Effects and Mechanisms of Chitosan and ChitosanOligosaccharide on Hepatic Lipogenesis and Lipid Peroxidation, Adipose Lipolysis, and Intestinal Lipid Absorption in Rats with High-Fat Diet-Induced Obesity
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Changes in Body Weight, Food Intake, Food Utilization Rate, and Tissue Weight
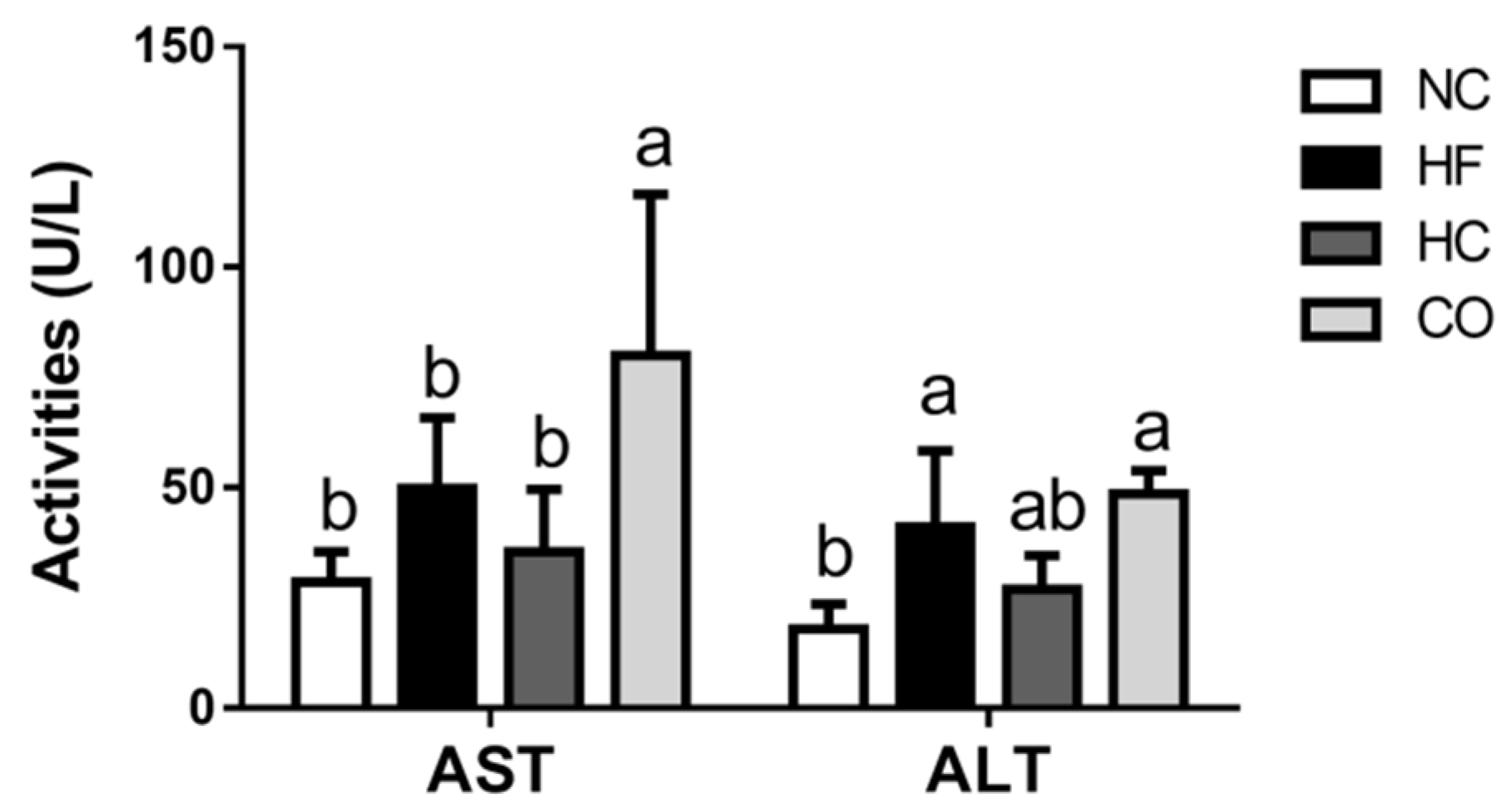
2.2. The Changes in Plasma Biochemical Indexes
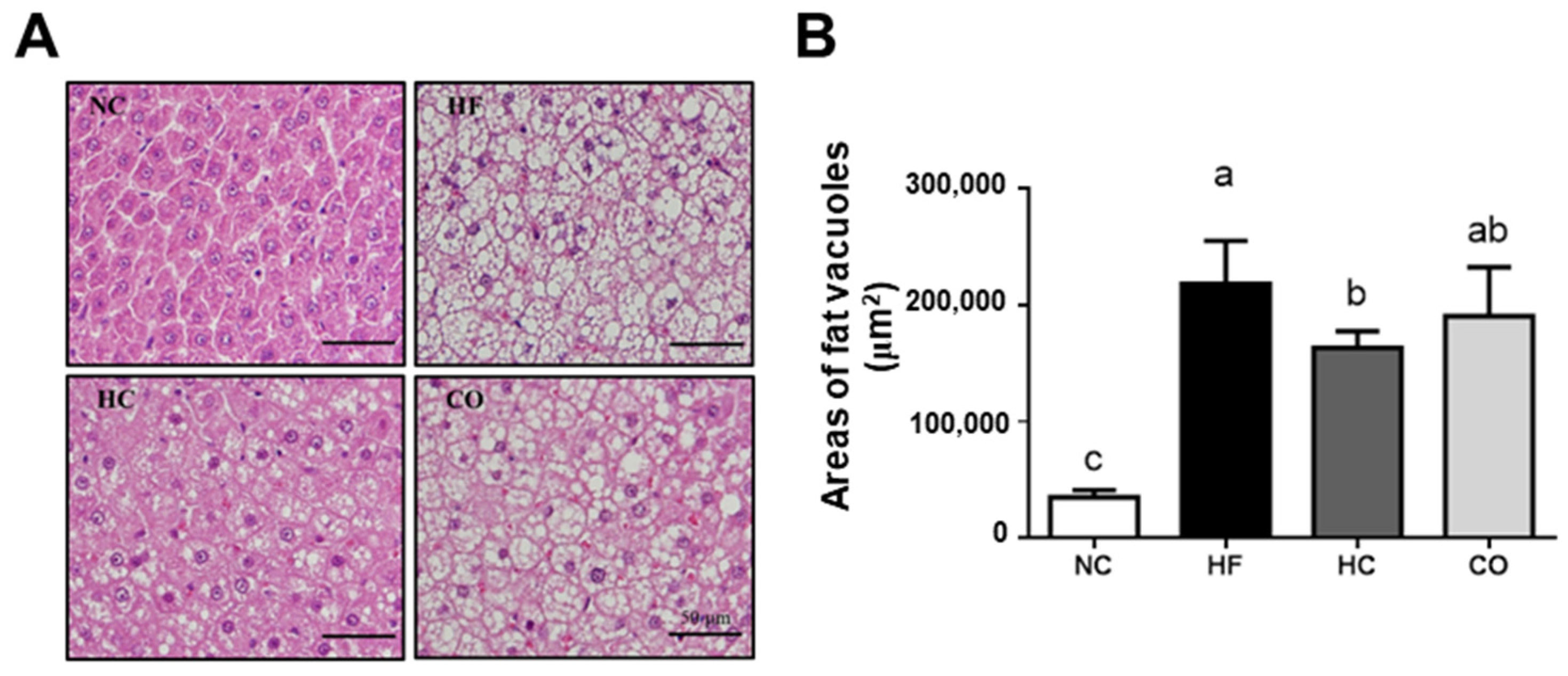
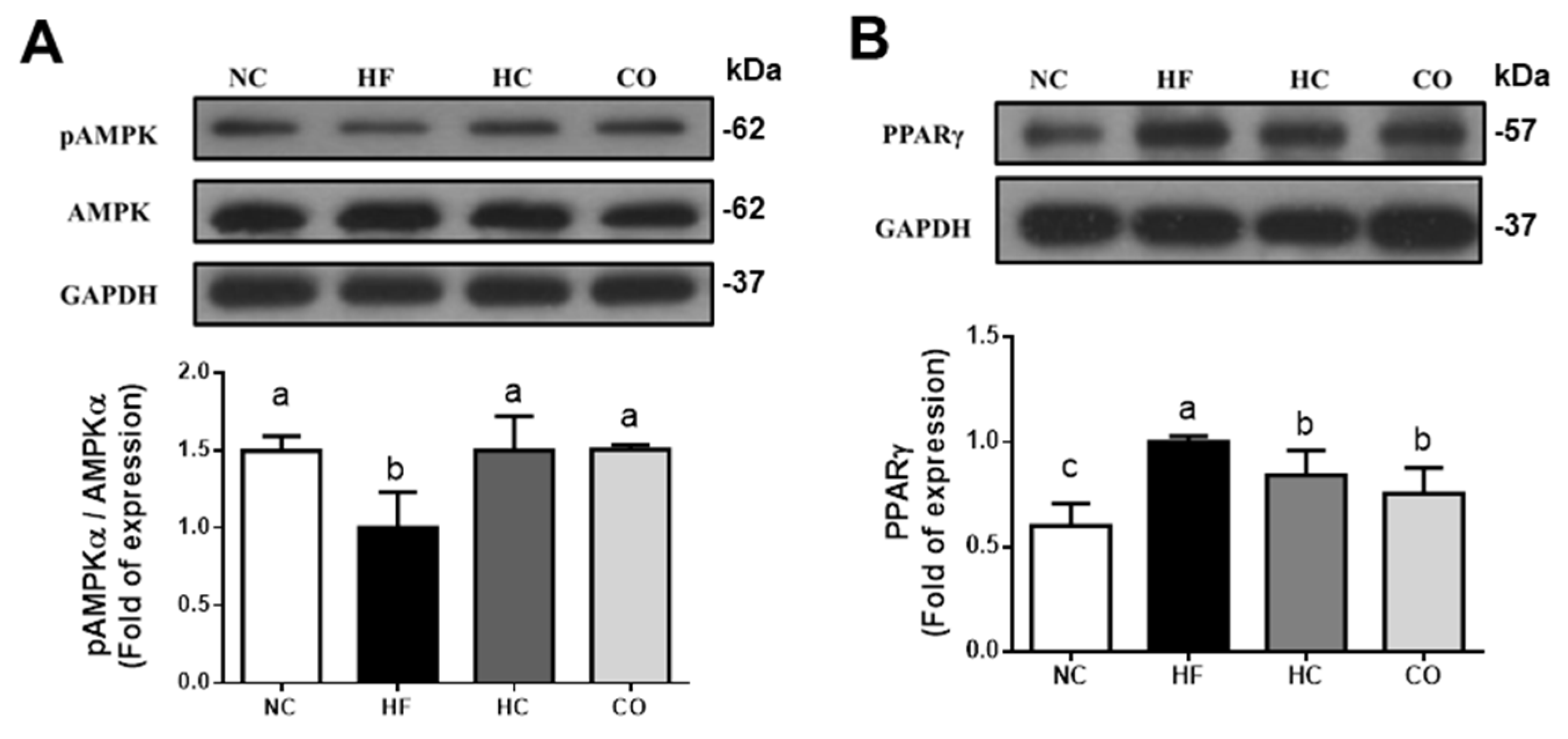
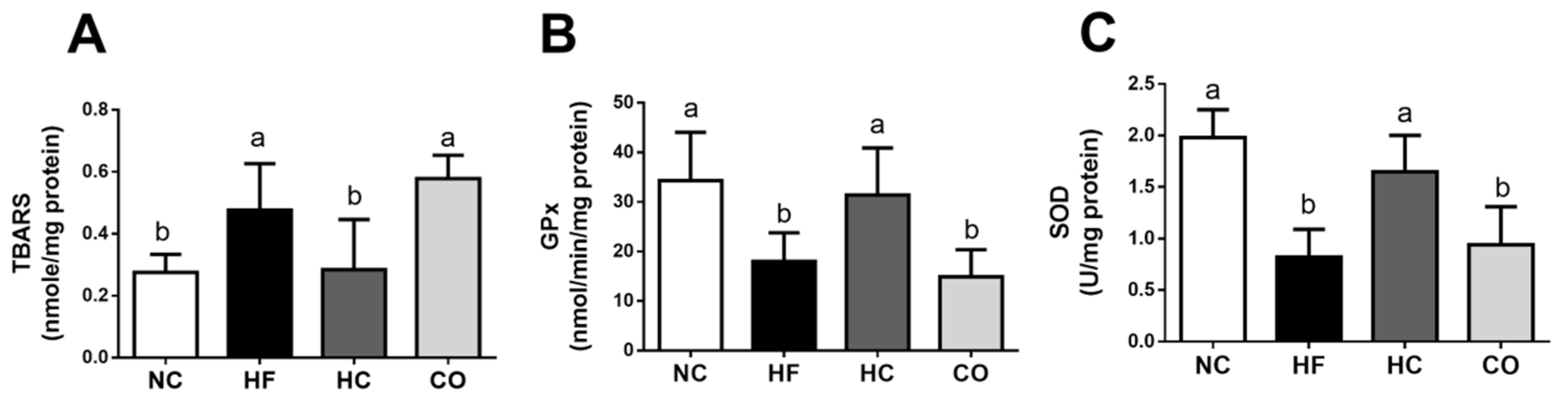
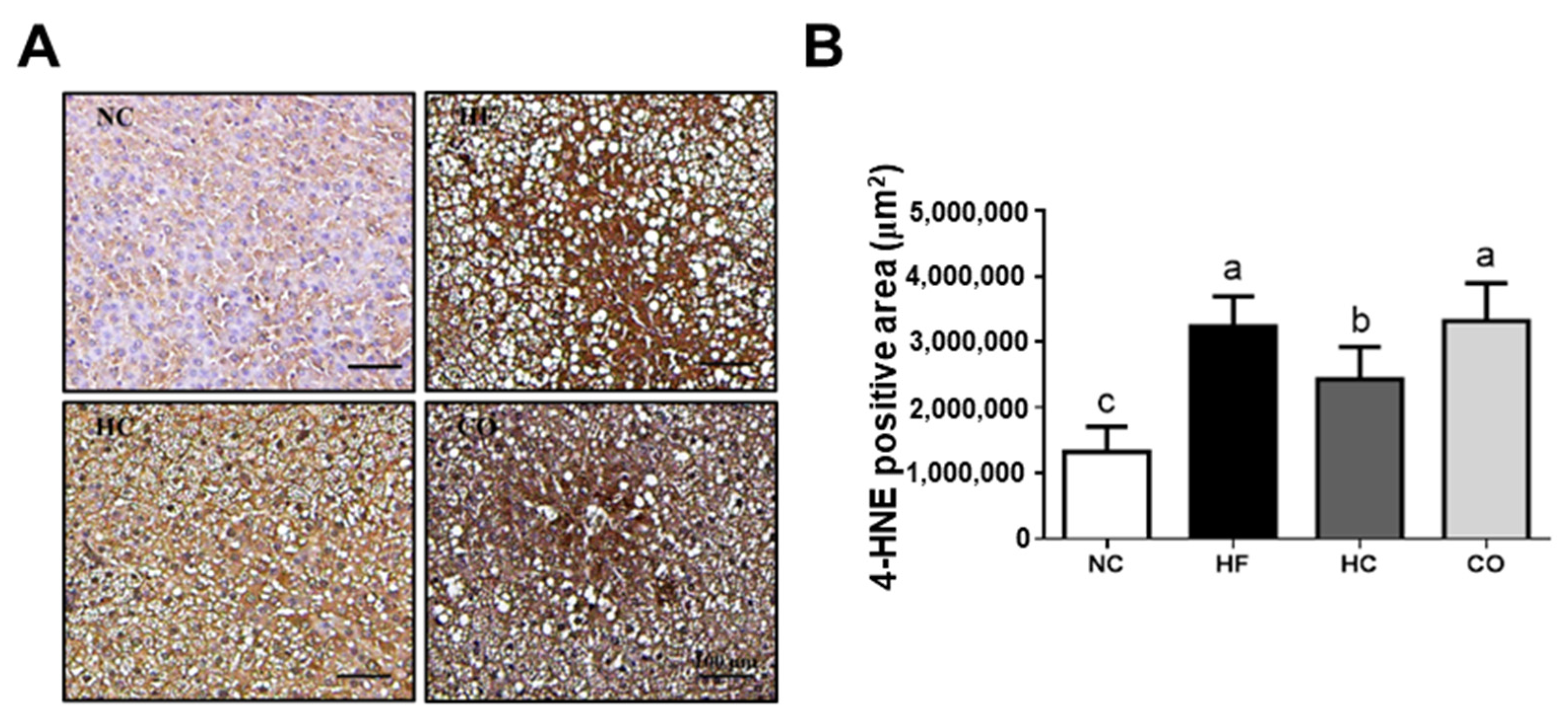
2.3. The Changes in Liver Lipids and Biochemical Indexes
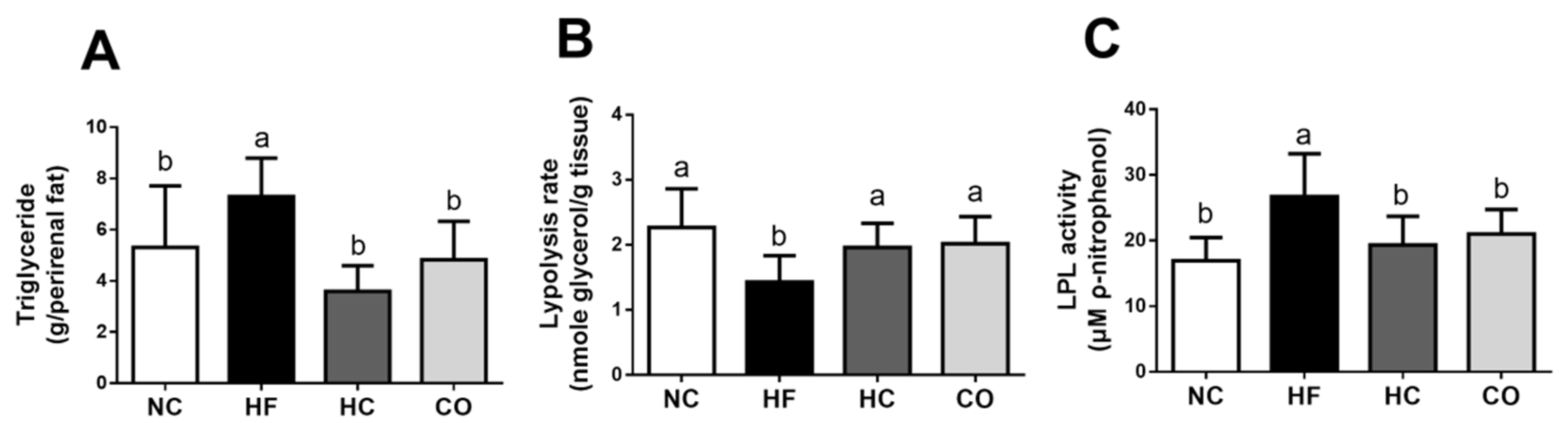
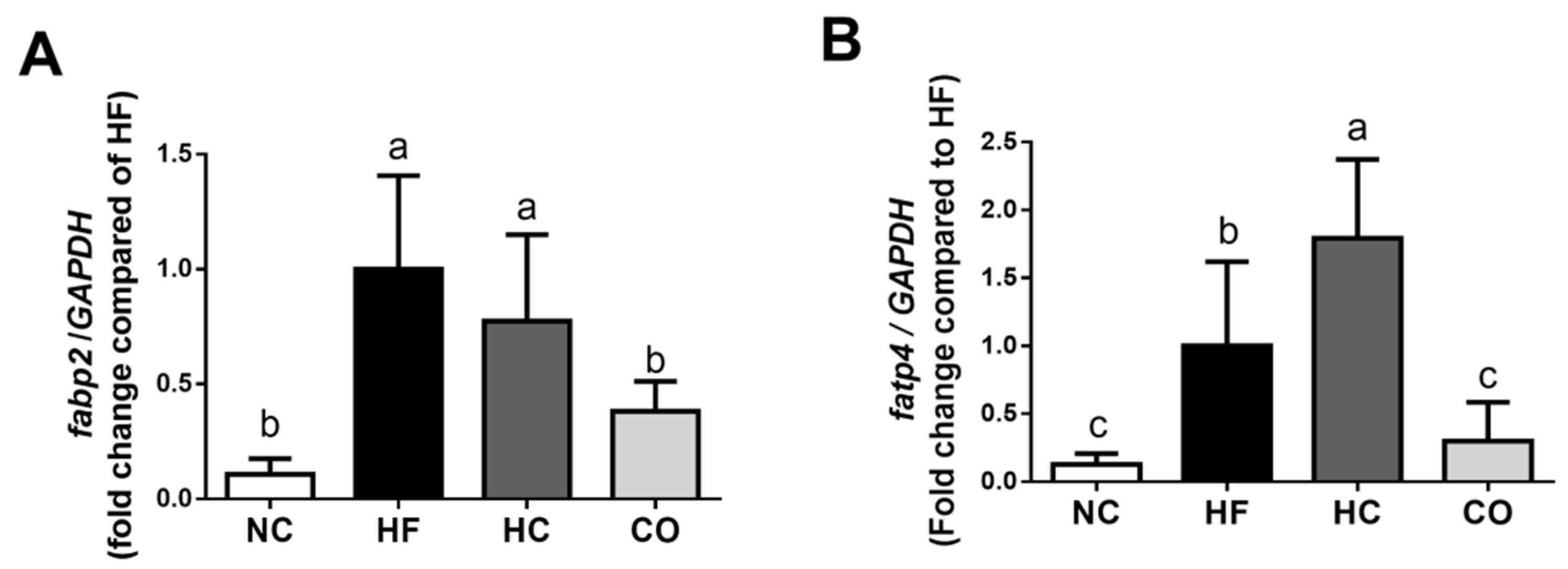
2.4. The Changes in Triglyceride, Lipolysis Rate, and Lipoprotein Lipase (LPL) Activity in the Perirenal Adipose Tissue, fabp2 and fatp4 mRNA Expression in the Small Intestinal Mucosa, and Fecal Weight and TC and TG Levels in Feces
3. Materials and Methods
3.1. Materials
3.2. Experimental Animals
3.3. Sampling Blood and Tissue
3.4. Analysis of Plasma Lipids, Liver Lipids, and Lipoproteins
3.5. Measurement of Activities of Plasma Aspartate Aminotransferase (AST), Plasma Alanine Aminotransferase (ALT), Liver Superoxide Dismutase (SOD), and Liver Glutathione Peroxidase (GPx) and Content of Liver Lipid Peroxide (Thiobarbituric Acid Reactive Substances, TBARS)
3.6. Detection of Lipolysis Rate and Lipoprotein Lipase (LPL) Activity
3.7. Measurement of Activities of Hepatic Acetyl-CoA Carboxylase (ACC), Hepatic Fatty Acid Synthase (FAS), and Hepatic HMG-CoA Reductase (HMGCR)
3.8. Analysis of Immunoblot
3.9. Analysis of Immunohistochemistry
3.10. Analysis of Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
3.11. Examination of Liver Histology
3.12. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 December 2020).
- Brunt, E.M. Nonalcoholic steatohepatitis: Definition and pathology. Semin. Liver Dis. 2001, 21, 3–16. [Google Scholar] [CrossRef]
- Petrović, G.; Bjelaković, G.; Benedeto-Stojanov, D.; Nagorni, A.; Brzački, V.; Marković-Živković, B. Obesity and metabolic syndrome as risk factors for the development of non-alcoholic fatty liver disease as diagnosed by ultrasound. Vojnosanit. Pregl. 2016, 73, 910–920. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Ilari, P.; Petrarulo, M. Solubility and structure of N-carboxy-methylchitosan. Int. J. Biol. Macromol. 1994, 16, 177–180. [Google Scholar] [CrossRef]
- Shepherd, R.; Reader, S.; Falshaw, A. Chitosan functional properties. Glycoconj. J. 1997, 14, 535–542. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Sudheesh Kumar, P.T.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Kim, S.K. Production of chitooligosaccharides using an ultrafiltration membrane reactor and their antibacterial activity. Carbohydr. Polym. 2000, 41, 133–141. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Cao, P.; Pan, H.; Ding, C.; Xiao, T.; Zhang, P.; Guo, J.; Su, Z. Anti-obese effect of glucosamine and chitosan oligosaccharide in high-fat diet-induced obese rats. Mar. Drugs 2015, 13, 2732–2756. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.T.; Huang, S.Y.; Chiang, M.T. A comparative study on hypoglycemic and hypocholesterolemic effects of high and low molecular weight chitosan in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2008, 46, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Chang, T.C.; Liu, S.H.; Chiang, M.T. The regulatory effects of fish oil and chitosan on hepatic lipogenic signals in high-fat diet-induced obese rats. J. Food Drug Anal. 2017, 25, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.H.; Chang, Y.H.; Chiang, M.T. Chitosan reduces gluconeogenesis and increases glucose uptake in skeletal muscle in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2010, 58, 5795–5800. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Cai, F.Y.; Chiang, M.T. Long-term feeding of chitosan ameliorates glucose and lipid metabolism in a high-fructose-diet-impaired rat model of glucose tolerance. Mar. Drugs 2015, 13, 7302–7313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.H.; Chiu, C.Y.; Shi, C.M.; Chiang, M.T. Functional comparison of high and low molecular weight chitosan on lipid metabolism and signals in high-fat diet-fed rats. Mar. Drugs 2018, 16, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, C.Y.; Yen, T.E.; Liu, S.H.; Chiang, M.T. Comparative effects and mechanisms of chitosan and its derivatives on hypercholesterolemia in high-fat diet-fed rats. Int. J. Mol. Sci. 2019, 21, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhang, J.; Xia, W. Hypocholesterolaemic effects of different chitosan samples in vitro and in vivo. Food Chem. 2008, 107, 419–425. [Google Scholar] [CrossRef]
- Ebihara, K.; Nakamoto, Y. Effect of the particle size of corn bran on the plasma cholesterol concentration, fecal output and cecal fermentation in rats. Nutr. Res. 2001, 21, 1509–1518. [Google Scholar] [CrossRef]
- Deuchi, K.; Kanauchi, O.; Imasato, Y.; Kobayashi, E. Decreasing effect of chitosan on the apparent fat digestibility by rats fed on a high-fat diet. Biosci. Biotechnol. Biochem. 1994, 58, 1613–1616. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Qin, C.; Wang, W.; Chi, W.; Li, W. Absorption and distribution of chitosan in mice after oral administration. Carbohydr. Polym. 2008, 71, 435–440. [Google Scholar] [CrossRef]
- Xu, G.; Huang, X.; Qiu, L.; Wu, J.; Hu, Y. Mechanism study of chitosan on lipid metabolism in hyperlipidemic rats. Asia Pac. J. Clin. Nutr. 2007, 16, 313–317. [Google Scholar]
- Zong, C.; Yu, Y.; Song, G.; Luo, T.; Li, L.; Wang, X.; Qin, S. Chitosan oligosaccharides promote reverse cholesterol transport and expression of scavenger receptor BI and CYP7A1 in mice. Exp. Biol. Med. 2012, 237, 194–200. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Chan, I.L.; Yang, T.H.; Liu, S.H.; Chiang, M.T. Supplementation of chitosan alleviates high-fat diet-enhanced lipogenesis in rats via adenosine monophosphate (AMP)-activated protein kinase activation and inhibition of lipogenesis-associated genes. J. Agric. Food Chem. 2015, 63, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Biddinger, S.B.; Almind, K.; Miyazaki, M.; Kokkotou, E.; Ntambi, J.M.; Kahn, C.R. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes 2005, 54, 1314–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbrini, E.; Mohammed, B.S.; Magkos, F.; Korenblat, K.M.; Patterson, B.W.; Klein, S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology 2008, 134, 424–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; He, L.; Ding, Y.; Liu, G. Mechanisms underlying different responses of plasma triglyceride to high-fat diets in hamsters and mice: Roles of hepatic MTP and triglyceride secretion. Biochem. Biophys. Res. Commun. 2010, 398, 619–626. [Google Scholar] [CrossRef]
- Loh, K.; Tam, S.; Murray-Segal, L.; Huynh, K.; Meikle, P.J.; Scott, J.W.; van Denderen, B.; Chen, Z.; Steel, R.; LeBlond, N.D.; et al. Inhibition of adenosine monophosphate-activated protein kinase-3-hydroxy-3-methylglutaryl coenzyme A reductase signaling leads to hypercholesterolemia and promotes hepatic steatosis and insulin resistance. Hepatol. Commun. 2018, 3, 84–98. [Google Scholar] [CrossRef] [Green Version]
- Chyau, C.C.; Wang, H.F.; Zhang, W.J.; Chen, C.C.; Huang, S.H.; Chang, C.C.; Peng, R.Y. Antrodan alleviates high-fat and high-fructose diet-induced fatty liver disease in C57BL/6 mice model via AMPK/Sirt1/SREBP-1c/PPARγ pathway. Int. J. Mol. Sci. 2020, 21, 360. [Google Scholar] [CrossRef] [Green Version]
- Matsusue, K.; Aibara, D.; Hayafuchi, R.; Matsuo, K.; Takiguchi, S.; Gonzalez, F.J.; Yamano, S. Hepatic PPARγ and LXRα independently regulate lipid accumulation in the livers of genetically obese mice. FEBS Lett. 2014, 588, 2277–2281. [Google Scholar] [CrossRef]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol. Metab. Syndr. 2011, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zhang, S.; Wang, X.; Yang, S.; Jiang, Q.; Xu, Y.; Xia, W. Transcriptome analysis of the effects of chitosan on the hyperlipidemia and oxidative stress in high-fat diet fed mice. Int. J. Biol. Macromol. 2017, 102, 104–110. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Gomes, A.P.; Varela, A.T.; Duarte, F.; Rolo, A.P.; Palmeira, C.M. Hepatic and skeletal muscle mitochondrial toxicity of chitosan oligosaccharides of normal and diabetic rats. Toxicol. Mech. Methods 2016, 26, 650–657. [Google Scholar] [CrossRef]
- Zhang, Y.; Ahmad, K.A.; Khan, F.U.; Yan, S.; Ihsan, A.U.; Ding, Q. Chitosan oligosaccharides prevent doxorubicin-induced oxidative stress and cardiac apoptosis through activating p38 and JNK MAPK mediated Nrf2/ARE pathway. Chem. Biol. Interact. 2019, 305, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Baier, L.J.; Bogardus, C.; Sacchettini, J.C. A polymorphism in the human intestinal fatty acid binding protein alters fatty acid transport across Caco-2 cells. J. Biol. Chem. 1996, 217, 10892–10896. [Google Scholar] [CrossRef] [Green Version]
- Milger, K.; Herrmann, T.; Becker, C.; Gotthardt, D.; Zickwolf, J.; Ehehalt, R.; Watkins, P.A.; Stremmel, W.; Füllekrug, J. Cellular uptake of fatty acids driven by the ER-localized acyl-CoA synthetase FATP4. J. Cell Sci. 2006, 119, 4678–4688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanauchi, O.; Deuchi, K.; Imasato, Y.; Shizukuishi, M.; Kobayashi, E. Mechanism for the inhibition of fat digestion by chitosan and for the synergistic effect of ascorbate. Biosci. Biotechnol. Biochem. 1995, 59, 786–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Research Council. Guide for the Care and Use of Laboratory Animals: Eighth Edition; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Carlson, S.E.; Goldfarb, S. A sensitive enzymatic method for the determination of free and esterified tissue cholesterol. Clin. Chim. Acta 1977, 79, 575–582. [Google Scholar] [CrossRef]
- Takehisa, F.; Suzuki, Y. Effect of guar gum and cholestyramine on plasma lipoprotein cholesterol in rats. J. Jpn. Soc. Nutr. Food Sci. 1990, 43, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Yen, Y.P.; Tsai, K.S.; Chen, Y.W.; Huang, C.F.; Yang, R.S.; Liu, S.H. Arsenic induces apoptosis in myoblasts through a reactive oxygen species-induced endoplasmic reticulum stress and mitochondrial dysfunction pathway. Arch. Toxicol. 2012, 86, 923–933. [Google Scholar] [CrossRef]
- Berger, J.J.; Barnard, R.J. Effect of diet on fat cell size and hormone-sensitive lipase activity. J. Appl. Physiol. 1999, 87, 227–232. [Google Scholar] [CrossRef]
- Kusunoki, M.; Hara, T.; Tsutsumi, K.; Nakamura, T.; Miyata, T.; Sakakibara, F.; Sakamoto, S.; Ogawa, H.; Nakaya, Y.; Storlien, L.H. The lipoprotein lipase activator, NO-1886, suppresses fat accumulation and insulin resistance in rats fed a high-fat diet. Diabetologia 2000, 43, 875–880. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.B.; Kekwick, R.G. Acetyl-coenzyme a carboxylase from avocado (Persea americana) plastids and spinach (Spinacia oleracea) chloroplasts. Biochem. J. 1980, 187, 667–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.H.; Yao, H.T.; Chiang, M.T. Red algae (Gelidium amansii) hot-water extract ameliorates lipid metabolism in hamsters fed a high-fat diet. J. Food Drug Anal. 2017, 25, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Nepokroeff, C.M.; Lakshmanan, M.R.; Porter, J.W. Fatty-acid synthase from rat liver. Methods Enzymol. 1975, 35, 37–44. [Google Scholar]
- Edwards, P.A.; Lemongello, D.; Kane, J.; Shechter, I.; Fogelman, A.M. Properties of purified rat hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and regulation of enzyme activity. J. Biol. Chem. 1980, 255, 3715–3725. [Google Scholar] [CrossRef]
- Liu, S.H.; Chiu, C.Y.; Huang, L.H.; Chiang, M.T. Resistant maltodextrin ameliorates altered hepatic lipid homeostasis via activation of AMP-activated protein kinase in a high-fat diet-fed rat model. Nutrients 2019, 11, 291. [Google Scholar] [CrossRef] [Green Version]
- Krüner, G.; Westernhagen, H.V. Sources of measurement error in assays of EROD activity of fish for biological effects monitoring. Helgol. Mar. Res. 1999, 53, 250–256. [Google Scholar]
- Chen, B.L.; Sheu, M.L.; Tsai, K.S.; Lan, K.C.; Guan, S.S.; Wu, C.T.; Chen, L.P.; Hung, K.Y.; Huang, J.W.; Chiang, C.K.; et al. CCAAT-enhancer-binding protein homologous protein deficiency attenuates oxidative stress and renal ischemia-reperfusion injury. Antioxid. Redox. Signal. 2015, 23, 1233–1245. [Google Scholar] [CrossRef] [Green Version]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Liu, S.H.; Chen, R.Y.; Chiang, M.T. Effects of chitosan oligosaccharide on plasma and hepatic lipid metabolism and liver histomorphology in normal Sprague-Dawley rats. Mar. Drugs 2020, 18, 408. [Google Scholar] [CrossRef]
- Bai, Y.; Zheng, J.; Yuan, X.; Jiao, S.; Feng, C.; Du, Y.; Liu, H.; Zheng, L. Chitosan oligosaccharides improve glucolipid metabolism disorder in liver by suppression of obesity-related inflammation and restoration of peroxisome proliferator-activated receptor gamma (PPARγ). Mar. Drugs 2018, 16, 455. [Google Scholar] [CrossRef] [Green Version]
| Parameters | NC | HF | HC | CO |
|---|---|---|---|---|
| Initial body weight (g) | 223.86 ± 5.17 a | 223.86 ± 7.92 a | 223.85 ± 6.11 a | 224.34 ± 5.09 a |
| Final body weight (g) | 527.07 ± 37.55 b | 602.45 ± 46.15 a | 537.28 ± 32.85 b | 557.56 ± 23.77 b |
| Body weight gain (g) | 304.01 ± 29.89 b | 377.99 ± 44.23 a | 314.14 ± 32.39 b | 331.49 ± 25.18 b |
| Food intake (g/day) | 27.07 ± 1.87 a | 25.79 ± 1.22 a,b | 24.12 ± 1.57 b | 24.89 ± 1.23 b |
| Food efficiency (%) | 11.22 ± 0.49 c | 14.61 ± 1.11 a | 13.00 ± 0.66 b | 13.33 ± 0.88 b |
| Parameters | NC | HF | HC | CO |
|---|---|---|---|---|
| Liver weight (g) | 15.18 ± 0.71 c | 28.31 ± 3.16 a | 21.57 ± 2.59 b | 27.19 ± 2.27 a |
| Relative liver weight (g/100 g BW) | 2.89 ± 0.10 c | 4.70 ± 0.31 a | 4.01 ± 0.35 b | 4.88 ± 0.45 a |
| Perirenal fat (g) | 10.56 ± 3.58 b | 13.85 ± 2.39 a | 8.57 ± 1.67 b | 10.57 ± 2.72 b |
| Relative Perirenal fat weight (g/100 g BW) | 2.00 ± 0.65 a,b | 2.30 ± 0.38 a | 1.59 ± 0.38 b | 1.88 ± 0.43 a,b |
| Epididymal fat (g) | 7.96 ± 1.93 b | 10.02 ± 1.62 a | 7.53 ± 1.69 b | 7.20 ± 1.80 b |
| Relative Epididymal fat weight (g/100 g BW) | 1.52 ± 0.36 a,b | 1.66 ± 0.22 a | 1.39 ± 0.29 a,b | 1.29 ± 0.28 b |
| Total adipose tissue weight (g) | 18.53 ± 5.38 b | 23.87 ± 3.85 a | 16.10 ± 3.27 b | 17.77 ± 4.10 b |
| Relative adipose tissue weight (g/100 g BW) | 3.52 ± 0.98 a,b | 3.97 ± 0.57 a | 2.98 ± 0.53 b | 3.17 ± 0.62 a,b |
| Small intestine weight (g) | 10.98 ± 0.94 a | 11.12 ± 1.05 a | 10.65 ± 1.42 a | 10.00 ± 1.07 a |
| Small intestine length (cm) | 135.63 ± 10.25 a | 132.00 ± 5.71 a | 131.56 ± 8.29 a | 132.44 ± 7.00 a |
| Cecal weight (g) | 8.14 ± 1.09 a | 6.37 ± 0.83 b | 8.46 ± 1.79 a | 5.51 ± 0.53 b |
| Cecal content weight (g) | 6.82 ± 1.03 a | 5.42 ± 0.80 b | 7.09 ± 1.72 a | 4.14 ± 0.44 c |
| Parameters | NC | HF | HC | CO |
|---|---|---|---|---|
| Total cholesterol (mg/dl) | 56.57 ± 10.43 b | 74.10 ± 21.30 a | 44.67 ± 4.50 b | 53.81 ± 11.32 b |
| HDL-C (mg/dl) | 39.43 ± 6.37 a | 26.86 ± 5.48 b | 28.92 ± 4.50 b | 24.33 ± 3.38 b |
| LDL-C + VLDL-C (mg/dl) | 17.14 ± 5.23 c | 47.23 ± 17.03 a | 15.74 ± 3.09 c | 29.49 ± 8.50 b |
| VLDL-C (mg/dl) | 10.37 ± 2.70 c | 25.09 ± 4.64 a | 8.29 ± 2.91 c | 15.89 ± 3.50 b |
| LDL-C (mg/dl) | 6.77 ± 3.40 b | 22.14 ± 14.83 a | 7.45 ± 3.43 b | 13.60 ± 6.02 a,b |
| TC / HDL-C (mg/dl) | 1.43 ± 0.10 c | 2.75 ± 0.39 a | 1.56 ± 0.15 c | 2.19 ± 0.26 b |
| HDL-C / (LDL-C + VLDL-C) | 2.46 ± 0.63 a | 0.60 ± 0.13 b | 1.97 ± 0.76 a | 0.88 ± 0.21 b |
| Triglyceride (mg/dl) | 59.96 ± 12.41 a | 30.87 ± 7.31 b | 16.34 ± 4.01 c | 22.22 ± 5.10 b,c |
| Blood glucose (mg/dl) | 216.7 ± 7.64 b | 239.6 ± 4.102 a | 208.0 ± 6.122 b | 202.6 ± 9.021 b |
| Parameters | NC | HF | HC | CO |
|---|---|---|---|---|
| Total cholesterol | ||||
| (mg/g liver) | 4.26 ± 0.64 c | 110.04 ± 12.57 a | 84.10 ± 8.06 b | 86.25 ± 6.30 b |
| (g/liver) | 0.06 ± 0.01 d | 3.11 ± 0.43 a | 1.82 ± 0.31 c | 2.34 ± 0.26 b |
| Triglyceride | ||||
| (mg/g liver) | 20.58 ± 6.21 c | 80.64 ± 13.59 a | 62.32 ± 11.16 b | 64.98 ± 16.59 b |
| (g/liver) | 0.31 ± 0.09 c | 2.31 ± 0.31 a | 1.35 ± 0.31 b | 1.75 ± 0.41 b |
| Acetyl-CoA carboxylase (ACC) (n mole NADPH/min/mg protein) | 0.64 ± 0.13 c | 2.32 ± 0.24 a | 1.21 ± 0.19 b | 1.36 ± 0.34 b |
| Fatty acid synthase (FAS) (n mole NADPH/min/mg protein) | 0.33 ± 0.13 c | 1.57 ± 0.64 a | 0.77 ± 0.22 b | 0.78 ± 0.33 b |
| HMG-CoA reductase (HMGCR) (n mole NADPH/min/mg protein) | 0.29 ± 0.009 b | 0.56 ± 0.18 a | 0.35 ± 0.10 b | 0.39 ± 0.14 b |
| Parameters | NC | HF | HC | CO |
|---|---|---|---|---|
| Feces wet weight (g/day) | 7.49 ± 1.70 a | 6.87 ± 0.96 a | 7.41 ± 0.69 a | 8.15 ± 1.25 a |
| Feces dry weight (g/day) | 5.80 ± 0.60 a | 5.49 ± 0.57 a | 5.79 ± 0.21 a | 5.96 ± 0.65 a |
| Total cholesterol | ||||
| (mg/g feces) | 6.02 ± 1.34 c | 10.56 ± 1.33 b | 12.21 ± 1.25 a | 10.84 ± 1.63 a,b |
| (mg/day) | 34.89 ± 8.08 c | 57.66 ± 6.63 b | 70.63 ± 7.23 a | 64.68 ± 12.05 a,b |
| Triglyceride | ||||
| (mg/g feces) | 5.69 ± 1.72 c | 6.68 ± 1.04 b,c | 8.69 ± 1.66 a | 7.64 ± 2.09 a,b |
| (mg/day) | 33.10 ± 10.49 c | 36.91 ± 8.12 b,c | 50.40 ± 10.30 a | 44.98 ± 10.40 a,b |
| Bile acid (μmol/day) | 25.5 ± 9.3 b | 25.3 ± 8.3 b | 39.1 ± 9.7 a | 25.9 ± 9.8 b |
| Ingredient (%) | NC | HF | HC | CO |
|---|---|---|---|---|
| Lard | ― | 10 | 10 | 10 |
| Cholesterol | ― | 0.5 | 0.5 | 0.5 |
| Cholic acid | ― | 0.1 | 0.1 | 0.1 |
| Chitosan oligosaccharides 1 | ― | ― | ― | 5 |
| Chitosan 2 | ― | ― | 5 | ― |
| Chow diet | 100 | 89.4 | 84.4 | 84.4 |
| Total calories (kcal/100 g) | 336.2 | 390.56 | 383.75 | 383.75 |
| Carbohydrates (% kcal) | 57.9 | 44.6 | 45.4 | 45.4 |
| Protein (% kcal) | 28.7 | 22.1 | 21.2 | 21.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-H.; Chen, R.-Y.; Chiang, M.-T. Effects and Mechanisms of Chitosan and ChitosanOligosaccharide on Hepatic Lipogenesis and Lipid Peroxidation, Adipose Lipolysis, and Intestinal Lipid Absorption in Rats with High-Fat Diet-Induced Obesity. Int. J. Mol. Sci. 2021, 22, 1139. https://doi.org/10.3390/ijms22031139
Liu S-H, Chen R-Y, Chiang M-T. Effects and Mechanisms of Chitosan and ChitosanOligosaccharide on Hepatic Lipogenesis and Lipid Peroxidation, Adipose Lipolysis, and Intestinal Lipid Absorption in Rats with High-Fat Diet-Induced Obesity. International Journal of Molecular Sciences. 2021; 22(3):1139. https://doi.org/10.3390/ijms22031139
Chicago/Turabian StyleLiu, Shing-Hwa, Rui-Yi Chen, and Meng-Tsan Chiang. 2021. "Effects and Mechanisms of Chitosan and ChitosanOligosaccharide on Hepatic Lipogenesis and Lipid Peroxidation, Adipose Lipolysis, and Intestinal Lipid Absorption in Rats with High-Fat Diet-Induced Obesity" International Journal of Molecular Sciences 22, no. 3: 1139. https://doi.org/10.3390/ijms22031139
APA StyleLiu, S. -H., Chen, R. -Y., & Chiang, M. -T. (2021). Effects and Mechanisms of Chitosan and ChitosanOligosaccharide on Hepatic Lipogenesis and Lipid Peroxidation, Adipose Lipolysis, and Intestinal Lipid Absorption in Rats with High-Fat Diet-Induced Obesity. International Journal of Molecular Sciences, 22(3), 1139. https://doi.org/10.3390/ijms22031139







