Abstract
Most investigations of iodine metabolism in humans and animals have focused on its role in thyroid function. However, considerable evidence indicates that iodine could also be implicated in the physiopathology of other organs. We review the literature that shows that molecular iodine (I2) exerts multiple and complex actions on the organs that capture it, not including its effects as part of thyroid hormones. This chemical form of iodine is internalized by a facilitated diffusion system that is evolutionary conserved, and its effects appear to be mediated by a variety of mechanisms and pathways. As an oxidized component, it directly neutralizes free radicals, induces the expression of type II antioxidant enzymes, or inactivates proinflammatory pathways. In neoplastic cells, I2 generates iodolipids with nuclear actions that include the activation of apoptotic pathways and the inhibition of markers related to stem cell maintenance, chemoresistance, and survival. Recently, I2 has been postulated as an immune modulator that depending on the cellular context, can function as an inhibitor or activator of immune responses. We propose that the intake of molecular iodine is increased in adults to at least 1 mg/day in specific pathologies to obtain the potential extrathyroid benefits described in this review.
1. Introduction
Iodine in its different chemical forms is captured and used by practically all living beings and is considered a micronutrient in chordates. In vertebrates, iodine is a component of thyroid hormones that is essential for the proper development and functioning of several organs, primarily in the nervous system [1]. However, a significant amount of iodine in the body is non-hormonal and is concentrated in extra-thyroid tissues, where its biological function is barely understood [2]. Several groups have postulated that iodine may have an ancestral antioxidant function in all the cells that concentrate it, from primitive algae to the most recent vertebrates [3,4]. In these cells, oxidized iodine can act as an electron donor neutralizing reactive oxygen species (ROS) or attach to the double bonds of some polyunsaturated fatty acids in cell membranes, making them less reactive to ROS [5]. In addition, it has been shown that iodine binds to lipids, such as arachidonic acid (AA), exerts apoptosis, and/or has differentiation effects on diverse epithelial cells [6,7,8]. Moreover, iodine is uptake and metabolized by immune cells, and depending on the physiological context, this halogen can act as an anti-inflammatory or proinflammatory agent [9,10]. The distribution and action of iodine in organisms depend on the chemical form of iodine that is ingested. For example, molecular iodine (I2) is not reduced to iodide (I−) in the blood before being absorbed in the gastrointestinal tract [11], induces differential effects [12], and its capture is 40% lower in the thyroid [13]. In fact, under conditions of iodine deficiency, I− appears to be more efficient than I2 in restoring the thyroid gland to normality in goiter stages, while I2 is more effective in decreasing mammary alterations secondary to iodine deficiency [14]. This article reviews different reports on the effects of iodine as an antioxidant, differentiator and immunomodulator, and does do not include the actions of thyroid hormones.
1.1. Safety Concentration
Iodine is a structural component of thyroid hormones, which are essential for differentiation of the nervous system during development and crucial regulators of energetic metabolism. Public health policies have been established to guarantee that populations consume the required amount of iodine to eradicate iodine deficiency disorders. According to the International Council for Control of Iodine Deficiency Disorders (ICCIDD), the recommended dietary allowance of iodine is 150–299 μg/day for normal thyroid functioning, and the maximum limit of iodine intake with the lowest observed adverse effect level (LOAEL) is 1700–1800 μg/day [15,16]. In 1988, the joint of Food and Agriculture Organization of the United Nations and WHO Expert Committee on Food Additives suggested the maximal upper level from all iodine sources of 1 mg/day would be safe for most of the population except those with iodine sensitivity or underlying thyroid disorders. The increased intake of iodide can also have interactions with medications such as lithium or sulfisoxazole [17], but similar studies with molecular iodine do not exist; see Table 1 [15,16]. However, several studies report that iodine supplements at moderately high concentration are well tolerated in euthyroid subjects and that only high doses (>30 mg/day), mainly as I−, generate hypothyroidism and goiter, which rapidly revert to normal when these individuals stop taking iodine at high concentrations, see Table 2 [15,18]. Other studies indicate that iodine per se participates in the physiopathology of various organs that uptake it, mainly the thyroid, mammary, prostate, pancreas, and ovaries, and potentially in the gastric, immune, and nervous systems [6]. Moreover, in its molecular form, iodine acts as an antioxidant throughout the body if ingested at concentrations higher than 1 mg/day [19,20]. Dose-response studies in humans have demonstrated that I2 at concentrations of 1 to 6 mg/day exhibited significant beneficial actions in benign pathologies like fibrocystic breast disease [21,22], prostatic hyperplasia [23] and polycystic ovaries (unpublished results). The treatments in these studies lasted from five weeks up to two years and did not have any side effects at these concentrations. Some of the dose-response studies also analyzed the highest concentration of iodine (9 and 12 mg/day) and showed the same benefits but accompanied, in some cases, by transient hypothyroidism and/or minor side effects like headache, sinusitis, acne or diarrhea. These effects disappeared when the high dose of supplemental iodine was suspended [24]. Antineoplastic action of the I2 supplement without harmful effects on the thyroid has also been observed in mammary and prostatic pathologies in preclinical (rodents and canines) and clinical protocols [25,26,27,28]. Although the thyroid captures 40% less I2 than I−, the acceptable upper limits for iodine intake during pregnancy are not well defined, and the consequences of excess iodine in newborns are not well documented [15], so the iodine intake in any of its forms above the upper limits is not recommended in pregnant women or infants.

Table 1.
Predisposing Risk Factors Associated with permanent Iodine-Induced Thyroid Dysfunction.

Table 2.
Sources and Effects of Excess Iodine.
1.2. Iodine in Normal Tissues
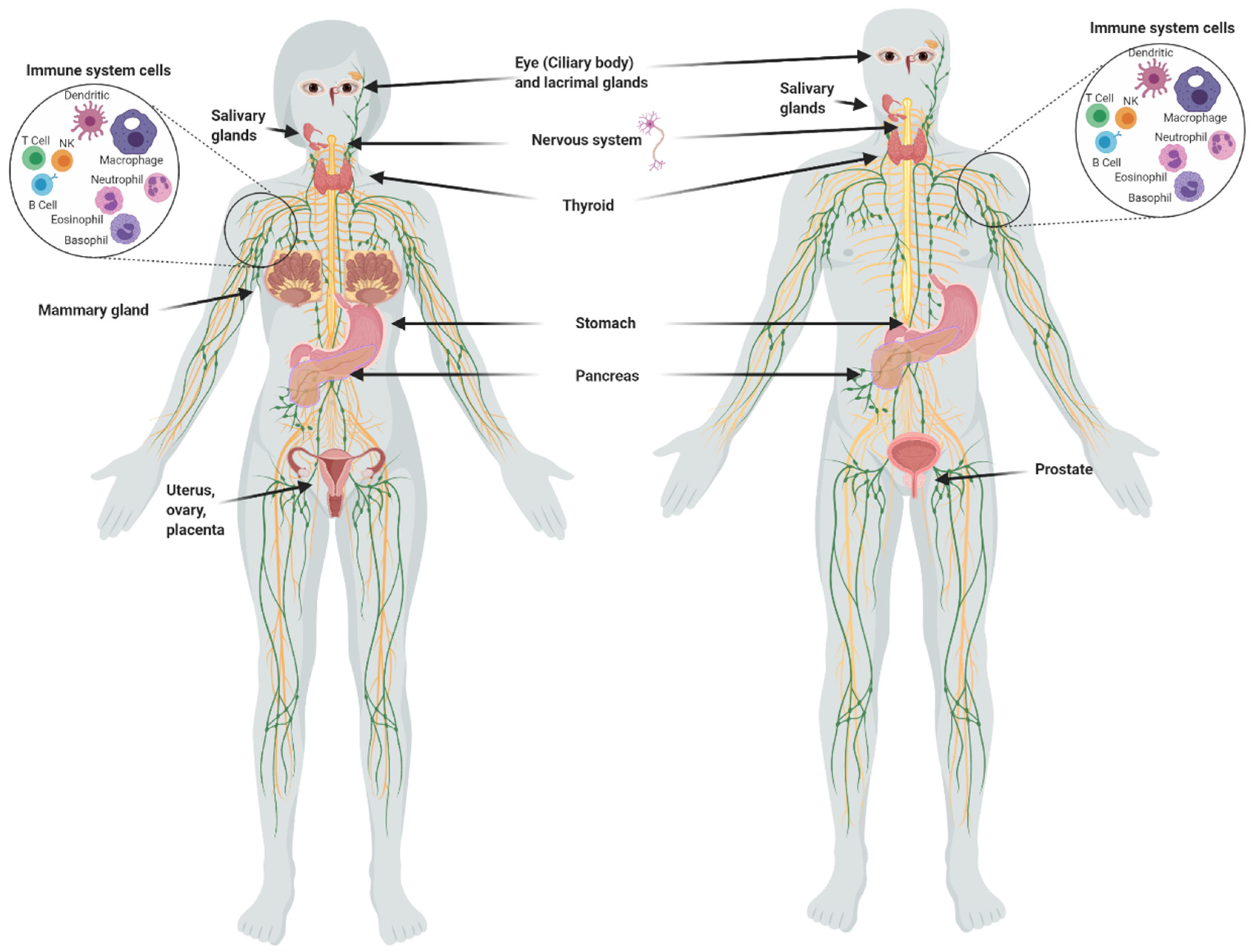
Although the main uptake of iodine takes place in the thyroid, many other organs take it up (Figure 1), including the salivary glands, gastric mucosa, lactating mammary gland, nervous system, choroid plexus, ciliary body of the eye, lacrimal gland, thymus, skin, placenta, ovary, uterus, prostate, and pancreas, and they can maintain or lose this ability in pathological conditions [1]. The I− transport system in many of these extrathyroidal tissues involves the expression of the sodium iodide symporter (NIS) and/or the anion exchanger Pendrin (PDS/SLC26A4). Recent studies have also demonstrated the direct participation of other transporters including anoctamin 1 (ANO1), cystic fibrosis transmembrane conductance regulator (CFTR) and sodium multivitamin transporter (SMVT) that are capable to take up I− [1]. On the other hand, various studies have shown that I2 is captured by an independent mechanism of NIS, PDS, Na+ and ATP, but it is saturable and depends on protein synthesis, suggesting a facilitated diffusion system [34]. This mechanism is similar to the one described in marine algae [35], indicating that I2 absorption could be an evolutionary conserved system. Indeed, we demonstrated that the thyroid, mammary gland, and prostate can accumulate both types of iodine, which are captured by different mechanisms. The thyroid, lactating mammary gland, and prostate exhibit a significant uptake of I−, which is internalized by NIS (inhibited by KClO4). Molecular iodine is taken up by these tissues, but also by others like the nubile mammary gland, and NIS does not participate in its internalization [36]. These findings agree with the notion that I2 contributes to maintaining the integrity of these organs. Iodine deficiency in rats is accompanied by ductal hyperplasia and perilobular fibrosis in the virgin mammary glands, and the supplement of I2 but not I− reverts these alterations [14]. Similarly, the supplement of I2 (3–6 mg/day) in patients with fibrocystic breast disease is accompanied by remission of symptoms, as well as significant anti-inflammatory effects [21,22]. Our group has found similar benefits in benign prostatic hyperplasia (BPH) in preclinical and clinical models [36]. In human patients with early BPH (Grade I and II), the supplement of 5 mg/day of Lugol’s solution (mix 1:3; I2:KI) for 8 months decreased the prostate-specific antigen (PSA) circulating levels and improved the urinary flow and symptoms scale [23]. These studies agree with epidemiological data that associate the low incidence of breast and prostate pathologies with the moderately high dietary intake of iodine in Asian countries [3,4,37]. These populations consume marine algae daily in their diet, which contain high amounts of iodine in various chemical forms such as I−, I2 and iodate (IO3)−. The average consumption of iodine in the Japanese population is 1200 to 5280 µg/day compared to 166 and 209 µg/day in the United Kingdom and the United States, respectively [33,38]. However, despite the high nutritional intake of iodine, Asia does not differ from the rest of the world in the prevalence of thyroid disorders [37].
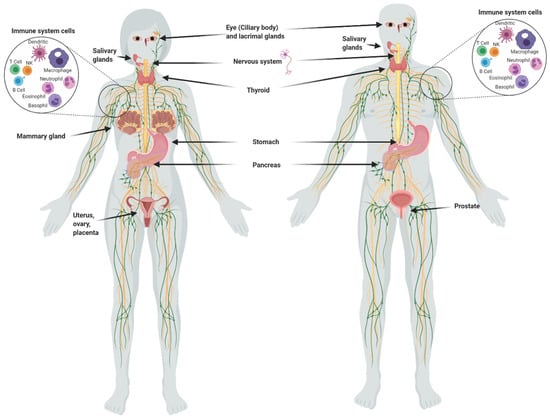
Figure 1.
Organs and tissues that take up iodine.
1.3. Antioxidant Effects
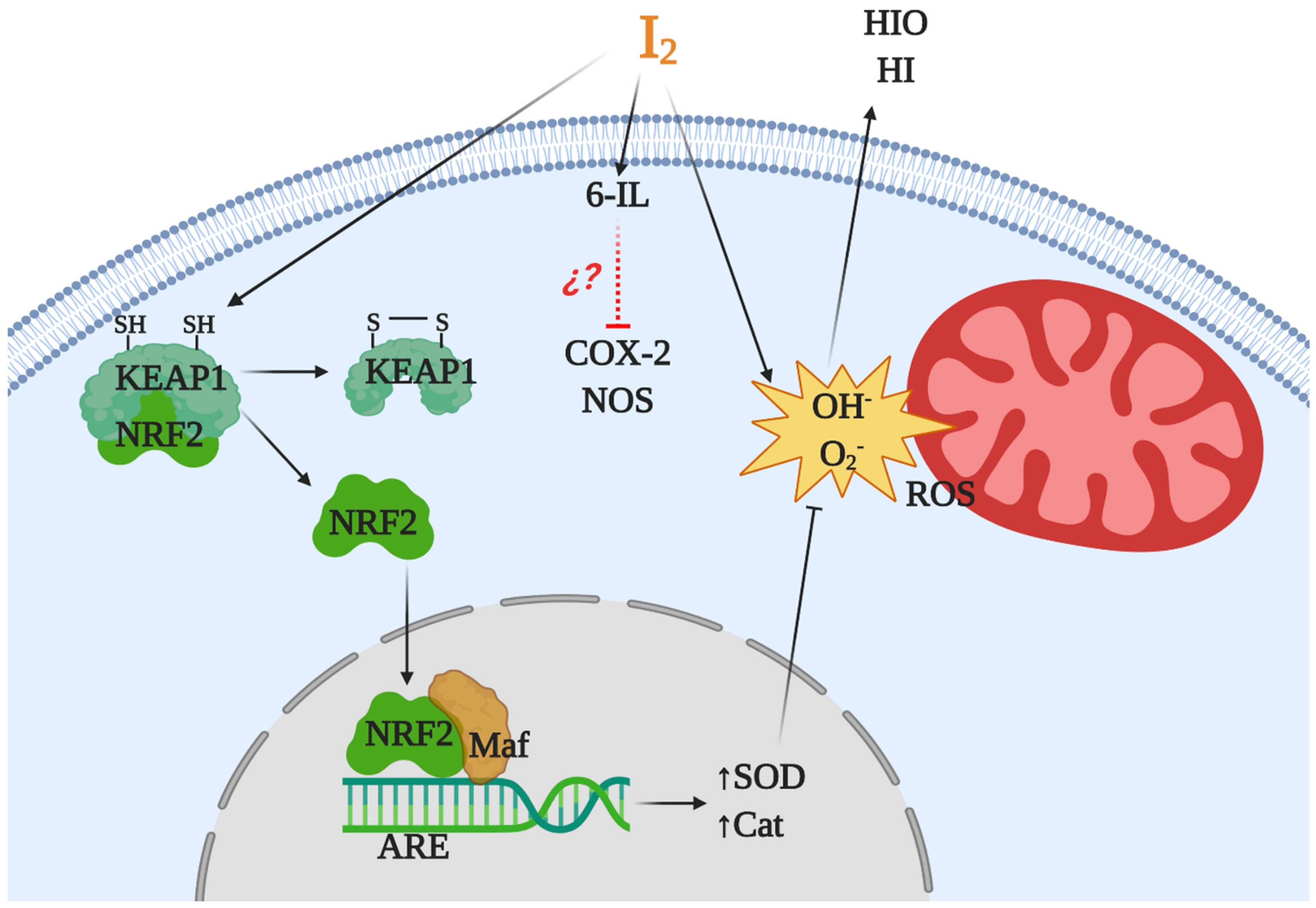
Iodine is considered an ancestral antioxidant and its action is conserved throughout phylogeny [2]. Laminaria brown algae contain an iodine concentration 300,000 times higher than any other living organism, and inorganic iodine acts as a scavenger of various reactive oxygen species (ROS) [35,39]. Similar antioxidant effects have been described in other photosynthetic organisms, as well as in some invertebrates such as polyps of the jellyfish Aurelia aurita and urchin larvae [40,41]. In vertebrates, micromolar amounts of iodine decrease damage by ROS, increasing the total antioxidant status in rat and human serum [20] and preventing lipid peroxidation in the eyes of rabbits [42] and in several tissues of vertebrates [43,44,45]. The iodine released by deiodination of thyroxine has been shown to be an antioxidant agent and an inhibitor of lipoperoxidation [43]. Molecular iodine supplements decrease lipid peroxidation in normal and tumor mammary tissues from rats with methyl nitrosourea (MNU)-induced mammary cancer [13] and prevent the cardiac damage induced by the antineoplastic agent doxorubicin when I2 (0.05% in drinking water) is administered 2 days before starting the antineoplastic treatment [19]. Moderate iodine diets improve the lipid profile in mice, increasing low density lipoprotein receptors and scavenger receptor class B type 1 (SR-B1) in liver [46]. Moreover, iodine supplementation decreased hypercholesterolemia in overweight women [47]. More recently, our group showed that a moderate I2 supplement prevented the pancreatic damage secondary to hypothyroidism by methimazole, normalizing thyroid hormone synthesis in the thyroid and preventing the oxidative status in pancreatic tissue [48]. Several studies suggest that iodine works by neutralizing ROS, or by acting as a free radical iodinating tyrosine, histidine, and double bonds of polyunsaturated fatty acids in cell membranes, making them less reactive to ROS [4,49]. However, the antioxidant effect of iodine could be more complex and include various mechanisms (Figure 2). In a model of prostatic hyperplasia, our group demonstrated that I2 supplements prevent testosterone-induced oxidative stress, decreasing lipoperoxidation but also inhibiting the activity of both nitric oxide synthase (NOS) and type 2 cyclooxygenase (Cox2). The I2 supplement also inhibit the formation of prostaglandins with equivalent intensity to that observed with Celecoxib (a specific Cox2 inhibitor). The effect on Cox2 inhibition can occur by deactivating the heme iron active site or as a competitor of its main substrate, arachidonic acid (AA). In the latter case, the formation of 6-iodolactone (6-IL) from AA can decrease the formation of prostaglandins, or 6-IL acts as a direct inhibitor of the enzyme [50]. Another recent proposal is the interaction of I2 with the nuclear factor erythroid-2-related factor-2 (Nrf2) pathway [51]. Nrf2 is a promoter of the antioxidant response to endogenous and exogenous stressors that trigger the expression of phase II protective antioxidant enzymes such as superoxide dismutase (SOD) and catalase (Cat) [52]. Under basal conditions, Nrf2 is anchored to the cytoplasm through the actin cytoskeleton-binding protein 1 (Keap1). Iodination of Keap1 results in the release and translocation of Nrf2 to the nucleus. After Nrf2 heterodimerizes with small Maf proteins and binds to the antioxidant response element (ARE), SOD and Cat become overexpressed [51].
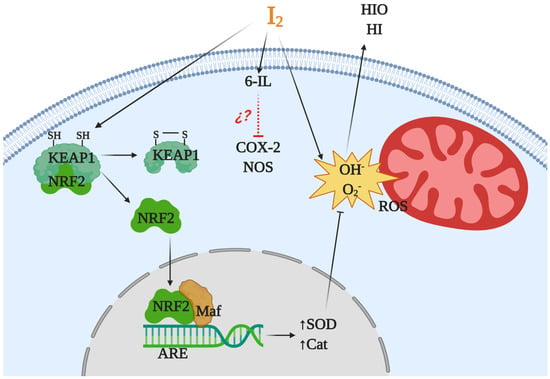
Figure 2.
Antioxidant mechanisms of molecular iodine (I2). I2 acts as a scavenger of a reactive oxygen species (ROS) like hydroxyl radicals (OH) or superoxide anions (O2) generating neutral components hypoiodous acid (HIO) or hydroiodic acid (HI). I2 in combination with arachidonic acid (AA), and generating the iodolipid 6-iodolactone (6-IL), inhibits the activity of proinflammatory enzymes like nitric oxide synthase (NOS) and cyclooxygenase type 2 (Cox2). In addition, the iodination of the cysteine-rich protein Keap1 releases and promotes the nuclear translocation of nuclear factor erythroid-2-related factor-2 (Nrf2) that with Maf activates the antioxidant response element (ARE), inducing overexpression of antioxidant enzymes type II like superoxide dismutase (SOD) and catalase (Cat).
1.4. Antiproliferative and Apoptotic Actions
Since the 1940s it has been known that iodine, in addition to be a structural part of thyroid hormones, also participates in the function and proliferation of thyroid cells. An excess of I− causes inhibitory actions that include decreased iodine organification and hormonal secretion, thyroglobulin proteolysis, decreased glucose and amino acid transport, protein and RNA biosynthesis, and significant inhibition of thyrocyte proliferation under both in vitro and in vivo conditions [49]. The specific mechanism by which iodine performs all these modifications has not been fully elucidated, but a multifaceted mechanism has been postulated and includes the participation of transforming growth factor beta-1 (TGF-β1), triiodothyronine (T3) and iodolipids such as 6-IL or 2-iodohexadecanal (2-IHDA). Moreover, all the inhibitory actions of iodine can be reversed with drugs that block the enzyme thyroid peroxidase (TPO), such as methylmercaptoimidazole (MMI) or propylthiouracil (PTU) [49]. In the presence of H2O2, TPO oxidizes I− and covalently binds it to proteins or lipids. The specific species of iodine generated by TPO has not been identified, but there are several candidates, such as I−, I0 (free radical of iodine), IO− (hypoiodite) and I2 [49]. Vitale et al. [53] showed that an excess of KI (10–50 mM) induces apoptosis in primary thyrocyte cells, but if TPO activity is blocked with PTU, the apoptotic effect of I− is eliminated. In addition, lung cancer cells (without absorption of natural iodide) transfected with NIS or NIS/TPO, a supplement of KI (30 mM), induced apoptosis only in cells transfected with NIS/TPO, indicating that oxidation of I− by TPO is required to exert apoptotic effects [54].
In terms of carcinogenesis, the overproduction of ROS, such as single oxygen (O2), superoxide anions (O2−), hydrogen peroxide (H2O2) and hydroxyl radicals (°OH), is a hallmark related to the etiology and progression of cancer [55]. ROS have a wide range of cellular and molecular effects resulting in mutagenicity, cytotoxicity, and changes in gene expression. The notion that I2 is the chemical form responsible for antineoplastic effects originates from the first descriptions of the consumption of seaweed or Lugol’s solution [4]. Previously, we mentioned that seaweeds contain iodine in several chemical forms although the exact proportion is not known [33]. Traditional Eastern breast cancer medicine has long used iodine-rich seaweeds as a cancer treatment to “soften” tumors and “reduce” nodulation [56]. The addition of small proportions (2 to 5%) of Laminaria angustata, Porphyra tenera or Laminaria religiosa to the diet significantly delays the occurrence of tumors in rats treated with the chemical carcinogen, 7,12-dimethylbenzanthracene (DMBA) [57,58]. The first report demonstrating that iodine exhibited an antineoplastic effect in extrathyroidal tissues was in the rat mammary cancer model induced by DMBA, using 0.05% Lugol’s solution [59]. Later, our group reported that in this model, KI, I2, or Lugol’s solution can induce antineoplastic actions. The protective effect of 0.1% KI is lost when the enzyme lactoperoxidase (LPO), which is present in mammary cancers, is inhibited by MMI, indicating that I− from KI needs to be oxidized to have the apoptotic effect [60]. In this study, our group also demonstrated that I2 prevents DMBA-induced DNA adduct formation in pre-malignant and cancer tissues. This finding is particularly relevant since LPO can oxidize natural or synthetic estrogens to catechol estrogens [61]. The resulting estrogenic quinones have been shown to react with DNA to form mutagenic adducts that can initiate or promote cancer [62]. This notion agrees with the report of Cavalieri’s group showing that higher levels of E2-DNA adducts are present in the urine of breast cancer patients and women at high risk for this disease [63].
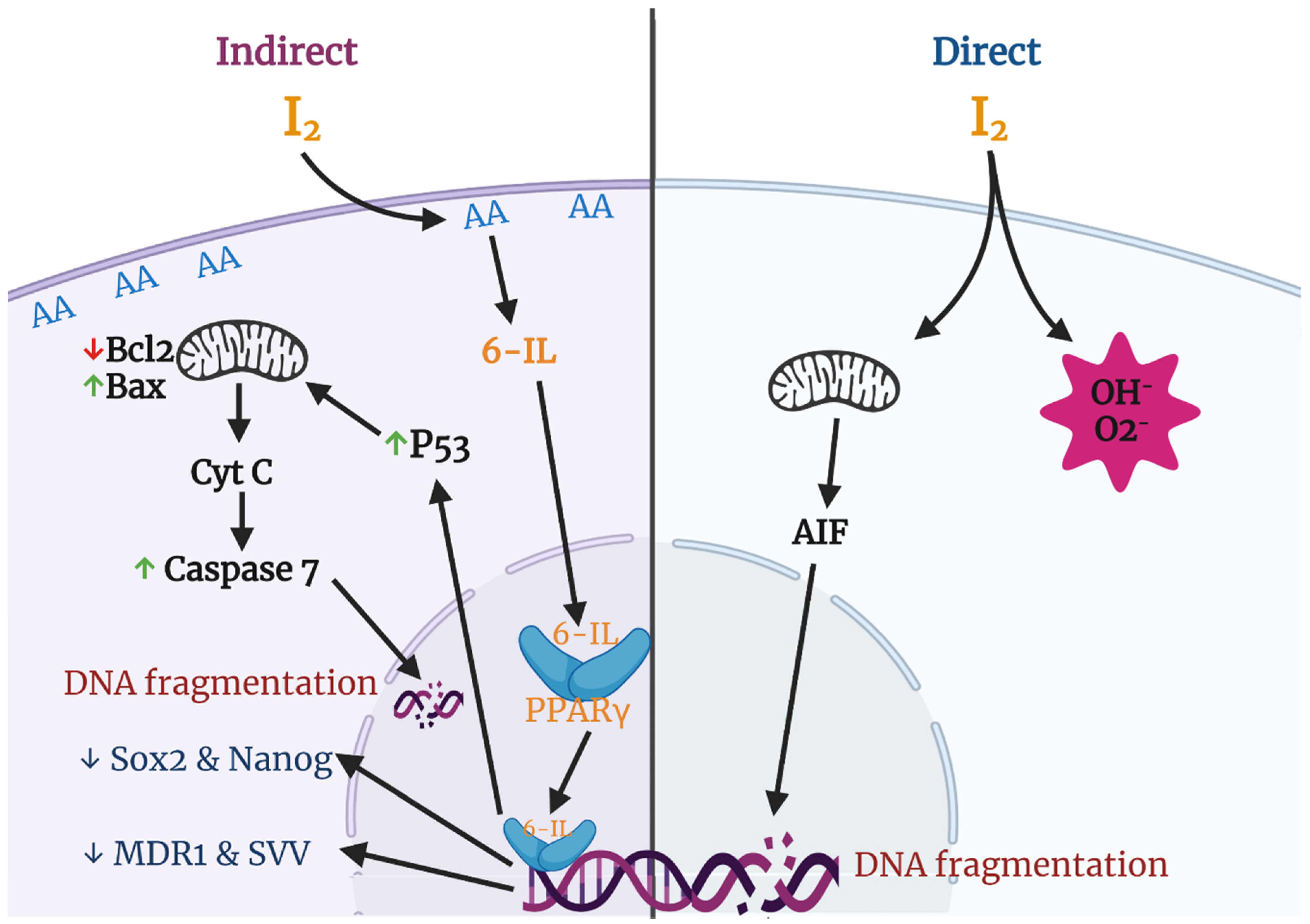
Various groups have described apoptosis effects of iodine in several cancer cell lines and proposed different mechanisms and pathways (Figure 3). The most studied effects include a direct action, where the oxidized iodine dissipates the mitochondrial membrane potential, thereby triggering mitochondrion-mediated apoptosis [64], and an indirect effect through iodolipids formation and the activation of peroxisome proliferator-activated receptors type gamma (PPARγ) [65].
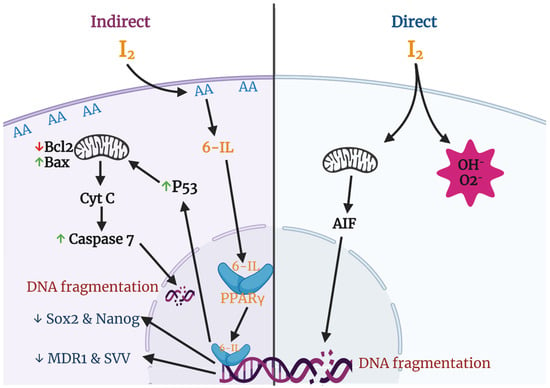
Figure 3.
Apoptotic and differentiation mechanisms of molecular iodine (I2). In the direct pathway the oxidized iodine dissipates the mitochondrial membrane potential triggering mitochondrion-mediated apoptosis. The indirect pathway includes the iodination of arachidonic acid (AA), generating 6- iodolactone (6-IL), and the activation of peroxisome proliferator-activated receptors type gamma (PPARγ). The activation of PPARγ could induce the p53-caspase apoptotic pathway and/or the inhibition of markers related to stem cell maintenance (Sox2, Nanog), chemoresistance (multidrug resistance protein 1; MDR1), and survival (Survivin; SVV).
It is well known that the mitochondrial membrane potential (MMP) is required for a variety of mitochondrial functions including protein import, ATP production, and regulation of metabolite transport. The mitochondrial intermembrane space contains proteins that can induce apoptosis involving caspases (e.g., cytochrome c) or execute a caspase-independent apoptotic death program through the apoptosis-inducing factor (AIF) or through the release and degradation of the antiapoptotic protein Survivin (SVV). The release of these factors requires abatement of the MMP, and thiol depletion is a powerful trigger [66,67]. Molecular iodine treatment is accompanied by depletion of cellular thiol content and dissipation of the MMP in estrogen-responsive (MCF-7) and non-responsive (MDA-MB-231) human cell lines. In addition, the pre-incubation of MCF-7 cells with N-acetylcysteine (NAC), a thiol-containing agent, prevents the apoptotic effect of I2 [64]. Comparative studies of mitochondria isolated from tumoral (TT) versus extra-tumoral (ET) human breast tissue showed that the I2 treatment increased mitochondrial permeability in TT and decreased it in ET, suggesting a differential sensitivity toward iodine in both physiological conditions [68].
The indirect action of I2 could be exerted by the formation 6-IL previously detected in thyroid tissue of rat, pig, horse, and human [69,70]. Although the specific iodinated components have not yet been characterized in other tissues, several studies have reported elevated prostaglandin levels in cancerous tissues compared to normal tissues [71]. Prostaglandins are produced from AA by Cox2, indicating high levels of AA in several tumors [72]. In relation to the mammary gland, we reported that MNU-induced tumors contain four times higher basal concentrations of AA, and after 0.05% I2 treatment, 6-IL levels were 15-fold higher than in normal mammary tissue, suggesting a role for 6-IL in the antiproliferative effect of I2 [65]. These findings have been corroborated in human cancer cell lines where lipids like 6-IL were identified after I2 treatment [73] or where the addition of I2 or 6-IL triggered apoptosis [74,75]. In this regard, the consistent observations that cancer cells are more sensitive to I2 than normal cells [73,74,75] led us to propose that the high concentration of AA in tumoral cells is the crucial component that, when iodinated, is responsible for the antiproliferative effect of I2 [65].
In the search for cellular mechanisms associated with iodine effects, studies from our laboratory demonstrated that both I2 and 6-IL supplementation significantly modified the expression of PPARs [76]. These receptors, originally associated with lipid metabolism regulation, are widely expressed and form part of the nuclear receptor family that binds thyroid hormones, steroids, and vitamins. To date, three isotypes called PPARα, PPARβ/δ, and PPARγ have been identified. These three subtypes display differential tissue distribution, and each is involved in specific functions such as early development, cell proliferation, differentiation, apoptosis, and metabolic homeostasis [77]. In our experiments, 20–200 μM I2 increased the expression of PPARγ mRNA and protein, decreased the expression of mRNA for PPARα, and had no effect on PPARβ/δ expression in MCF-7 cells. We also showed that 6-IL is a specific agonist of PPARγ with an in vitro affinity 6 times higher than AA [76]. These findings agree with the observation that the affinity and selectivity of the PPARγ isoform for some fatty acids is increased by the conformational changes resulting from the incorporation of halogens (phenyl acetate < phenyl butyrate < p-chlorophenyl acetate < p-iodophenyl butyrate) [78]. Moreover, recent reports have shown that antineoplastic effects of iodine or iodolipids are exerted on different types of cells that can take up I2 and exhibit apoptotic induction by PPARγ agonists. Such cells include prostate, lung carcinoma, pancreas carcinoma, melanoma, glioblastoma, and neuroblastoma cells [79].
1.5. Effects on Cellular Differentiation
Another possible effect of iodine is the induction of cellular differentiation. Iodine plays a central role in thyroid physiology by maintaining the integrity of thyroid epithelium [23]. Epidemiological studies associating iodine intake and thyroid cancer have led to controversy. Some suggested that chronic iodine deficiency is firmly established as a risk factor for follicular thyroid cancer, whereas others suggested that iodine supplementation programs could increase the incidence of papillary thyroid cancer in chronic iodine-deficient populations [80]. In relation to differentiation induction, cancer studies have shown that moderate iodine supplements prevent the transformation from differentiated to anaplastic thyroid cancer, the most aggressive type of thyroid cancer with a median survival of 4–12 months from the time of diagnosis [81,82]. In vivo studies of mammary cancer (MNU-induced model, xenografts in the nu/nu Foxn1 mouse, and canine and human patients) have shown that I2 treatment increases the expression of NIS, PDS, LPO, and/or estrogen alpha receptors (ERα) and reduces the invasive and metastatic inducers like vascular endothelial growth factor (VEGF), urokinase-type plasminogen activator (uPA), Bcl2, and SVV, indicating a consistent effect on differentiation [25,26,28,83]. Similar results were obtained in trophoblast cells exposed to iodine supplementation increasing the synthesis of chorionic gonadotropin [84]. Moreover, studies related to stemness markers in breast and cervical cancer cells indicated that I2 supplementation decreased the stem cell-like population, transforming the survival cells into non-invasive type cells incapable of generating xenografts [85,86]. In both conditions PPARγ receptors were increased.
1.6. Immune Modulator
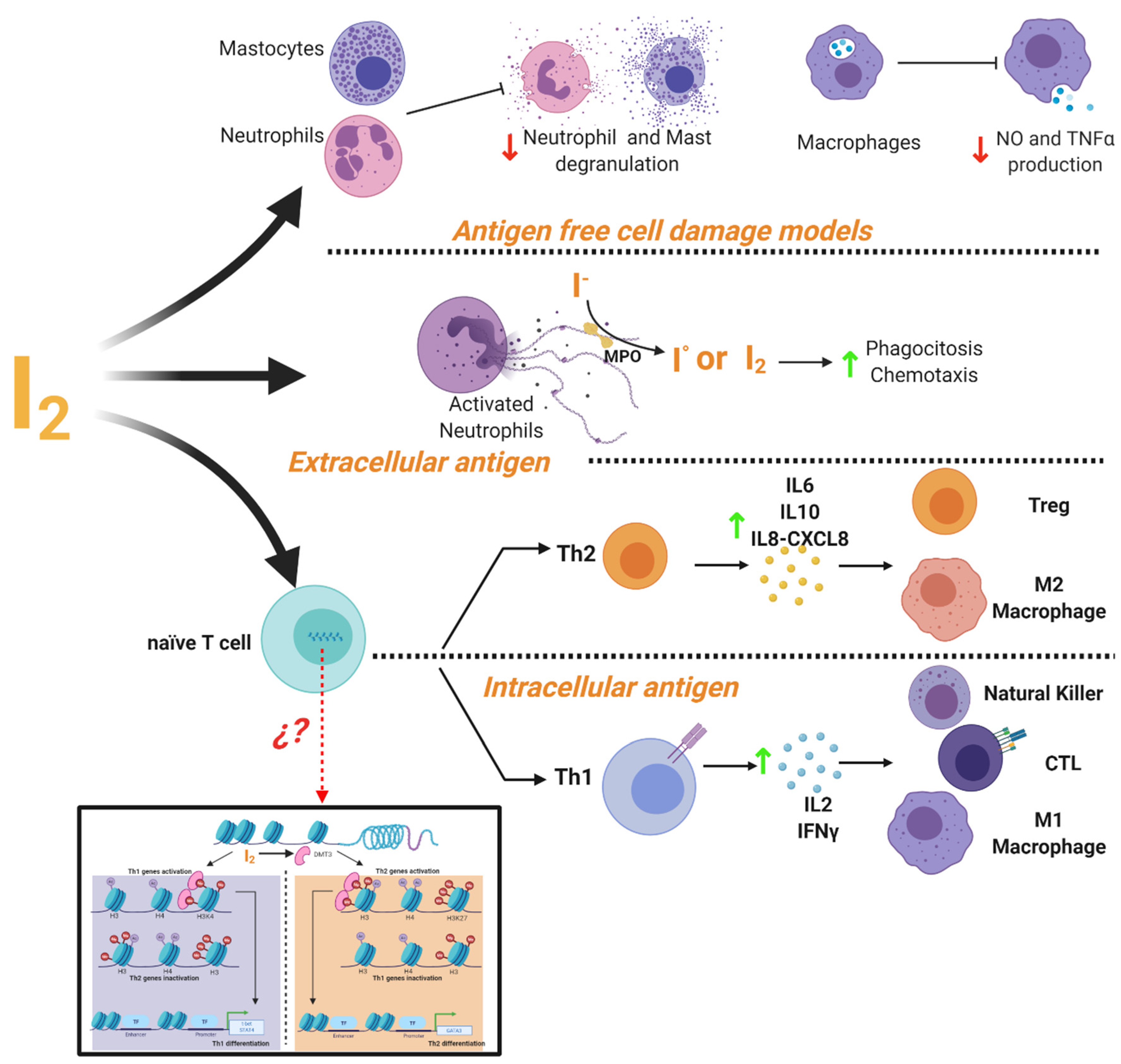
It is widely accepted that iodine exerts important actions on the immune system [87]. The thymus, as well as many immune cells, have the ability to capture and metabolize iodine [88,89]. This element can act as an inhibitor or activator of the immune response depending on the cellular context (Figure 4). In cell damage models induced by non-infectious agents it has been shown that iodine prevents the inflammatory response with a radical scavenging effect. It has described that iodine promoted inhibition of ROS production and oxygen consumption of human polymorphonuclear leukocytes [90], decreased neutrophil chemotaxis [91] and inhibited human complement, mast cell degranulation, nitric oxide and TNF-α production by murine and human macrophages [9,92]. Iodine is oxidized by myeloperoxidase into free radical iodine and is used to kill bacteria [93]. This ancestral defense mechanism has been documented in Roseolovirus spp. bacteria as a defense against other species [94]. In addition, iodide supplementation increases granulocyte chemotaxis to inflamed areas and improves phagocytosis of bacteria [95]. Besides, under adaptive immune response, micromolar concentrations of iodine improve the Th2 response of leukocytes from normal subjects preactivated with PMA, increasing the release of IL6, IL8-CXCL8 and IL10 [9]. Marani and Venturi described that in the central region of Italy, partial iodine deficiency (urinary excretion of 4 mg/day) is enough to maintain euthyroid conditions, but it is accompanied by serious alterations in the immune response of school-age children. Lugol’s oral solution supplement (2 mg/week) for 8 months restored the normal immune response evaluated by skin tests [96]. Similar responses to Lugol’s supplement were observed in patients with infections in which the immune system was compromised, such as granulomatous, lepromatous, syphilitic, and fungal tuberculous lesions [97].
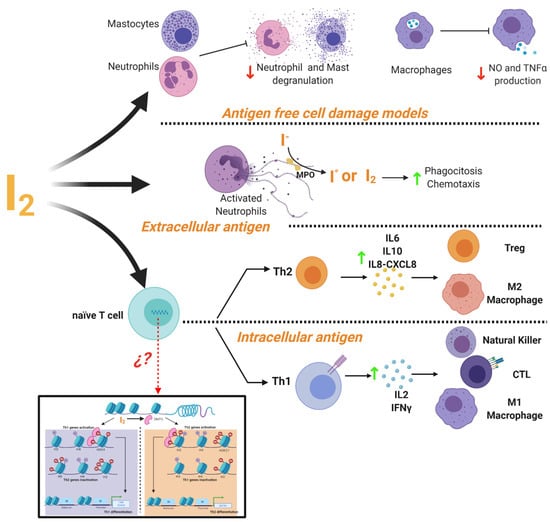
Figure 4.
Mechanisms of immune modulation by molecular iodine (I2). Evidence showed that I2 the mediated activation or inhibition of immune responses depending on the cellular context. In a cell damage environment without antigens iodine supplementation inhibits the degranulation of neutrophil and mast cells, as well as the production of macrophages NO and TNFα. In response to extracellular antigens (e.g., bacteria or fungi), I2 improves the phagocytosis and chemotaxis of activated neutrophils. It also activates the Th2 response and the increase of cytokine secretion (IL-6, IL-10, IL-8-CXCL8) promoting the activation and differentiation of Treg lymphocytes and M2 macrophages. In response to intracellular antigens (e.g., virus-infected cells and cancer cells), I2 activates the Th1 response and increases the cytokine production of IL-2 and IFNγ, promoting the activation and proliferation of effector cells (NK, CTL and M1 macrophages). This differential response might be associated with epigenetic modifications facilitated by the I2-mediated activation of the demethylase enzyme (DMT3) that regulates the Th fate by activating and/or inactivating the specific genes for the differentiation of Th1 (T-bet and STAT4) or Th2 (GATA3).
Regarding the participation of iodine in the immune response against intracellular antigens (virus or cancer), studies of our group have shown that I2 supplementation, alone or in co-administration with conventional chemotherapies, is accompanied by the activation of anti-tumor immune responses in several models (rodents, dogs and humans). The remaining tumors from these treatments, which contain multiple necrotic areas, also show CD8+ immune cell infiltration [13,26,28]. Transcriptomic analysis of human samples showed that I2 supplementation up-regulated Th1 and Th17 pathways including differentiation by activation of T-cell receptor, cytotoxicity by NK and CD8+, lymphocyte migration and formation of tertiary lymphoid structures [26,98]. In accordance with these data, micromolar supplementation of I2 improves the Th1 response in leukocytes from normal subjects by increasing the release of IL2 and IFN-γ cytokines [9].
The signaling pathways underlying these immune responses have not been deeply studied. However, the activation of PPARγ receptors might be involved, since these receptors participate in the modulation of several immune cells [99]; although it is generally accepted that PPARγ exerts anti-inflammatory responses on infections, its participation in the antitumoral immune response has also been suggested. One report using cyclophosphamide metronomic therapy showed the activation of the immune cascade, and PPARγ was proposed as the main inducer [100]. Our group identified a similar activation through breast cancer RNAseq analysis; PPARγ was positively correlated with changes in IRF1, STAT1, and IRF4 [26]. Another possibility currently explored in our laboratory is that I2 as an oxidized agent can exert epigenetic modifications associated with the activation of important demethylase enzymes like DMT3 [101], unpublished results). In fact, other natural antioxidants, such as resveratrol or curcumin, exert part of their effects by modifying the methylation/demethylation equilibrium genes for the differentiation of Th1 (T-bet and STAT4) or Th2 (GATA3) [102].
2. Discussion/Conclusions
Molecular iodine in vertebrates acts in the following ways:
- as an ancestral antioxidant by combining or competing with free radicals for membrane lipids, proteins, and DNA, increasing the expression or activity of antioxidant enzymes, or inactivating proinflammatory pathways stabilizing cellular redox status;
- as an inducer of antiproliferative, differentiation or apoptotic mechanisms by modulating mitochondrial potential or forming iodolipids and activating nuclear receptors;
- as an immune modulator acting directly on specific immune cells; and
- as a constituent part of thyroid hormones.
Although iodine excess is generally well tolerated, it may induce physiological changes in susceptible groups, particularly those previously exposed to iodine deficiency, thyroid defunctions, pregnant women, or infants. We propose that molecular iodine intake be increased in adults to at least 1 mg/day in specific pathologies to obtain the potential extrathyroidal benefits described in the present review.
Funding
This research was partially funded by PAPIIT-UNAM, grant numbers 203919, 205920; and DGAPA postdoctoral fellowship award for Irasema Mendieta.
Acknowledgments
We thank Laura Inés García and Carlos S. Flores for their technical assistance; Nuri Aranda and Lourdes Lara for academic support; Francisco Javier Valles and Rafael Silva for bibliographic assistance; Alberto Lara, Omar Gonzalez, Ramon Martinez, and Maria Eugenia Rosas-Alatorre for computer assistance; Jessica Gonzalez Norris for proofreading and English language editing.
Conflicts of Interest
The authors declare no conflict of interest to declare.
References
- De la Vieja, A.; Santisteban, P. Role of iodide metabolism in physiology and cancer. Endocr. Relat. Cancer 2018, 25, R225–R245. [Google Scholar] [CrossRef]
- Venturi, S. Evolutionary significance of iodine. Curr. Chem. Biol. 2011, 5, 155–162. [Google Scholar]
- Cann, S.A.; Van Netten, J.P.; Van Netten, C. Hypothesis: Iodine, selenium and the development of breast cancer. Cancer Causes Control 2000, 11, 121–127. [Google Scholar] [CrossRef]
- Smyth, P.P.A. Role of iodine in antioxidant defence in thyroid and breast disease. BioFactors 2003, 19, 121–130. [Google Scholar] [CrossRef]
- Venturi, S.; Venturi, M. Iodine in Evolution of Salivary Glands and in Oral Health. Nutr. Health 2009, 20, 119–134. [Google Scholar] [CrossRef]
- Aceves, C.; Anguiano, B.; Delgado, G. The Extrathyronine Actions of Iodine as Antioxidant, Apoptotic, and Differentiation Factor in Various Tissues. Thyroid 2013, 23, 938–946. [Google Scholar] [CrossRef]
- Juvenal, G.J.; Thomasz, L.; Oglio, R.; Perona, M.; Pisarev, M.A.; Rossich, L.; Salvarredi, L. Thyroid: Iodine beyond the thyronines. Curr. Chem. Biol. 2011, 5, 163–167. [Google Scholar]
- Torremante, E.P.; Rosner, H. Antiproliferative effects of molecular iodine in cancers. Curr. Chem. Biol. 2011, 5, 168–176. [Google Scholar]
- Bilal, M.Y.; Dambaeva, S.; Kwak-Kim, J.; Gilman-Sachs, A.; Beaman, K.D. A role for iodide and thyroglobulin in modulating the function of human immune cells. Front. Immunol. 2017, 8, 1573. [Google Scholar] [CrossRef]
- Moore, K.; Thomas, A.; Harding, K.G. Iodine released from the wound dressing iodosorb modulates the secretion of cytokines by human macrophages responding to bacterial lipopolysaccharide. Int. J. Biochem. Cell Biol. 1997, 29, 163–171. [Google Scholar] [CrossRef]
- Thrall, K.D.; Bull, R. Differences in the distribution of iodine and iodide in the Sprague-Dawley rat. Fundament. Appl. Toxicol. 1990, 15, 75–81. [Google Scholar] [CrossRef]
- Sherer, T.T.; Thrall, K.D.; Bull, R.J. Comparison of toxicity induced by iodine and iodide in male and female rats. J. Toxicol. Environ. Health Part A 1991, 32, 89–101. [Google Scholar] [CrossRef] [PubMed]
- García-Solís, P.; Alfaro, Y.; Anguiano, B.; Delgado, G.; Guzman, R.C.; Nandi, S.; Aceves, C. Inhibition of N-methyl-N-nitrosourea-induced mammary carcinogenesis by molecular iodine (I2) but not by iodide (I−) treatment: Evidence that I2 prevents cancer promotion. Mol. Cell. Endocrinol. 2005, 236, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Eskin, B.A.; Grotkowski, C.E.; Connolly, C.P.; Ghent, W.R. Different tissue responses for iodine and iodide in rat thyroid and mammary glands. Biol. Trace Elem. Res. 1995, 49, 9–19. [Google Scholar] [CrossRef]
- Farebrother, J.; Zimmermann, M.B.; Andersson, M. Excess iodine intake: Sources, assessment, and effects on thyroid function. Ann. N. Y. Acad. Sci. 2019, 1446, 44–65. [Google Scholar] [CrossRef]
- WHO. Progress towards the Elimination of Iodine Deficiency Disorders (IDD). 1999. Available online: https://apps.who.int/iris/handle/10665/65931 (accessed on 1 September 2020).
- Backer, H.; Hollowell, J. Use of iodine for water disinfection: Iodine toxicity and maximum recommended dose. Environ. Health Perspect. 2000, 108, 679–684. [Google Scholar] [CrossRef]
- Bürgi, H. Iodine excess. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 107–115. [Google Scholar] [CrossRef]
- Alfaro, Y.; Delgado, G.; Cárabez-Trejo, A.; Anguiano, B.; Aceves, C. Iodine and doxorubicin, a good combination for mammary cancer treatment: Antineoplastic adjuvancy, chemoresistance inhibition, and cardioprotection. Mol. Cancer 2013, 12, 45. [Google Scholar] [CrossRef]
- Winkler, R.; Griebenow, S.; Wonisch, W. Effect of iodide on total antioxidant status of human serum. Cell Biochem. Funct. 2000, 18, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Ghent, W.R.; Eskin, B.A.; Low, D.A.; Hill, L.P. Iodine replacement in fibrocystic disease of the breast. Can. J. Surg. 1993, 36, 453–460. [Google Scholar]
- Kessler, J.H. The Effect of Supraphysiologic Levels of Iodine on Patients with Cyclic Mastalgia. Breast J. 2004, 10, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Anguiano, B.; Ledezma, O.; Juárez, M.; Nunez, F.; Aceves, C. Therapeutic effect of iodine on human benign prostatic hyperplasia. In Proceedings of the 14th International Thyroid Congress, Paris, France, 11–16 September 2010. [Google Scholar]
- Kessler, J. Are there Side Effects when Using Supraphysiologic Levels of Iodine in Treatment Regimens? Compr. Handb. Iodine 2009, 801, 801–810. [Google Scholar] [CrossRef]
- Anguiano, B.; García-Solís, P.; Delgado, G.; Velasco, C.A. Uptake and Gene Expression with Antitumoral Doses of Iodine in Thyroid and Mammary Gland: Evidence that Chronic Administration Has No Harmful Effects. Thyroid 2007, 17, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vega, A.; Vega-Riveroll, L.; Ayala, T.; Peralta, G.; Torres-Martel, J.M.; Rojas, J.; Mondragón, P.; Domínguez, A.; De Obaldía, R.; Avecilla-Guerrero, C.; et al. Adjuvant Effect of Molecular Iodine in Conventional Chemotherapy for Breast Cancer. Randomized Pilot Study. Nutrients 2019, 11, 1623. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Caltzontzin, P.; Delgado, G.; Aceves, C.; Anguiano, B. Iodine Uptake and Prostate Cancer in the TRAMP Mouse Model. Mol. Med. 2013, 19, 409–416. [Google Scholar] [CrossRef]
- Zambrano-Estrada, X.; Landaverde-Quiroz, B.; Dueñas-Bocanegra, A.A.; De Paz-Campos, M.A.; Hernández-Alberto, G.; Solorio-Perusquia, B.; Trejo-Mandujano, M.; Pérez-Guerrero, L.; Delgado-González, E.; Anguiano, B.; et al. Molecular iodine/doxorubicin neoadjuvant treatment impair invasive capacity and attenuate side effect in canine mammary cancer. BMC Vet. Res. 2018, 14, 87. [Google Scholar] [CrossRef]
- Yeğinsu, A.; Karamustafaoglu, A.; Ozugurlu, F.; Etikan, I. Iodopovidone pleurodesis does not effect thyroid function in normal adults. Interact. Cardiovasc. Thorac. Surg. 2007, 6, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Bogazzi, F.; Tomisti, L.; Bartalena, L.; Aghini-Lombardi, F.; Martino, E. Amiodarone and the thyroid: A 2012 update. J. Endocrinol. Investig. 2012, 35, 340–348. [Google Scholar]
- Rhee, C.M.; Bhan, I.; Alexander, E.K.; Brunelli, S.M. Association Between Iodinated Contrast Media Exposure and Incident Hyperthyroidism and Hypothyroidism. Arch. Intern. Med. 2012, 172, 153. [Google Scholar] [CrossRef]
- Michikawa, T.; Inoue, M.; Shimazu, T.; Sawada, N.; Iwasaki, M.; Sasazuki, S.; Yamaji, T.; Tsugane, S.; Japan Public Health Center-Based Prospective Study Group. Seaweed consumption and the risk of thyroid cancer in women: The Japan Public Health Center-based Prospective Study. Eur. J. Cancer Prev. 2012, 21, 254–260. [Google Scholar] [CrossRef]
- Nagataki, S. The Average of Dietary Iodine Intake due to the Ingestion of Seaweeds is 1.2 mg/day in Japan. Thyroid 2008, 18, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Helguera, O.; Anguiano, B.; Delgado, G.; Aceves, C. Uptake and antiproliferative effect of molecular iodine in the MCF-7 breast cancer cell line. Endocr. Relat. Cancer 2006, 13, 1147–1158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Küpper, F.C.; Schweigert, N.; Gall, E.A.; Legendre, J.-M.; Vilter, H.; Kloareg, B. Iodine uptake in Laminariales involves extracellular, haloperoxidase-mediated oxidation of iodide. Planta 1998, 207, 163–171. [Google Scholar] [CrossRef]
- Anguiano, B.; Aceves, C. Iodine in mammary and prostate pathologies. Curr. Chem. Biol. 2011, 5, 177–182. [Google Scholar]
- Kamangar, F.; Dores, G.M.; Anderson, W.F. Patterns of Cancer Incidence, Mortality, and Prevalence Across Five Continents: Defining Priorities to Reduce Cancer Disparities in Different Geographic Regions of the World. J. Clin. Oncol. 2006, 24, 2137–2150. [Google Scholar] [CrossRef] [PubMed]
- Zava, T.T.; Zava, D.T. Assessment of Japanese iodine intake based on seaweed consumption in Japan: A literature-based analysis. Thyroid Res. 2011, 4, 14. [Google Scholar] [CrossRef]
- Küpper, F.C.; Carpenter, L.J.; McFiggans, G.B.; Palmer, C.J.; Waite, T.J.; Boneberg, E.-M.; Woitsch, S.; Weiller, M.; Abela, R.; Grolimund, D.; et al. Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proc. Natl. Acad. Sci. USA 2008, 105, 6954–6958. [Google Scholar] [CrossRef]
- Berking, S.; Czech, N.; Gerharz, M.; Herrmann, K.; Hoffmann, U.; Raifer, H.; Sekul, G.; Siefker, B.; Sommerei, A.; Vedder, F. A newly discovered oxidant defence system and its involvement in the development of Aurelia aurita (Scyphozoa, Cnidaria): Reactive oxygen species and elemental iodine control medusa formation. Int. J. Dev. Biol. 2005, 49, 969–976. [Google Scholar] [CrossRef]
- Miller, A.E.M.; Heyland, A. Iodine accumulation in sea urchin larvae is dependent on peroxide. J. Exp. Biol. 2012, 216, 915–926. [Google Scholar] [CrossRef]
- Elstner, E.; Adamczyk, R.; Kröner, R.; Furch, A. The uptake of potassium iodide and its effect as an antioxidant in isolated rabbit eyes. Int. J. Ophthalmol. 1985, 191, 122–126. [Google Scholar]
- Cocchi, M.; Venturi, S. Iodide, antioxidant function and omega-6 and omega-3 fatty acids: A new hypothesis of biochemical cooperation? Prog. Nutr. 2000, 2, 15–19. [Google Scholar]
- Tseng, Y.-C.L.; Latham, K.R. Iodothyronines: Oxidative deiodination by hemoglobin and inhibition of lipid peroxidation. Lipids 1984, 19, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.B.; Khayat, G.B. Studies on the prevention of cholesterol atherosclerosis in rabbits. The influence of thyroidectomy upon the protective action of potassium iodide. J. Exp. Med. 1993, 58, 127. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-J.; Ye, Y.; Sun, F.-J.; Tian, E.-J.; Chen, Z.-P. The Impact of Dietary Iodine Intake on Lipid Metabolism in Mice. Biol. Trace Elem. Res. 2010, 142, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Herter-Aeberli, I.; Cherkaoui, M.; El Ansari, N.; Rohner, R.; Stinca, S.; Chabaa, L.; Von Eckardstein, A.; Aboussad, A.; Zimmermann, M. Iodine Supplementation Decreases Hypercholesterolemia in Iodine-Deficient, Overweight Women: A Randomized Controlled Trial. J. Nutr. 2015, 145, 2067–2075. [Google Scholar] [CrossRef]
- Rodríguez-Castelán, J.; Delgado-González, E.; Rodríguez-Benítez, E.; Castelán, F.; Cuevas-Romero, E.; Aceves, C. SAT-561 Protective Effect of Moderated Dose of Iodine in Pancreatic Alterations during Hypothyroidism. J. Endocr. Soc. 2019, 3, SAT-561. [Google Scholar] [CrossRef]
- Gärtner, R. Autoregulation of thyroid growth and function by iodine: Independent regulation of the thyroid gland by iodocompounds. In Comprehensive Handbook of Iodine: Nutritional, Biochemical, Pathological and Therapeutic Aspects, 1st ed.; Academic Press/Elsevier: Cambridge, MA, USA, 2009; pp. 243–247. [Google Scholar]
- Quintero-García, M.; Delgado-González, E.; Sánchez-Tusie, A.; Vázquez, M.; Aceves, C.; Anguiano, B. Iodine prevents the increase of testosterone-induced oxidative stress in a model of rat prostatic hyperplasia. Free Radic. Biol. Med. 2018, 115, 298–308. [Google Scholar] [CrossRef]
- Greenwald, M.B.-Y.; Frušić-Zlotkin, M.; Soroka, Y.; Ben-Sasson, S.; Bianco-Peled, H.; Kohen, R. A novel role of topical iodine in skin: Activation of the Nrf2 pathway. Free Radic. Biol. Med. 2017, 104, 238–248. [Google Scholar] [CrossRef]
- Paunkov, A.; Chartoumpekis, D.V.; Ziros, P.G.; Sykiotis, G.P. A Bibliometric Review of the Keap1/Nrf2 Pathway and its Related Antioxidant Compounds. Antioxidants 2019, 8, 353. [Google Scholar] [CrossRef]
- Vitale, M.; Di Matola, T.; D’Ascoli, F.; Salzano, S.; Bogazzi, F.; Fenzi, G.; Rossi, G. Iodide excess induces apoptosis in thyroid cells through a p53-independent mechanism involving oxidative stress. Endocrinology 2000, 141, 598–605. [Google Scholar] [CrossRef]
- Zhang, L.; Sharma, S.; Zhu, L.X.; Kogai, T.; Hershman, J.M.; Brent, G.A.; Dubinett, S.M.; Huang, M. Nonradioactive iodide effectively induces apoptosis in genetically modified lung cancer cells. Cancer Res. 2003, 63, 5065–5072. [Google Scholar] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Teas, J.; Harbison, M.L.; Gelman, R.S. Dietary seaweed (Laminaria) and mammary carcinogenesis in rats. Cancer Res. 1984, 44, 2758–2761. [Google Scholar] [PubMed]
- Funahashi, H.; Imai, T.; Tanaka, Y.; Tsukamura, K.; Hayakawa, Y.; Kikumori, T.; Mase, T.; Itoh, T.; Nishikawa, M.; Hayashi, H.; et al. Wakame Seaweed Suppresses the Proliferation of 7,12-Dimethylbenz(a)-anthracene-induced Mammary Tumors in Rats. Jpn. J. Cancer Res. 1999, 90, 922–927. [Google Scholar] [CrossRef]
- Yamamoto, I.; Maruyama, H.; Moriguchi, M. The effect of dietary seaweeds on 7,12-dimethylbenz[a]anthracene-induced mammary tumorigenesis in rats. Cancer Lett. 1987, 35, 109–118. [Google Scholar] [CrossRef]
- Kato, N. Suppressive effect of iodine preparations on proliferation of DMBA-induced breast cancer in rat. J. Jpn. Soc. Cancer Ther. 1994, 29, 582. [Google Scholar]
- Soriano, O.; Delgado, G.; Anguiano, B.; Petrosyan, P.; Molina-Servín, E.D.; Gonsebatt, M.E.; Aceves, C. Antineoplastic effect of iodine and iodide in dimethylbenz[a]anthracene-induced mammary tumors: Association between lactoperoxidase and estrogen-adduct production. Endocr. Relat. Cancer 2011, 18, 529–539. [Google Scholar] [CrossRef]
- Løvstad, R.A. A kinetic study on the lactoperoxidase catalyzed oxidation of estrogens. BioMetals 2006, 19, 587–592. [Google Scholar] [CrossRef]
- Cavalieri, E.L.; Stack, D.E.; Devanesan, P.D.; Todorovic, R.; Dwivedy, I.; Higginbotham, S.; Johansson, S.L.; Patil, K.D.; Gross, M.L.; Gooden, J.K.; et al. Molecular origin of cancer: Catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc. Natl. Acad. Sci. USA 1997, 94, 10937–10942. [Google Scholar] [CrossRef]
- Cavalieri, E.L.; Rogan, E.G. Depurinating estrogen-DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol. 2010, 6, 75–91. [Google Scholar] [CrossRef]
- Shrivastava, A.; Tiwari, M.; Sinha, R.A.; Kumar, A.; Balapure, A.K.; Bajpai, V.K.; Sharma, R.; Mitra, K.; Tandon, A.; Godbole, M.M. Molecular Iodine Induces Caspase-Independent Apoptosis in Human Breast Carcinoma Cells Involving the Mitochondria-mediated Pathway. J. Biol. Chem. 2006, 281, 19762–19771. [Google Scholar] [CrossRef]
- Aceves, C.; García-Solís, P.; Omar, A.-H.; Vega-Riveroll, L.; Delgado, G.; Anguiano, B. Antineoplastic effect of iodine in mammary cancer: Participation of 6-iodolactone (6-IL) and peroxisome proliferator-activated receptors (PPAR). Mol. Cancer 2009, 8, 33. [Google Scholar] [CrossRef]
- Chen, X.; Duan, N.; Zhang, C.; Zhang, W. Survivin and Tumorigenesis: Molecular Mechanisms and Therapeutic Strategies. J. Cancer 2016, 7, 314–323. [Google Scholar] [CrossRef]
- Yang, C.F.; Shen, H.M.; Ong, C.N. Intracellular Thiol Depletion Causes Mitochondrial Permeability Transition in Ebselen-Induced Apoptosis. Arch. Biochem. Biophys. 2000, 380, 319–330. [Google Scholar] [CrossRef]
- Upadhyay, G.; Singh, R.; Sharma, R.; Balapure, A.K.; Godbole, M.M. Differential action of iodine on mitochondria from human tumoral- and extra-tumoral tissue in inducing the release of apoptogenic proteins. Mitochondrion 2002, 2, 199–210. [Google Scholar] [CrossRef]
- Boeynaems, J.; Hubbard, W. Transformation of arachidonic acid into an iodolactone by the rat thyroid. J. Biol. Chem. 1980, 255, 9001–9004. [Google Scholar] [CrossRef]
- Dugrillon, A.; Uedelhoven, W.M.; Pisarev, M.A.; Bechtner, G.; Gärtner, R. Identification of δ-Iodolactone in Iodide Treated Human Goiter and Its Inhibitory Effect on Proliferation of Human Thyroid Follicles. Horm. Metab. Res. 1994, 26, 465–469. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N. Role of prostanoids in gastrointestinal cancer. J. Clin. Investig. 2018, 128, 2732–2742. [Google Scholar] [CrossRef]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of Phytonutrient Modulation of Cyclooxygenase-2 (COX-2) and Inflammation Related to Cancer. Nutr. Cancer 2018, 70, 350–375. [Google Scholar] [CrossRef]
- Arroyo-Helguera, O.; Rojas, E.; Delgado, G.; Aceves, C. Signaling pathways involved in the antiproliferative effect of molecular iodine in normal and tumoral breast cells: Evidence that 6-iodolactone mediates apoptotic effects. Endocr. Relat. Cancer 2008, 15, 1003–1011. [Google Scholar] [CrossRef]
- Aranda, N.; Sosa, S.; Delgado, G.; Aceves, C.; Anguiano, B. Uptake and antitumoral effects of iodine and 6-iodolactone in differentiated and undifferentiated human prostate cancer cell lines. Prostate 2012, 73, 31–41. [Google Scholar] [CrossRef]
- Rösner, H.; Möller, W.; Groebner, S.; Torremante, P. Antiproliferative/cytotoxic effects of molecular iodine, povidone-iodine and Lugol’s solution in different human carcinoma cell lines. Oncol. Lett. 2016, 12, 2159–2162. [Google Scholar] [CrossRef]
- Nuñez-Anita, R.; Arroyo-Helguera, O.; Cajero-Juárez, M.; López-Bojorquez, L.; Aceves, C. A complex between 6-iodolactone and the peroxisome proliferator-activated receptor type gamma may mediate the antineoplasic effect of iodine in mammary cancer. Prostaglandins Other Lipid Mediat. 2009, 89, 34–42. [Google Scholar] [CrossRef]
- Reka, A.K.; Kurapati, H.; Narala, V.R.; Bommer, G.T.; Chen, J.; Standiford, T.J.; Keshamouni, V.G. Peroxisome Proliferator-Activated Receptor-γ Activation Inhibits Tumor Metastasis by Antagonizing Smad3-Mediated Epithelial-Mesenchymal Transition. Mol. Cancer Ther. 2010, 9, 3221–3232. [Google Scholar] [CrossRef]
- Samid, D.; Wells, M.; Greene, M.E.; Shen, W.; Palmer, C.N.; Thibault, A. Peroxisome proliferator-activated receptor γ as a novel target in cancer therapy: Binding and activation by an aromatic fatty acid with clinical antitumor activity. Clin. Cancer Res. 2000, 6, 933–941. [Google Scholar]
- Reka, A.K.; Goswami, M.T.; Krishnapuram, R.; Standiford, T.J.; Keshamouni, V.G. Molecular cross-regulation between PPAR-γ and other signaling pathways: Implications for lung cancer therapy. Lung Cancer 2011, 72, 154–159. [Google Scholar] [CrossRef]
- Dijkstra, B.; Prichard, R.S.; Lee, A.; Kelly, L.M.; Smyth, P.P.A.; Crotty, T.; McDermott, E.W.; Hill, A.D.K.; O’Higgins, N.J. Changing patterns of thyroid carcinoma. Ir. J. Med. Sci. 2007, 176, 87–90. [Google Scholar] [CrossRef]
- Maso, L.D.; Bosetti, C.; La Vecchia, C.; Franceschi, S. Risk factors for thyroid cancer: An epidemiological review focused on nutritional factors. Cancer Causes Control 2008, 20, 75–86. [Google Scholar] [CrossRef]
- Knobel, M.; Medeiros-Neto, G. Relevance of iodine intake as a reputed predisposing factor for thyroid cancer. Arq. Bras. Endocrinol. Metabol. 2007, 51, 701–712. [Google Scholar] [CrossRef]
- Mendieta, I.; Nuñez-Anita, R.E.; Nava-Villalba, M.; Zambrano-Estrada, X.; Delgado-González, E.; Anguiano, B.; Aceves, C. Molecular iodine exerts antineoplastic effects by diminishing proliferation and invasive potential and activating the immune response in mammary cancer xenografts. BMC Cancer 2019, 19, 261. [Google Scholar] [CrossRef]
- Olivo-Vidal, Z.E.; Rodríguez, R.C.; Arroyo-Helguera, O. Iodine Affects Differentiation and Migration Process in Trophoblastic Cells. Biol. Trace Elem. Res. 2015, 169, 180–188. [Google Scholar] [CrossRef]
- Bigoni-Ordóñez, G.D.; Ortiz-Sánchez, E.; Rosendo-Chalma, P.; Valencia-González, H.A.; Aceves, C.; García-Carrancá, A. Molecular iodine inhibits the expression of stemness markers on cancer stem-like cells of established cell lines derived from cervical cancer. BMC Cancer 2018, 18, 928. [Google Scholar] [CrossRef]
- Bontempo, A.; Ugalde-Villanueva, B.; Delgado-González, E.; Rodríguez, Á.L.; Aceves, C. Molecular iodine impairs chemoresistance mechanisms, enhances doxorubicin retention and induces downregulation of the CD44+/CD24+ and E-cadherin+/vimentin+ subpopulations in MCF-7 cells resistant to low doses of doxorubicin. Oncol. Rep. 2017, 38, 2867–2876. [Google Scholar] [CrossRef]
- Stone, O.J. The role of the primitive sea in the natural selection of iodides as a regulating factor in inflammation. Med. Hypotheses 1988, 25, 125–129. [Google Scholar] [CrossRef]
- Ullberg, S.; Ewaldsson, B. Distribution of Radio-Iodine Studied by Whole-Body Autoradiography. Acta Radiol. Ther. Phys. Biol. 1964, 2, 24–32. [Google Scholar] [CrossRef]
- Venturi, S.; Venturi, M. Iodine, thymus, and immunity. Nutrition 2009, 25, 977–979. [Google Scholar] [CrossRef]
- Miyachi, Y.; Niwa, Y. Effects of potassium iodide, colchicine and dapsone on the generation of polymorphonuclear leukocyte-derived oxygen intermediates. Br. J. Dermatol. 1982, 107, 209–214. [Google Scholar] [CrossRef]
- Honma, K.; Saga, K.; Onodera, H.; Takahashi, M. Potassium iodide inhibits neutrophil chemotaxis. Acta Derm. Venereol. 1990, 70, 247–249. [Google Scholar]
- Beukelman, C.; Berg, A.V.D.; Hoekstra, M.; Uhl, R.; Reimer, K.; Mueller, S. Anti-inflammatory properties of a liposomal hydrogel with povidone-iodine (Repithel®) for wound healing in vitro. Burns 2008, 34, 845–855. [Google Scholar] [CrossRef]
- Klebanoff, S.J.; Kettle, A.J.; Rosen, H.; Winterbourn, C.C.; Nauseef, W.M. Myeloperoxidase: A front-line defender against phagocytosed microorganisms. J. Leukoc. Biol. 2013, 93, 185–198. [Google Scholar] [CrossRef]
- Zhao, D.; Lim, C.-P.; Miyanaga, K.; Tanji, Y. Iodine from bacterial iodide oxidization by Roseovarius spp. inhibits the growth of other bacteria. Appl. Microbiol. Biotechnol. 2012, 97, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, P.L.; Alsagoff, S.A.L.; El-Kafrawi, H.Y.; Pyon, J.-K.; Wa, C.T.C.; Villa, M.A. Povidone iodine in wound healing: A review of current concepts and practices. Int. J. Surg. 2017, 44, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Marani, L.; Venturi, S.; Masala, R. Role of iodine in delayed immune response. Ir. J. Med. Sci. 1985, 21, 864. [Google Scholar]
- Costa, R.O.; De Macedo, P.M.; Carvalhal, A.; Bernardes-Engemann, A.R. Use of potassium iodide in Dermatology: Updates on an old drug. An. Bras. Dermatol. 2013, 88, 396–402. [Google Scholar] [CrossRef]
- Cuenca-Micó, O. Efectos del Yodo Molecular/Quimioterapia en los Patrones de Metilación de Células Inmunes Asociadas a Tumores de Cáncer Mamario. Ph.D. Thesis, UNAM Institute of Neurobiology (Instituto de Neurobiología UNAM), Juriquilla, Querétaro, Mexico, 2020. [Google Scholar]
- Luongo, D.; Bergamo, P.; Rossi, M. Effects of conjugated linoleic acid on growth and cytokine expression in Jurkat T cells. Immunol. Lett. 2003, 90, 195–201. [Google Scholar] [CrossRef]
- Doloff, J.C.; Waxman, D.J. Transcriptional profiling provides insights into metronomic cyclophosphamide-activated, innate immune-dependent regression of brain tumor xenografts. BMC Cancer 2015, 15, 375. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Q. Epigenetic Alterations in Cellular Immunity: New Insights into Autoimmune Diseases. Cell. Physiol. Biochem. 2017, 41, 645–660. [Google Scholar] [CrossRef]
- Gan, Z.; Wei, W.; Wu, J.; Zhao, Y.; Zhang, L.; Wang, T.; Gan, Z. Resveratrol and Curcumin Improve Intestinal Mucosal Integrity and Decrease m6A RNA Methylation in the Intestine of Weaning Piglets. ACS Omega 2019, 4, 17438–17446. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).