Assessing Kidney Graft Viability and Its Cells Metabolism during Machine Perfusion
Abstract
1. Introduction
2. Pathogenesis of the Ischemic Injury
3. Consequences of Ischemic Reperfusion Injury
3.1. Delayed Graft Function
3.2. Acute Rejection
4. Preservation Techniques
4.1. Static Cold Storage
4.2. Dynamic Preservation
4.2.1. Hypothermic Machine Perfusion
4.2.2. Normothermic Machine Perfusion
5. Viability Assessment via Machine Perfusion
- (1)
- During NMP macroscopic appearance of the perfused graft is available: the quality assessment score (QAS), is based on macroscopic appearance, mean renal blood flow and total urine output [41]. Kidneys are graded 1–5, with 1–3 scores considered suitable for transplantation: score 1 indicates the least injury and 5 the most severe. More in details, the score is built up by a combination of the perfusion assessment parameters within 60 min from the start: grade I, excellent perfusion or global pink appearance; grade II, moderate perfusion with patchy pink/purple appearance which either remains or improves during NMP; grade III, poor perfusion, consisting of global mottling and purple/black appearance constantly throughout NMP. In addition, thresholds of renal blood flow (<50 mL per min per 100 g) and total urine output (<43 mL per min per 100 g) gives additional single points each to be combined with the macroscopic grades (I-III) for the final assessment score.
- (2)
- Pressure, flow and resistance readings measured during MP are used as viability assessors, although they cannot be considered as stand-alone criteria, since their relative predictive value is low. The rationale for the use of perfusion parameters stands on the structure of the renal vascular system itself, very rich in capillary network with filtration function [77]. The release of vasoconstrictors from this capillary network (single one-layer endothelium) following the ischemic and inflammatory insults, determines accumulation of erythrocytes and microthrombosis, eventually leading to a diminished flow and increased resistance in the graft [26]. Furthermore, the hypoxia is directly responsible for endothelium cell activation, synergically favoring a pro-coagulant and pro-inflammatory phenotype of the renal vasculature, with consequent disruption of the blood flow, and increased leukocyte infiltration, with a further decline in kidney function. On this basis, increased renal vascular resistance and low intraparenchymal flow are expressions of tissue damage.
- (3)
- Glucose consumption: the difference between the concentration into the arterial inflow and venous outflow could estimate the aerobic respiration and energy activity of the kidney cells. Several ways to measure glucose consumption have been described, including metabolic profiles via noninvasive MR spectroscopy [78]. The rationale lies on the estimation of cells viability in view of their metabolic utilization of carbohydrate energy sources, as it physiologically occurs when the organ is within the human body. The pattern of shutting down metabolically is peculiar of the kidney suffering from oxidative stress and shifting towards anaerobic energy production, while renal perfusion decreases.
- (4)
- Oxygen consumption: the blood concentration of oxygen is measured to indirectly assess mitochondrial activity: there is a linear relationship between Na+ reabsorption and oxygen consumption, in fact Na+ reabsorption is mediated by an energy-dependent (Na+/K+ ATP-pump) process [12]. Recent studies have shown that oxygen administration during HMP increases oxygen consumption from the cells and improves kidney function (GFR) in the transplanted kidneys [28]. There are a variety of formulas currently used that differ on the parameters to be considered [55]. On a separate note, it is of relevance to estimate the calculation according to the temperature range, as in fact previously mentioned, cell metabolism is slowed down by the reduced temperature, therefore the oxygen requirement at hypothermic conditions if different from that at body temperature; furthermore, oxygen consumption during NMP is dependent on the oxygen concentrations offered to the kidney itself [79].
- (5)
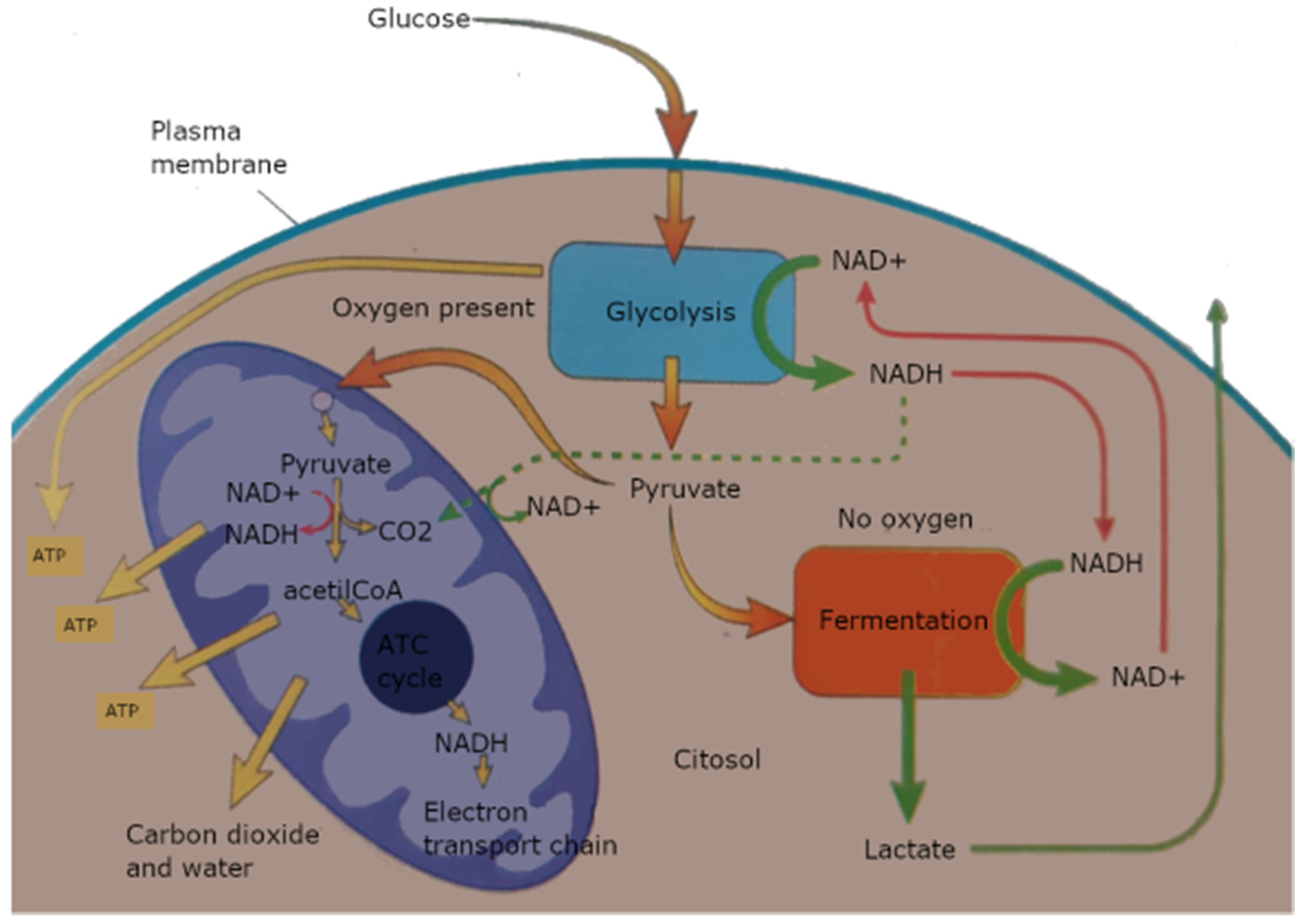
- Measurements of final glycolysis products. A lack of oxygen causes accumulation of peculiar metabolites [80]: succinate/pyruvate, NADH, lactate (Figure 1). The measurement of tissue damage and estimated anaerobic metabolism is a major feature within ischemic organs, with correlation to the extent of warm ischemia time, as for example in the case of DCDs.
- (6)
- Measurement of ATP depletion or ATP/ADP ratio, as the key feature to determine if cell metabolism is predominantly oxidative or glycolytic. With the Na+/K+ ATPases block, the influx of free Ca2+ into the cells and the activation of phospholipases are direct consequences of the fall of ATP production [12]. Another indirect effect is also the increase concentration of transition metals as free iron, since its binding into the carrier proteins (transferrin, ferritin) is inhibited, too by the energy depletion. In this situation, there is also activation of the oxygen free radicals cascade, generating a vicious cycle in which the production of Nitric Oxide (NO), another commonly used measurement of cell viability, increases too [81]. NO has also a direct effect on vasoconstriction, thus relating to perfusion dynamics.
- (7)
- Viability of the kidney during machine perfusion can also be measured by sampling the perfusate for biomarkers of cellular injury [82]. In the hypothermic setting, the most commonly used are glutathione S-transferase (GST), as total-GST (t-GST) or its isoforms (alpha-GST and pi-GST), fatty acid binding protein (FABP), lactate dehydrogenase (LDH) and lactate levels. In the normothermic scenario, the most utilized are neutrophil gelatinase–associated lipocalin (NGAL) and endothelin-1 [39,83].
- (8)
- Microdialysis: a tissue sampling technique using a small (normally 600 μm diameter) probe with a semipermeable membrane at the tip. The inside of the membrane is perfused to maintain concentration gradient across the membrane between the extracellular fluid and the probe. This creates a dialysate stream specular to the tissue concentrations of analytes, as for example glucose and lactate. There is evidence in literature of real time in vivo monitoring, demonstrating that using online microdialysis can provide information on the metabolic state of organs during preservation [84].
- (9)
- mRNA profiling: defective postreperfusion metabolic recovery directly associates with incident delayed graft function and there is evidence of some ischemia induced omics that could be used as predictors of tissue injury [85]. Specific mRNA expression of several glycolytic and gluconeogenic enzymes could evaluate renal glucose metabolism or the degree of inflammation and cytokine production, secondary to the ischemic insult.
- (10)
- Flavin mononucleotide (FMN) levels in the acellular perfusate after 30 min of hypothermic perfusion, as a result of damaged mitochondria releasing their content into cytoplasm [44]. Physiologically, FMN is non-covalently bound to a subunit of the mitochondrial complex I and its dissociation with release at a cytoplasmatic level is an effect of the ischemic injury, where the MPT is damaged, with ROS production and increased toxicity [86].
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | acute rejection |
| AKI | acute kidney injury |
| ATN | acute tubular necrosis ATN |
| ATP | adenosine-triphosphate |
| DCD | donation after circulatory death |
| DGF | delayed graft function |
| ECD | extended criteria donor |
| ESRD | end stage renal disease |
| G6PD | 6-phosphate dehydrogenase |
| MP | machine perfusion |
| HMP | hypothermic machine perfusion |
| MPT | mitochondrial permeability transition |
| NADH | nicotinamide adenine dinucleotide hydride |
| NADPH | adenine dinucleotide phosphate |
| NMP | normothermic machine perfusion |
| NO | nitric oxide |
| 6PG | 6-phosphogluconate dehydrogenase |
| PFI | perfusion flow index |
| PNF | primary non function |
| PPP | pentose phosphate pathway |
| QAS | quality assessment score |
| ROS | reactive oxygen species |
| SCS | static cold storage |
| WIT | warm ischemic time |
References
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.; Held, P.J.; Port, F.K. Comparison of Mortality in All Patients on Dialysis, Patients on Dialysis Awaiting Transplantation, and Recipients of a First Cadaveric Transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, D.; Kościelska-Kasprzak, K.; Chudoba, P.; Hałoń, A.; Mazanowska, O.; Gomółkiewicz, A.; Dzięgiel, P.; Drulis-Fajdasz, D.; Myszka, M.; Lepiesza, A.; et al. The influence of warm ischemia elimination on kidney injury during transplantation–clinical and molecular study. Sci. Rep. 2016, 6, 36118. [Google Scholar] [CrossRef] [PubMed]
- Peters-Sengers, H.; Houtzager, J.H.E.; Heemskerk, M.B.A.; Idu, M.M.; Minnee, R.C.; Klaasen, R.W.; Joor, S.E.; Hagenaars, J.A.M.; Rebers, P.M.; Van Der Heide, J.J.H.; et al. DCD donor hemodynamics as predictor of outcome after kidney transplantation. Arab. Archaeol. Epigr. 2018, 18, 1966–1976. [Google Scholar] [CrossRef] [PubMed]
- Port, F.K.; Bragg-Gresham, J.L.; Metzger, R.A.; Dykstra, D.M.; Gillespie, B.W.; Young, E.W.; Delmonico, F.L.; Wynn, J.J.; Merion, R.M.; Wolfe, R.A.; et al. Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors1. Transplantation 2002, 74, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.N.; Boyne, D.J.; Quinn, R.R.; Austin, P.C.; Hemmelgarn, B.R.; Campbell, P.; Knoll, G.A.; Tibbles, L.A.; Yilmaz, S.; Quan, H.; et al. Mortality and Morbidity in Kidney Transplant Recipients with a Failing Graft: A Matched Cohort Study. Can. J. Kidney Health Dis. 2020, 7, 2054358120908677. [Google Scholar] [CrossRef]
- Bellini, M.I.; Charalampidis, S.; Herbert, P.E.; Bonatsos, V.; Crane, J.; Muthusamy, A.; Dor, F.J.M.F.; Papalois, V. Cold Pulsatile Machine Perfusion versus Static Cold Storage in Kidney Transplantation: A Single Centre Experience. BioMed Res. Int. 2019, 2019, 7435248. [Google Scholar] [CrossRef]
- Sung, R.S.; Christensen, L.L.; Leichtman, A.B.; Greenstein, S.M.; Distant, D.A.; Wynn, J.J.; Stegall, M.D.; Delmonico, F.L.; Port, F.K. Determinants of Discard of Expanded Criteria Donor Kidneys: Impact of Biopsy and Machine Perfusion. Arab. Archaeol. Epigr. 2008, 8, 783–792. [Google Scholar] [CrossRef]
- Bellini, M.I.; D’Andrea, V. Organ preservation: Which temperature for which organ? J. Int. Med Res. 2019, 47, 2323–2325. [Google Scholar] [CrossRef]
- Murray, J.E. Reflections on the first successful kidney transplantation. World J. Surg. 1982, 6, 372–376. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.-Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.I.; Yiu, J.; Nozdrin, M.; Papalois, V. The Effect of Preservation Temperature on Liver, Kidney, and Pancreas Tissue ATP in Animal and Preclinical Human Models. J. Clin. Med. 2019, 8, 1421. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Meng, X.-F.; Zhang, C. Role of NADPH Oxidase-Mediated Reactive Oxygen Species in Podocyte Injury. BioMed Res. Int. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, Y.; Chabrashvili, T.; Aslam, S.; Conde, L.J.B.; Umans, J.G.; Wilcox, C.S. Role of Oxidative Stress in Endothelial Dysfunction and Enhanced Responses to Angiotensin II of Afferent Arterioles from Rabbits Infused with Angiotensin II. J. Am. Soc. Nephrol. 2003, 14, 2783–2789. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Fernández-Marcos, P.J.; Nóbrega-Pereira, S. NADPH: New oxygen for the ROS theory of aging. Oncotarget 2016, 7, 50814–50815. [Google Scholar] [CrossRef]
- Shankland, S.J. The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int. 2006, 69, 2131–2147. [Google Scholar] [CrossRef]
- Chawla, L.S.; Kimmel, P.L. Acute kidney injury and chronic kidney disease: An integrated clinical syndrome. Kidney Int. 2012, 82, 516–524. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Griffin, K.A.; Lan, R.; Geng, H.; Saikumar, P.; Bidani, A.K. Acute kidney injury: A springboard for progression in chronic kidney disease. Am. J. Physiol. Physiol. 2010, 298, F1078–F1094. [Google Scholar] [CrossRef]
- Yarlagadda, S.G.; Coca, S.G.; Garg, A.X.; Doshi, M.; Poggio, E.; Marcus, R.J.; Parikh, C.R. Marked variation in the definition and diagnosis of delayed graft function: A systematic review. Nephrol. Dial. Transplant. 2008, 23, 2995–3003. [Google Scholar] [CrossRef]
- De Oliveira, B.D.; Xu, K.; Shen, T.H.; Callahan, M.; Kiryluk, K.; D’Agati, V.D.; Tatonetti, N.P.; Barasch, J.; Devarajan, P. Molecular nephrology: Types of acute tubular injury. Nat. Rev. Nephrol. 2019, 15, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Perico, N.; Cattaneo, D.; Sayegh, M.H.; Remuzzi, G. Delayed graft function in kidney transplantation. Lancet 2004, 364, 1814–1827. [Google Scholar] [CrossRef]
- Bellini, M.I.; Courtney, A.E.; McCaughan, J.A. Living Donor Kidney Transplantation Improves Graft and Recipient Survival in Patients with Multiple Kidney Transplants. J. Clin. Med. 2020, 9, 2118. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, C. The impact of cold ischemia time on renal transplant outcome. Kidney Int. 2015, 87, 272–275. [Google Scholar] [CrossRef]
- Bellini, M.I.; Nozdrin, M.; Yiu, J.; Papalois, V. Machine Perfusion for Abdominal Organ Preservation: A Systematic Review of Kidney and Liver Human Grafts. J. Clin. Med. 2019, 8, 1221. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, J.; Xia, T.C.; Xu, R.; He, X.; Xia, Y. Preservation Solutions for Kidney Transplantation: History, Advances and Mechanisms. Cell Transplant. 2019, 28, 1472–1489. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Hunter, J.P.; Nicholson, M.L. Cold Ischaemic Injury in Kidney Transplantation; IntechOpen: London, UK, 2012. [Google Scholar]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, H.S.; Leemkolk, F.E.M.V.D.; Leuvenink, H.G.D.; Knight, S.R.; Pirenne, J.; Ploeg, R.J.; Abramowicz, D.; et al. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662. [Google Scholar] [CrossRef]
- Thompson, E.R.; Bates, L.; Ibrahim, I.K.; Sewpaul, A.; Stenberg, B.; McNeill, A.; Figueiredo, R.; Girdlestone, T.; Wilkins, G.C.; Wang, L.; et al. Novel delivery of cellular therapy to reduce ischemia reperfusion injury in kidney transplantation. Arab. Archaeol. Epigr. 2020. [Google Scholar] [CrossRef]
- Ravaioli, M.; De Pace, V.; Angeletti, A.; Comai, G.; Vasuri, F.; Baldassarre, M.; Maroni, L.; Odaldi, F.; Fallani, G.; Caraceni, P.; et al. Hypothermic Oxygenated New Machine Perfusion System in Liver and Kidney Transplantation of Extended Criteria Donors:First Italian Clinical Trial. Sci. Rep. 2020, 10, 6063. [Google Scholar] [CrossRef]
- Van Rijn, R.; Karimian, N.; Matton, A.P.M.; Burlage, L.C.; Westerkamp, A.C.; Berg, A.P.V.D.; De Kleine, R.H.J.; De Boer, M.T.; Lisman, T.; Porte, R.J. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. BJS 2017, 104, 907–917. [Google Scholar] [CrossRef]
- Schlegel, A.; Kron, P.; Graf, R.; Dutkowski, P.; Clavien, P.-A. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J. Hepatol. 2014, 61, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-F.; Dong, Q.; Zhang, T. Effects of Static Cold Storage and Hypothermic Machine Perfusion on Oxidative Stress Factors, Adhesion Molecules, and Zinc Finger Transcription Factor Proteins Before and After Liver Transplantation. Ann. Transplant. 2017, 22, 96–100. [Google Scholar] [CrossRef] [PubMed]
- De Beule, J.; Jochmans, I. Kidney Perfusion as an Organ Quality Assessment Tool—Are We Counting Our Chickens Before They Have Hatched? J. Clin. Med. 2020, 9, 879. [Google Scholar] [CrossRef] [PubMed]
- Dirito, J.R.; Hosgood, S.A.; Tietjen, G.T.; Nicholson, M.L. The future of marginal kidney repair in the context of normothermic machine perfusion. Arab. Archaeol. Epigr. 2018, 18, 2400–2408. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Thompson, E.; Moore, T.; Wilson, C.H.; Nicholson, M.L. Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. Br. J. Surg. 2018, 105, 388–394. [Google Scholar] [CrossRef]
- Kaths, J.M.; Echeverri, J.; Chun, Y.M.; Cen, J.Y.; Goldaracena, N.; Linares, I.; Dingwell, L.S.; Yip, P.M.; John, R.; Bagli, D.; et al. Continuous Normothermic Ex Vivo Kidney Perfusion Improves Graft Function in Donation After Circulatory Death Pig Kidney Transplantation. Transplantation 2017, 101, 754–763. [Google Scholar] [CrossRef]
- Kaminski, J.; Delpech, P.-O.; Kaaki-Hosni, S.; Promeyrat, X.; Hauet, T.; Hannaert, P. Oxygen Consumption by Warm Ischemia-Injured Porcine Kidneys in Hypothermic Static and Machine Preservation. J. Surg. Res. 2019, 242, 78–86. [Google Scholar] [CrossRef]
- Weissenbacher, A.; Faro, L.L.; Boubriak, O.; Soares, M.F.; Roberts, I.S.; Hunter, J.P.; Voyce, D.; Mikov, N.; Cook, A.; Ploeg, R.J.; et al. Twenty-four–hour normothermic perfusion of discarded human kidneys with urine recirculation. Arab. Archaeol. Epigr. 2019, 19, 178–192. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Nicholson, M.L. First in Man Renal Transplantation after Ex Vivo Normothermic Perfusion. Transplantation 2011, 92, 735–738. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Barlow, A.D.; Hunter, J.P.; Nicholson, M.L. Ex vivo normothermic perfusion for quality assessment of marginal donor kidney transplants. Br. J. Surg. 2015, 102, 1433–1440. [Google Scholar] [CrossRef]
- Bellini, M.I.; Paoletti, F.; Herbert, P.E. Obesity and bariatric intervention in patients with chronic renal disease. J. Int. Med. Res. 2019, 47, 2326–2341. [Google Scholar] [CrossRef] [PubMed]
- Peters-Sengers, H.; Berger, S.P.; Heemskerk, M.B.; Al Arashi, D.; Van Der Heide, J.J.H.; Hemke, A.C.; Berge, I.J.T.; Idu, M.M.; Betjes, M.G.; Van Zuilen, A.D.; et al. Stretching the Limits of Renal Transplantation in Elderly Recipients of Grafts from Elderly Deceased Donors. J. Am. Soc. Nephrol. 2016, 28, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Thompson, E.; Bates, L.; Pither, T.L.; Hosgood, S.A.; Nicholson, M.L.; Watson, C.J.; Wilson, C.; Fisher, A.J.; Ali, S.; et al. Flavin Mononucleotide as a Biomarker of Organ Quality—A Pilot Study. Transplant. Direct 2020, 6, e600. [Google Scholar] [PubMed]
- Dos Santos, V.G.; Ramos-Muñoz, E.; García-Bermejo, M.L.; Ruiz-Hernández, M.; Rodríguez-Serrano, E.M.; Saiz-González, A.; Martínez-Perez, A.; Burgos-Revilla, F.J. MicroRNAs in Kidney Machine Perfusion Fluid as Novel Biomarkers for Graft Function. Normalization Methods for miRNAs Profile Analysis. Transplant. Proc. 2019, 51, 307–310. [Google Scholar] [CrossRef]
- Juriasingani, S.; Akbari, M.; Chan, J.Y.; Whiteman, M.; Sener, A. H2S supplementation: A novel method for successful organ preservation at subnormothermic temperatures. Nitric Oxide 2018, 81, 57–66. [Google Scholar] [CrossRef]
- Gregorini, M.; Corradetti, V.; Pattonieri, E.F.; Rocca, C.; Milanesi, S.; Peloso, A.; Canevari, S.; De Cecco, L.; Dugo, M.; Avanzini, M.A.; et al. Perfusion of isolated rat kidney with Mesenchymal Stromal Cells/Extracellular Vesicles prevents is chaemic injury. J. Cell. Mol. Med. 2017, 21, 3381–3393. [Google Scholar] [CrossRef]
- van Smaalen, T.C.; Beurskens, D.M.; Hoogland, E.P.; Winkens, B.; Christiaans, M.H.; Reutelingsperger, C.P.; van Heurn, L.W.; Nicolaes, G.A.F. Presence of Cytotoxic Extracellular Histones in Machine Perfusate of Donation after Circulatory Death Kidneys. Transplantation 2017, 101, e93–e101. [Google Scholar] [CrossRef]
- Hamaoui, K.; Aftab, A.; Gowers, S.A.N.; Boutelle, M.; Cook, T.; Rudd, D.; Dobson, G.P.; Papalois, V. An ex vivo comparison of adenosine and lidocaine solution and University of Wisconsin solution for hypothermic machine perfusion of porcine kidneys: Potential for development. J. Surg. Res. 2017, 208, 219–229. [Google Scholar] [CrossRef]
- Sevinc, M.; Stamp, S.; Ling, J.; Carter, N.; Talbot, D.; Sheerin, N. Ex Vivo Perfusion Characteristics of Donation After Cardiac Death Kidneys Predict Long-Term Graft Survival. Transplant. Proc. 2016, 48, 3251–3260. [Google Scholar] [CrossRef]
- Schopp, I.; Reissberg, E.; Lüer, B.; Efferz, P.; Minor, T. Controlled Rewarming after Hypothermia: Adding a New Principle to Renal Preservation. Clin. Transl. Sci. 2015, 8, 475–478. [Google Scholar] [CrossRef]
- Guy, A.J.; Nath, J.; Cobbold, M.; Ludwig, C.; Tennant, D.A.; Inston, N.; Ready, A.R. Metabolomic Analysis of Perfusate During Hypothermic Machine Perfusion of Human Cadaveric Kidneys. Transplantation 2015, 99, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Gómez, V.; Orosa, A.; Rivera, M.; Diez-Nicolás, V.; Hevia, V.; Alvarez, S.; Carracedo, D.; Ramos, E.; Burgos, F.J. Resistance Index Determination in the Pre and Post Kidney Transplantation Time Points in Graft Dysfunction Diagnosis. Transplant. Proc. 2015, 47, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Buchs, J.-B.; Buehler, L.; Moll, S.; Ruttimann, R.; Nastasi, A.; Kasten, J.; Morel, P.; Lazeyras, F. DCD Pigs’ Kidneys Analyzed by MRI to Assess Ex Vivo Their Viability. Transplantation 2013, 97, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Bunegin, L.; Tolstykh, G.P.; Gelineau, J.F.; Cosimi, A.B.; Anderson, L.M. Oxygen Consumption During Oxygenated Hypothermic Perfusion as a Measure of Donor Organ Viability. ASAIO J. 2013, 59, 427–432. [Google Scholar] [CrossRef] [PubMed]
- De Vries, E.E.; Boer, J.; Hoogland, P.; Krivitski, N.M.; Snoeijs, M.G.; Van Heurn, E. Measurement of Renovascular Circulating Volume During Hypothermic Organ Perfusion. Transplantation 2012, 94, 511. [Google Scholar] [CrossRef]
- Gallinat, A.; Lüer, B.; Swoboda, S.; Rauen, U.; Paul, A.; Minor, T. Use of the new preservation solution Custodiol-N supplemented with dextran for hypothermic machine perfusion of the kidney. Cryobiology 2013, 66, 131–135. [Google Scholar] [CrossRef]
- Nagelschmidt, M.; Minor, T.; Gallinat, A.; Moers, C.; Jochmans, I.; Pirenne, J.; Ploeg, R.J.; Paul, A.; Treckmann, J.W. Lipid peroxidation products in machine perfusion of older donor kidneys. J. Surg. Res. 2013, 180, 337–342. [Google Scholar] [CrossRef]
- Hoogland, E.R.P.; De Vries, E.E.; Christiaans, M.H.L.; Winkens, B.; Snoeijs, M.G.J.; Van Heurn, L.W.E. The Value of Machine Perfusion Biomarker Concentration in DCD Kidney Transplantations. Transplantation 2013, 95, 603–610. [Google Scholar] [CrossRef]
- Wilson, C.H.; Wyrley-Birch, H.; Vijayanand, D.; Leea, A.; Carter, N.; Haswell, M.; Cunningham, A.C.; Talbot, D. The influence of perfusion solution on renal graft viability assessment. Transplant. Res. 2012, 1, 18. [Google Scholar] [CrossRef]
- Jochmans, I.; Moers, C.; Smits, J.M.; Leuvenink, H.G.D.; Treckmann, J.W.; Paul, A.; Rahmel, A.; Squifflet, J.-P.; Van Heurn, E.; Monbaliu, D.; et al. The Prognostic Value of Renal Resistance During Hypothermic Machine Perfusion of Deceased Donor Kidneys. Arab. Archaeol. Epigr. 2011, 11, 2214–2220. [Google Scholar] [CrossRef]
- Tolstykh, G.P.; Gelineau, J.F.; Maier, L.M.; Bunegin, L. Novel portable hypothermic pulsatile perfusion preservation technology: Improved viability and function of rodent and canine kidneys. Ann. Transplant. 2010, 15, 35–43. [Google Scholar] [PubMed]
- Weegman, B.; Kirchner, V.; Scott, W.E.; Avgoustiniatos, E.; Suszynski, T.; Ferrer-Fàbrega, J.; Rizzari, M.; Kidder, L.; Kandaswamy, R.; Sutherland, D.; et al. Continuous Real-time Viability Assessment of Kidneys Based on Oxygen Consumption. Transplant. Proc. 2010, 42, 2020–2023. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, N.; Konno, Y.; Jyojima, Y.; Akashi, I.; Iwamoto, H.; Hama, K.; Hiirano, T.; Nagao, T. Machine Perfusion Preservation for Kidney Grafts With a High Creatinine From Uncontrolled Donation After Cardiac Death. Transplant. Proc. 2010, 42, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Koetting, M.; Frotscher, C.; Minor, T. Hypothermic reconditioning after cold storage improves postischemic graft function in isolated porcine kidneys. Transpl. Int. 2009, 23, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Sohrabi, S.; Reddy, M.; Carter, N.; Ahmed, A.; Talbot, D. Dual Transplantation of Marginal Kidneys From Nonheart Beating Donors Selected Using Machine Perfusion Viability Criteria. J. Urol. 2008, 179, 2305–2309. [Google Scholar] [CrossRef]
- Bagul, A.; Hosgood, S.A.; Kaushik, M.; Kay, M.D.; Waller, H.L.; Nicholson, M.L. Experimental renal preservation by normothermic resuscitation perfusion with autologous blood. Br. J. Surg. 2008, 95, 111–118. [Google Scholar] [CrossRef]
- Maathuis, M.-H.J.; Manekeller, S.; Van Der Plaats, A.; Leuvenink, H.G.D.; A’t Hart, N.; Lier, A.B.; Rakhorst, G.; Ploeg, R.J.; Minor, T. Improved Kidney Graft Function After Preservation Using a Novel Hypothermic Machine Perfusion Device. Ann. Surg. 2007, 246, 982–991. [Google Scholar] [CrossRef]
- Wilson, C.H.; Asher, J.; Gupta, A.; Vijayanand, D.; Wyrley-Birch, H.; Stamp, S.; Rix, D.; Soomro, N.; Manas, D.; Jaques, B.; et al. Comparison of HTK and Hypertonic Citrate to Intraarterial Cooling in Human Non–Heart-Beating Kidney Donors. Transplant. Proc. 2007, 39, 351–352. [Google Scholar] [CrossRef]
- Baicu, S.C.; Taylor, M.J.; Brockbank, K.G.M. Modulating biochemical perturbations during 72-hour machine perfusion of kidneys: Role of preservation solution. Cryobiology 2007, 54, 114–120. [Google Scholar] [CrossRef]
- De Vries, B.; Snoeijs, M.G.J.; Von Bonsdorff, L.; Van Heurn, L.W.E.; Parkkinen, J.; Buurman, W.A. Redox-Active Iron Released During Machine Perfusion Predicts Viability of Ischemically Injured Deceased Donor Kidneys. Arab. Archaeol. Epigr. 2006, 6, 2686–2693. [Google Scholar] [CrossRef]
- Minor, T.; Sitzia, M.; Dombrowski, F. Kidney transplantation from non-heart-beating donors after oxygenated low-flow machine perfusion preservation with histidine? tryptophan? ketoglutarate solution. Transpl. Int. 2004, 17, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Gok, M.A.; Shenton, B.K.; Buckley, P.E.; Peaston, R.; Cornell, C.; Soomro, N.; Jaques, B.C.; Manas, D.M.; Talbot, D. How to improve the quality of kidneys from non-heart-beating donors: A randomised controlled trial of thrombolysis in non–heart-beating donors. Transplantation 2003, 76, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Brook, N.R.; Knight, A.J.; Nicholson, M.L. Intra-renal resistance reflects warm ischaemic damage, and is further increased by static cold storage: A model of non-heart-beating donor kidneys. Med. Sci. Monit. 2003, 9, 271–275. [Google Scholar]
- Balupuri, S.; Mantle, D.; Mohamed, M.; Shenton, B.; Gok, M.; Soomro, N.; Manas, D.; Kirby, J.A.; Talbot, D. Machine perfusion and viability assessment of non–heart-beating donor kidneys—a single-centre result. Transplant. Proc. 2001, 33, 1119–1120. [Google Scholar] [CrossRef]
- Polak, W.; Danielewicz, R.; Kwiatkowski, A.; Kosieradzki, M.; Michalak, G.; Węgrowicz-Rebandel, I.; Wałaszewski, J.; Rowiński, W.; Wȩgrowicz-Rebandel, I. Pretransplant evaluation of renal viability by glutathione S-transferase in machine perfusate. Transplant. Proc. 2000, 32, 171–172. [Google Scholar] [CrossRef]
- Nordling, S.; Brännström, J.; Carlsson, F.; Lu, B.; Salvaris, E.; Wanders, A.; Buijs, J.; Estrada, S.; Tolmachev, V.; Cowan, P.J.; et al. Enhanced protection of the renal vascular endothelium improves early outcome in kidney transplantation: Preclinical investigations in pig and mouse. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Abramowicz, D.; Oberbauer, R.; Heemann, U.; Viklicky, O.; Peruzzi, L.; Mariat, C.; Crespo, M.; Budde, K.; Oniscu, G.C. Recent advances in kidney transplantation: A viewpoint from the Descartes advisory board. Nephrol. Dial. Transplant. 2018, 33, 1699–1707. [Google Scholar] [CrossRef]
- Patel, K.; Smith, T.B.; Neil, D.A.H.; Thakker, A.; Tsuchiya, Y.; Higgs, E.B.; Hodges, N.J.; Ready, A.R.; Nath, J.; Ludwig, C. The Effects of Oxygenation on Ex Vivo Kidneys Undergoing Hypothermic Machine Perfusion. Transplantation 2019, 103, 314–322. [Google Scholar] [CrossRef]
- O’Neill, L.A. Biochemistry: Succinate strikes. Nature 2014, 515, 350–351. [Google Scholar] [CrossRef]
- Thuillier, R.; Hauet, T. Impact of Hypothermia and Oxygen Deprivation on the Cytoskeleton in Organ Preservation Models. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Guzzi, F.; Knight, S.R.; Ploeg, R.J.; Hunter, J.P. A systematic review to identify whether perfusate biomarkers produced during hypothermic machine perfusion can predict graft outcomes in kidney transplantation. Transpl. Int. 2020, 33, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Nicholson, M.L. An Assessment of Urinary Biomarkers in a Series of Declined Human Kidneys Measured During Ex Vivo Normothermic Kidney Perfusion. Transplantation 2017, 101, 2120–2125. [Google Scholar] [CrossRef] [PubMed]
- Hamaoui, K.; Gowers, S.A.N.; Damji, S.; Rogers, M.; Leong, C.L.; Hanna, G.; Darzi, A.; Boutelle, M.; Papalois, V. Rapid sampling microdialysis as a novel tool for parenchyma assessment during static cold storage and hypothermic machine perfusion in a translational ex vivo porcine kidney model. J. Surg. Res. 2016, 200, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Wijermars, L.G.M.; Schaapherder, A.F.; De Vries, D.K.; Verschuren, L.; Wust, R.C.I.; Kostidis, S.; Mayboroda, O.A.; Prins, F.; Ringers, J.; Bierau, J.; et al. Defective postreperfusion metabolic recovery directly associates with incident delayed graft function. Kidney Int. 2016, 90, 181–191. [Google Scholar] [CrossRef]
- Ten, V.; Galkin, A. Mechanism of mitochondrial complex I damage in brain ischemia/reperfusion injury. A hypothesis. Mol. Cell. Neurosci. 2019, 100, 103408. [Google Scholar] [CrossRef]
| Author | Donor Type | Perfusion Type | Viability Assessment | Model |
|---|---|---|---|---|
| Wang et al. [44] | DCD/ECD | NMP | Macroscopic appearance, mean renal blood flow and total urine output; FMN in the perfusate | Human |
| Gomez-Dos-Santos et al. [45] | ECD | HMP | miRNA in the perfusate | Human |
| Bellini et al. [6] | SCD/DCD | HMP | Perfusion dynamics | Human |
| Weissenbacher et al. [39] | DBD/DCD | NMP | Perfusion parameters; NGAL and KIM-1 levels in the perfusate; pO2 and pCO2 levels; glucose measurement; lactate levels; urine production and sodium levels in perfusate and urine | Human |
| Juriasingani et al. [46] | DCD | SNM | Fluorescent marker that binds to double-stranded DNA | Animal (pig) |
| Gregorini et al. [47] | DCD | – | Lactate, LDH, MDA, glucose and pyruvate in perfusate samples, RNA in the perfusate | Animal (rat) |
| Van Smaalen et al. [48] | DCD | HMP | Extracellular histone (H3) in perfusate samples | Human |
| Hamaoui et al. [49] | DCD | HMP | Creatinine clearance, oxygen, glucose consumption, lactate, microdialysis | Animal (pig) |
| Sevinc et al. [50] | DCD | HMP | Perfusion parameters; GST levels in the perfusate | Human |
| Hosgood et al. [41] | Kidneys retrieved, but not implanted | NMP | Macroscopic perfusion, perfusion parameters, urine output | Human |
| Schopp et al. [51] | SCD | COR | Oxygen consumption, total content of NAD, functional activity of caspase 9 in mitochondria | Animal (pig) |
| Guy et al. [52] | SCD/DCD/ECD | HMP | Perfusion parameters; metabolomic profile via nuclear magnetic resonance: glucose, inosine, leucine and gluconate concentrations. | Human |
| Gomez et al. [53] | ECD | HMP | Perfusion parameters | Human |
| Buchs et al. [54] | DCD | HMP | ATP levels via Magnetic Resonance Imaging | Animal (pig) |
| Bunegin et al. [55] | ECD | HMP | Perfusion parameters; oxygen consumption | Human |
| De Vries E. et al. [56] | DCD | HMP | Renovasculature circulating volume | Human |
| Gallinat et al. [57] | SCD | HMP | Perfusion parameters, oxygen consumption, urine production, clearance of creatinine | Animal (pig) |
| Nagelsch et al. [58] | ECD | HMP | GST, LPOP, lactate and LDH levels in the perfusate | Human |
| Hoogland et al. [59] | DCD | HMP | GST, LDH, H-FABP, redox-active iron, IL-18, and NGAL in the perfusate | Human |
| Wilson et al. [60] | DCD | HMP | Perfusion parameters; GST in perfusate samples and mitochondrial electron microscopy | Animal (rat) |
| Jochmans et al. [61] | SCD, ECD and DCD | HMP | Perfusion parameters | Human |
| Tolstykh et al. [62] | SCD | HMP/NMP | Oxygen consumption, potassium-hydrogen gradient, perfusion parameters, GFR, fluorescence to investigate cell membrane viability | Animal (rat and dog) |
| Weegman et al. [63] | DCD | HMP | Oxygen consumption | Animal (pig) |
| Matsuno et al. [64] | DCD | HMP | Perfusion parameters | Human |
| Koetting et al. [65] | SCD | HMP | Oxygen consumption, LDH in perfusate, creatinine and urea concentrations, functional activity of caspase 3 | Animal (pig) |
| Navarro et al. [66] | DCD | HMP | Perfusion parameters | Human |
| Bagul et al. [67] | DCD | HMP/NMP | Perfusion parameters, oxygen consumption, ATP levels, Von Willebrand factor | Animal (pig) |
| Maathius et al. [68] | DCD | HMP | Perfusion parameters, TBARS, NAG and AAP activity in urine, microcirculation, mRNA, histology | Animal (pig) |
| Wilson et al. [69] | DCD | HMP | Perfusion parameters, perfusate enzyme viability assay (GST), perfusate pH and lactate concentrations. | Human |
| Baicu et al. [70] | DCD | HMP | Kidney weight, perfusion parameters, glutamate and ammonium in the perfusate | Animal (pig) |
| De Vries B. et al. [71] | DCD | HMP | Redox-active iron, LDH, GST and hemoglobin concentrations | Human |
| Minor et al. [72] | DCD | HMP | Electron microscopy (vascular endothelium), ATP levels in tissue homogenates | Animal (pig) |
| Gok et al. [73] | DCD | HMP | Perfusion parameters; biomarkers of tubular injury: GST, AAP and FABP levels. | Human |
| Brook et al. [74] | DCD | HMP | Perfusion parameters, | Animal (pig) |
| Balupuri et al. [75] | DCD | HMP | GST levels in the perfusate | Human |
| Polak et al. [76] | SCD | HMP | Perfusion parameters, LDH, lactate level, pH and samples of perfusate for α- and π-GST. | Human |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellini, M.I.; Tortorici, F.; Amabile, M.I.; D’Andrea, V. Assessing Kidney Graft Viability and Its Cells Metabolism during Machine Perfusion. Int. J. Mol. Sci. 2021, 22, 1121. https://doi.org/10.3390/ijms22031121
Bellini MI, Tortorici F, Amabile MI, D’Andrea V. Assessing Kidney Graft Viability and Its Cells Metabolism during Machine Perfusion. International Journal of Molecular Sciences. 2021; 22(3):1121. https://doi.org/10.3390/ijms22031121
Chicago/Turabian StyleBellini, Maria Irene, Francesco Tortorici, Maria Ida Amabile, and Vito D’Andrea. 2021. "Assessing Kidney Graft Viability and Its Cells Metabolism during Machine Perfusion" International Journal of Molecular Sciences 22, no. 3: 1121. https://doi.org/10.3390/ijms22031121
APA StyleBellini, M. I., Tortorici, F., Amabile, M. I., & D’Andrea, V. (2021). Assessing Kidney Graft Viability and Its Cells Metabolism during Machine Perfusion. International Journal of Molecular Sciences, 22(3), 1121. https://doi.org/10.3390/ijms22031121








