Low Protein Expression of both ATRX and ZNRF3 as Novel Negative Prognostic Markers of Adult Adrenocortical Carcinoma
Abstract
1. Introduction
2. Results
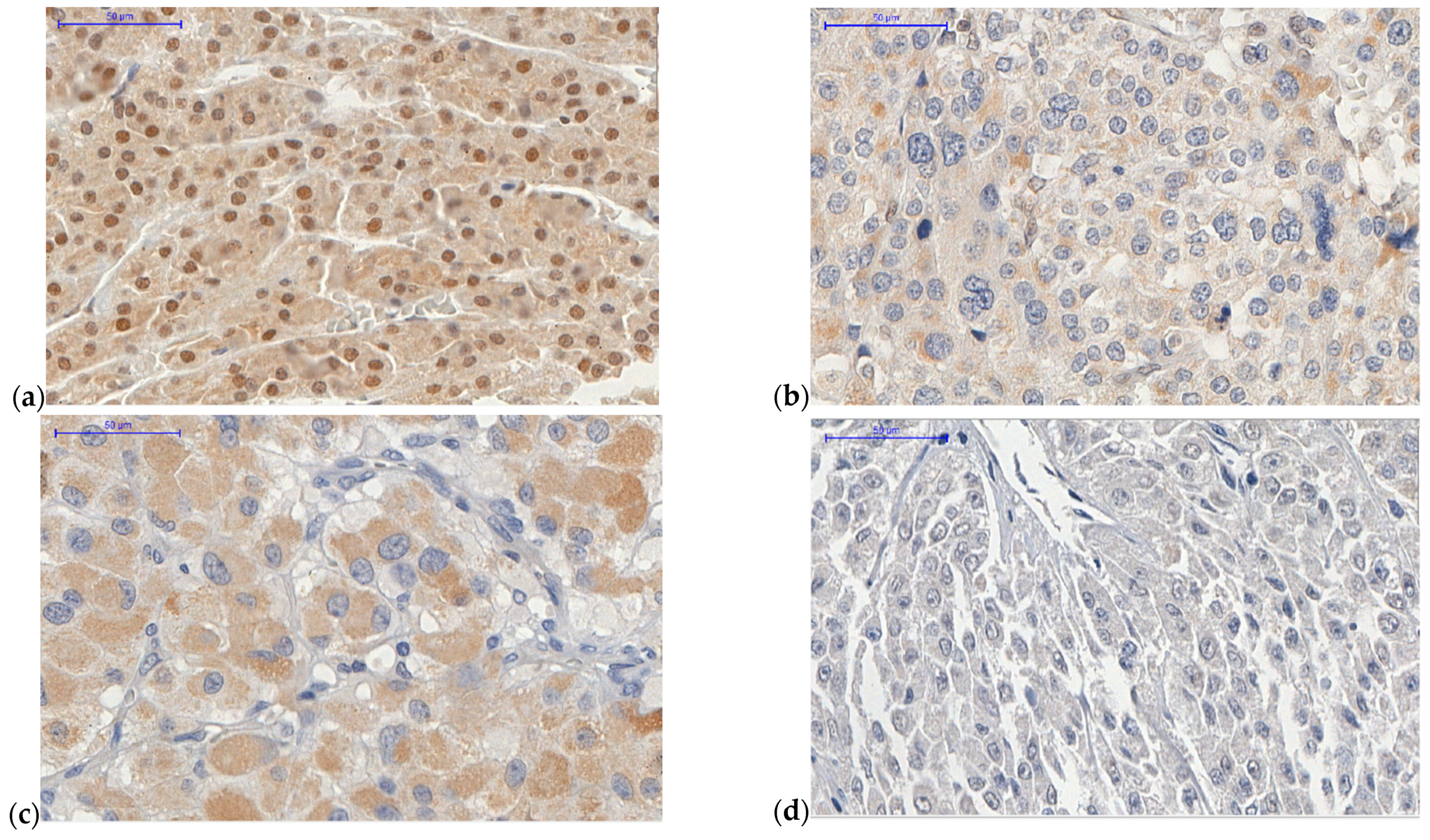
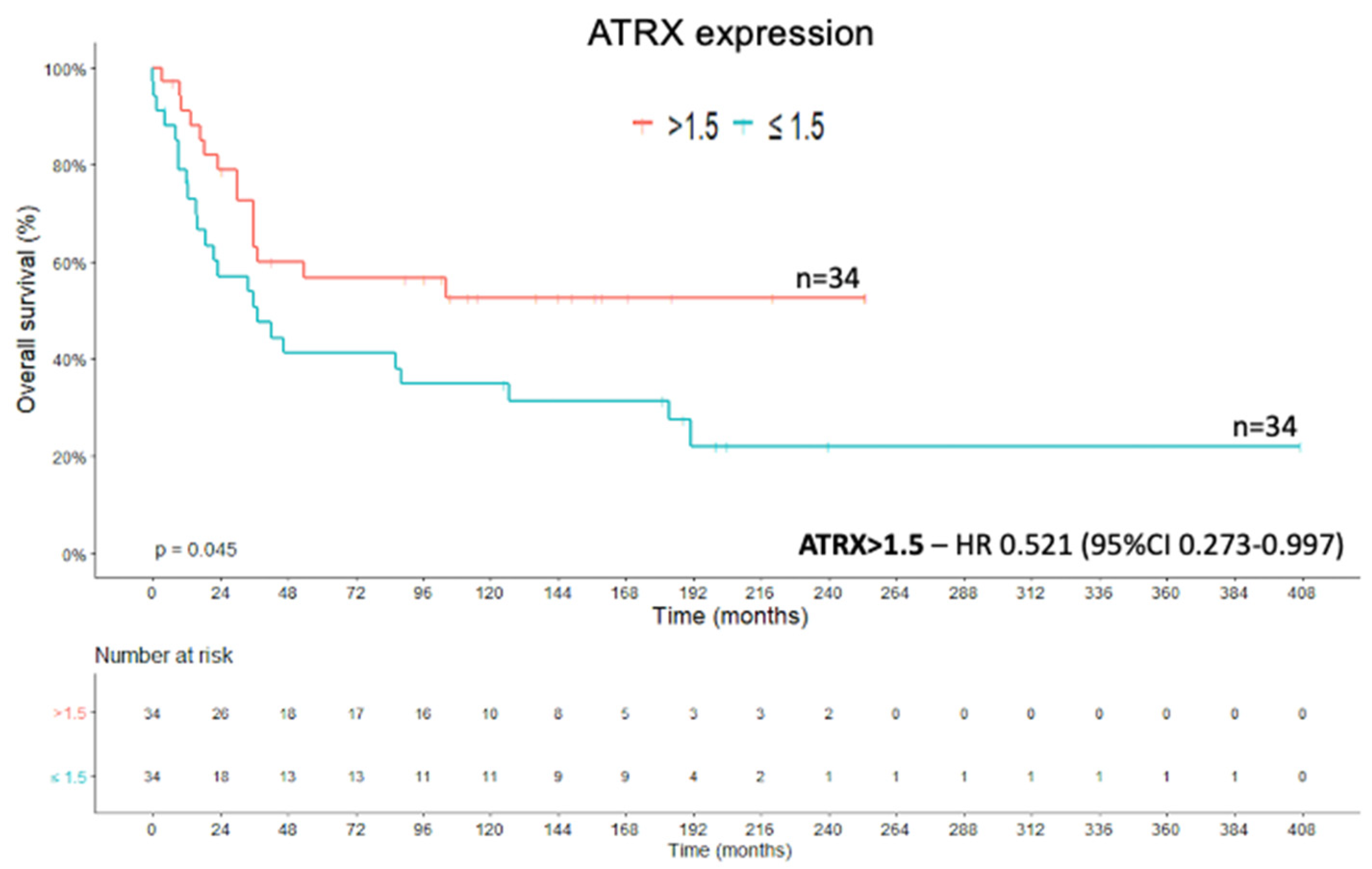
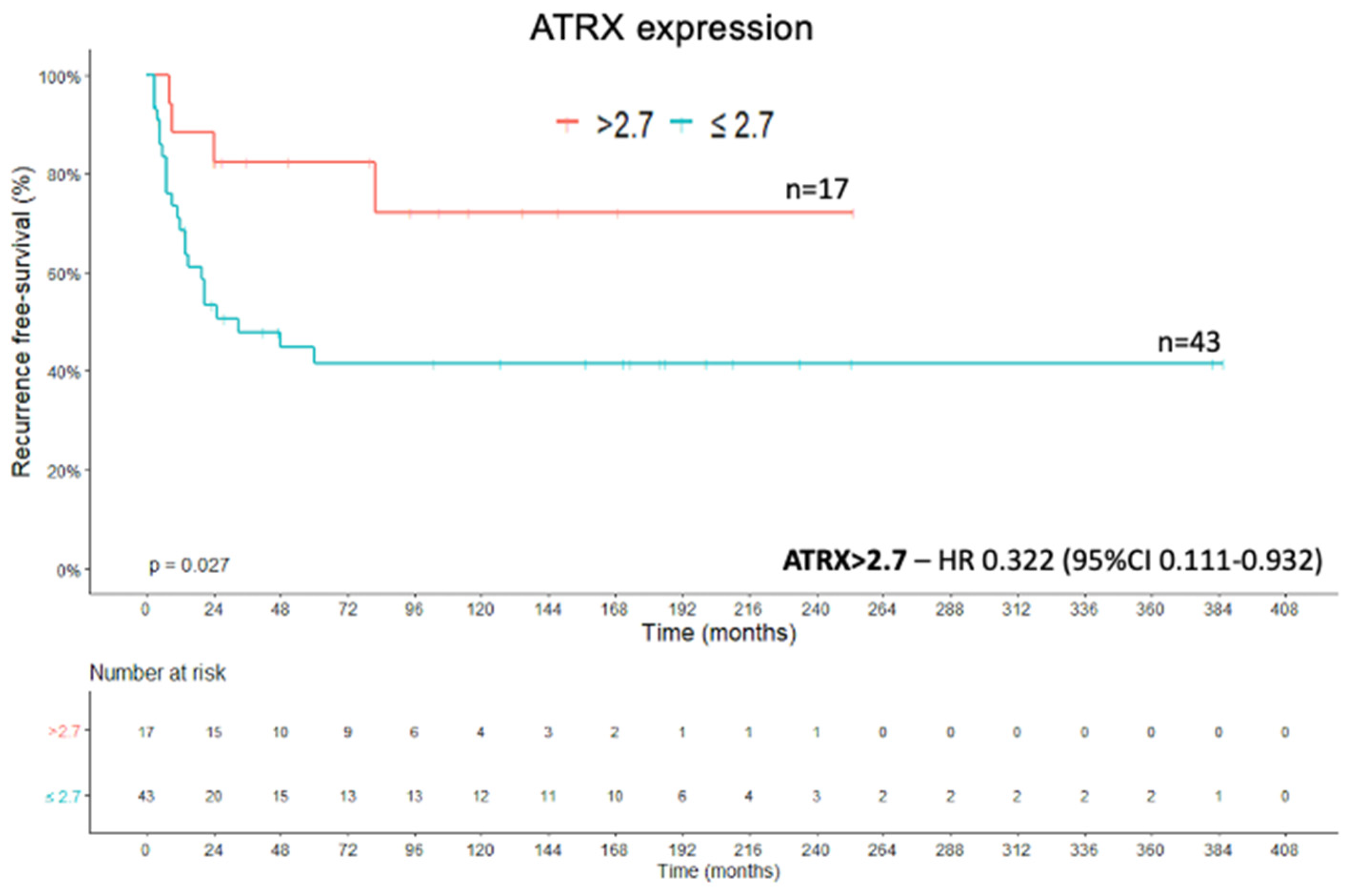
2.1. ATRX Protein
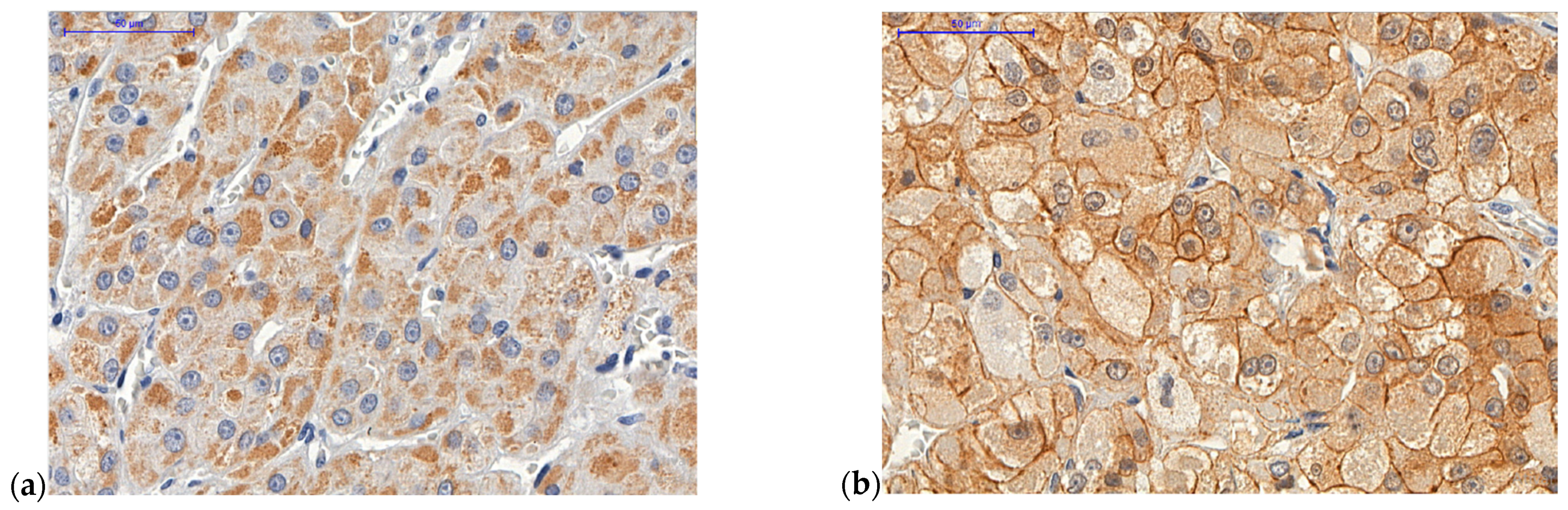
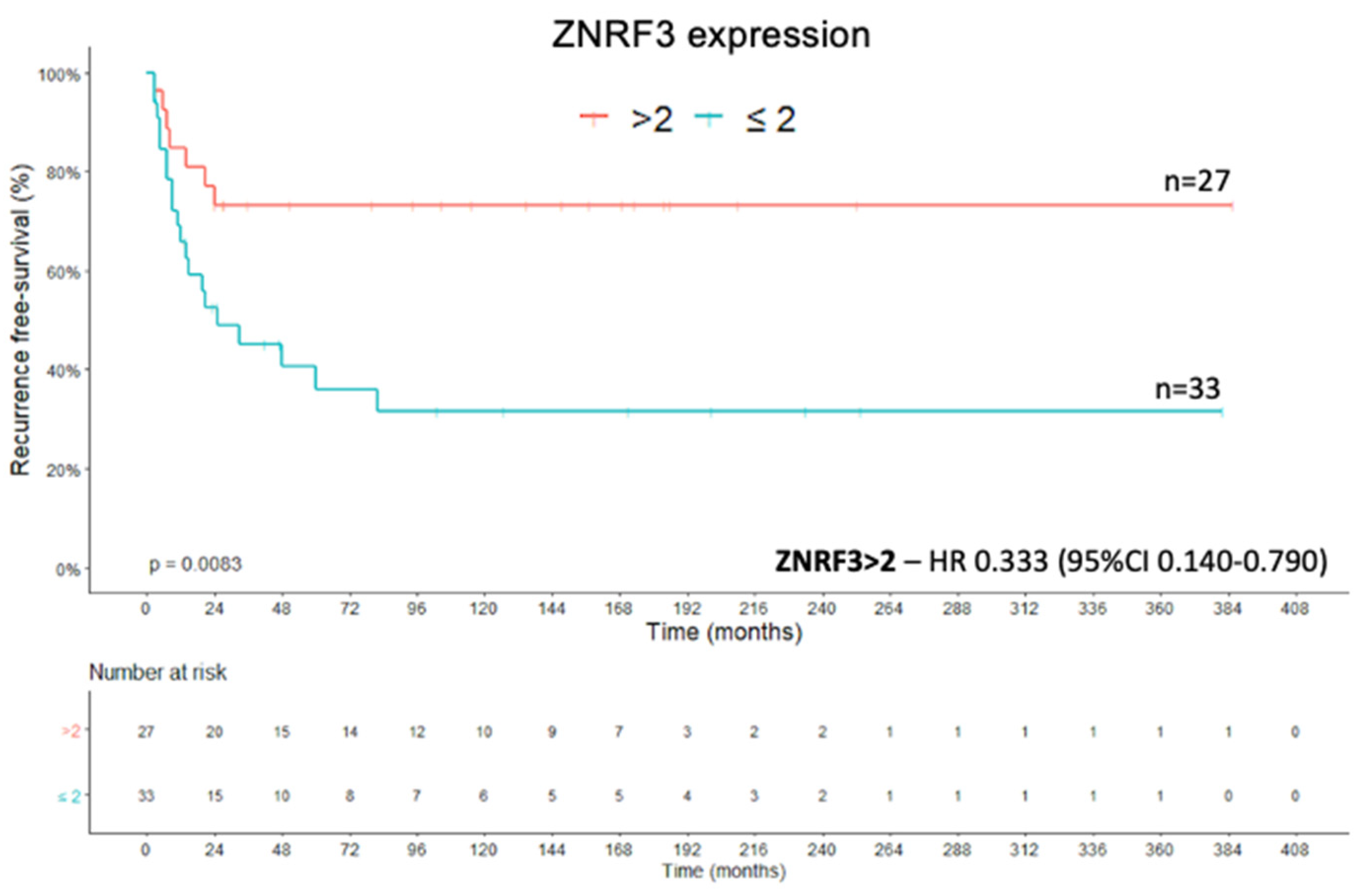
2.2. ZNRF3 Protein
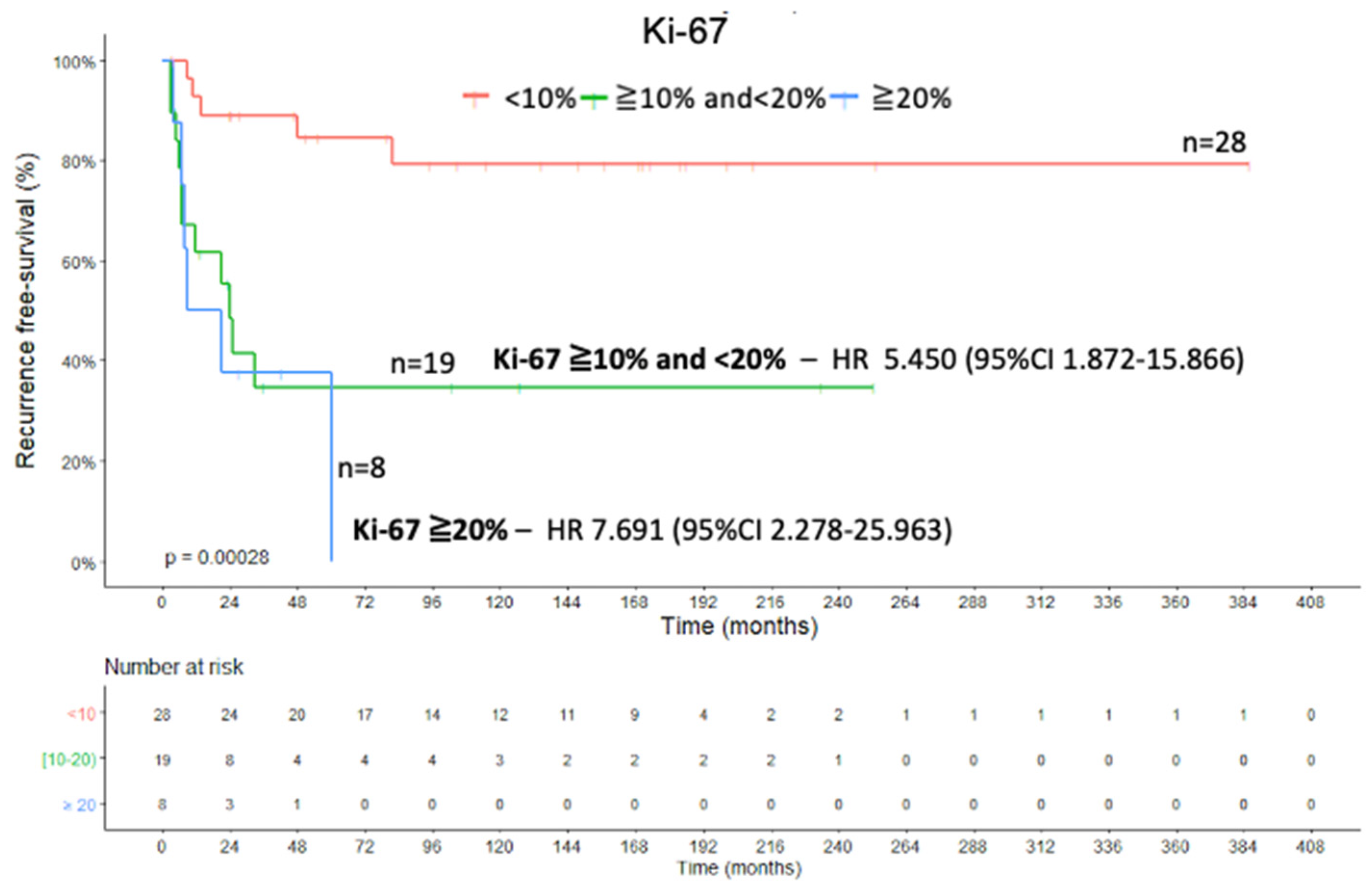
2.3. Ki-67 Proliferation Marker and Combined Analysis
2.4. Recurrence-Free Survival
2.5. Other Variables with Prognostic Value
3. Discussion
4. Materials and Methods
4.1. Tissue Microarrays (TMA) and Immunohistochemistry
Analysis of Immunohistochemistry
4.2. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dackiw, A.P.B.; Lee, J.E.; Gagel, R.F.; Evans, D.B. Adrenal cortical carcinoma. World J. Surg. 2001, 25, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Kebebew, E.; Reiff, E.; Duh, Q.Y.; Clark, O.H.; McMillan, A. Extent of disease at presentation and outcome for adrenocortical carcinoma: Have we made progress? World J. Surg. 2006, 30, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Shen, W.T.; Elaraj, D.; Bentrern, D.J.; Winchester, D.J.; Kebebew, E. Adrenocortical Carcinoma in the United States Treatment Utilization and Prognostic Factors. Cancer 2008, 113, 3130–3136. [Google Scholar] [CrossRef]
- Icard, P.; Goudet, P.; Charpenay, C.; Andreassian, B.; Carnaille, B.; Chapuis, Y.; Cougard, P.; Henry, J.-F.; Proye, C. Adrenocortical carcinomas: Surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J. Surg. 2000, 25, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Johanssen, S.; Quinkler, M.; Bucsky, P.; Willenberg, H.S.; Beuschlein, F.; Terzolo, M.; Mueller, H.-H.; Hahner, S.; Allolio, B.; et al. Limited Prognostic Value of the 2004 International Union Against Cancer Staging Classification for Adrenocortical Carcinomas. Cancer 2009, 115, 243–250. [Google Scholar] [CrossRef]
- Fassnacht, M.; Libe, R.; Kroiss, M.; Allolio, B. Adrenocortical carcinoma: A clinician’s update. Nat. Rev. Endocrinol. 2011, 7, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Terzolo, M.; Allolio, B.; Baudin, E.; Haak, H.; Berruti, A.; Welin, S.; Schade-Brittinger, C.; Lacroix, A.; Jarzab, B.; et al. Combination Chemotherapy in Advanced Adrenocortical Carcinoma. N. Engl. J. Med. 2012, 366, 2189–2197. [Google Scholar] [CrossRef]
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; De Krijger, R.R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018, 179, G1–G46. [Google Scholar] [CrossRef]
- Else, T.; Kim, A.C.; Sabolch, A.; Raymond, V.M.; Kandathil, A.; Caoili, E.M.; Jolly, S.; Miller, B.S.; Giordano, T.J.; Hammer, G.D. Adrenocortical Carcinoma. Endocr. Rev. 2014, 35, 282–326. [Google Scholar] [CrossRef]
- Weiss, L.M. Comparative histologic-study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am. J. Surg. Pathol. 1984, 8, 163–169. [Google Scholar] [CrossRef]
- Luton, J.P.; Cerdas, S.; Billaud, L.; Thomas, G.; Guilhaume, B.; Bertagna, X.; Laudat, M.-H.; Louvel, A.; Chapuis, Y.; Blondeau, P.; et al. Clinical-features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N. Engl. J. Med. 1990, 322, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, L.J.; Weiss, L.M. New developments in the pathological diagnosis of adrenal-cortical neoplasms—A review. Am. J. Clin. Pathol. 1992, 97, 73–83. [Google Scholar] [CrossRef] [PubMed]
- McNicol, A.M.; Struthers, A.J.; Nolan, C.E.; Hermans, J.; Haak, H.R. Proliferation in adrenocortical tumors: Correlation with clinical outcome and p53 status. Endocr. Pathol. 1997, 8, 29–36. [Google Scholar] [CrossRef]
- Vassilopoulou-Sellin, R.; Schultz, P.N. Adrenocortical carcinoma—Clinical outcome at the end of the 20th century. Cancer 2001, 92, 1113–1121. [Google Scholar] [CrossRef]
- Stojadinovic, A.; Ghossein, R.A.; Hoos, A.; Nissan, A.; Marshall, D.; Dudas, M.; Cordon-Cardo, C.; Jaques, D.P.; Brennan, M.F. Adrenocortical carcinoma: Clinical, morphologic, and molecular characterization. J. Clin. Oncol. 2002, 20, 941–950. [Google Scholar] [CrossRef]
- Aubert, S.; Wacrenier, A.; Leroy, X.; Devos, P.; Carnaille, B.; Proye, C.; Wemeau, J.L.; Lecomte-Houcke, M.; Leteurtre, E. Weiss system revisited—A clinicopathologic and immunohistochemical study of 49 adrenocortical tumors. Am. J. Surg. Pathol. 2002, 26, 1612–1619. [Google Scholar] [CrossRef]
- Stojadinovic, A.; Brennan, M.F.; Hoos, A.; Omeroglu, A.; Leung, D.H.Y.; Dudas, M.E.; Nissan, A.; Cordon-Cardo, C.; Ghossein, R.A. Adrenocortical adenoma and carcinoma: Histopathological and molecular comparative analysis. Mod. Pathol. 2003, 16, 742–751. [Google Scholar] [CrossRef]
- Sturgeon, C.; Shen, W.T.; Clark, O.H.; Duh, Q.Y.; Kebebew, E. Risk assessment in 457 adrenal cortical carcinomas: How much does tumor size predict the likelihood of malignancy? J. Am. Coll. Surg. 2006, 202, 423–430. [Google Scholar] [CrossRef]
- Assie, G.; Antoni, G.; Tissier, F.; Caillou, B.; Abiven, G.; Gicquel, C.; Leboulleux, S.; Travagli, J.-P.; Dromain, C.; Bertagna, X.; et al. Prognostic parameters of metastatic adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2007, 92, 148–154. [Google Scholar] [CrossRef]
- Morimoto, R.; Satoh, F.; Murakami, O.; Suzuki, T.; Abe, T.; Tanemoto, M.; Abe, M.; Urno, A.; Ishidoya, S.; Arai, Y.; et al. Immunohistochemistry of a proliferation marker Ki-67/MIB1 in adrenocortical carcinomas: Ki-67/MIB1 Labeling index is a predictor for recurrence of adrenocortical carcinomas. Endocr. J. 2008, 55, 49–55. [Google Scholar] [CrossRef]
- Lau, S.K.; Weiss, L.M. The Weiss system for evaluating adrenocortical neoplasms: 25 years later. Hum. Pathol. 2009, 40, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.Q.; Soares, I.C.; Ribeiro, T.C.; Fragoso, M.; Marins, L.V.; Wakamatsu, A.; Ressio, R.A.; Nishi, M.Y.; Jorge, A.A.L.; Lerario, A.M.; et al. Steroidogenic Factor 1 Overexpression and Gene Amplification Are More Frequent in Adrenocortical Tumors from Children than from Adults. J. Clin. Endocrinol. Metab. 2010, 95, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.; Lampron, A.; Mazzuco, T.L.; Chapman, A.; Bourdeau, I. Characterization of Differential Gene Expression in Adrenocortical Tumors Harboring β-Catenin (CTNNB1) Mutations. J. Clin. Endocrinol. Metab. 2011, 96, E1206–E1211. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux, S.; Grabar, S.; Fassnacht, M.; Ragazzon, B.; Launay, P.; Libe, R.; Chokri, I.; Audebourg, A.; Royer, B.; Sbiera, S.; et al. β-Catenin Activation Is Associated with Specific Clinical and Pathologic Characteristics and a Poor Outcome in Adrenocortical Carcinoma. Clin. Cancer Res. 2011, 17, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Ramirez, M.; Jasim, S.; Feng, L.; Ejaz, S.; Deniz, F.; Busaidy, N.; Waguespack, S.G.; Naing, A.; Sircar, K.; Wood, C.G.; et al. Adrenocortical carcinoma: Clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur. J. Endocrinol. 2013, 169, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Fassnacht, M.; Haak, H.; Else, T.; Baudin, E.; Sperone, P.; Kroiss, M.; Kerkhofs, T.; Williams, A.R.; Ardito, A.; et al. Prognostic Role of Overt Hypercortisolism in Completely Operated Patients with Adrenocortical Cancer. Eur. Urol. 2014, 65, 832–838. [Google Scholar] [CrossRef]
- Assié, G.; Letouzé, E.; Fassnacht, M.; Jouinot, A.; Luscap, W.; Barreau, O.; Omeiri, H.; Rodriguez, S.; Perlemoine, K.; René-Corail, F.; et al. Integrated genomic characterization of adrenocortical carcinoma. Nat. Genet. 2014, 46, 607–612. [Google Scholar] [CrossRef]
- Beuschlein, F.; Weigel, J.; Saeger, W.; Kroiss, M.; Wild, V.; Daffara, F.; Libé, R.; Ardito, A.; Al Ghuzlan, A.; Quinkler, M.; et al. Major Prognostic Role of Ki-67 in Localized Adrenocortical Carcinoma After Complete Resection. J. Clin. Endocrinol. Metab. 2015, 100, 841–849. [Google Scholar] [CrossRef]
- Libé, R.; Borget, I.; Ronchi, C.L.; Zaggia, B.; Kroiss, M.; Kerkhofs, T.; Bertherat, J.; Volante, M.; Quinkler, M.; Chabre, O.; et al. Prognostic factors in stage III-IV adrenocortical carcinomas (ACC): An European Network for the Study of Adrenal Tumor (ENSAT) study. Ann. Oncol. 2015, 26, 2119–2125. [Google Scholar] [CrossRef]
- Pennanen, M.; Heiskanen, I.; Sane, T.; Remes, S.; Mustonen, H.; Haglund, C.; Arola, J. Helsinki score-a novel model for prediction of metastases in adrenocortical carcinomas. Hum. Pathol. 2015, 46, 404–410. [Google Scholar] [CrossRef]
- Zheng, S.; Cherniack, A.D.; Dewal, N.; Moffitt, R.A.; Danilova, L.; Murray, B.A.; Lerario, A.M.; Else, T.; Knijnenburg, T.A.; Ciriello, G.; et al. Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell. 2016, 29, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Mete, O.; Gucer, H.; Kefeli, M.; Asa, S.L. Diagnostic and Prognostic Biomarkers of Adrenal Cortical Carcinoma. Am. J. Surg. Pathol. 2018, 42, 201–213. [Google Scholar] [CrossRef]
- Mete, O.; Asa, S.L.; Giordano, T.J.; Papotti, M.; Sasano, H.; Volante, M. Immunohistochemical Biomarkers of Adrenal Cortical Neoplasms. Endocr. Pathol. 2018, 29, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Libe, R. Clinical and molecular prognostic factors in adrenocortical carcinoma. Minerva Endocrinol. 2019, 44, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Thomas, A.J.; Ganeshan, D.M.; Blair, K.J.; Lall, C.; Lee, J.T.; Morshid, A.I.; Habra, M.A.; Elsayes, K.M. Adrenal cortical carcinoma: Pathology, genomics, prognosis, imaging features, and mimics with impact on management. Abdom. Radiol. 2020, 45, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.M.; Chen, X.; Easton, J.; Finkelstein, D.; Liu, Z.; Pounds, S.; Rodriguez-Galindo, C.; Lund, T.C.; Mardis, E.R.; Wilson, R.K.; et al. Genomic landscape of paediatric adrenocortical tumours. Nat. Commun. 2015, 6, 10. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Program. Available online: https://www.cancer.gov/tcga (accessed on 3 January 2021).
- Giles, R.H.; van Es, J.H.; Clevers, H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta 2003, 1653, 1–24. [Google Scholar] [CrossRef]
- Lacombe, A.M.F.; Soares, I.C.; Mariani, B.M.D.P.; Nishi, M.Y.; Bezerra-Neto, J.E.; Charchar, H.D.S.; Brondani, V.B.; Tanno, F.Y.; Srougi, V.; Chambo, J.L.; et al. Sterol O-Acyl Transferase 1 as a Prognostic Marker of Adrenocortical Carcinoma. Cancers 2020, 12, 247. [Google Scholar] [CrossRef]
- Fearon, E.R.; Spence, J.R. Cancer Biology: A New RING to Wnt Signaling. Curr. Biol. 2012, 22, R849–R851. [Google Scholar] [CrossRef][Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Cesare, A.J.; Reddel, R.R. Alternative lengthening of telomeres: Models, mechanisms and implications. Nat. Rev. Genet. 2010, 11, 319–330. [Google Scholar] [CrossRef]
- Watson, J.D. Origin of concatemeric T7 DNA. Nat. New Biol. 1972, 239, 197. [Google Scholar] [CrossRef]
- Olovnikov, A.M. Theory of marginotomy—Incomplete copying of template margin in enzymic-synthesis of polynucleotides and biological significance of phenomenon. J. Theor. Biol. 1973, 41, 181–190. [Google Scholar] [CrossRef]
- Shay, J.W. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov. 2016, 6, 584–593. [Google Scholar] [CrossRef]
- Der-Sarkissian, H.; Bacchetti, S.; Cazes, L.; Londono-Vallejo, J.A. The shortest telomeres drive karyotype evolution in transformed cells. Oncogene 2004, 23, 1221–1228. [Google Scholar] [CrossRef]
- Watson, L.A.; Goldberg, H.; Berube, N.G. Emerging roles of ATRX in cancer. Epigenomics 2015, 7, 1365–1378. [Google Scholar] [CrossRef]
- Clynes, D.; Higgs, D.R.; Gibbons, R.J. The chromatin remodeller ATRX: A repeat offender in human disease. Trends Biochem. Sci. 2013, 38, 461–466. [Google Scholar] [CrossRef]
- Drane, P.; Ouararhni, K.; Depaux, A.; Shuaib, M.; Hamiche, A. The death-associated protein DAXX is a novel histone chaperone involved in the replication-independent deposition of H3.3. Genes Dev. 2010, 24, 1253–1265. [Google Scholar] [CrossRef]
- Elsasser, S.J.; Huang, H.D.; Lewis, P.W.; Chin, J.W.; Allis, C.D.; Patel, D.J. DAXX envelops a histone H3.3-H4 dimer for H3.3-specific recognition. Nature 2012, 491, 560. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.D.; Banaszynski, L.A.; Noh, K.-M.; Lewis, P.W.; Elsaesser, S.J.; Stadler, S.; Dewell, S.; Law, M.; Guo, X.; Li, X.; et al. Distinct Factors Control Histone Variant H3.3 Localization at Specific Genomic Regions. Cell 2010, 140, 678–691. [Google Scholar] [CrossRef]
- Lovejoy, C.A.; Li, W.; Reisenweber, S.; Thongthip, S.; Bruno, J.; De Lange, T.; De, S.; Petrini, J.H.J.; Sung, P.A.; Jasin, M.; et al. Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway. PLoS Genet. 2012, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Bower, K.; Napier, C.E.; Cole, S.L.; Dagg, R.A.; Lau, L.M.S.; Duncan, E.L.; Moy, E.L.; Reddel, R.R. Loss of Wild-Type ATRX Expression in Somatic Cell Hybrids Segregates with Activation of Alternative Lengthening of Telomeres. PLoS ONE 2012, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Barthel, F.P.; Wei, W.; Tang, M.; Martinez-Ledesma, E.; Hu, X.; Amin, S.B.; Akdemir, K.C.; Seth, S.; Song, X.; Wang, Q.; et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat. Genet. 2017, 49, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Wiestler, B.; Capper, D.; Holland-Letz, T.; Von Deimling, A.; Pfister, S.M.; Platten, M.; Weller, M.; Wick, W. ATRX loss refines the classification of anaplastic glioma and is a favorable prognostic marker. Cancer Res. 2013, 73, 1. [Google Scholar]
- Marinoni, I.; Kurrer, A.S.; Vassella, E.; Dettmer, M.; Rudolph, T.; Banz, V.M.; Hunger, F.; Pasquinelli, S.; Speel, E.; Perren, A. Loss of DAXX and ATRX Are Associated With Chromosome Instability and Reduced Survival of Patients With Pancreatic Neuroendocrine Tumors. Gastroenterology 2014, 146, 453. [Google Scholar] [CrossRef] [PubMed]
- Liau, J.-Y.; Lee, J.-C.; Tsai, J.-H.; Yang, C.-Y.; Liu, T.-L.; Ke, Z.-L.; Hsu, H.-H.; Jeng, Y.-M. Comprehensive screening of alternative lengthening of telomeres phenotype and loss of ATRX expression in sarcomas. Mod. Pathol. 2015, 28, 1545–1554. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Liau, J.-Y.; Huang, W.-J.; Chang, Y.-T.; Chang, M.-C.; Lee, J.-C.; Tsai, J.-H.; Su, Y.-N.; Hung, C.-C.; Jeng, Y.-M. Targeted next-generation sequencing of cancer genes identified frequent TP53 and ATRX mutations in leiomyosarcoma. Am. J. Transl. Res. 2015, 7, 2072–2081. [Google Scholar]
- Cai, J.; Chen, J.; Zhang, W.; Yang, P.; Zhang, C.; Li, M.; Yao, K.; Wang, H.; Li, Q.; Jiang, C.; et al. Loss of ATRX, associated with DNA methylation pattern of chromosome end, impacted biological behaviors of astrocytic tumors. Oncotarget 2015, 6, 18105–18115. [Google Scholar] [CrossRef][Green Version]
- Cai, J.; Zhu, P.; Zhang, C.; Li, Q.; Wang, Z.; Li, G.; Wang, G.; Yang, P.; Li, J.; Han, B.; et al. Detection of ATRX and IDH1-R132H immunohistochemistry in the progression of 211 paired gliomas. Oncotarget 2016, 7, 16384–16395. [Google Scholar] [CrossRef]
- Singhi, A.D.; Liu, T.-C.; Roncaioli, J.L.; Cao, D.; Zeh, H.J.; Zureikat, A.H.; Tsung, A.; Marsh, J.W.; Lee, K.K.; Hogg, M.E.; et al. Alternative Lengthening of Telomeres and Loss of DAXX/ATRX Expression Predicts Metastatic Disease and Poor Survival in Patients with Pancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2017, 23, 600–609. [Google Scholar] [CrossRef]
- Ren, X.L.; Tu, C.; Tang, Z.C.; Ma, R.F.; Li, Z.H. Alternative lengthening of telomeres phenotype and loss of ATRX expression in sarcomas (Review). Oncol. Lett. 2018, 15, 7489–7496. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.; Itchins, M.; De Reuver, P.R.; Arena, J.; Clarkson, A.; Sheen, A.; Sioson, L.; Cheung, V.; Perren, A.; Nahm, C.; et al. ATRX loss is an independent predictor of poor survival in pancreatic neuroendocrine tumors. Hum. Pathol. 2018, 82, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Apicelli, A.J.; Eulo, V.; Pekmezci, M.; Hirbe, A.C.; Dahiya, S. Aberrant ATRX Protein Expression is Associated with Worse Survival in NF1-Associated Malignant Peripheral Nerve Sheath Tumors. Mod. Pathol. 2018, 31, 661. [Google Scholar]
- Terra, S.; Xie, H.; Boland, J.M.; Mansfield, A.S.; Molina, J.R.; Roden, A.C. Loss of ATRX expression predicts worse prognosis in pulmonary carcinoid tumors. Hum. Pathol. 2019, 94, 78–85. [Google Scholar] [CrossRef]
- Hong, X.; Qiao, S.; Li, F.; Wang, W.; Jiang, R.; Wu, H.; Chen, H.; Liu, L.; Peng, J.; Wang, J.; et al. Whole-genome sequencing reveals distinct genetic bases for insulinomas and non-functional pancreatic neuroendocrine tumours: Leading to a new classification system. Gut 2020, 69, 877–887. [Google Scholar] [CrossRef]
- Klaus, A.; Birchmeier, W. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 2008, 8, 387–398. [Google Scholar] [CrossRef]
- Hao, H.-X.; Xie, Y.; Zhang, Y.; Charlat, O.; Oster, E.; Avello, M.; Lei, H.; Mickanin, C.; Liu, D.; Ruffner, H.; et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nat. Cell Biol. 2012, 485, 195–200. [Google Scholar] [CrossRef]
- Basham, K.J.; Rodriguez, S.; Turcu, A.F.; Lerario, A.M.; Logan, C.Y.; Rysztak, M.R.; Gomez-Sanchez, C.E.; Breault, D.T.; Koo, B.K.; Clevers, H.; et al. A ZNRF3-dependent Wnt/β-catenin signaling gradient is required for adrenal homeostasis. Genes. Dev. 2019, 33, 209–220. [Google Scholar] [CrossRef]
- Zhou, Y.; Lan, J.; Wang, W.; Shi, Q.; Lan, Y.; Cheng, Z.; Guan, H. ZNRF3 acts as a tumour suppressor by the Wnt signalling pathway in human gastric adenocarcinoma. J. Mol. Histol. 2013, 44, 555–563. [Google Scholar] [CrossRef]
- Qin, H.Z.; Cai, A.Z.; Xi, H.Q.; Yuan, J.; Chen, L. ZNRF3 Induces Apoptosis of Gastric Cancer Cells by Antagonizing Wnt and Hedgehog Signaling. Cell Biochem. Biophys. 2015, 73, 361–367. [Google Scholar] [CrossRef]
- Yu, N.; Zhu, H.; Tao, Y.; Huang, Y.; Song, X.; Zhou, Y.; Li, Y.; Pei, Q.; Tan, Q.; Pei, H. Association between prognostic survival of human colorectal carcinoma and ZNRF3 expression. Onco Targets Ther. 2016, 9, 6679–6687. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bond, C.E.; McKeone, D.M.; Kalimutho, M.; Bettington, M.L.; Pearson, S.-A.; Dumenil, T.D.; Wockner, L.F.; Burge, M.; Leggett, B.A.; Whitehall, V.L. RNF43 and ZNRF3 are commonly altered in serrated pathway colorectal tumorigenesis. Oncotarget 2016, 7, 70589–70600. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.W.; Yang, Z.L.; Fan, Y.B.; Zheng, Q. ZNRF3 is downregulated in papillary thyroid carcinoma and suppresses the proliferation and invasion of papillary thyroid cancer cells. Tumor Biol. 2016, 37, 12665–12672. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.C.; Jiang, X.G.; Yu, Z.J.; He, G.Q.; Ning, H.Y.; Wu, Z.X.; Cai, Y.; Yu, H.; Chen, A. ZNRF3 contributes to the growth of lung carcinoma via inhibiting Wnt/β-catenin pathway and is regulated by miR-93. Tumor Biol. 2016, 37, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Zhao, J.; Ma, J.; Wang, C.; Zhang, C.; Jiang, H.; Cheng, J.; Gao, R.; Zhou, X. miR-146b-5p promotes invasion and metastasis contributing to chemoresistance in osteosarcoma by targeting zinc and ring finger 3. Oncol. Rep. 2016, 35, 275–283. [Google Scholar] [CrossRef]
- Wang, Z.W.; Wang, Y.L.; Ren, H.T.; Jin, Y.Y.; Guo, Y. ZNRF3 Inhibits the Invasion and Tumorigenesis in Nasopharyngeal Carcinoma Cells by Inactivating the Wnt/β-Catenin Pathway. Oncol. Res. 2017, 25, 571–577. [Google Scholar] [CrossRef]
- Qiao, G.E.; Dai, C.G.; He, Y.; Shi, J.J.; Xu, C.F. Effects of miR-106b-3p on cell proliferation and epithelial-mesenchymal transition, and targeting of ZNRF3 in esophageal squamous cell carcinoma. Int. J. Mol. Med. 2019, 43, 1817–1829. [Google Scholar] [CrossRef]
- Berbegall, A.P.; Villamen, E.; Tadeo, I.; Martinsson, T.; Canete, A.; Castel, V.; Navarro, S.; Noguera, R. Neuroblastoma after Childhood: Prognostic Relevance of Segmental Chromosome Aberrations, ATRX Protein Status, and Immune Cell Infiltration. Neoplasia 2014, 16, 471–480. [Google Scholar] [CrossRef]
- Davidson, B.; McFadden, E.; Holth, A.; Brunetti, M.; Florenes, V.A. Death domain-associated protein (DAXX) expression is associated with poor survival in metastatic high-grade serous carcinoma. Virchows Arch. 2020, 477, 857–864. [Google Scholar] [CrossRef]
- Berruti, A.; Terzolo, M.; Sperone, P.; Pia, A.; Della Casa, S.; Gross, D.J.; Carnaghi, C.; Casali, P.; Porpiglia, F.; Mantero, F.; et al. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: A large prospective phase II trial. Endocr. Relat. Cancer 2005, 12, 657–666. [Google Scholar] [CrossRef]
- Abiven, G.; Coste, J.; Groussin, L.; Anract, P.; Tissier, F.; Legmann, P.; Dousset, B.; Bertagna, X.; Bertherat, J. Clinical and biological features in the prognosis of adrenocortical cancer: Poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J. Clin. Endocrinol. Metab. 2006, 91, 2650–2655. [Google Scholar] [CrossRef] [PubMed]
- Margonis, G.A.; Kim, Y.; Tran, T.B.; Postlewait, L.M.; Maithel, S.K.; Wang, T.S.; Glenn, J.A.; Hatzaras, I.; Shenoy, R.; Phay, J.E.; et al. Outcomes after resection of cortisol-secreting adrenocortical carcinoma. Am. J. Surg. 2016, 211, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Vanbrabant, T.; Fassnacht, M.; Assie, G.; Dekkers, O.M. Influence of hormonal functional status on survival in adrenocortical carcinoma: Systematic review and meta-analysis. Eur. J. Endocrinol. 2018, 179, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Clements, W.M.; Wang, J.; Sarnaik, A.; Kim, O.J.; MacDonald, J.; Fenoglio-Preiser, C.; Groden, J.; Lowy, A.M. β-catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002, 62, 3503–3506. [Google Scholar]
- De La Coste, A.; Romagnolo, B.; Billuart, P.; Renard, C.A.; Buendia, M.A.; Soubrane, O.; Fabre, M.; Chelly, J.; Beldjord, C.; Khan, A.; et al. Somatic mutations of the β-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc. Natl. Acad. Sci. USA 1998, 95, 8847–8851. [Google Scholar] [CrossRef]
- Hao, H.-X.; Jiang, X.; Cong, F. Control of Wnt Receptor Turnover by R-spondin-ZNRF3/RNF43 Signaling Module and Its Dysregulation in Cancer. Cancers 2016, 8, 25. [Google Scholar] [CrossRef]
- Faillot, S.; Assie, G. The genomics of adrenocortical tumors. Eur. J. Endocrinol. 2016, 174, R249–R265. [Google Scholar] [CrossRef]
- Espiard, S.; Bertherat, J. The genetics of adrenocortical tumors. Endocrinol. Metab. Clin. N. Am. 2015, 44, 311–334. [Google Scholar] [CrossRef]
- Tissier, F.; Cavard, C.; Groussin, L.; Perlemoine, K.; Fumey, G.; Hagnere, A.M.; Rene-Corail, F.; Jullian, E.; Gicquel, C.; Bertagna, X.; et al. Mutations of β-catenin in adrenocortical tumors: Activation of the wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005, 65, 7622–7627. [Google Scholar] [CrossRef]
- Gomes, D.C.; Leal, L.F.; Mermejo, L.M.; Scrideli, C.A.; Martinelli, C.E.; Fragoso, M.C.B.V.; Latronico, A.C.; Tone, L.G.; Tucci, S.; Yunes, J.A.; et al. Sonic Hedgehog Signaling Is Active in Human Adrenal Cortex Development and Deregulated in Adrenocortical Tumors. J. Clin. Endocrinol. Metab. 2014, 99, E1209–E1216. [Google Scholar] [CrossRef]
- Terzolo, M.; Boccuzzi, A.; Bovio, S.; Cappia, S.; De Giuli, P.; Alì, A.; Paccotti, P.; Porpiglia, F.; Fontana, D.; Angeli, A. Immunohistochemical assessment of Ki-67 in the differential diagnosis of adrenocortical tumors. Urology 2001, 57, 176–182. [Google Scholar] [CrossRef]
- Sasano, H.; Suzuki, T.; Moriya, T. Recent advances in histopathology and immunohistochemistry of adrenocortical carcinoma. Endocr. Pathol. 2007, 17, 345–354. [Google Scholar] [CrossRef]
- Xie, Y.; Tan, Y.; Yang, C.; Zhang, X.; Xu, C.; Qiao, X.; Xu, J.; Tian, S.; Fang, C.; Kang, C. Omics-based integrated analysis identified ATRX as a biomarker associated with glioma diagnosis and prognosis. Cancer Biol. Med. 2019, 16, 784–796. [Google Scholar]
- Ikemura, M.; Shibahara, J.; Mukasa, A.; Takayanagi, S.; Aihara, K.; Saito, N.; Aburatani, H.; Fukayama, M. Utility of ATRX immunohistochemistry in diagnosis of adult diffuse gliomas. Histopathology 2016, 69, 260–267. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Gerges, N.; Korshunov, A.; Sabha, N.; Khuong-Quang, D.-A.; Fontebasso, A.M.; Fleming, A.; Hadjadj, D.; Schwartzentruber, J.; Majewski, J.; et al. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012, 124, 615–625. [Google Scholar] [CrossRef]
- Duregon, E.; Volante, M.; Bollito, E.; Goia, M.; Buttigliero, C.; Zaggia, B.; Berruti, A.; Scagliotti, G.V.; Papotti, M. Pitfalls in the diagnosis of adrenocortical tumors: A lesson from 300 consultation cases. Hum. Pathol. 2015, 46, 1799–1807. [Google Scholar] [CrossRef]
- Faria, A.M.; Sbiera, S.; Ribeiro, T.C.; Soares, I.C.; Mariani, B.M.; Freire, D.S.; De Sousa, G.R.; Lerário, A.M.; Ronchi, C.L.; Deutschbein, T.; et al. Expression of LIN28 and its regulatory microRNAs in adult adrenocortical cancer. Clin. Endocrinol. 2015, 82, 481–488. [Google Scholar] [CrossRef]
- De Sousa, G.R.V.; Soares, I.C.; Faria, A.M.; Domingues, V.B.; Wakamatsu, A.; Lerário, A.M.; Alves, V.A.F.; Zerbini, M.C.N.; De Mendonça, B.B.; Fragoso, M.C.B.V.; et al. DAX1 Overexpression in Pediatric Adrenocortical Tumors: A Synergic Role with SF1 in Tumorigenesis. Horm. Metab. Res. 2015, 47, 656–661. [Google Scholar] [CrossRef]
- De Sousa, G.R.V.; Ribeiro, T.C.; Faria, A.M.; Mariani, B.M.P.; Lerario, A.M.; Zerbini, M.C.N.; Soares, I.C.; Wakamatsu, A.; Alves, V.A.F.; Mendonca, B.B.; et al. Low DICER1 expression is associated with poor clinical outcome in adrenocortical carcinoma. Oncotarget 2015, 6, 22724–22733. [Google Scholar] [CrossRef]
- Shi, S.R.; Key, M.E.; Kalra, K.L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues—An enhancement method for immunohistochemical staining based on microwave-oven heating of tissue-sections. J. Histochem. Cytochem. 1991, 39, 741–748. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. Measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Granja, S.; Longatto, A.; Faria, A.M.; Fragoso, M.; Lovisolo, S.M.; Bonatelli, M.; Costa, R.F.A.; Lerario, A.M.; Almeida, M.Q.; et al. GLUT1 expression in pediatric adrenocortical tumors: A promising candidate to predict clinical behavior. Oncotarget 2017, 8, 63835–63845. [Google Scholar] [CrossRef] [PubMed]
- Heaton, J.H.; Wood, M.A.; Kim, A.C.; Lima, L.O.; Barlaskar, F.M.; Almeida, M.Q.; Fragoso, M.C.B.V.; Kuick, R.; Lerario, A.M.; Simon, D.P.; et al. Progression to Adrenocortical Tumorigenesis in Mice and Humans through Insulin-Like Growth Factor 2 and β-Catenin. Am. J. Pathol. 2012, 181, 1017–1033. [Google Scholar] [CrossRef]
- Tadjine, M.; Lampron, A.; Ouadi, L.; Horvath, A.; Stratakis, C.A.; Bourdeau, I. Detection of somatic β-catenin mutations in primary pigmented nodular adrenocortical disease (PPNAD). Clin. Endocrinol. 2008, 69, 367–373. [Google Scholar] [CrossRef]
- Lausen, B.; Schumacher, M. Maximally selected rank statistics. Biometrics 1992, 48, 73–85. [Google Scholar] [CrossRef]
- Cox, D.R. Regression models and life-tables. J. R. Stat. Soc. 1972, 34, 187. [Google Scholar] [CrossRef]
- Schoenfeld, D. Partial residuals for the proportional hazards regression-model. Biometrika 1982, 69, 239–241. [Google Scholar] [CrossRef]




| Variables | Parameters | Values—N (%) |
|---|---|---|
| Gender | Female | 63 (76.8%) |
| Male | 19 (23.2%) | |
| Age | Median | 38.17 y |
| Average | 42.05 y | |
| Range | 15.38–85.46 y | |
| Clinical presentation | Hypercortisolism | 51 (66.2%) |
| No hypercortisolism | 10 (13%) | |
| Silent tumor | 16 (20.8%) | |
| Not available | 5 | |
| ENSAT * staging system | 1 | 9 (11.2%) |
| 2 | 36 (44.4%) | |
| 3 | 18 (22.2%) | |
| 4 | 18 (22.2%) | |
| Not available | 1 | |
| Tumor weight | Median | 445 g |
| Average | 707.46 g | |
| Range | 10–2600 g | |
| Tumor size | Median | 11 cm |
| Average | 11.59 cm | |
| Range | 2.2–23 cm | |
| Weiss system | 3 | 15 (19.5%) |
| 4 | 16 (20.8%) | |
| 5 | 6 (7.8%) | |
| 6 | 12 (15.6%) | |
| 7 | 11 (14.3%) | |
| 8 | 15 (19.5%) | |
| 9 | 2 (2.5%) | |
| Modified Weiss system | <3 | 15 (22.4%) |
| 3 | 13 (19.4%) | |
| 4 | 6 (8.9%) | |
| 5 | 11 (16.4%) | |
| 6 | 8 (11.9%) | |
| 7 | 14 (20.8%) | |
| Not available | 15 | |
| Helsinki system | ≤8.5 | 21 (32.3%) |
| >8.5 | 44 (67.7%) | |
| Not available | 17 | |
| Ki-67 proliferation marker | <10% | 31 (43%) |
| ≥10 and <20% | 26 (36.1%) | |
| ≥20% | 15 (20.9%) | |
| Not available | 10 | |
| Outcome | Death | 39 |
| Alive | 31 | |
| Not available | 12 | |
| Global survival | Median | 39.58 months |
| Average | 85.35 months | |
| Range | 1.4–406.83 months |
| Protein Expression | Compartment of ACC Cells | Parameters | Values | Description |
|---|---|---|---|---|
| ATRX | Nuclear | Extent | 0 | No immunoreactive cells |
| 1 | <25% immunoreactive cells | |||
| 2 | 25–50% immunoreactive cells | |||
| 3 | 51–75% immunoreactive cells | |||
| 4 | >75% immunoreactive cells | |||
| ZNRF3 | Cytoplasmic | Extent | 0 | No immunoreactive cells |
| 1 | <25% immunoreactive cells | |||
| 2 | 25–50% immunoreactive cells | |||
| 3 | 51–75% immunoreactive cells | |||
| 4 | >75% immunoreactive cells | |||
| Intensity | 0 | Negative | ||
| 1 | Weak | |||
| 2 | Intermediate | |||
| 3 | Strong |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brondani, V.B.; Lacombe, A.M.F.; Mariani, B.M.d.P.; Montenegro, L.; Soares, I.C.; Bezerra-Neto, J.E.; Tanno, F.Y.; Srougi, V.; Chambo, J.L.; Mendonca, B.B.; et al. Low Protein Expression of both ATRX and ZNRF3 as Novel Negative Prognostic Markers of Adult Adrenocortical Carcinoma. Int. J. Mol. Sci. 2021, 22, 1238. https://doi.org/10.3390/ijms22031238
Brondani VB, Lacombe AMF, Mariani BMdP, Montenegro L, Soares IC, Bezerra-Neto JE, Tanno FY, Srougi V, Chambo JL, Mendonca BB, et al. Low Protein Expression of both ATRX and ZNRF3 as Novel Negative Prognostic Markers of Adult Adrenocortical Carcinoma. International Journal of Molecular Sciences. 2021; 22(3):1238. https://doi.org/10.3390/ijms22031238
Chicago/Turabian StyleBrondani, Vania Balderrama, Amanda Meneses Ferreira Lacombe, Beatriz Marinho de Paula Mariani, Luciana Montenegro, Iberê Cauduro Soares, João Evangelista Bezerra-Neto, Fabio Yoshiaki Tanno, Victor Srougi, José Luis Chambo, Berenice Bilharinho Mendonca, and et al. 2021. "Low Protein Expression of both ATRX and ZNRF3 as Novel Negative Prognostic Markers of Adult Adrenocortical Carcinoma" International Journal of Molecular Sciences 22, no. 3: 1238. https://doi.org/10.3390/ijms22031238
APA StyleBrondani, V. B., Lacombe, A. M. F., Mariani, B. M. d. P., Montenegro, L., Soares, I. C., Bezerra-Neto, J. E., Tanno, F. Y., Srougi, V., Chambo, J. L., Mendonca, B. B., Almeida, M. Q., Zerbini, M. C. N., & Fragoso, M. C. B. V. (2021). Low Protein Expression of both ATRX and ZNRF3 as Novel Negative Prognostic Markers of Adult Adrenocortical Carcinoma. International Journal of Molecular Sciences, 22(3), 1238. https://doi.org/10.3390/ijms22031238







