Molecular Mechanism of Autosomal Recessive Long QT-Syndrome 1 without Deafness
Abstract
1. Introduction
2. Results
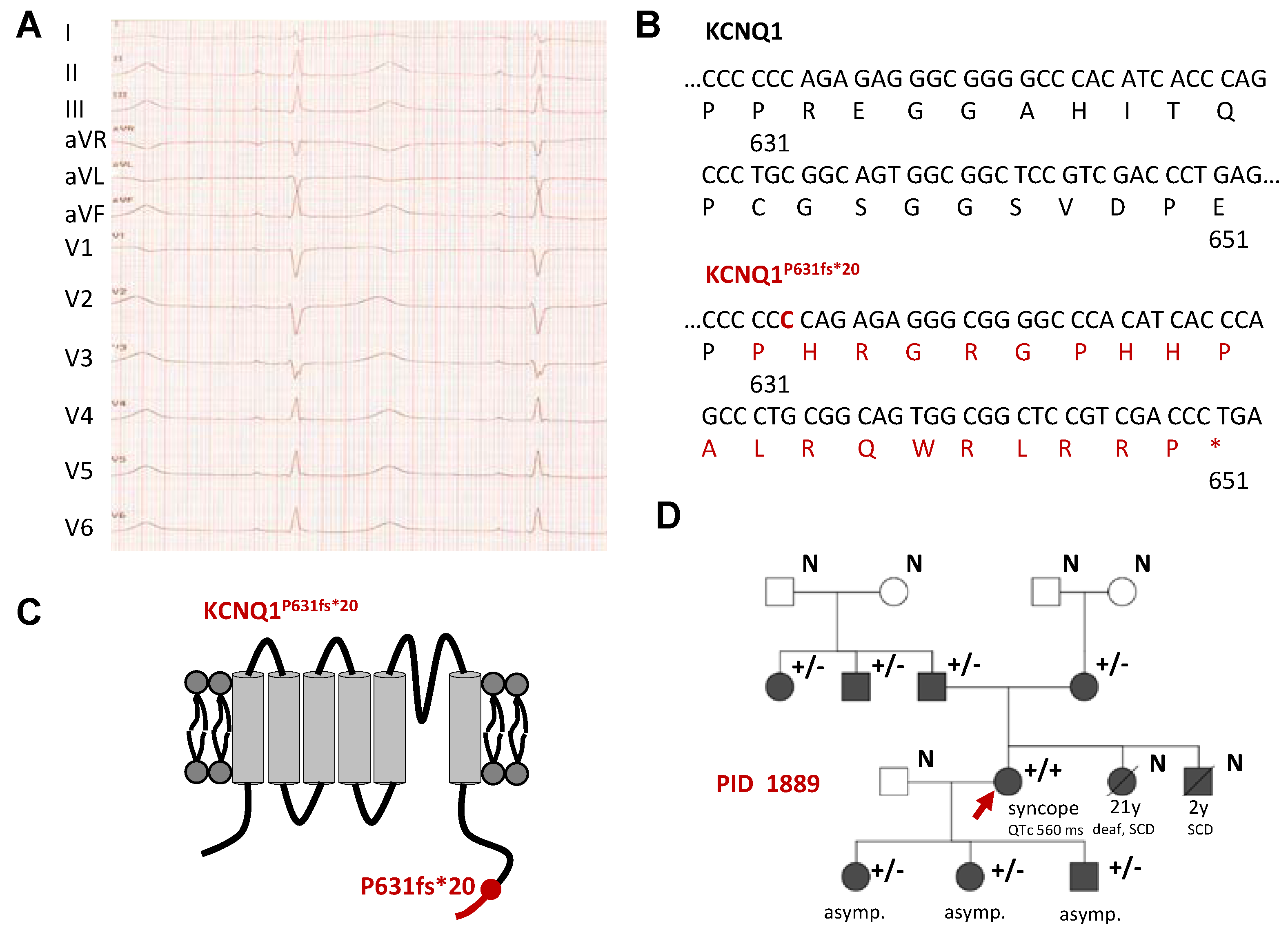
2.1. Identification of a Homozygous KCNQ1 Gene Mutation in a Family with LQTS without Hearing Impairment
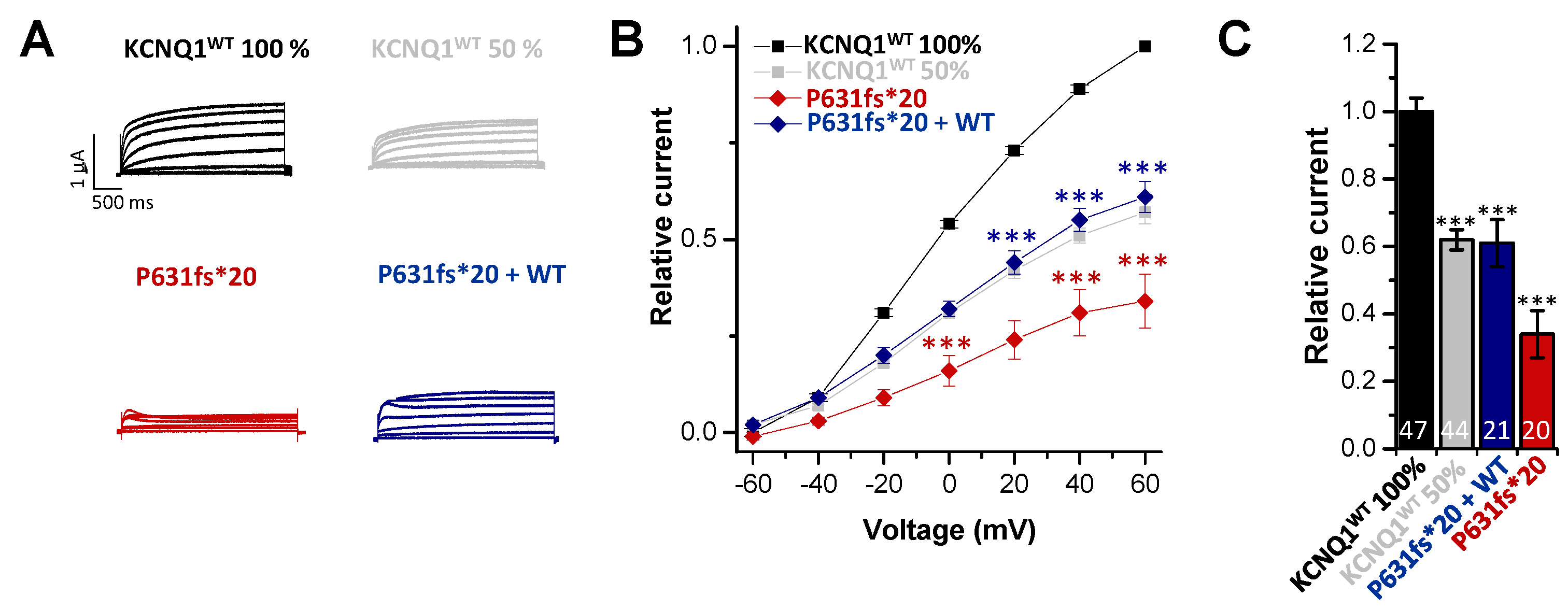
2.2. Electrophysiological Characterization of the KCNQ1P631fs*20 Variant Revealed a Loss-of-Function in Both Homozygous and Heterozygous states
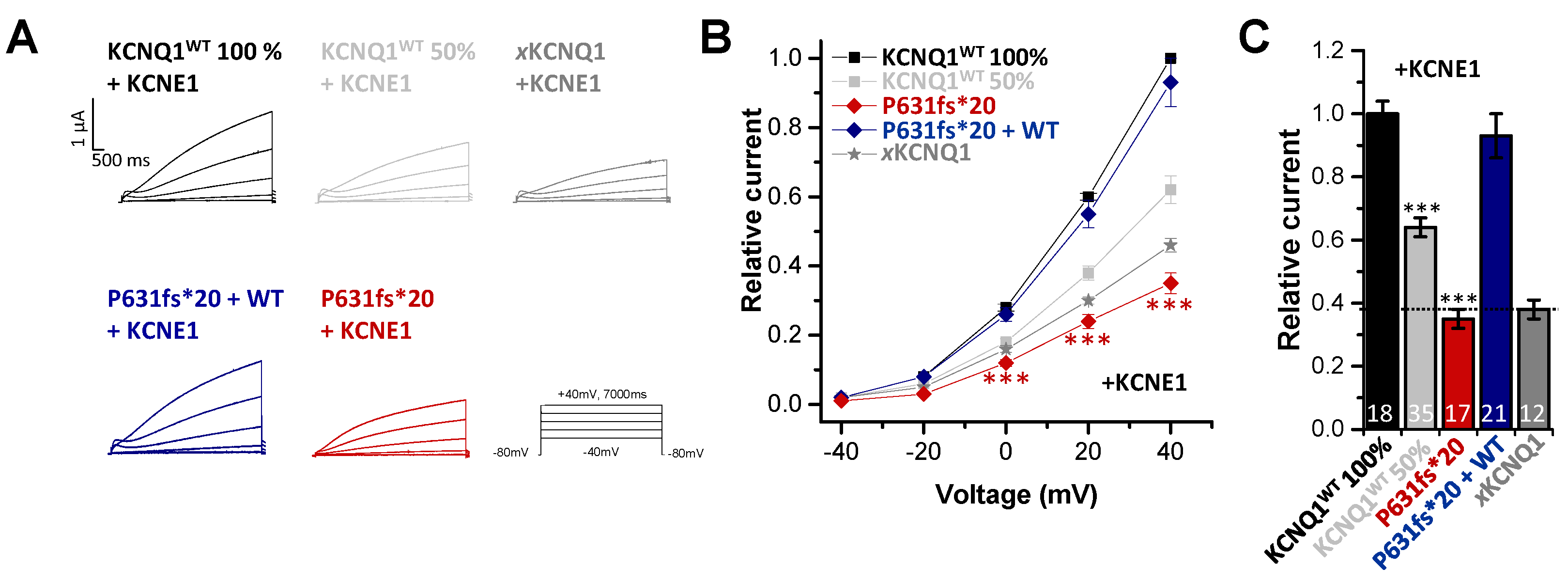
2.3. In Electrophysiological Recordings, KCNE1 Rescued the KCNQ1P631fs*20 Loss-of-Function Present in the Heteromeric State with KCNQ1WT
2.4. The KCNQ1P631fs*20 Mutant does Not alter the Gating of Homomeric and Heteromeric IKs Channel Complexes
2.5. Action Potential Modelling Predicts a QT Prolongation Exclusively Present In Homozygous Patients
3. Discussion
4. Materials and Methods
4.1. Clinical Evaluation
4.2. Molecular Biology
4.3. Electrophysiology
4.4. Action Potential Modelling
4.5. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanguinetti, M.C.; Jurkiewicz, N.K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990, 96, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.W.; Noble, D. A transition state theory approach to the kinetics of conductance changes in excitable membranes. J. Membr. Biol. 1969, 1, 248–273. [Google Scholar] [CrossRef]
- Marx, S.O.; Kurokawa, J.; Reiken, S.; Motoike, H.; D’Armiento, J.; Marks, A.R.; Kass, R.S. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science 2002, 295, 496–499. [Google Scholar] [CrossRef]
- Sanguinetti, M.C.; Curran, M.E.; Zou, A.; Shen, J.; Spector, P.S.; Atkinson, D.L.; Keating, M.T. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature 1996, 384, 80–83. [Google Scholar] [CrossRef]
- Barhanin, J.; Lesage, F.; Guillemare, E.; Fink, M.; Lazdunski, M.; Romey, G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature 1996, 384, 78–80. [Google Scholar] [CrossRef]
- Snyders, D.J. Structure and function of cardiac potassium channels. Cardiovasc. Res. 1999, 42, 377–390. [Google Scholar] [CrossRef]
- MacKinnon, R. Pore loops: An emerging theme in ion channel structure. Neuron 1995, 14, 889–892. [Google Scholar] [CrossRef][Green Version]
- Wrobel, E.; Tapken, D.; Seebohm, G. The KCNE Tango—How KCNE1 Interacts with Kv7.1. Front. Pharmacol. 2012, 3, 142. [Google Scholar] [CrossRef]
- Bendahhou, S.; Marionneau, C.; Haurogne, K.; Larroque, M.M.; Derand, R.; Szuts, V.; Escande, D.; Demolombe, S.; Barhanin, J. In vitro molecular interactions and distribution of KCNE family with KCNQ1 in the human heart. Cardiovasc. Res. 2005, 67, 529–538. [Google Scholar] [CrossRef]
- Warth, R.; Barhanin, J. The multifaceted phenotype of the knockout mouse for the KCNE1 potassium channel gene. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R639–R648. [Google Scholar] [CrossRef]
- Chouabe, C.; Neyroud, N.; Guicheney, P.; Lazdunski, M.; Romey, G.; Barhanin, J. Properties of KvLQT1 K+ channel mutations in Romano-Ward and Jervell and Lange-Nielsen inherited cardiac arrhythmias. EMBO J. 1997, 16, 5472–5479. [Google Scholar] [CrossRef] [PubMed]
- Neyroud, N.; Tesson, F.; Denjoy, I.; Leibovici, M.; Donger, C.; Barhanin, J.; Faure, S.; Gary, F.; Coumel, P.; Petit, C.; et al. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat. Genet. 1997, 15, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, M.C.; Knollmann, B.C.; Ebert, S.N.; Vary, J.C., Jr.; Greene, A.E.; Franz, M.R.; Grinberg, A.; Huang, S.P.; Pfeifer, K. Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange-Nielsen Syndrome. Proc. Natl. Acad. Sci. USA 2001, 98, 2526–2531. [Google Scholar] [CrossRef]
- Bleich, M.; Warth, R. The very small-conductance K+ channel KvLQT1 and epithelial function. Pflüg. Arch. Eur. J. Physiol. 2000, 440, 202–206. [Google Scholar] [CrossRef]
- Forge, A.; Wright, T. The molecular architecture of the inner ear. Br. Med. Bull. 2002, 63, 5–24. [Google Scholar] [CrossRef]
- Nicolas, M.; Dememes, D.; Martin, A.; Kupershmidt, S.; Barhanin, J. KCNQ1/KCNE1 potassium channels in mammalian vestibular dark cells. Heart Res. 2001, 153, 132–145. [Google Scholar] [CrossRef]
- Vetter, D.E.; Mann, J.R.; Wangemann, P.; Liu, J.; McLaughlin, K.J.; Lesage, F.; Marcus, D.C.; Lazdunski, M.; Heinemann, S.F.; Barhanin, J. Inner ear defects induced by null mutation of the isk gene. Neuron 1996, 17, 1251–1264. [Google Scholar] [CrossRef]
- Moss, A.J. Long QT Syndrome. JAMA 2003, 289, 2041–2044. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Stramba-Badiale, M.; Crotti, L.; Pedrazzini, M.; Besana, A.; Bosi, G.; Gabbarini, F.; Goulene, K.; Insolia, R.; Mannarino, S.; et al. Prevalence of the congenital long-QT syndrome. Circulation 2009, 120, 1761–1767. [Google Scholar] [CrossRef]
- Napolitano, C.; Priori, S.G.; Schwartz, P.J.; Bloise, R.; Ronchetti, E.; Nastoli, J.; Bottelli, G.; Cerrone, M.; Leonardi, S. Genetic testing in the long QT syndrome: Development and validation of an efficient approach to genotyping in clinical practice. JAMA 2005, 294, 2975–2980. [Google Scholar] [CrossRef]
- Splawski, I.; Shen, J.; Timothy, K.W.; Lehmann, M.H.; Priori, S.; Robinson, J.L.; Moss, A.J.; Schwartz, P.J.; Towbin, J.A.; Vincent, G.M.; et al. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation 2000, 102, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Tester, D.J.; Will, M.L.; Haglund, C.M.; Ackerman, M.J. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm 2005, 2, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, Z.A.; Wilde, A.A. IKs in heart and hearing, the ear can do with less than the heart. Circ. Cardiovasc. Genet. 2013, 6, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Giudicessi, J.R.; Ackerman, M.J. Prevalence and potential genetic determinants of sensorineural deafness in KCNQ1 homozygosity and compound heterozygosity. Circ. Cardiovasc. Genet. 2013, 6, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Jervell, A.; Lange-Nielsen, F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am. Heart J. 1957, 54, 59–68. [Google Scholar] [CrossRef]
- Schulze-Bahr, E.; Wang, Q.; Wedekind, H.; Haverkamp, W.; Chen, Q.; Sun, Y.; Rubie, C.; Hordt, M.; Towbin, J.A.; Borggrefe, M.; et al. KCNE1 mutations cause jervell and Lange-Nielsen syndrome. Nat. Genet. 1997, 17, 267–268. [Google Scholar] [CrossRef]
- Splawski, I.; Timothy, K.W.; Vincent, G.M.; Atkinson, D.L.; Keating, M.T. George, M. Cober Lecturer: Mark T. Keating. Molecular basis of the long-QT syndrome associated with deafness. Proc. Assoc. Am. Physicians 1997, 109, 504–511. [Google Scholar] [PubMed]
- Nishimura, M.; Ueda, M.; Ebata, R.; Utsuno, E.; Ishii, T.; Matsushita, K.; Ohara, O.; Shimojo, N.; Kobayashi, Y.; Nomura, F. A novel KCNQ1 nonsense variant in the isoform-specific first exon causes both jervell and Lange-Nielsen syndrome 1 and long QT syndrome 1: A case report. BMC Med. Genet. 2017, 18, 66. [Google Scholar] [CrossRef]
- Neyroud, N.; Richard, P.; Vignier, N.; Donger, C.; Denjoy, I.; Demay, L.; Shkolnikova, M.; Pesce, R.; Chevalier, P.; Hainque, B.; et al. Genomic organization of the KCNQ1 K+ channel gene and identification of C-terminal mutations in the long-QT syndrome. Circ. Res. 1999, 84, 290–297. [Google Scholar] [CrossRef]
- Novotny, T.; Kadlecova, J.; Janousek, J.; Gaillyova, R.; Bittnerova, A.; Florianova, A.; Sisakova, M.; Toman, O.; Chroust, K.; Papousek, I.; et al. The homozygous KCNQ1 gene mutation associated with recessive Romano-Ward syndrome. Pacing Clin. Electrophysiol. 2006, 29, 1013–1015. [Google Scholar] [CrossRef]
- O’Hara, T.; Virag, L.; Varro, A.; Rudy, Y. Simulation of the undiseased human cardiac ventricular action potential: Model formulation and experimental validation. PLoS Comput. Biol. 2011, 7, e1002061. [Google Scholar] [CrossRef]
- Sato, A.; Arimura, T.; Makita, N.; Ishikawa, T.; Aizawa, Y.; Ushinohama, H.; Aizawa, Y.; Kimura, A. Novel mechanisms of trafficking defect caused by KCNQ1 mutations found in long QT syndrome. J. Biol. Chem. 2009, 284, 35122–35133. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Kanda, V.A.; Choi, E.; Panaghie, G.; Roepke, T.K.; Gaeta, S.A.; Christini, D.J.; Lerner, D.J.; Abbott, G.W. MinK-dependent internalization of the IKs potassium channel. Cardiovasc. Res. 2009, 82, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Xu Parks, X.; Qudsi, H.; Braun, C.; Lopes, C.M.B. The auxiliary subunit KCNE1 regulates KCNQ1 channel response to sustained calcium-dependent PKC activation. PLoS ONE 2020, 15, e0237591. [Google Scholar] [CrossRef]
- Krumerman, A.; Gao, X.; Bian, J.S.; Melman, Y.F.; Kagan, A.; McDonald, T.V. An LQT mutant minK alters KvLQT1 trafficking. Am. J. Of Physiol.-Cell Physiol. 2004, 286, C1453–C1463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bianchi, L.; Priori, S.G.; Napolitano, C.; Surewicz, K.A.; Dennis, A.T.; Memmi, M.; Schwartz, P.J.; Brown, A.M. Mechanisms of I(Ks) suppression in LQT1 mutants. Am. J. Physiol.-Heart Circ. Physiol. 2000, 279, H3003–H3011. [Google Scholar] [CrossRef]
- Goldenberg, I.; Zareba, W.; Moss, A.J. Long QT Syndrome. Curr. Probl. Cardiol. 2008, 33, 629–694. [Google Scholar] [CrossRef]
- Rush, S.; Larsen, H. A practical algorithm for solving dynamic membrane equations. IEEE Trans. Bio-Med. Eng. 1978, 25, 389–392. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oertli, A.; Rinné, S.; Moss, R.; Kääb, S.; Seemann, G.; Beckmann, B.-M.; Decher, N. Molecular Mechanism of Autosomal Recessive Long QT-Syndrome 1 without Deafness. Int. J. Mol. Sci. 2021, 22, 1112. https://doi.org/10.3390/ijms22031112
Oertli A, Rinné S, Moss R, Kääb S, Seemann G, Beckmann B-M, Decher N. Molecular Mechanism of Autosomal Recessive Long QT-Syndrome 1 without Deafness. International Journal of Molecular Sciences. 2021; 22(3):1112. https://doi.org/10.3390/ijms22031112
Chicago/Turabian StyleOertli, Annemarie, Susanne Rinné, Robin Moss, Stefan Kääb, Gunnar Seemann, Britt-Maria Beckmann, and Niels Decher. 2021. "Molecular Mechanism of Autosomal Recessive Long QT-Syndrome 1 without Deafness" International Journal of Molecular Sciences 22, no. 3: 1112. https://doi.org/10.3390/ijms22031112
APA StyleOertli, A., Rinné, S., Moss, R., Kääb, S., Seemann, G., Beckmann, B.-M., & Decher, N. (2021). Molecular Mechanism of Autosomal Recessive Long QT-Syndrome 1 without Deafness. International Journal of Molecular Sciences, 22(3), 1112. https://doi.org/10.3390/ijms22031112






