U.; Rannug, A. Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 4479–4484. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.A.; Elferink, C.J. Timing is everything: Consequences of transient and sustained AhR activity. Biochem. Pharmacol. 2009, 77, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.V.; Boverhof, D.R.; Burgoon, L.D.; Fielden, M.R.; Zacharewski, T.R. Comparative analysis of dioxin response elements in human, mouse and rat genomic sequences. Nucleic Acids Res. 2004, 32, 4512–4523. [Google Scholar] [CrossRef] [PubMed]
- Frericks, M.; Meissner, M.; Esser, C. Microarray analysis of the AHR system: Tissue-specific flexibility in signal and target genes. Toxicol. Appl. Pharmacol. 2007, 220, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Wakx, A.; Nedder, M.; Tomkiewicz-Raulet, C.; Dalmasso, J.; Chissey, A.; Boland, S.; Vibert, F.; Degrelle, S.A.; Fournier, T.; Coumoul, X.; et al. Expression, localization, and activity of the aryl hydrocarbon receptor in the human placenta. Int. J. Mol. Sci. 2018, 19, 3762. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Marín, N.; Barrasa, E.; Morales-Hernández, A.; Paniagua, B.; Blanco-Fernández, G.; Merino, J.M.; Fernández-Salguero, P.M. Dioxin receptor adjusts liver regeneration after acute toxic injury and protects against liver carcinogenesis. Sci. Rep. 2017, 7, 10420. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.V.; Su, G.H.; Reddy, J.K.; Simon, M.C.; Bradfield, C.A. Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proc. Natl. Acad. Sci. USA 1996, 93, 6731–6736. [Google Scholar] [CrossRef] [PubMed]
- Carreira, V.S.; Fan, Y.; Wang, Q.; Zhang, X.; Kurita, H.; Ko, C.I.; Naticchioni, M.; Jiang, M.; Koch, S.; Medvedovic, M.; et al. Ah receptor signaling controls the expression of cardiac development and homeostasis genes. Toxicol. Sci. 2015, 147, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Krock, B.L.; Eisinger-Mathason, T.S.; Giannoukos, D.N.; Shay, J.E.; Gohil, M.; Lee, D.S.; Nakazawa, M.S.; Sesen, J.; Skuli, N.; Simon, M.C. The aryl hydrocarbon receptor nuclear translocator is an essential regulator of murine hematopoietic stem cell viability. Blood 2015, 125, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, Q.; Ma, Y.; Li, L.; Yu, K.; Zhang, Z.; Chen, G.; Li, X.; Xiao, W.; Xu, P.; et al. Aryl hydrocarbon receptor activation modulates intestinal epithelial barrier function by maintaining tight junction integrity. Int. J. Biol. Sci. 2018, 14, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Garmyn, M.; van Laethem, A. The Aryl hydrocarbon receptor: An illuminating effector of the UVB response. Sci. STKE 2007, 2007, pe49. [Google Scholar] [CrossRef] [PubMed]
- Levine-Fridman, A.; Chen, L.; Elferink, C.J. Cytochrome P4501A1 promotes G1 phase cell cycle progression by controlling aryl hydrocarbon receptor activity. Mol. Pharmacol. 2004, 65, 461–469. [Google Scholar] [CrossRef]
- Ishihara, Y.; Kado, S.Y.; Hoeper, C.; Harel, S.; Vogel, C.F.A. Role of NF-kB RelB in Aryl hydrocarbon receptor-mediated ligand specific effects. Int. J. Mol. Sci. 2019, 20, 2652. [Google Scholar] [CrossRef]
- Kim, D.W.; Gazourian, L.; Quadri, S.A.; Romieu-Mourez, R.; Sherr, D.H.; Sonenshein, G.E. The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene 2000, 19, 5498–5506. [Google Scholar] [CrossRef]
- Kimura, A.; Naka, T.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc. Natl. Acad. Sci. USA 2008, 105, 9721–9726. [Google Scholar] [CrossRef]
- Swedenborg, E.; Pongratz, I. AhR and ARNT modulate ER signaling. Toxicology 2010, 268, 132–138. [Google Scholar] [CrossRef]
- Evans, B.R.; Karchner, S.I.; Allan, L.L.; Pollenz, R.S.; Tanguay, R.L.; Jenny, M.J.; Sherr, D.H.; Hahn, M.E. Repression of aryl hydrocarbon receptor (AHR) signaling by AHR repressor: Role of DNA binding and competition for AHR nuclear translocator. Mol. Pharmacol. 2008, 73, 387–398. [Google Scholar] [CrossRef]
- Hahn, M.E.; Karchner, S.I.; Evans, B.R.; Franks, D.G.; Merson, R.R.; Lapseritis, J.M. Unexpected diversity of aryl hydrocarbon receptors in non-mammalian vertebrates: Insights from comparative genomics. J. Exp. Zool. A Comp. Exp. Biol. 2006, 305, 693–706. [Google Scholar] [CrossRef]
- Wilson, S.R.; Joshi, A.D.; Elferink, C.J. The tumor suppressor Kruppel-like factor 6 is a novel aryl hydrocarbon receptor DNA binding partner. J. Pharmacol. Exp. Ther. 2013, 345, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.P.; Li, H.; Mitchell, K.A.; Joshi, A.D.; Elferink, C.J. Ah receptor-mediated suppression of liver regeneration through NC-XRE-driven p21Cip1 expression. Mol. Pharmacol. 2014, 85, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.D.; Carter, D.E.; Harper, T.A., Jr.; Elferink, C.J. Aryl hydrocarbon receptor-dependent stanniocalcin 2 induction by cinnabarinic acid provides cytoprotection against endoplasmic reticulum and oxidative stress. J. Pharmacol. Exp. Ther. 2015, 353, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.; Sciullo, E.; Matsumura, F. Involvement of RelB in aryl hydrocarbon receptor-mediated induction of chemokines. Biochem. Biophys. Res. Commun. 2007, 363, 722–726. [Google Scholar] [CrossRef]
- Ge, N.L.; Elferink, C.J. A direct interaction between the aryl hydrocarbon receptor and retinoblastoma protein. Linking dioxin signaling to the cell cycle. J. Biol. Chem. 1998, 273, 22708–22713. [Google Scholar] [CrossRef]
- Huang, G.; Elferink, C.J. A novel nonconsensus xenobiotic response element capable of mediating aryl hydrocarbon receptor-dependent gene expression. Mol. Pharmacol. 2012, 81, 338–347. [Google Scholar] [CrossRef]
- Fritsche, E.; Schäfer, C.; Calles, C.; Bernsmann, T.; Bernshausen, T.; Wurm, M.; Hübenthal, U.; Cline, J.E.; Hajimiragha, H.; Schroeder, P.; et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc. Natl. Acad. Sci. USA 2007, 104, 8851–8856. [Google Scholar] [CrossRef]
- Soshilov, A.; Denison, M.S. Role of the Per/Arnt/Sim domains in ligand-dependent transformation of the aryl hydrocarbon receptor. J. Biol. Chem. 2008, 283, 32995–33005. [Google Scholar] [CrossRef]
- Fernandez-Salguero, P.M.; Hilbert, D.M.; Rudikoff, S.; Ward, J.M.; Gonzalez, F.J. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol. Appl. Pharmacol. 1996, 140, 173–179. [Google Scholar] [CrossRef]
- Kolluri, S.K.; Weiss, C.; Koff, A.; Göttlicher, M. p27(Kip1) induction and inhibition of proliferation by the intracellular Ah receptor in developing thymus and hepatoma cells. Genes. Dev. 1999, 13, 1742–1753. [Google Scholar] [CrossRef]
- Pang, P.H.; Lin, Y.H.; Lee, Y.H.; Hou, H.H.; Hsu, S.P.; Juan, S.H. Molecular mechanisms of p21 and p27 induction by 3-methylcholanthrene, an aryl-hydrocarbon receptor agonist, involved in antiproliferation of human umbilical vascular endothelial cells. J. Cell Physiol. 2008, 215, 161–171. [Google Scholar] [CrossRef]
- Barnes-Ellerbe, S.; Knudsen, K.E.; Puga, A. 2,3,7,8-Tetrachlorodibenzo-p-dioxin blocks androgen-dependent cell proliferation of LNCaP cells through modulation of pRB phosphorylation. Mol. Pharmacol. 2004, 66, 502–511. [Google Scholar] [CrossRef]
- Huang, G.; Elferink, C.J. Multiple mechanisms are involved in Ah receptor-mediated cell cycle arrest. Mol. Pharmacol. 2005, 67, 88–96. [Google Scholar] [CrossRef]
- Marlowe, J.L.; Knudsen, E.S.; Schwemberger, S.; Puga, A. The aryl hydrocarbon receptor displaces p300 from E2F-dependent promoters and represses S phase-specific gene expression. J. Biol. Chem. 2004, 279, 29013–29022. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Smith, R., 3rd; Safe, S. Aryl hydrocarbon receptor gene silencing with small inhibitory RNA differentially modulates Ah-responsiveness in MCF-7 and HepG2 cancer cells. Mol. Pharmacol. 2003, 63, 1373–1381. [Google Scholar] [CrossRef]
- Watabe, Y.; Nazuka, N.; Tezuka, M.; Shimba, S. Aryl hydrocarbon receptor functions as a potent coactivator of E2F1-dependent trascription activity. Biol. Pharm. Bull. 2010, 33, 389–397. [Google Scholar] [CrossRef]
- Pierre, S.; Bats, A.S.; Chevallier, A.; Bui, L.C.; Ambolet-Camoit, A.; Garlatti, M.; Aggerbeck, M.; Barouki, R.; Coumoul, X. Induction of the Ras activator Son of Sevenless 1 by environmental pollutants mediates their effects on cellular proliferation. Biochem. Pharmacol. 2011, 81, 304–313. [Google Scholar] [CrossRef]
- Mulero-Navarro, S.; Pozo-Guisado, E.; Pérez-Mancera, P.A.; Alvarez-Barrientos, A.; Catalina-Fernández, I.; Hernández-Nieto, E.; Sáenz-Santamaria, J.; Martínez, N.; Rojas, J.M.; Sánchez-García, I.; et al. Immortalized mouse mammary fibroblasts lacking dioxin receptor have impaired tumorigenicity in a subcutaneous mouse xenograft model. J. Biol. Chem. 2005, 280, 28731–28741. [Google Scholar] [CrossRef]
- Diry, M.; Tomkiewicz, C.; Koehle, C.; Coumoul, X.; Bock, K.W.; Barouki, R.; Transy, C. Activation of the dioxin/aryl hydrocarbon receptor (AhR) modulates cell plasticity through a JNK-dependent mechanism. Oncogene 2006, 25, 5570–5574. [Google Scholar] [CrossRef]
- Hanieh, H.; Mohafez, O.; Hairul-Islam, V.I.; Alzahrani, A.; Ismail, M.B.; Thirugnanasambantham, K. Novel aryl hydrocarbon receptor agonist suppresses migration and invasion of breast cancer cells. PLoS ONE 2016, 11, e0167650. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.H.; Lee, S.O.; Pfent, C.; Safe, S. The aryl hydrocarbon receptor ligand omeprazole inhibits breast cancer cell invasion and metastasis. BMC Cancer 2014, 14, 498. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Saito, N.; Zhao, S.; Hiruta, N.; Park, Y.; Bujo, H.; Nemoto, K.; Kanno, Y. Heregulin-induced cell migration is promoted by aryl hydrocarbon receptor in HER2-overexpressing breast cancer cells. Exp. Cell Res. 2018, 366, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Stanford, E.A.; Ramirez-Cardenas, A.; Wang, Z.; Novikov, O.; Alamoud, K.; Koutrakis, P.; Mizgerd, J.P.; Genco, C.A.; Kukuruzinska, M.; Monti, S.; et al. Role for the aryl hydrocarbon receptor and diverse ligands in oral squamous cell carcinoma migration and tumorigenesis. Mol. Cancer Res. 2016, 14, 696–706. [Google Scholar] [CrossRef]
- Vogel, C.F.; Sciullo, E.; Li, W.; Wong, P.; Lazennec, G.; Matsumura, F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol. Endocrinol. 2007, 21, 2941–2955. [Google Scholar] [CrossRef]
- Tsuji, G.; Takahara, M.; Uchi, H.; Matsuda, T.; Chiba, T.; Takeuchi, S.; Yasukawa, F.; Moroi, Y.; Furue, M. Identification of ketoconazole as an AhR-Nrf2 activator in cultured human keratinocytes: The basis of its anti-inflammatory effect. J. Investig. Dermatol. 2012, 132, 59–68. [Google Scholar] [CrossRef]
- Haarmann-Stemmann, T.; Abel, J.; Fritsche, E.; Krutmann, J. The AhR-Nrf2 pathway in keratinocytes: On the road to chemoprevention? J. Investig. Dermatol. 2012, 132, 7–9. [Google Scholar] [CrossRef]
- Puga, A.; Hoffer, A.; Zhou, S.; Bohm, J.M.; Leikauf, G.D.; Shertzer, H.G. Sustained increase in intracellular free calcium and activation of cyclooxygenase-2 expression in mouse hepatoma cells treated with dioxin. Biochem. Pharmacol. 1997, 54, 1287–1296. [Google Scholar] [CrossRef]
- Li, W.; Matsumura, F. Significance of the nongenomic, inflammatory pathway in mediating the toxic action of TCDD to induce rapid and long-term cellular responses in 3T3-L1 adipocytes. Biochemistry 2008, 47, 13997–14008. [Google Scholar] [CrossRef]
- Matsumura, F. The significance of the nongenomic pathway in mediating inflammatory signaling of the dioxin-activated Ah receptor to cause toxic effects. Biochem. Pharmacol. 2009, 77, 608–626. [Google Scholar] [CrossRef]
- Vorrink, S.U.; Severson, P.L.; Kulak, M.V.; Futscher, B.W.; Domann, F.E. Hypoxia perturbs aryl hydrocarbon receptor signaling and CYP1A1 expression induced by PCB 126 in human skin and liver-derived cell lines. Toxicol. Appl. Pharmacol. 2014, 274, 408–416. [Google Scholar] [CrossRef]
- Wong, W.J.; Richardson, T.; Seykora, J.T.; Cotsarelis, G.; Simon, M.C. Hypoxia-inducible factors regulate filaggrin expression and epidermal barrier function. J. Investig. Dermatol. 2015, 135, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Mathew, L.K.; Sengupta, S.S.; Ladu, J.; Andreasen, E.A.; Tanguay, R.L. Crosstalk between AHR and Wnt signaling through R-Spondin1 impairs tissue regeneration in zebrafish. FASEB J. 2008, 22, 3087–3096. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.M.; Denison, M.S. Analysis of the antiestrogenic activity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in human ovarian carcinoma BG-1 cells. Mol. Pharmacol. 2002, 61, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Wang, F.; Porter, W.; Duan, R.; McDougal, A. Ah receptor agonists as endocrine disruptors: Antiestrogenic activity and mechanisms. Toxicol. Lett. 1998, 102–103, 343–347. [Google Scholar] [CrossRef]
- Denison, M.S.; Soshilov, A.A.; He, G.; DeGroot, D.E.; Zhao, B. Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci. 2011, 124, 1–22. [Google Scholar] [CrossRef]
- Esser, C.; Bargen, I.; Weighardt, H.; Haarmann-Stemmann, T.; Krutmann, J. Functions of the aryl hydrocarbon receptor in the skin. Semin. Immunopathol. 2013, 35, 677–691. [Google Scholar] [CrossRef]
- Schmidt, J.V.; Bradfield, C.A. Ah receptor signaling pathways. Annu. Rev. Cell Dev. Biol. 1996, 12, 55–89. [Google Scholar] [CrossRef]
- Dere, E.; Forgacs, A.L.; Zacharewski, T.R.; Burgoon, L.D. Genome-wide computational analysis of dioxin response element location and distribution in the human, mouse, and rat genomes. Chem. Res. Toxicol. 2011, 24, 494–504. [Google Scholar] [CrossRef]
- Mimura, J.; Ema, M.; Sogawa, K.; Fujii-Kuriyama, Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes. Dev. 1999, 13, 20–25. [Google Scholar] [CrossRef]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef]
- Tsuji, G.; Hashimoto-Hachiya, A.; Kiyomatsu-Oda, M.; Takemura, M.; Ohno, F.; Ito, T.; Morino-Koga, S.; Mitoma, C.; Nakahara, T.; Uchi, H.; et al. Aryl hydrocarbon receptor activation restores filaggrin expression via OVOL1 in atopic dermatitis. Cell Death Dis. 2017, 8, e2931. [Google Scholar] [CrossRef] [PubMed]
- Jux, B.; Kadow, S.; Esser, C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J. Immunol. 2009, 182, 6709–6717. [Google Scholar] [CrossRef] [PubMed]
- Tauchi, M.; Hida, A.; Negishi, T.; Katsuoka, F.; Noda, S.; Mimura, J.; Hosoya, T.; Yanaka, A.; Aburatani, H.; Fujii-Kuriyama, Y.; et al. Constitutive expression of aryl hydrocarbon receptor in keratinocytes causes inflammatory skin lesions. Mol. Cell Biol. 2005, 25, 9360–9368. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hirota, K.; Westendorf, A.M.; Buer, J.; Dumoutier, L.; Renauld, J.C.; Stockinger, B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 2008, 453, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Influence of light on aryl hydrocarbon receptor signaling and consequences in drug metabolism, physiology and disease. Expert Opin. Drug Metab. Toxicol. 2011, 7, 1267–1293. [Google Scholar] [CrossRef]
- Peng, F.; Xue, C.H.; Hwang, S.K.; Li, W.H.; Chen, Z.; Zhang, J.Z. Exposure to fine particulate matter associated with senile lentigo in Chinese women: A cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 355–360. [Google Scholar] [CrossRef]
- Guo, Y.L.; Yu, M.L.; Hsu, C.C.; Rogan, W.J. Chloracne, goiter, arthritis, and anemia after polychlorinated biphenyl poisoning: 14-year follow-Up of the Taiwan Yucheng cohort. Environ. Health Perspect. 1999, 107, 715–719. [Google Scholar] [CrossRef]
- Caputo, R.; Monti, M.; Ermacora, E.; Carminati, G.; Gelmetti, C.; Gianotti, R.; Gianni, E.; Puccinelli, V. Cutaneous manifestations of tetrachlorodibenzo-p-dioxin in children and adolescents. Follow-up 10 years after the Seveso, Italy, accident. J. Am. Acad. Dermatol. 1988, 19, 812–819. [Google Scholar] [CrossRef]
- Furue, M.; Uenotsuchi, T.; Urabe, K.; Ishikawa, T.; Kuwabara, M. Overview of Yusho. J. Dermatol. Sci. Suppl. 2005, 1, 3–10. [Google Scholar] [CrossRef]
- Mitoma, C.; Mine, Y.; Utani, A.; Imafuku, S.; Muto, M.; Akimoto, T.; Kanekura, T.; Furue, M.; Uchi, H. Current skin symptoms of Yusho patients exposed to high levels of 2,3,4,7,8-pentachlorinated dibenzofuran and polychlorinated biphenyls in 1968. Chemosphere 2015, 137, 45–51. [Google Scholar] [CrossRef]
- Dandekar, A.; Mendez, R.; Zhang, K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol. Biol. 2015, 1292, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Haarmann-Stemmann, T.; Bothe, H.; Abel, J. Growth factors, cytokines and their receptors as downstream targets of arylhydrocarbon receptor (AhR) signaling pathways. Biochem. Pharmacol. 2009, 77, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Puga, A.; Ma, C.; Marlowe, J.L. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem. Pharmacol. 2009, 77, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Takahara, M.; Uchi, H.; Takeuchi, S.; Mitoma, C.; Moroi, Y.; Furue, M. An environmental contaminant, benzo(a)pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J. Dermatol. Sci. 2011, 62, 42–49. [Google Scholar] [CrossRef]
- Fortes, C.; Mastroeni, S.; Leffondré, K.; Sampogna, F.; Melchi, F.; Mazzotti, E.; Pasquini, P.; Abeni, D. Relationship between smoking and the clinical severity of psoriasis. Arch. Dermatol. 2005, 141, 1580–1584. [Google Scholar] [CrossRef]
- Kohda, F.; Takahara, M.; Hachiya, A.; Takei, K.; Tsuji, G.; Yamamura, K.; Furue, M. Decrease of reactive oxygen species and reciprocal increase of nitric oxide in human dermal endothelial cells by Bidens pilosa extract: A possible explanation of its beneficial effect on livedo vasculopathy. J. Derm. Sci. 2013, 72, 75–77. [Google Scholar] [CrossRef]
- Han, S.G.; Han, S.S.; Toborek, M.; Hennig, B. EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicol. Appl. Pharmacol. 2012, 261, 181–188. [Google Scholar] [CrossRef]
- Yin, Y.; Li, W.; Son, Y.O.; Sun, L.; Lu, J.; Kim, D.; Wang, X.; Yao, H.; Wang, L.; Pratheeshkumar, P.; et al. Quercitrin protects skin from UVB-induced oxidative damage. Toxicol. Appl. Pharmacol. 2013, 269, 89–99. [Google Scholar] [CrossRef]
- Niestroy, J.; Barbara, A.; Herbst, K.; Rode, S.; van Liempt, M.; Roos, P.H. Single and concerted effects of benzo[a]pyrene and flavonoids on the AhR and Nrf2-pathway in the human colon carcinoma cell line Caco-2. Toxicol. In Vitro 2011, 25, 671–683. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Chiu-Leung, L.C.; Lin, S.M.; Leung, L.K. The citrus flavonone hesperetin attenuates the nuclear translocation of aryl hydrocarbon receptor. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 210, 57–64. [Google Scholar] [CrossRef]
- Mohammadi-Bardbori, A.; Bengtsson, J.; Rannug, U.; Rannug, A.; Wincent, E. Quercetin, resveratrol, and curcumin are indirect activators of the aryl hydrocarbon receptor (AHR). Chem. Res. Toxicol. 2012, 25, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Van den Bogaard, E.H.; Bergboer, J.G.; Vonk-Bergers, M.; van Vlijmen-Willems, I.M.; Hato, S.V.; van der Valk, P.G.; Schröder, J.M.; Joosten, I.; Zeeuwen, P.L.; Schalkwijk, J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J. Clin. Investig. 2013, 123, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Roelofzen, J.H.; Aben, K.K.; Oldenhof, U.T.; Coenraads, P.J.; Alkemade, H.A.; van de Kerkhof, P.C.; van der Valk, P.G.; Kiemeney, L.A. No increased risk of cancer after coal tar treatment in patients with psoriasis or eczema. J. Investig. Dermatol. 2010, 130, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Ulzii, D.; Vu, Y.H.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T. Pathogenesis of atopic dermatitis: Current paradigm. Iran. J. Immunol. 2019, 16, 97–107. [Google Scholar] [CrossRef]
- Iwamoto, K.; Moriwaki, M.; Miyake, R.; Hide, M. Staphylococcus aureus in atopic dermatitis: Strain-specific cell wall proteins and skin immunity. Allergol. Int. 2019, 68, 309–315. [Google Scholar] [CrossRef]
- Yu, J.; Luo, Y.; Zhu, Z.; Zhou, Y.; Sun, L.; Gao, J.; Sun, J.; Wang, G.; Yao, X.; Li, W. A tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor. J. Allergy Clin. Immunol. 2019, 143, 2108–2119.e12. [Google Scholar] [CrossRef]
- Hong, C.H.; Lee, C.H.; Yu, H.S.; Huang, S.K. Benzopyrene, a major polyaromatic hydrocarbon in smoke fume, mobilizes Langerhans cells and polarizes Th2/17 responses in epicutaneous protein sensitization through the aryl hydrocarbon receptor. Int. Immunopharmacol. 2016, 36, 111–117. [Google Scholar] [CrossRef]
- Kiyomatsu-Oda, M.; Uchi, H.; Morino-Koga, S.; Furue, M. Protective role of 6-formylindolo[3,2-b]carbazole (FICZ), an endogenous ligand for arylhydrocarbon receptor, in chronic mite-induced dermatitis. J. Dermatol. Sci. 2018, 90, 284–294. [Google Scholar] [CrossRef]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int. J. Mol. Sci. 2019, 20, 5424. [Google Scholar] [CrossRef]
- Kim, H.O.; Kim, J.H.; Chung, B.Y.; Choi, M.G.; Park, C.W. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp. Dermatol. 2014, 23, 278–281. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Liu, P.; Mu, Z.L.; Zhang, J.Z. Aryl hydrocarbon receptor expression in serum, peripheral blood mononuclear cells, and skin lesions of patients with atopic dermatitis and its correlation with disease severity. Chin. Med. J. (Engl.) 2020, 133, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Mitoma, C.; Hashimoto-Hachiya, A.; Uchi, H.; Takahara, M.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. Antioxidant soybean tar Glyteer rescues T-helper-mediated downregulation of filaggrin expression via aryl hydrocarbon receptor. J. Dermatol. 2015, 42, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Edamitsu, T.; Taguchi, K.; Kobayashi, E.H.; Okuyama, R.; Yamamoto, M. Aryl Hydrocarbon receptor directly regulates artemin gene expression. Mol. Cell Biol. 2019, 39, e00190-19. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, T.; Ogawa, E.; Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Fujimura, T.; Aiba, S.; Nakayama, K.; Okuyama, R.; et al. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat. Immunol. 2017, 18, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Morino-Koga, S.; Uchi, H.; Mitoma, C.; Wu, Z.; Kiyomatsu, M.; Fuyuno, Y.; Nagae, K.; Yasumatsu, M.; Suico, M.A.; Kai, H.; et al. 6-Formylindolo[3,2-b]Carbazole accelerates skin wound healing via activation of ERK, but not aryl hydrocarbon receptor. J. Investig. Dermatol. 2017, 137, 2217–2226. [Google Scholar] [CrossRef]
- Takemura, M.; Nakahara, T.; Hashimoto-Hachiya, A.; Furue, M.; Tsuji, G. Glyteer, soybean tar, impairs IL-4/Stat6 signaling in murine bone marrow-derived dendritic cells: The basis of its therapeutic effect on atopic dermatitis. Int. J. Mol. Sci. 2018, 19, 1169. [Google Scholar] [CrossRef]
- Smits, J.P.H.; Ederveen, T.H.A.; Rikken, G.; van den Brink, N.J.M.; van Vlijmen-Willems, I.M.J.J.; Boekhorst, J.; Kamsteeg, M.; Schalkwijk, J.; van Hijum, S.A.F.T.; Zeeuwen, P.L.J.M.; et al. Targeting the cutaneous microbiota in atopic dermatitis by coal tar via AHR-dependent induction of antimicrobial peptides. J. Investig. Dermatol. 2020, 140, 415–424.e10. [Google Scholar] [CrossRef]
- Smith, S.H.; Jayawickreme, C.; Rickard, D.J.; Nicodeme, E.; Bui, T.; Simmons, C.; Coquery, C.M.; Neil, J.; Pryor, W.M.; Mayhew, D.; et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J. Investig. Dermatol. 2017, 137, 2110–2119. [Google Scholar] [CrossRef]
- Kamata, M.; Tada, Y. Safety of biologics in psoriasis. J. Dermatol. 2018, 45, 279–286. [Google Scholar] [CrossRef]
- Cibrian, D.; Saiz, M.L.; de la Fuente, H.; Sánchez-Díaz, R.; Moreno-Gonzalo, O.; Jorge, I.; Ferrarini, A.; Vázquez, J.; Punzón, C.; Fresno, M.; et al. CD69 controls the uptake of L-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis. Nat. Immunol. 2016, 17, 985–996. [Google Scholar] [CrossRef]
- Qiu, J.; Heller, J.J.; Guo, X.; Chen, Z.M.; Fish, K.; Fu, Y.X.; Zhou, L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 2012, 36, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Hirota, K.; Cua, D.J.; Stockinger, B.; Veldhoen, M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 2009, 31, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Shimauchi, T.; Hirakawa, S.; Suzuki, T.; Yasuma, A.; Majima, Y.; Tatsuno, K.; Yagi, H.; Ito, T.; Tokura, Y. Serum interleukin-22 and vascular endothelial growth factor serve as sensitive biomarkers but not as predictors of therapeutic response to biologics in patients with psoriasis. J. Dermatol. 2013, 40, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Witte, E.; Wallace, E.; Döcke, W.D.; Kunz, S.; Asadullah, K.; Volk, H.D.; Sterry, W.; Sabat, R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: A potential role in psoriasis. Eur. J. Immunol. 2006, 36, 1309–1323. [Google Scholar] [CrossRef] [PubMed]
- Cochez, P.M.; Michiels, C.; Hendrickx, E.; Van Belle, A.B.; Lemaire, M.M.; Dauguet, N.; Warnier, G.; de Heusch, M.; Togbe, D.; Ryffel, B.; et al. AhR modulates the IL-22-producing cell proliferation/recruitment in imiquimod-induced psoriasis mouse model. Eur. J. Immunol. 2016, 46, 1449–1459. [Google Scholar] [CrossRef]
- Wolk, K.; Haugen, H.S.; Xu, W.; Witte, E.; Waggie, K.; Anderson, M.; Vom Baur, E.; Witte, K.; Warszawska, K.; Philipp, S.; et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J. Mol. Med. (Berl.) 2009, 87, 523–536. [Google Scholar] [CrossRef]
- di Meglio, P.; Duarte, J.H.; Ahlfors, H.; Owens, N.D.L.; Li, Y.; Villanova, F.; Tosi, I.; Hirota, K.; Nestle, F.O.; Mrowietz, U.; et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity 2014, 40, 989–1001. [Google Scholar] [CrossRef]
- Goldsmith, Z.G.; Dhanasekaran, D.N. G protein regulation of MAPK networks. Oncogene 2007, 26, 3122–3142. [Google Scholar] [CrossRef]
- Walczak, K.; Turski, W.A.; Rajtar, G. Kynurenic acid inhibits colon cancer proliferation in vitro: Effects on signaling pathways. Amino Acids. 2014, 46, 2393–2401. [Google Scholar] [CrossRef]
- Beránek, M.; Fiala, Z.; Kremláček, J.; Andrýs, C.; Krejsek, J.; Hamáková, K.; Palička, V.; Borská, L. Serum levels of aryl hydrocarbon receptor, cytochromes P450 1A1 and 1B1 in patients with exacerbated psoriasis vulgaris. Folia. Biol. (Praha.) 2018, 64, 97–102. [Google Scholar]
- Tian, S.; Krueger, J.G.; Li, K.; Jabbari, A.; Brodmerkel, C.; Lowes, M.A.; Suárez-Fariñas, M. Meta-analysis derived (MAD) transcriptome of psoriasis defines the “core” pathogenesis of disease. PLoS ONE 2012, 7, e44274. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsson, J.E.; Ding, J.; Johnston, A.; Tejasvi, T.; Guzman, A.M.; Nair, R.P.; Voorhees, J.J.; Abecasis, G.R.; Elder, J.T. Assessment of the psoriatic transcriptome in a large sample: Additional regulated genes and comparisons with in vitro models. J. Investig. Dermatol. 2010, 130, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef]
- Wei, Y.D.; Bergander, L.; Rannug, U.; Rannug, A. Regulation of CYP1A1 transcription via the metabolism of the tryptophan-derived 6-formylindolo[3,2-b]carbazole. Arch. Biochem. Biophys. 2000, 383, 99–107. [Google Scholar] [CrossRef]
- di Meglio, P.; Perera, G.K.; Nestle, F.O. The multitasking organ: Recent insights into skin immune function. Immunity 2011, 35, 857–869. [Google Scholar] [CrossRef]
- van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, J.; Lin, Y.; Zhang, C.; Li, W.; Qiao, H.; Fu, M.; Dang, E.; Wang, G. Aryl hydrocarbon receptor in cutaneous vascular endothelial cells restricts psoriasis development by negatively regulating neutrophil recruitment. J. Investig. Dermatol. 2020, 140, 1233–1243.e9. [Google Scholar] [CrossRef]
- Nakamura, M.; Ueda, Y.; Hayashi, M.; Kato, H.; Furuhashi, T.; Morita, A. Tobacco smoke-induced skin pigmentation is mediated by the aryl hydrocarbon receptor. Exp. Dermatol. 2013, 22, 556–558. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Fisher, D.E. MITF and UV responses in skin: From pigmentation to addiction. Pigment Cell Melanoma Res. 2019, 32, 224–236. [Google Scholar] [CrossRef]
- Luecke, S.; Backlund, M.; Jux, B.; Esser, C.; Krutmann, J.; Rannug, A. The aryl hydrocarbon receptor (AHR), a novel regulator of human melanogenesis. Pigment Cell Melanoma Res. 2010, 23, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Alam, S.; Singh, K.P.; Kumar, M.; Gupta, S.K.; Ansari, K.M. Aryl hydrocarbon receptor activation contributes to benzanthrone-induced hyperpigmentation via modulation of melanogenic signaling pathways. Chem. Res. Toxicol. 2017, 30, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Rashighi, M.; Harris, J.E. Vitiligo pathogenesis and emerging treatments. Dermatol. Clin. 2017, 35, 257–265. [Google Scholar] [CrossRef]
- Rekik, R.; Ben Hmid, A.; Lajnef, C.; Zamali, I.; Zaraa, I.; Ben Ahmed, M. Aryl hydrocarbon receptor (AhR) transcription is decreased in skin of vitiligo patients. Int. J. Dermatol. 2017, 56, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Haarmann-Stemmann, T.; Esser, C.; Krutmann, J. The janus-faced role of aryl hydrocarbon receptor signaling in the skin: Consequences for prevention and treatment of skin disorders. J. Investig. Dermatol. 2015, 135, 2572–2576. [Google Scholar] [CrossRef]
- Dwivedi, M.; Kemp, E.H.; Laddha, N.C.; Mansuri, M.S.; Weetman, A.P.; Begum, R. Regulatory T cells in vitiligo: Implications for pathogenesis and therapeutics. Autoimmun. Rev. 2015, 14, 49–56. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zaraa, I.; Rekik, R.; Elbeldi-Ferchiou, A.; Kourda, N.; Belhadj Hmida, N.; Abdeladhim, M.; Karoui, O.; Ben Osman, A.; Mokni, M.; et al. Functional defects of peripheral regulatory T lymphocytes in patients with progressive vitiligo. Pigment Cell Melanoma Res. 2012, 25, 99–109. [Google Scholar] [CrossRef]
- Taher, Z.A.; Lauzon, G.; Maguiness, S.; Dytoc, M.T. Analysis of interleukin-10 levels in lesions of vitiligo following treatment with topical tacrolimus. Br. J. Dermatol. 2009, 161, 654–659. [Google Scholar] [CrossRef]
- Wang, X.; Li, K.; Liu, L.; Shi, Q.; Song, P.; Jian, Z.; Guo, S.; Wang, G.; Li, C.; Gao, T. AHR promoter variant modulates its transcription and downstream effectors by allele-specific AHR-SP1 interaction functioning as a genetic marker for vitiligo. Sci. Rep. 2015, 5, 13542. [Google Scholar] [CrossRef]
- Eguchi, H.; Hayashi, S.I.; Watanabe, J.; Gotoh, O.; Kawajiri, K. Molecular cloning of the human ah receptor gene promoter. Biochem. Biophys. Res. Commun. 1994, 203, 615–622. [Google Scholar] [CrossRef]
- Behfarjam, F.; Jadali, Z. Vitiligo patients show significant up-regulation of aryl hydrocarbon receptor transcription factor. An. Bras. Dermatol. 2018, 93, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Lee, K.M.; Vujkovic-Cvijin, I.; Ucmak, D.; Farahnik, B.; Abrouk, M.; Nakamura, M.; Zhu, T.H.; Bhutani, T.; Wei, M.; et al. The role of IL-17 in vitiligo: A review. Autoimmun. Rev. 2016, 15, 397–404. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Salem, M.A.; Gibbons, N.C.; Martinez, A.; Slominski, R.; Lüdemann, J.; Rokos, H. Blunted epidermal L-tryptophan metabolism in vitiligo affects immune response and ROS scavenging by Fenton chemistry, part 1: Epidermal H2O2/ONOO(-)-mediated stress abrogates tryptophan hydroxylase and dopa decarboxylase activities, leading to low serotonin and melatonin levels. FASEB J. 2012, 26, 2457–2470. [Google Scholar] [CrossRef]
- Saurat, J.H.; Kaya, G.; Saxer-Sekulic, N.; Pardo, B.; Becker, M.; Fontao, L.; Mottu, F.; Carraux, P.; Pham, X.C.; Barde, C.; et al. The cutaneous lesions of dioxin exposure: Lessons from the poisoning of Victor Yushchenko. Toxicol. Sci. 2012, 125, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Tsuji, G. Chloracne and hyperpigmentation caused by exposure to hazardous aryl hydrocarbon receptor ligands. Int. J. Environ. Res. Public Health 2019, 16, 4864. [Google Scholar] [CrossRef] [PubMed]
- Panteleyev, A.A.; Bickers, D.R. Dioxin-induced chloracne--reconstructing the cellular and molecular mechanisms of a classic environmental disease. Exp. Dermatol. 2006, 15, 705–730. [Google Scholar] [CrossRef] [PubMed]
- Suskind, R.R. Chloracne, “the hallmark of dioxin intoxication”. Scand. J. Work Environ. Health 1985, 11, 165–171. [Google Scholar] [CrossRef]
- Van den Bogaard, E.H.; Podolsky, M.A.; Smits, J.P.; Cui, X.; John, C.; Gowda, K.; Desai, D.; Amin, S.G.; Schalkwijk, J.; Perdew, G.H.; et al. Genetic and pharmacological analysis identifies a physiological role for the AHR in epidermal differentiation. J. Investig. Dermatol. 2015, 135, 1320–1328. [Google Scholar] [CrossRef]
- Fernandez-Salguero, P.M.; Ward, J.M.; Sundberg, J.P.; Gonzalez, F.J. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet. Pathol. 1997, 34, 605–614. [Google Scholar] [CrossRef]
- Morokuma, S.; Tsukimori, K.; Hori, T.; Kato, K.; Furue, M. The vernix caseosa is the main site of dioxin excretion in the human foetus. Sci. Rep. 2017, 7, 739. [Google Scholar] [CrossRef]
- Hu, T.; Wang, D.; Yu, Q.; Li, L.; Mo, X.; Pan, Z.; Zouboulis, C.C.; Peng, L.; Xia, L.; Ju, Q. Aryl hydrocarbon receptor negatively regulates lipid synthesis and involves in cell differentiation of SZ95 sebocytes in vitro. Chem. Biol. Interact. 2016, 258, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Ju, Q.; Fimmel, S.; Hinz, N.; Stahlmann, R.; Xia, L.; Zouboulis, C.C. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters sebaceous gland cell differentiation in vitro. Exp. Dermatol. 2011, 20, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, J.; Song, J.; Liang, P.; Zheng, K.; Xiao, G.; Liu, L.; Zouboulis, C.C.; Lei, T. Particulate matter 2.5 regulates lipid synthesis and inflammatory cytokine production in human SZ95 sebocytes. Int. J. Mol. Med. 2017, 40, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Pan, Z.; Yu, Q.; Mo, X.; Song, N.; Yan, M.; Zouboulis, C.C.; Xia, L.; Ju, Q. Benzo(a)pyrene induces interleukin (IL)-6 production and reduces lipid synthesis in human SZ95 sebocytes via the aryl hydrocarbon receptor signaling pathway. Environ. Toxicol. Pharmacol. 2016, 43, 54–60. [Google Scholar] [CrossRef]
- Muku, G.E.; Blazanin, N.; Dong, F.; Smith, P.B.; Thiboutot, D.; Gowda, K.; Amin, S.; Murray, I.A.; Perdew, G.H. Selective Ah receptor ligands mediate enhanced SREBP1 proteolysis to restrict lipogenesis in sebocytes. Toxicol. Sci. 2019, 171, 146–158. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.M.; Coenraads, P.J.; Ji, Z.Y.; Chen, X.; Dong, L.; Ma, X.M.; Han, W.; Tang, N.J. Abnormal expression of MAPK, EGFR, CK17 and TGk in the skin lesions of chloracne patients exposed to dioxins. Toxicol. Lett. 2011, 201, 230–234. [Google Scholar] [CrossRef][Green Version]
- Sutter, C.H.; Yin, H.; Li, Y.; Mammen, J.S.; Bodreddigari, S.; Stevens, G.; Cole, J.A.; Sutter, T.R. EGF receptor signaling blocks aryl hydrocarbon receptor-mediated transcription and cell differentiation in human epidermal keratinocytes. Proc. Natl. Acad. Sci. USA 2009, 106, 4266–4271. [Google Scholar] [CrossRef]
- Kypriotou, M.; Huber, M.; Hohl, D. The human epidermal differentiation complex: Cornified envelope precursors, S100 proteins and the ‘fused genes’ family. Exp. Dermatol. 2012, 21, 643–649. [Google Scholar] [CrossRef]
- Loertscher, J.A.; Sattler, C.A.; Allen-Hoffmann, B.L. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters the differentiation pattern of human keratinocytes in organotypic culture. Toxicol. Appl. Pharmacol. 2001, 175, 121–129. [Google Scholar] [CrossRef]
- Muenyi, C.S.; Carrion, S.L.; Jones, L.A.; Kennedy, L.H.; Slominski, A.T.; Sutter, C.H.; Sutter, T.R. Effects of in utero exposure of C57BL/6J mice to 2,3,7,8-tetrachlorodibenzo-p-dioxin on epidermal permeability barrier development and function. Environ. Health Perspect. 2014, 122, 1052–1058. [Google Scholar] [CrossRef][Green Version]
- Panteleyev, A.A.; Thiel, R.; Wanner, R.; Zhang, J.; Roumak, V.S.; Paus, R.; Neubert, D.; Henz, B.M.; Rosenbach, T. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCCD) affects keratin 1 and keratin 17 gene expression and differentially induces keratinization in hairless mouse skin. J. Investig. Dermatol. 1997, 108, 330–335. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kennedy, L.H.; Sutter, C.H.; Carrion, S.L.; Tran, Q.T.; Bodreddigari, S.; Kensicki, E.; Mohney, R.P.; Sutter, T.R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated production of reactive oxygen species is an essential step in the mechanism of action to accelerate human keratinocyte differentiation. Toxicol. Sci. 2013, 132, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Pohjanvirta, R. The AH Receptor in Biology and Toxicology; John Wiley & Sons: New York, NY, USA, 2012; pp. 485–497. [Google Scholar] [CrossRef]
- Furness, S.G.; Lees, M.J.; Whitelaw, M.L. The dioxin (aryl hydrocarbon) receptor as a model for adaptive responses of bHLH/PAS transcription factors. FEBS Lett. 2007, 581, 3616–3625. [Google Scholar] [CrossRef]
- Contador-Troca, M.; Alvarez-Barrientos, A.; Barrasa, E.; Rico-Leo, E.M.; Catalina-Fernández, I.; Menacho-Márquez, M.; Bustelo, X.R.; García-Borrón, J.C.; Gómez-Durán, A.; Sáenz-Santamaría, J.; et al. The dioxin receptor has tumor suppressor activity in melanoma growth and metastasis. Carcinogenesis 2013, 34, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Reagan-Shaw, S.; Wu, J.; Longley, B.J.; Ahmad, N. Chemoprevention of skin cancer by grape constituent resveratrol: Relevance to human disease? FASEB J. 2005, 19, 1193–1195. [Google Scholar] [CrossRef]
- Gawkrodger, D.J. Occupational skin cancers. Occup. Med. (Lond.) 2004, 54, 458–463. [Google Scholar] [CrossRef]
- Richard, G.; Puisieux, A.; Caramel, J. Antagonistic functions of EMT-inducers in melanoma development: Implications for cancer cell plasticity. Cancer Cell Microenviron. 2014, e61. [Google Scholar] [CrossRef]
- Kostyuk, V.A.; Potapovich, A.I.; Lulli, D.; Stancato, A.; De Luca, C.; Pastore, S.; Korkina, L. Modulation of human keratinocyte responses to solar UV by plant polyphenols as a basis for chemoprevention of non-melanoma skin cancers. Curr. Med. Chem. 2013, 20, 869–879. [Google Scholar]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef]
- Chahal, H.S.; Lin, Y.; Ransohoff, K.J.; Hinds, D.A.; Wu, W.; Dai, H.J.; Qureshi, A.A.; Li, W.Q.; Kraft, P.; Tang, J.Y.; et al. Genome-wide association study identifies novel susceptibility loci for cutaneous squamous cell carcinoma. Nat. Commun. 2016, 7, 12048. [Google Scholar] [CrossRef]
- Vogeley, C.; Esser, C.; Tüting, T.; Krutmann, J.; Haarmann-Stemmann, T. Role of the aryl hydrocarbon receptor in environmentally induced skin aging and skin carcinogenesis. Int. J. Mol. Sci. 2019, 20, 6005. [Google Scholar] [CrossRef] [PubMed]
- Kalmes, M.; Hennen, J.; Clemens, J.; Blömeke, B. Impact of aryl hydrocarbon receptor (AhR) knockdown on cell cycle progression in human HaCaT keratinocytes. Biol. Chem. 2011, 392, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Pollet, M.; Shaik, S.; Mescher, M.; Krutmann, J.; Haarmann-Stemmann, T. The AHR represses nucleotide excision repair and apoptosis and contributes to UV-induced skin carcinogenesis. Cell Death Differ. 2018, 25, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, E.F.; Kopparapu, P.R.; Koch, D.C.; Jang, H.S.; Phillips, J.L.; Tanguay, R.L.; Kerkvliet, N.I.; Kolluri, S.K. The aryl hydrocarbon receptor mediates leflunomide-induced growth inhibition of melanoma cells. PLoS ONE 2012, 7, e40926. [Google Scholar] [CrossRef]
- Roman, A.C.; Carvajal-Gonzalez, J.M.; Rico-Leo, E.M.; Fernandez-Salguero, P.M. Dioxin receptor deficiency impairs angiogenesis by a mechanism involving VEGF-A depletion in the endothelium and transforming growth factor-beta overexpression in the stroma. J. Biol. Chem. 2009, 284, 25135–25148. [Google Scholar] [CrossRef]
- Villano, C.M.; Murphy, K.A.; Akintobi, A.; White, L.A. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces matrix metalloproteinase (MMP) expression and invasion in A2058 melanoma cells. Toxicol. Appl. Pharmacol. 2006, 210, 212–224. [Google Scholar] [CrossRef]
- Helferich, W.G.; Denison, M.S. Ultraviolet photoproducts of tryptophan can act as dioxin agonists. Mol. Pharmacol. 1991, 40, 674–678. [Google Scholar]
- Walczak, K.; Wnorowski, A.; Turski, W.A.; Plech, T. Kynurenic acid and cancer: Facts and controversies. Cell Mol. Life Sci. 2020, 77, 1531–1550. [Google Scholar] [CrossRef]
- Cella, M.; Colonna, M. Aryl hydrocarbon receptor: Linking environment to immunity. Semin. Immunol. 2015, 27, 310–314. [Google Scholar] [CrossRef]
- Turski, M.P.; Turska, M.; Zgrajka, W.; Bartnik, M.; Kocki, T.; Turski, W.A. Distribution, synthesis, and absorption of kynurenic acid in plants. Planta Med. 2011, 77, 858–864. [Google Scholar] [CrossRef]
- Turski, M.P.; Turska, M.; Zgrajka, W.; Kuc, D.; Turski, W.A. Presence of kynurenic acid in food and honeybee products. Amino Acids 2009, 36, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, M.E.; Franks, D.G.; Woodin, B.R.; Jenny, M.J.; Garrick, R.A.; Behrendt, L.; Hahn, M.E.; Stegeman, J.J. The tryptophan photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) binds multiple AHRs and induces multiple CYP1 genes via AHR2 in zebrafish. Chem. Biol. Interact. 2009, 181, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Park, S.L.; Justiniano, R.; Williams, J.D.; Cabello, C.M.; Qiao, S.; Wondrak, G.T. The tryptophan-derived endogenous aryl hydrocarbon receptor ligand 6-Formylindolo[3,2-b]Carbazole is a nanomolar UVA photosensitizer in epidermal keratinocytes. J. Investig. Dermatol. 2015, 135, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, H.; DelTito, B.J., Jr.; Matgouranis, P.M.; Das, M.; Asokan, P.; Bickers, D.R. Additive effects of ultraviolet B and crude coal tar on cutaneous carcinogen metabolism: Possible relevance to the tumorigenicity of the Goeckerman regimen. J. Investig. Dermatol. 1986, 87, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Matsui, M.S.; Mukhtar, H. Ultraviolet-B exposure of human skin induces cytochromes P450 1A1 and 1B1. J. Investig. Dermatol. 2000, 114, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Rannug, A.; Fritsche, E. The aryl hydrocarbon receptor and light. Biol. Chem. 2006, 387, 1149–1157. [Google Scholar] [CrossRef]
- Murai, M.; Tsuji, G.; Hashimoto-Hachiya, A.; Kawakami, Y.; Furue, M.; Mitoma, C. An endogenous tryptophan photo-product, FICZ, is potentially involved in photo-aging by reducing TGF-β-regulated collagen homeostasis. J. Dermatol. Sci. 2018, 89, 19–26. [Google Scholar] [CrossRef]
- Mengoni, M.; Braun, A.D.; Gaffal, E.; Tüting, T. The aryl hydrocarbon receptor promotes inflammation-induced dedifferentiation and systemic metastatic spread of melanoma cells. Int. J. Cancer 2020, 147, 2902–2913. [Google Scholar] [CrossRef]
- Ikuta, T.; Kawajiri, K. Zinc finger transcription factor Slug is a novel target gene of aryl hydrocarbon receptor. Exp. Cell Res. 2006, 312, 3585–3594. [Google Scholar] [CrossRef]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef]
- Bessede, A.; Gargaro, M.; Pallotta, M.T.; Matino, D.; Servillo, G.; Brunacci, C.; Bicciato, S.; Mazza, E.M.; Macchiarulo, A.; Vacca, C.; et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 2014, 511, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Nuti, R.; Gargaro, M.; Matino, D.; Dolciami, D.; Grohmann, U.; Puccetti, P.; Fallarino, F.; Macchiarulo, A. Ligand binding and functional selectivity of L-tryptophan metabolites at the mouse aryl hydrocarbon receptor (mAhR). J. Chem. Inf. Model. 2014, 54, 3373–3383. [Google Scholar] [CrossRef] [PubMed]
- Pilotte, L.; Larrieu, P.; Stroobant, V.; Colau, D.; Dolusic, E.; Frédérick, R.; De Plaen, E.; Uyttenhove, C.; Wouters, J.; Masereel, B.; et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc. Natl. Acad. Sci. USA 2012, 109, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Litzenburger, U.M.; Opitz, C.A.; Sahm, F.; Rauschenbach, K.J.; Trump, S.; Winter, M.; Ott, M.; Ochs, K.; Lutz, C.; Liu, X.; et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget 2014, 5, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Wirthgen, E.; Hoeflich, A. Endotoxin-induced tryptophan degradation along the kynurenine pathway: The role of indolamine 2,3-Dioxygenase and aryl hydrocarbon receptor-mediated immunosuppressive effects in endotoxin tolerance and cancer and its implications for immunoparalysis. J. Amino Acids 2015, 2015, 973548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Patel, A.; Chu, C.; Jiang, W.; Wang, L.; Welty, S.E.; Moorthy, B.; Shivanna, B. Aryl hydrocarbon receptor is necessary to protect fetal human pulmonary microvascular endothelial cells against hyperoxic injury: Mechanistic roles of antioxidant enzymes and RelB. Toxicol. Appl. Pharmacol. 2015, 286, 92–101. [Google Scholar] [CrossRef]
- Baglole, C.J.; Maggirwar, S.B.; Gasiewicz, T.A.; Thatcher, T.H.; Phipps, R.P.; Sime, P.J. The aryl hydrocarbon receptor attenuates tobacco smoke-induced cyclooxygenase-2 and prostaglandin production in lung fibroblasts through regulation of the NF-kappaB family member RelB. J. Biol. Chem. 2008, 283, 28944–28957. [Google Scholar] [CrossRef]
- Sekine, H.; Mimura, J.; Oshima, M.; Okawa, H.; Kanno, J.; Igarashi, K.; Gonzalez, F.J.; Ikuta, T.; Kawajiri, K.; Fujii-Kuriyama, Y. Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol. Cell. Biol. 2009, 29, 6391–6400. [Google Scholar] [CrossRef]
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814. [Google Scholar] [CrossRef]
- Walczak, K.; Turski, W.A.; Rzeski, W. Kynurenic acid enhances expression of p21 Waf1/Cip1 in colon cancer HT-29 cells. Pharmacol. Rep. 2012, 64, 745–750. [Google Scholar] [CrossRef]
- Walczak, K.; Langner, E.; Makuch-Kocka, A.; Szelest, M.; Szalast, K.; Marciniak, S.; Plech, T. Effect of tryptophan-derived Ahr ligands, kynurenine, kynurenic acid and FICZ, on proliferation, cell cycle regulation and cell death of melanoma cells-in vitro studies. Int. J. Mol. Sci. 2020, 21, 7946. [Google Scholar] [CrossRef] [PubMed]
- Turski, M.P.; Turska, M.; Paluszkiewicz, P.; Parada-Turska, J.; Oxenkrug, G.F. Kynurenic Acid in the digestive system-new facts, new challenges. Int. J. Tryptophan. Res. 2013, 6, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Swanbeck, G.; Wennersten, G.; Nilsson, R. Participation of singlet state excited oxygen in photohemolysis induced by kynurenic acid. Acta Derm. Venereol. 1974, 54, 433–436. [Google Scholar] [PubMed]
- Wennersten, G.; Brunk, U. Cellular aspects of phototoxic reactions induced by kynurenic acid. I. Establishment of an experimental model utilizing in vitro cultivated cells. Acta Derm. Venereol. 1977, 57, 201–209. [Google Scholar]
- Vogel, C.F.A.; Ishihara, Y.; Campbell, C.E.; Kado, S.Y.; Nguyen-Chi, A.; Sweeney, C.; Pollet, M.; Haarmann-Stemmann, T.; Tuscano, J.M. A protective role of aryl hydrocarbon receptor repressor in inflammation and tumor growth. Cancers (Basel) 2019, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47(W1), W556–W560. [Google Scholar] [CrossRef]
- Théate, I.; van Baren, N.; Pilotte, L.; Moulin, P.; Larrieu, P.; Renauld, J.C.; Hervé, C.; Gutierrez-Roelens, I.; Marbaix, E.; Sempoux, C.; et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol. Res. 2015, 3, 161–172. [Google Scholar] [CrossRef]
- Labadie, B.W.; Bao, R.; Luke, J.J. Reimagining IDO Pathway inhibition in cancer immunotherapy via downstream focus on the tryptophan-kynurenine-aryl hydrocarbon axis. Clin. Cancer Res. 2019, 25, 1462–1471. [Google Scholar] [CrossRef]
- Vlachos, C.; Schulte, B.M.; Magiatis, P.; Adema, G.J.; Gaitanis, G. Malassezia-derived indoles activate the aryl hydrocarbon receptor and inhibit Toll-like receptor-induced maturation in monocyte-derived dendritic cells. Br. J. Dermatol. 2012, 167, 496–505. [Google Scholar] [CrossRef]
- Gaitanis, G.; Velegraki, A.; Magiatis, P.; Pappas, P.; Bassukas, I.D. Could Malassezia yeasts be implicated in skin carcinogenesis through the production of aryl-hydrocarbon receptor ligands? Med. Hypotheses 2011, 77, 47–51. [Google Scholar] [CrossRef]


| Alterations in Cellular Functions | Biological Effect | Type of Cell/ Mouse Model | Reference | |
|---|---|---|---|---|
| Cell metabolism |
| AHR-deficient mice | [39] | |
| Cell proliferation | Inhibition |
| 5L cells | [40] |
| HUVEC | [41] | |||
| LNCaP cells | [42] | ||
| BP8 5L HEK293 | [43] | |||
| Hepa-1c1c7 MCF-7 | [44] | ||
| HepG2 | [45] | ||
| Stimulation |
| MCF-7 | [45] | |
| A549 | [46] | ||
| HepG2 | [47] | ||
| Cell migration * |
| T-FGM-AHR−/− myofibroblasts | [48] | |
| MCF-7 HepG2 | [49] | ||
| MDA-MB-231 T47D | [50] | ||
| MDA-MB-231 | [51] | ||
| MCF-7 | [52] | ||
| HSC-3 CAL27 | [53] | ||
| Regulation of Signaling Pathways and Nuclear Receptors | ||||
| NF-κB signaling pathway |
| U937 macrophages | [54] | |
| B6 mice | [25] | ||
| Nuclear factor-erythroid 2-related factor-2 (Nrf2) signaling pathway |
| NHEK | [55,56] | |
| Calcium-dependent signaling pathways |
| Hepa-1 | [57] | |
| 3T3-L1 | [58,59] | ||
| Hypoxia-induced factor (HIF) |
| HepG2 HaCaT | [60] | |
| HEK Krt14-Cre+ mice | [61] | ||
| Zebrafish caudal fin regeneration model | [62] | ||
| Estrogen and retinoid receptors |
| BG1 | [63] | |
| MCF-7 | Reviewed in [28,64] | ||
| Substance | Outcome | Cell Type | References |
|---|---|---|---|
| Ketoconazole |
| NHEK | [55] |
| Bidens pilosa |
| Human dermal endothelial cells | [86] |
| Epigallocatechin gallate |
| Primary vascular endothelial cells | [87] |
| Quercitrin |
| JB6 cells | [88] |
| Quercetin, kaempferol |
| Caco2 | [89] |
| Cinnamaldehyde |
| HaCaT | [11] |
| Cynaropicrin (Cynara scolymus) |
| NHEK | [10] |
| Opuntia ficus indica |
| HNEK | [9] |
| Hesperetin |
| MCF-7 | [90] |
| Quercetin, resveratrol, curcumin |
| HaCaT | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szelest, M.; Walczak, K.; Plech, T. A New Insight into the Potential Role of Tryptophan-Derived AhR Ligands in Skin Physiological and Pathological Processes. Int. J. Mol. Sci. 2021, 22, 1104. https://doi.org/10.3390/ijms22031104
Szelest M, Walczak K, Plech T. A New Insight into the Potential Role of Tryptophan-Derived AhR Ligands in Skin Physiological and Pathological Processes. International Journal of Molecular Sciences. 2021; 22(3):1104. https://doi.org/10.3390/ijms22031104
Chicago/Turabian StyleSzelest, Monika, Katarzyna Walczak, and Tomasz Plech. 2021. "A New Insight into the Potential Role of Tryptophan-Derived AhR Ligands in Skin Physiological and Pathological Processes" International Journal of Molecular Sciences 22, no. 3: 1104. https://doi.org/10.3390/ijms22031104
APA StyleSzelest, M., Walczak, K., & Plech, T. (2021). A New Insight into the Potential Role of Tryptophan-Derived AhR Ligands in Skin Physiological and Pathological Processes. International Journal of Molecular Sciences, 22(3), 1104. https://doi.org/10.3390/ijms22031104





