Effects of Vitrification on the Blastocyst Gene Expression Profile in a Porcine Model
Abstract
:1. Introduction
2. Results
2.1. Embryo Collection and Embryo Viability
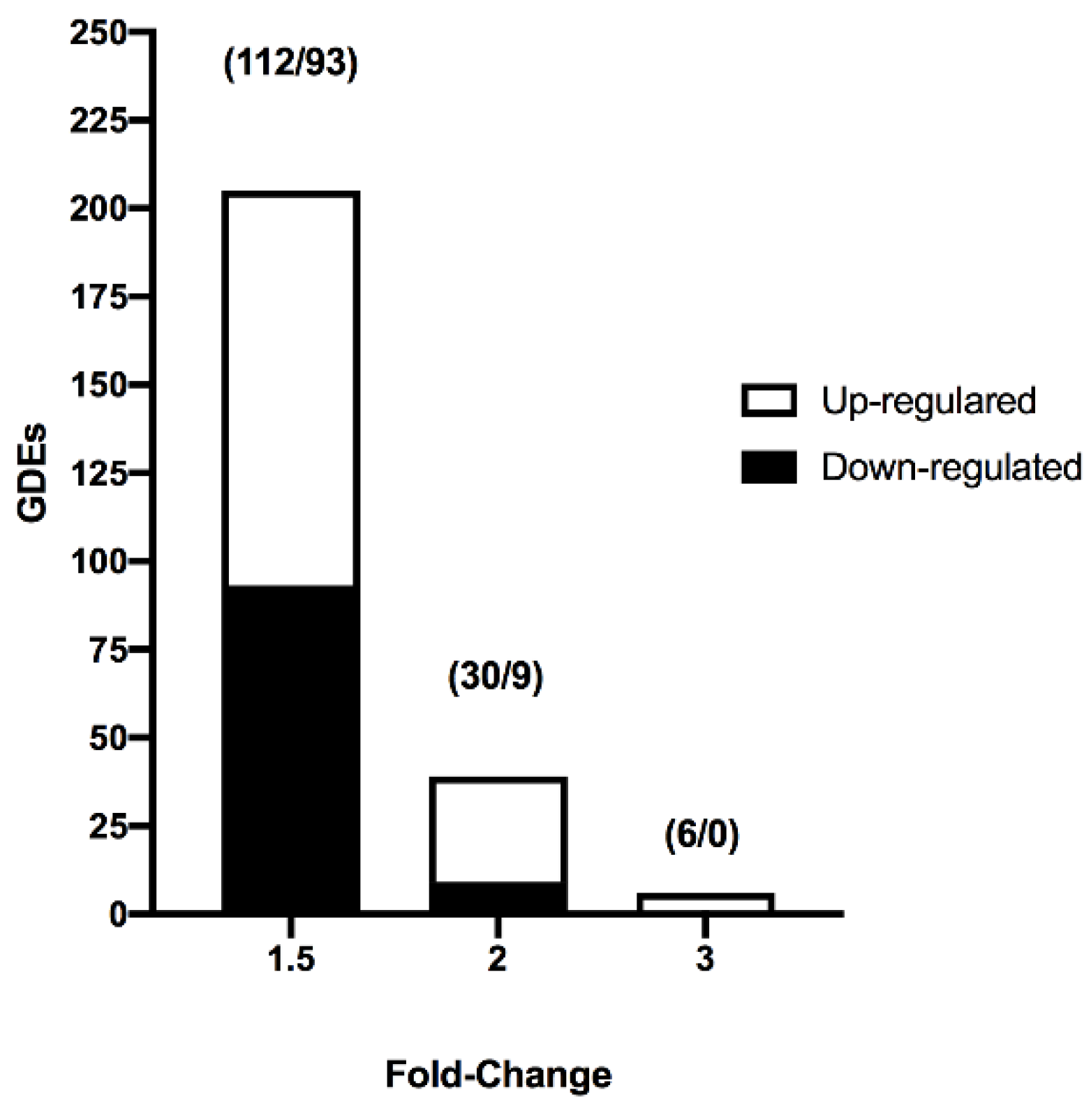
2.2. Transcriptome Profiles of Vitrified-Warmed Blastocysts
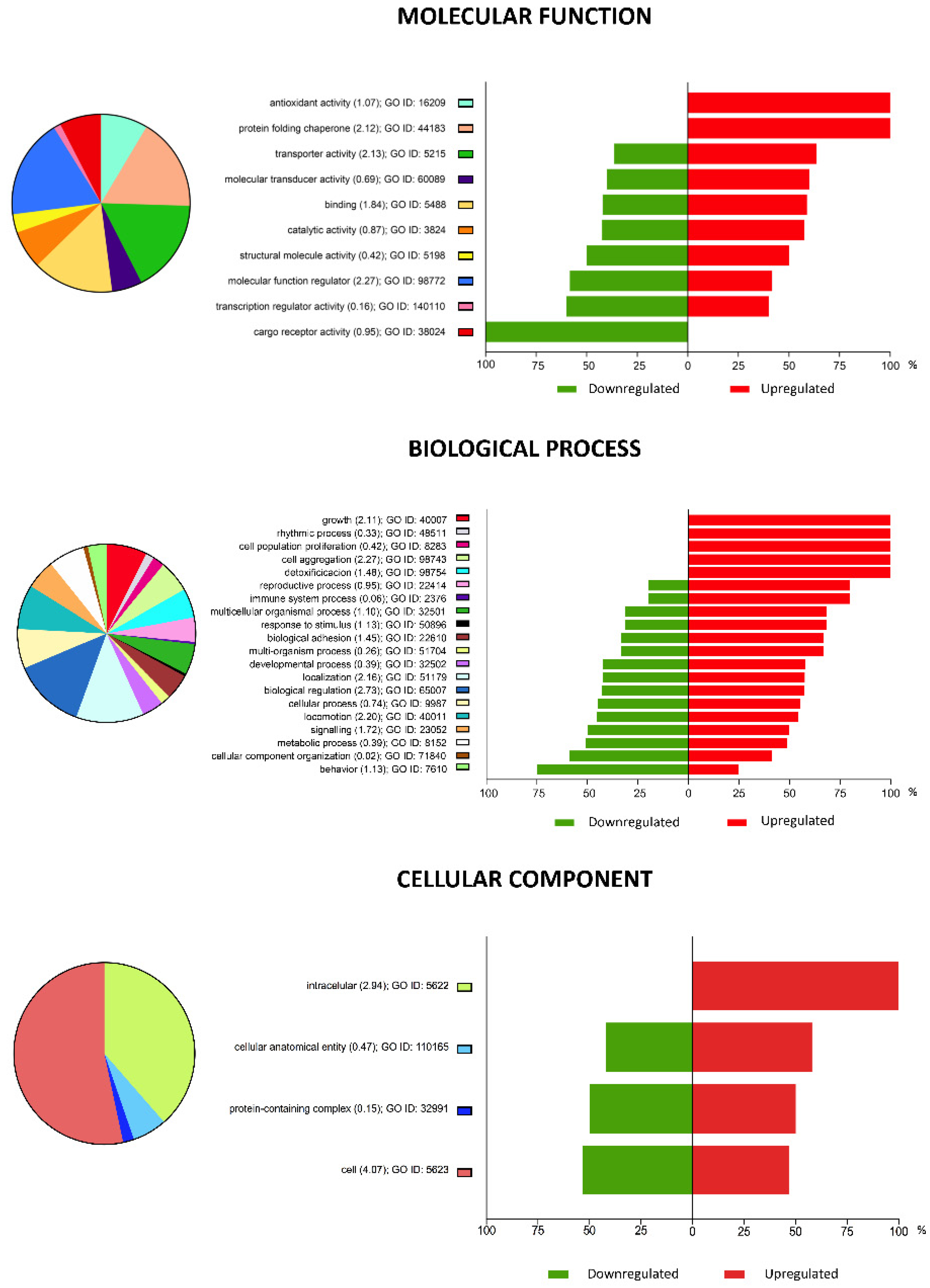
2.3. Gene Ontology (GO) Enrichment Analysis of DEGs in Vitrified Blastocysts
2.4. Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analysis of DEGs in Vitrified Blastocysts
2.5. Validation of Microarray Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Detection of Estrus and Artificial Insemination
4.4. Embryo Recovery and Assessment
4.5. Vitrification and Warming
4.6. In Vitro Embryo Culture and Evaluation of In Vitro Embryo Viability Post-Warming
4.7. Sample Preparation and Microarray Hybridization
4.8. Microarray Data Analysis
4.9. Quantitative Real-Time PCR (RT-qPCR) Analysis
4.10. Experimental Design
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adamson, G.D.; de Mouzon, J.; Chambers, G.M.; Zegers-Hochschild, F.; Mansour, R.; Ishihara, O.; Banker, M.; Dyer, S. International Committee for Monitoring Assisted Reproductive Technology: World report on assisted reproductive technology, 2011. Fertil. Steril. 2018, 110, 1067–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servick, K. Unsettled questions trail IVF’s success. Science 2014, 345, 744–746. [Google Scholar] [CrossRef]
- Rinaudo, P.; Wang, E. Fetal Programming and Metabolic Syndrome. Annu. Rev. Physiol. 2012, 74, 107–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duranthon, V.; Chavatte-Palmer, P. Long term effects of ART: What do animals tell us? Mol. Reprod. Dev. 2018, 85, 348–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perleberg, C.; Kind, A.; Schnieke, A. Genetically engineered pigs as models for human disease. DMM Dis. Model. Mech. 2018, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinritz, S.N.; Mosenthin, R.; Weiss, E. Use of pigs as a potential model for research into dietary modulation of the human gut microbiota. Nutr. Res. Rev. 2013, 26, 191–209. [Google Scholar] [CrossRef] [Green Version]
- Canovas, S.; Ivanova, E.; Romar, R.; García-Martínez, S.; Soriano-Úbeda, C.; García-Vázquez, F.A.; Saadeh, H.; Andrews, S.; Kelsey, G.; Coy, P. DNA methylation and gene expression changes derived from assisted reproductive technologies can be decreased by reproductive fluids. Elife 2017, 6, 1–24. [Google Scholar] [CrossRef]
- Vajta, G. Vitrification in ART: Past, present, and future. Theriogenology 2020, 150, 276–279. [Google Scholar] [CrossRef]
- Nagy, Z.P.; Shapiro, D.; Chang, C.C. Vitrification of the human embryo: A more efficient and safer in vitro fertilization treatment. Fertil. Steril. 2020, 113, 241–247. [Google Scholar] [CrossRef]
- Vajta, G. Vitrification in human and domestic animal embryology: Work in progress. Reprod. Fertil. Dev. 2013, 25, 719–727. [Google Scholar] [CrossRef]
- Bergh, C.; Wennerholm, U.B. Obstetric outcome and long-term follow up of children conceived through assisted reproduction. Best Pract. Res. Clin. Obstet. Gynaecol. 2012, 26, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.A.; Cuello, C.; Parrilla, I.; Martinez, C.A.; Nohalez, A.; Vazquez, J.L.; Vazquez, J.M.; Roca, J.; Gil, M.A. Recent advances toward the practical application of embryo transfer in pigs. Theriogenology 2016, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Cuello, C.; Berthelot, F.; Delaleu, B.; Venturi, E.; Pastor, L.M.; Vazquez, J.M.; Roca, J.; Martinat-Botte, F.; Martinez, E.A. The effectiveness of the stereomicroscopic evaluation of embryo quality in vitrified-warmed porcine blastocysts: An ultrastructural and cell death study. Theriogenology 2007, 67, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Fabian, D.; Gjørret, J.O.; Berthelot, F. Ultrastructure and cell death of in vivo derived and vitrified porcine blastocysts. Mol. Reprod. Dev. 2005, 70, 155–165. [Google Scholar] [CrossRef]
- Aksu, D.A.; Agca, C.; Aksu, S.; Bagis, H.; Akkoc, T.; Caputcu, A.T.; Arat, S.; Taskin, A.C.; Kizil, S.H.; Karasahin, T.; et al. Gene expression profiles of vitrified in vitro- and in vivo-derived bovine blastocysts. Mol. Reprod. Dev. 2012, 79, 613–625. [Google Scholar] [CrossRef]
- Bartolac, L.K.; Lowe, J.L.; Koustas, G.; Grupen, C.G.; Sjöblom, C. Vitrification, not cryoprotectant exposure, alters the expression of developmentally important genes in in vitro produced porcine blastocysts. Cryobiology 2018, 80, 70–76. [Google Scholar] [CrossRef]
- Castillo-Martín, M.; Yeste, M.; Pericuesta, E.; Morató, R.; Gutiérrez-Adán, A.; Bonet, S. Effects of vitrification on the expression of pluripotency, apoptotic and stress genes in in vitro-produced porcine blastocysts. Reprod. Fertil. Dev. 2014, 27, 1072–1081. [Google Scholar] [CrossRef]
- Chen, Y.N.; Dai, J.J.; Wu, C.F.; Zhang, S.S.; Sun, L.W.; Zhang, D.F. Apoptosis and developmental capacity of vitrified parthenogenetic pig blastocysts. Anim. Reprod. Sci. 2018, 198, 137–144. [Google Scholar] [CrossRef]
- de Oliveira Leme, L.; Dufort, I.; Spricigo, J.F.W.; Braga, T.F.; Sirard, M.A.; Franco, M.M.; Dode, M.A.N. Effect of vitrification using the Cryotop method on the gene expression profile of in vitro-produced bovine embryos. Theriogenology 2016, 85, 724–733.e1. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, J.; Dufort, I.; Robert, C.; Dias, F.C.F.; Anzar, M. Transcriptomic difference in bovine blastocysts following vitrification and slow freezing at morula stage. PLoS ONE 2017, 12, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Mantione, K.J.; Kream, R.M.; Kuzelova, H.; Ptacek, R.; Raboch, J.; Samuel, J.M.; Stefano, G.B. Comparing bioinformatic gene expression profiling methods: Microarray and RNA-Seq. Med. Sci. Monit. Basic Res. 2014, 20, 138–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruiswijk, F.; Labuschagne, C.F.; Vousden, K.H. P53 in survival, death and metabolic health: A lifeguard with a licence to kill. Nat. Rev. Mol. Cell Biol. 2015, 16, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, T.; Nakagawara, A. Role of p53 in cell death and human cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Lin, E.C.; Chen, S.C.; Huang, S.C.; Liu, B.H.; Yu, Y.H.; Chen, C.C.; Yang, C.C.; Lien, C.Y.; Wang, Y.H.; et al. Differential gene expression between the porcine morula and blastocyst. Reprod. Domest. Anim. 2012, 47, 69–81. [Google Scholar] [CrossRef]
- Tanaka, T.S.; Ko, M.S.H. A global view of gene expression in the preimplantation mouse embryo: Morula versus blastocyst. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115, 85–91. [Google Scholar] [CrossRef]
- Jia, B.; Xiang, D.; Liu, S.; Zhang, B.; Shao, Q.; Hong, Q.; Quan, G.; Wu, G. Theriogenology TMT-based quantitative proteomic analysis of cumulus cells derived from vitri fi ed porcine immature oocytes following in vitro maturation. Theriogenology 2020, 152, 8–17. [Google Scholar] [CrossRef]
- Niehrs, S.P.C. Dkk3 is required for TGF-b signaling during Xenopus mesoderm induction. Differentiation 2007, 75, 957–967. [Google Scholar] [CrossRef]
- Zhao, X.; Hao, H.; Du, W.; Zhu, H. Effect of vitrification on the MicroRNA transcriptome in mouse blastocysts. PLoS ONE 2015, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Beyer, T.A.; Narimatsu, M.; Weiss, A.; David, L.; Wrana, J.L. The TGFβ superfamily in stem cell biology and early mammalian embryonic development. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 2268–2279. [Google Scholar] [CrossRef]
- Mullen, A.C.; Wrana, J.L. TGF-β family signaling in embryonic and somatic stem-cell renewal and differentiation. Cold Spring Harb. Perspect. Biol. 2017, 9, 1–32. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, C.R.; Massagué, J. A self-enabling TGFβ response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol. Cell 2003, 11, 915–926. [Google Scholar] [CrossRef]
- Clavería, C.; Giovinazzo, G.; Sierra, R.; Torres, M. Myc-driven endogenous cell competition in the early mammalian embryo. Nature 2013, 500, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Lu, X. Live or let die: The cell’s response to p53. Nat. Rev. Cancer 2002, 2, 594–604. [Google Scholar] [CrossRef] [Green Version]
- Gambino, V.; De Michele, G.; Venezia, O.; Migliaccio, P.; Dall’Olio, V.; Bernard, L.; Minardi, S.P.; Fazia, M.A.D.; Bartoli, D.; Servillo, G.; et al. Oxidative stress activates a specific p53 transcriptional response that regulates cellular senescence and aging. Aging Cell 2013, 12, 435–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvajal, L.A.; Manfredi, J.J. Another fork in the road—Life or death decisions by the tumour suppressor p53. EMBO Rep. 2013, 14, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Kader, A.; Agarwal, A.; Abdelrazik, H.; Sharma, R.K.; Ahmady, A.; Falcone, T. Evaluation of post-thaw DNA integrity of mouse blastocysts after ultrarapid and slow freezing. Fertil. Steril. 2009, 91, 2087–2094. [Google Scholar] [CrossRef]
- Tran, S.; Wang, L.; Le, J.; Guan, J.; Wu, L.; Zou, J.; Wang, Z.; Wang, J.; Wang, F.; Chen, X.; et al. Altered methylation of the DNA repair gene MGMT is associated with neural tube defects. J. Mol. Neurosci. 2012, 47, 42–51. [Google Scholar] [CrossRef]
- Jaroudi, S.; SenGupta, S. DNA repair in mammalian embryos. Mutat. Res. Rev. Mutat. Res. 2007, 635, 53–77. [Google Scholar] [CrossRef]
- Kuscu, N.; Celik-Ozenci, C. FOXO1, FOXO3, and FOXO4 are differently expressed during mouse oocyte maturation and preimplantation embryo development. Gene Expr. Patterns 2015, 18, 16–20. [Google Scholar] [CrossRef]
- Bazer, F.; Geister, R.; Thatcher, W.; Roberts, R. The establishmentand maintenance of pregnancy. In Control of Pig Reproduction; Cole, D.J.A., Foxcroft, G.R., Eds.; Butter-Worth Scientific: London, UK, 1982; pp. 227–252. [Google Scholar]
- Meyer, A.E.; Pfeiffer, C.A.; Brooks, K.E.; Spate, L.D.; Benne, J.A.; Cecil, R.; Samuel, M.S.; Murphy, C.N.; Behura, S.; Mclean, M.K.; et al. New perspective on conceptus estrogens in maternal recognition and pregnancy establishment in the pig. Biol. Reprod. 2019, 101, 148–161. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Iny Stein, T.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards suite: From gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef] [PubMed]
- Houghton, F.D. Role of gap junctions during early embryo development. Reproduction 2002, 129, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.P. Mammalian HspB1 (Hsp27) is a molecular sensor linked to the physiology and environment of the cell. Cell Stress Chaperones 2017, 22, 517–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pursel, V.G.; Johnson, L.A. Freezing of boar spermatozoa: Fertilizing capacity with concentrated semen and a new thawing procedure. J. Anim. Sci. 1975, 40, 99–102. [Google Scholar] [CrossRef]
- Martinez, E.A.; Angel, M.A.; Cuello, C.; Sanchez-Osorio, J.; Gomis, J.; Parrilla, I.; Vila, J.; Colina, I.; Diaz, M.; Reixach, J.; et al. Successful Non-Surgical Deep Uterine Transfer of Porcine Morulae after 24 Hour Culture in a Chemically Defined Medium. PLoS ONE 2014, 9, e104696. [Google Scholar] [CrossRef]
- Funahashi, H.; Ekwall, H.; Rodriguez-Martinez, H. Zona Reaction in Porcine Oocytes Fertilized In Vivo and In Vitro as Seen with Scanning Electron Microscopy1. Biol. Reprod. 2000, 63, 1437–1442. [Google Scholar] [CrossRef] [Green Version]
- Martinez, E.A.; Martinez, C.A.; Nohalez, A.; Sanchez-Osorio, J.; Vazquez, J.M.; Roca, J.; Parrilla, I.; Gil, M.A.; Cuello, C. Nonsurgical deep uterine transfer of vitrified, in vivo-derived, porcine embryos is as effective as the default surgical approach. Sci. Rep. 2015, 5, 10587. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.M. Photographic illustrations of embryo developmental stage and quality codes. In Manual of the International Embryo Transfer Society; Stringfellow, D.A., Seidel, S.M., Eds.; International Embryo Transfer Society. IETS: Savoy, IL, USA, 1998; pp. 167–170. [Google Scholar]
- Cuello, C.; Martinez, C.A.; Nohalez, A.; Parrilla, I.; Roca, J.; Gil, M.A.; Martinez, E.A. Effective vitrification and warming of porcine embryos using a pH-stable, chemically defined medium. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Cuello, C.; Gil, M.; Parrilla, I.; Tornel, J.; Vázquez, J.; Roca, J.; Berthelot, F.; Martinat-Botté, F.; Martínez, E.A. In vitro development following one-step dilution of OPS-vitrified porcine blastocysts. Theriogenology 2004, 62, 1144–1152. [Google Scholar] [CrossRef]
- Sanchez-Osorio, J.; Cuello, C.; Gil, M.A.; Almiñana, C.; Parrilla, I.; Caballero, I.; Garcia, E.M.; Vazquez, J.M.; Roca, J.; Martinez, E.A. Factors affecting the success rate of porcine embryo vitrification by the Open Pulled Straw method. Anim. Reprod. Sci. 2008, 108, 334–344. [Google Scholar] [CrossRef]
- Petters, R.M.; Wells, K.D. Culture of pig embryos. J. Reprod. Fertil. Suppl. 1993, 48, 61–73. [Google Scholar] [PubMed]
- Bolstad, B.M.; Irizarry, R.A.; Åstrand, M.; Speed, T.P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003, 19, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pages, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 45e. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-J.; Jang, S.-J.; Lee, S.-C.; Park, J.-S.; Jeon, R.-H.; Subbarao, R.B.; Bharti, D.; Shin, J.-K.; Park, B.-W.; Rho, G.-J. Selection of reference genes for quantitative real-time polymerase chain reaction in porcine embryos. Reprod. Fertil. Dev. 2017, 29, 357. [Google Scholar] [CrossRef] [Green Version]



| Biological Function | Type | Enrichment Score | Enrichment p-Value | % Genes in Group That are Present * |
|---|---|---|---|---|
| Protein kinase C-activating G protein-coupled receptor signalling pathway | BP | 8.1 | 0.0003 | 37.5 |
| ATPase regulator activity | MF | 7.7 | 0.0004 | 33.3 |
| Glucose transmembrane transport | BP | 7.4 | 0.0006 | 30 |
| Muscle cell cellular homeostasis | BP | 7.1 | 0.0008 | 27.3 |
| Hexose transmembrane transport | BP | 7.1 | 0.0008 | 27.3 |
| Positive regulation of smooth muscle cell chemotaxis | BP | 6.9 | 0.0010 | 66.7 |
| Monosaccharide transmembrane transport | BP | 6.6 | 0.0014 | 23.1 |
| Manganese ion transport | BP | 6.3 | 0.0019 | 50 |
| Manganese ion transmembrane transport | BP | 6.3 | 0.0019 | 50 |
| Hexose transmembrane transporter | MF | 6.3 | 0.0019 | 50 |
| Pathway Name | Pathway ID | Enrichment Score | Enrichment p-Value | Altered Genes (%) * | Gene List |
|---|---|---|---|---|---|
| TGFβ signaling pathway | kegg_pathway_161 | 6.7 | 0.0012 | 4.9 | BMPR1B, ID4, SMAD3, TGFB1 |
| p53 signaling pathway | kegg_pathway_133 | 5.1 | 0.0062 | 4.6 | CDKN1A, FAS, ZMAT3 |
| FoxO signaling pathway | kegg_pathway_126 | 5.0 | 0.0065 | 3.1 | CDKN1A, GABARAPL1, SMAD3, TGFB1 |
| Other types of O-glycan biosynthesis | kegg_pathway_44 | 4.9 | 0.0077 | 8.7 | B3GLCT, ST6GAL1 |
| Cellular senescence | kegg_pathway_154 | 4.4 | 0.0117 | 2.6 | CDKN1A, MAP2K6, SMAD3, TGFB1 |
| Glycerophospholipid metabolism | kegg_pathway_55 | 4.1 | 0.0173 | 3.2 | DGKA, MBOAT1, PLA2G16 |
| MAPK signaling pathway | kegg_pathway_115 | 3.7 | 0.0238 | 1.8 | DUSP6, FAS, HSPB1, MAP2K6, TGFB1 |
| Cell cycle | kegg_pathway_131 | 3.4 | 0.0324 | 2.5 | CDKN1A, SMAD3, TGFB1 |
| Signaling pathways regulating pluripotency | kegg_pathway_174 | 3.2 | 0.0420 | 2.2 | BMPR1B, ID4, SMAD3 |
| Glycerolipid metabolism | kegg_pathway_52 | 3.1 | 0.0455 | 3.4 | DGKA, MBOAT1 |
| Pathway Name | Pathway ID | Enrichment Score | Enrichment p-Value | Altered Genes (%) * | Gene List |
|---|---|---|---|---|---|
| Steroid biosynthesis | kegg_pathway_12 | 5.7 | 0.0035 | 10.5 | DHCR24, MSMO1 |
| TGF-beta signaling pathway | kegg_pathway_161 | 5.0 | 0.0066 | 3.7 | BAMBI, MYC, RBL1 |
| cGMP-PKG signaling pathway | kegg_pathway_120 | 5.0 | 0.0067 | 2.5 | AKT1, ITPR1, PLN, RGS2 |
| Gap junction | kegg_pathway_173 | 4.9 | 0.0073 | 3.5 | HTR2B, ITPR1, MAP3K2 |
| Gene Symbol | Accession Number | Primer | Size | Efficiency | |
|---|---|---|---|---|---|
| PAIP1 | XM_003483818.4 | Forward (5’-3’) | AATGCCCCTGAATTTTACCC | 192 | 92.95 |
| Reverse (5’-3’) | ATCTGTTGTAACCCAGCCATTT | ||||
| MYC | NM_001005154.1 | Forward (5’-3’) | TCGGACTCTCTGCTCTCCTC | 157 | 102.05 |
| Reverse (5’-3’) | GCTGCCTCTTTTCCACAGAA | ||||
| TP53INP1 | XM_001925224 | Forward (5’-3’) | GCTGCCTCTTTTCCACAGAA | 184 | 91.44 |
| Reverse (5’-3’) | TAAGATTTTGGCGACGAAGG | ||||
| MGMT | XM_005671579.3 | Forward (5’-3’) | GGTCCAGAGGAGATGATGGA | 208 | 94.23 |
| Reverse (5’-3’) | GGGCTGCTAACTGCTGGTAA | ||||
| DKK3 | XM_005661123.3 | Forward (5’-3’) | AAGACACGCAGCACAAACTG | 163 | 84.56 |
| Reverse (5’-3’) | AAGACACGCAGCACAAACTG | ||||
| PPIA * | XM_021078519.1 | Forward (5’-3’) | AGAAGTCTGAATGGGTTCCTCA | 100 | 98.12 |
| Reverse (5’-3’) | CCAACCACTCAGTCTTGGCA | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuello, C.; Martinez, C.A.; Cambra, J.M.; Parrilla, I.; Rodriguez-Martinez, H.; Gil, M.A.; Martinez, E.A. Effects of Vitrification on the Blastocyst Gene Expression Profile in a Porcine Model. Int. J. Mol. Sci. 2021, 22, 1222. https://doi.org/10.3390/ijms22031222
Cuello C, Martinez CA, Cambra JM, Parrilla I, Rodriguez-Martinez H, Gil MA, Martinez EA. Effects of Vitrification on the Blastocyst Gene Expression Profile in a Porcine Model. International Journal of Molecular Sciences. 2021; 22(3):1222. https://doi.org/10.3390/ijms22031222
Chicago/Turabian StyleCuello, Cristina, Cristina A. Martinez, Josep M. Cambra, Inmaculada Parrilla, Heriberto Rodriguez-Martinez, Maria A. Gil, and Emilio A. Martinez. 2021. "Effects of Vitrification on the Blastocyst Gene Expression Profile in a Porcine Model" International Journal of Molecular Sciences 22, no. 3: 1222. https://doi.org/10.3390/ijms22031222
APA StyleCuello, C., Martinez, C. A., Cambra, J. M., Parrilla, I., Rodriguez-Martinez, H., Gil, M. A., & Martinez, E. A. (2021). Effects of Vitrification on the Blastocyst Gene Expression Profile in a Porcine Model. International Journal of Molecular Sciences, 22(3), 1222. https://doi.org/10.3390/ijms22031222









