Synthesis and Spectral Characteristics Investigation of the 2D-2D vdWs Heterostructure Materials
Abstract
1. Introduction
2. Results and Discussion
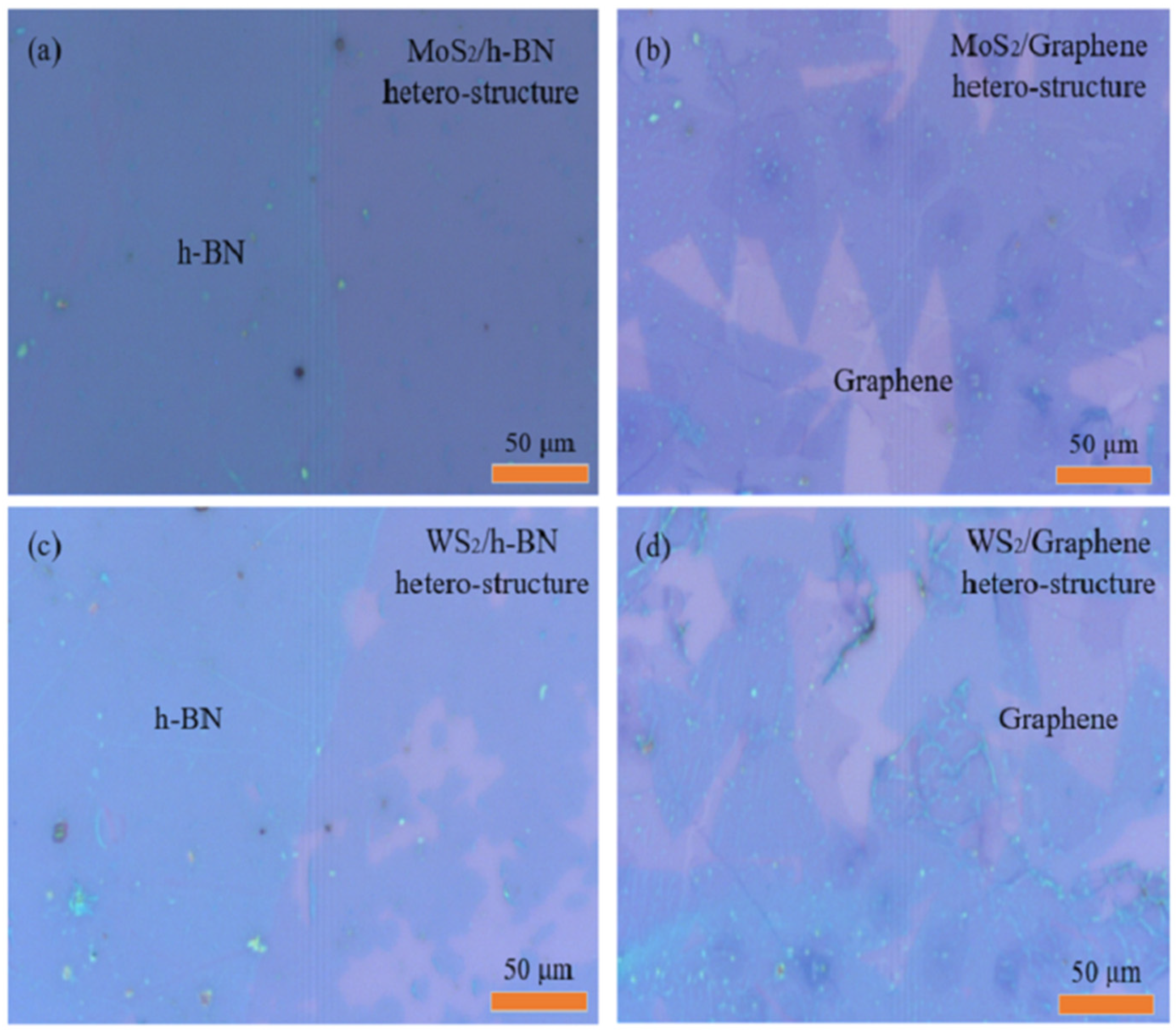
2.1. Optical Micrographs of 2D-2D vdWs Heterostructure Materials
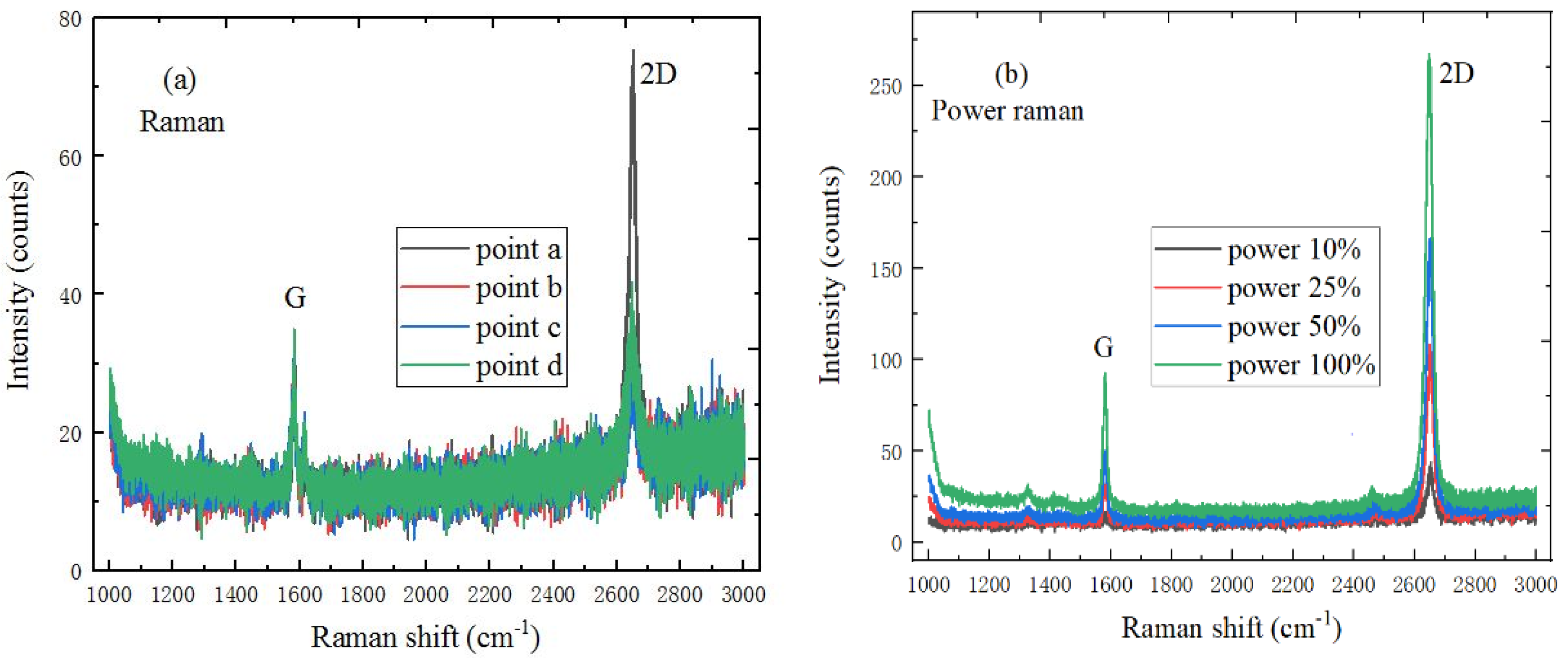
2.2. The Test Experiment Results of 2D Materials
2.3. The Test Experiment Results of 2D-2D vdWs Heterostructure Materials
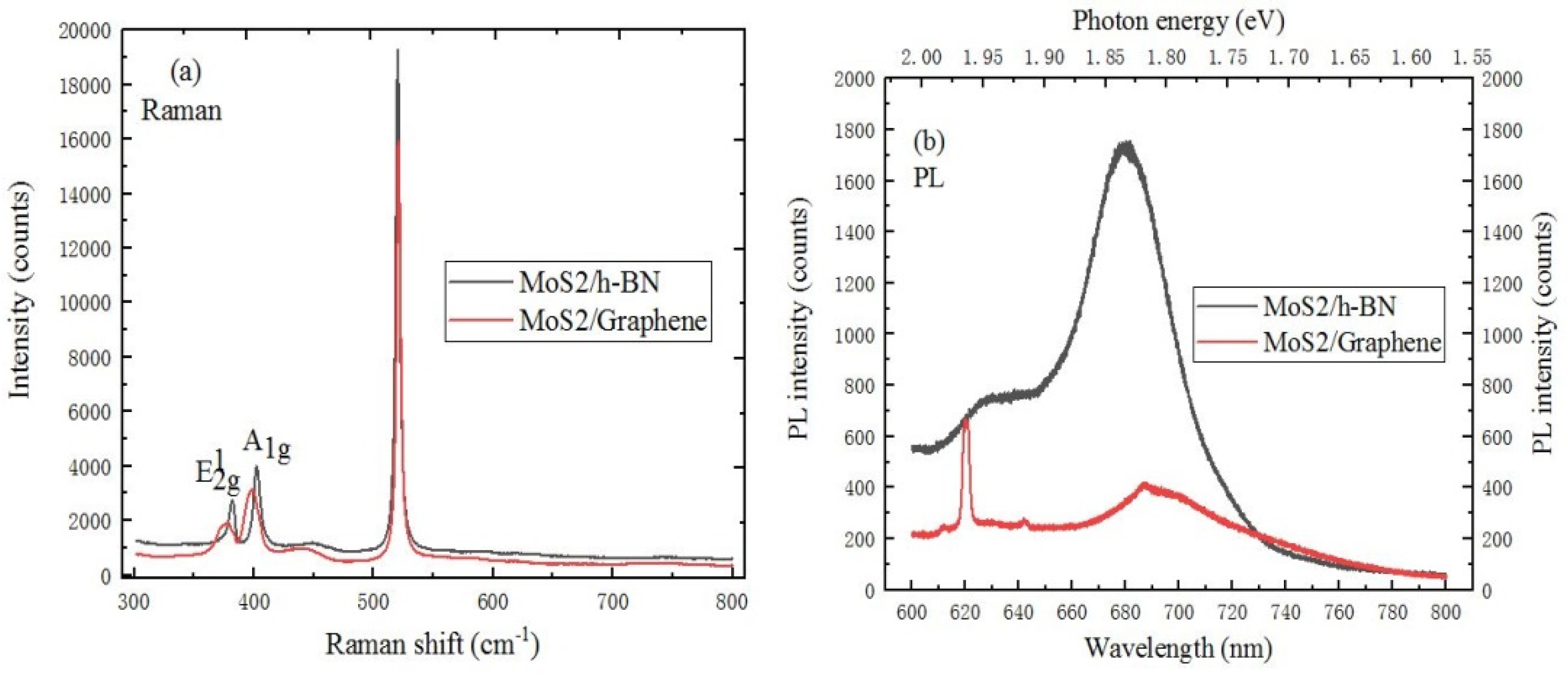
2.3.1. The Characterization of MoS2/h-BN Heterostructure
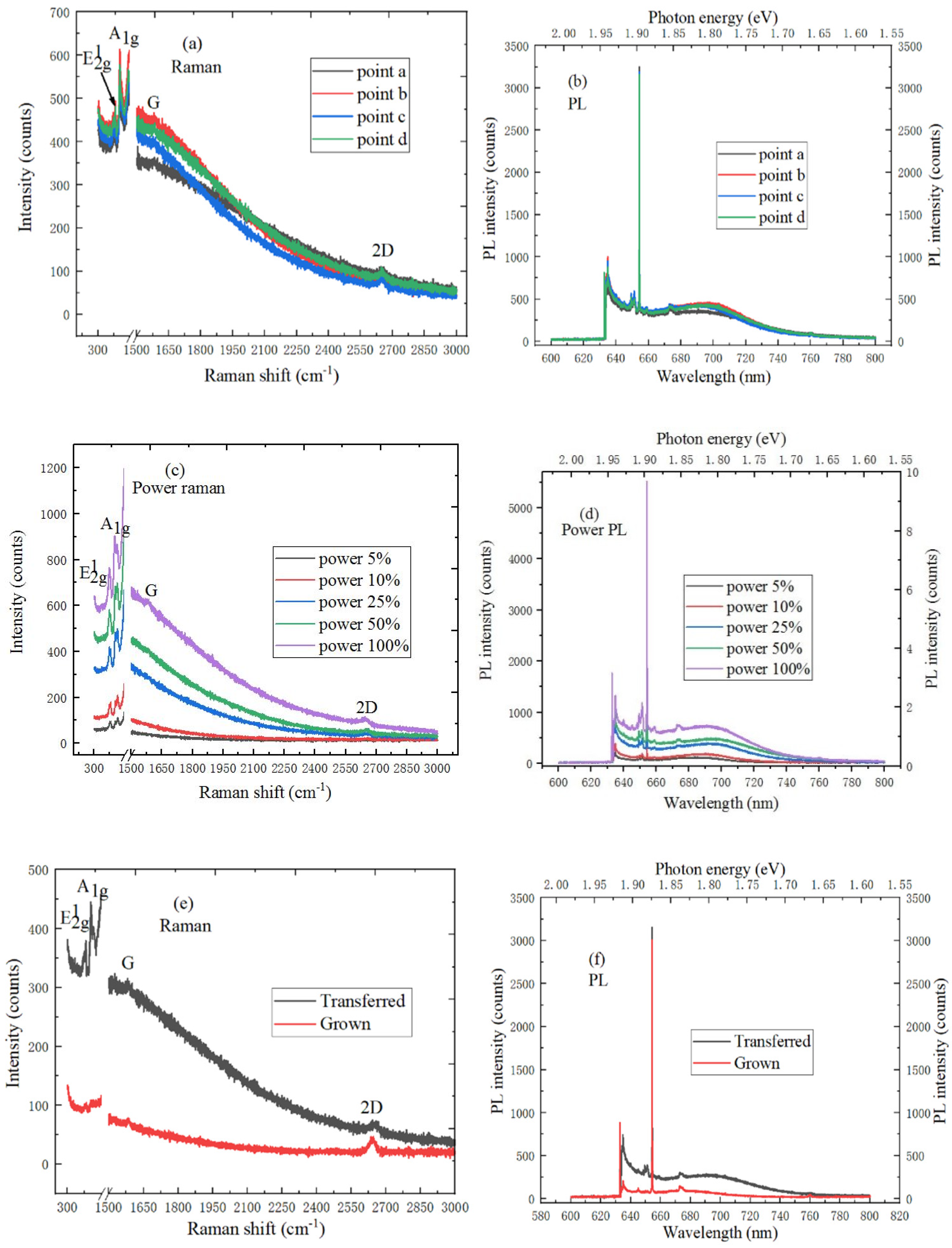
2.3.2. The Characterization of MoS2/Graphene Heterostructure
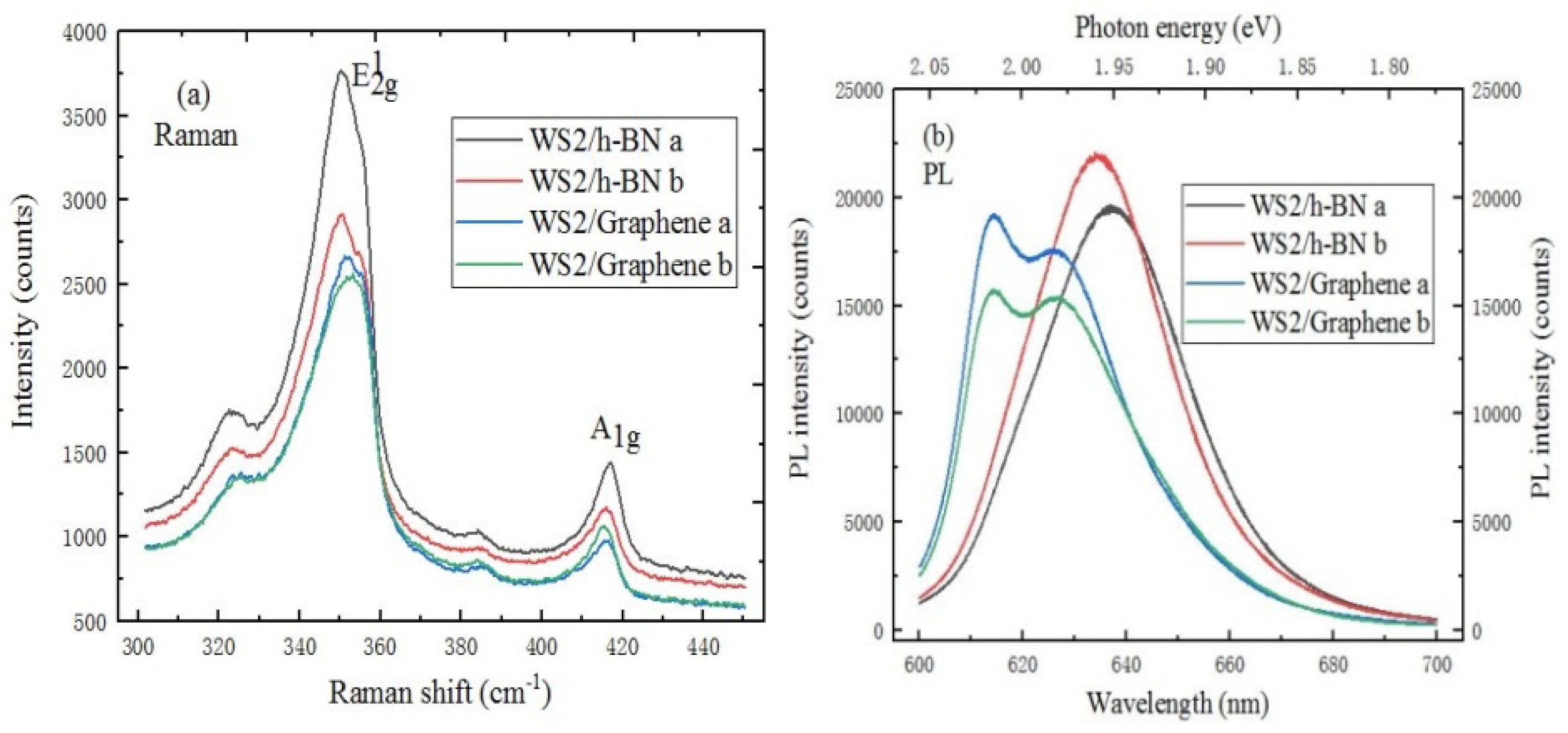
2.3.3. The Characterization of WS2/hBN Heterostructure
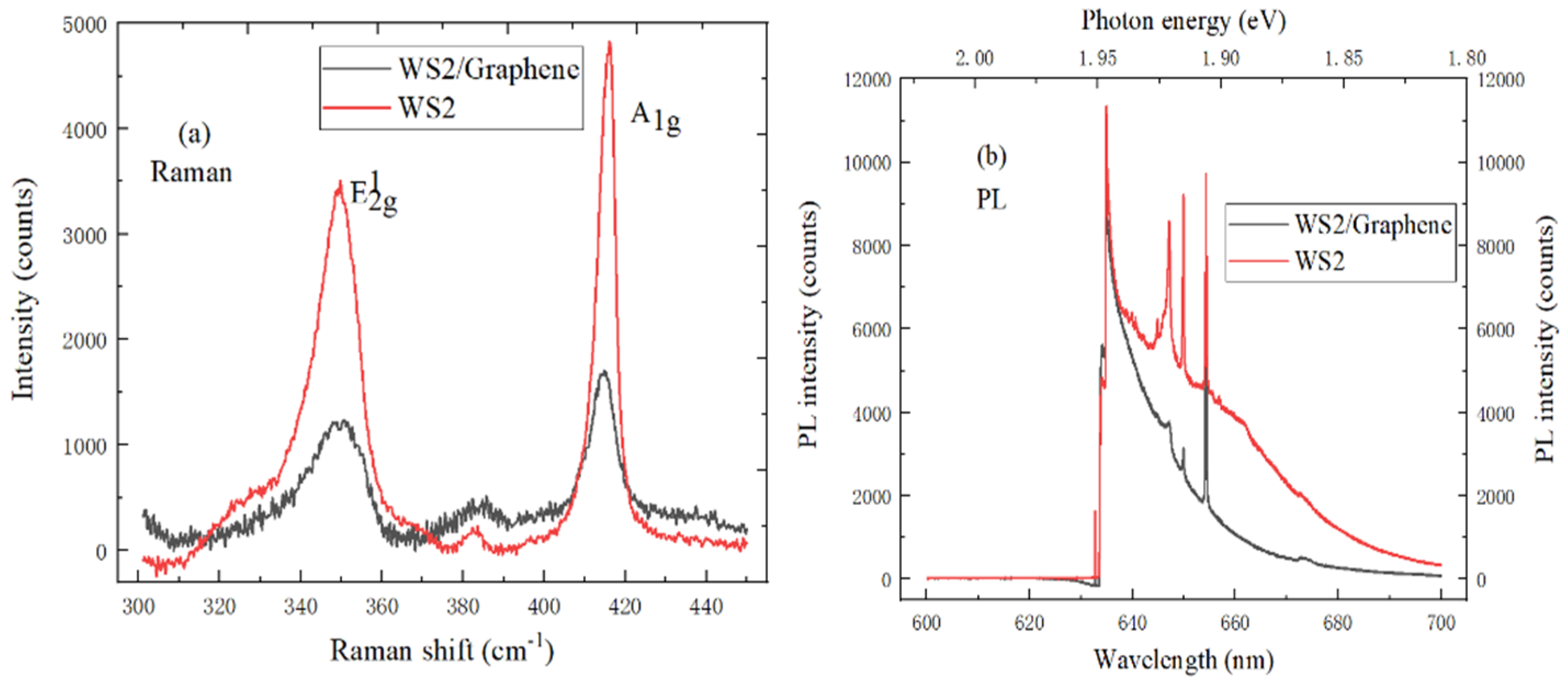
2.3.4. The Characterization of WS2/Graphene Heterostructure
3. Materials and Methods
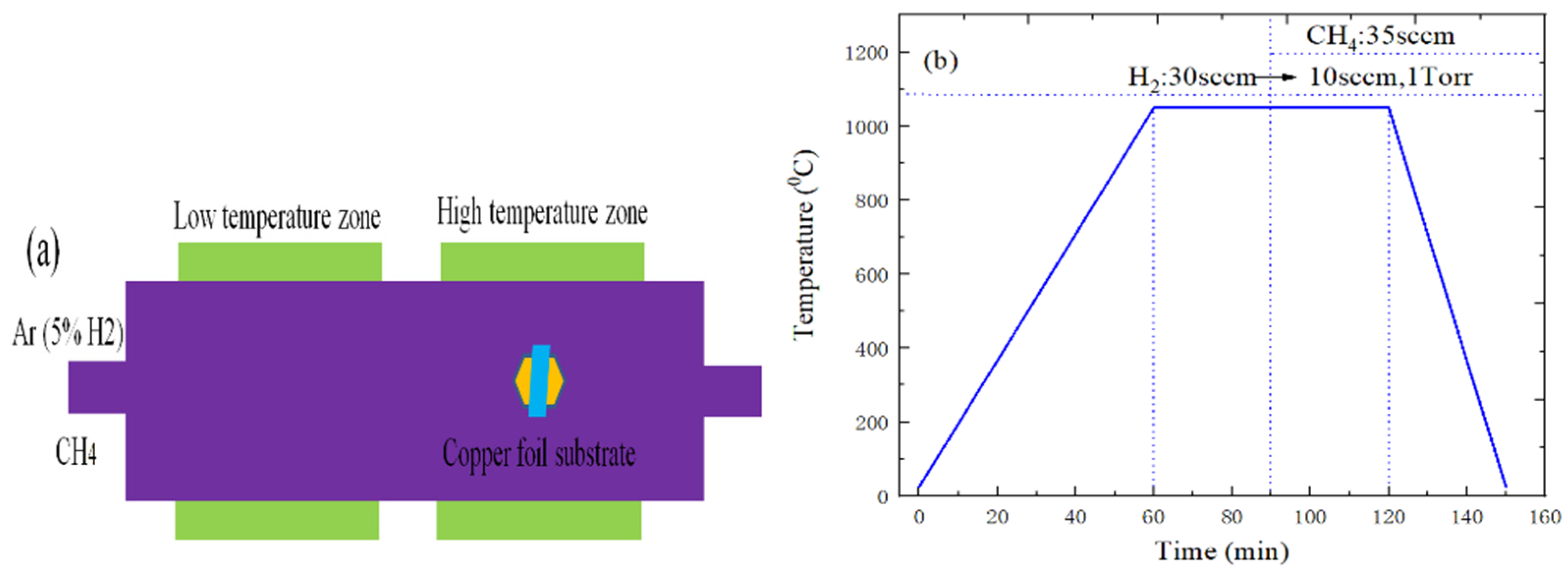
3.1. Preparation of Graphene and h-BN Materials
3.2. The Transfer of Graphene or h-BN
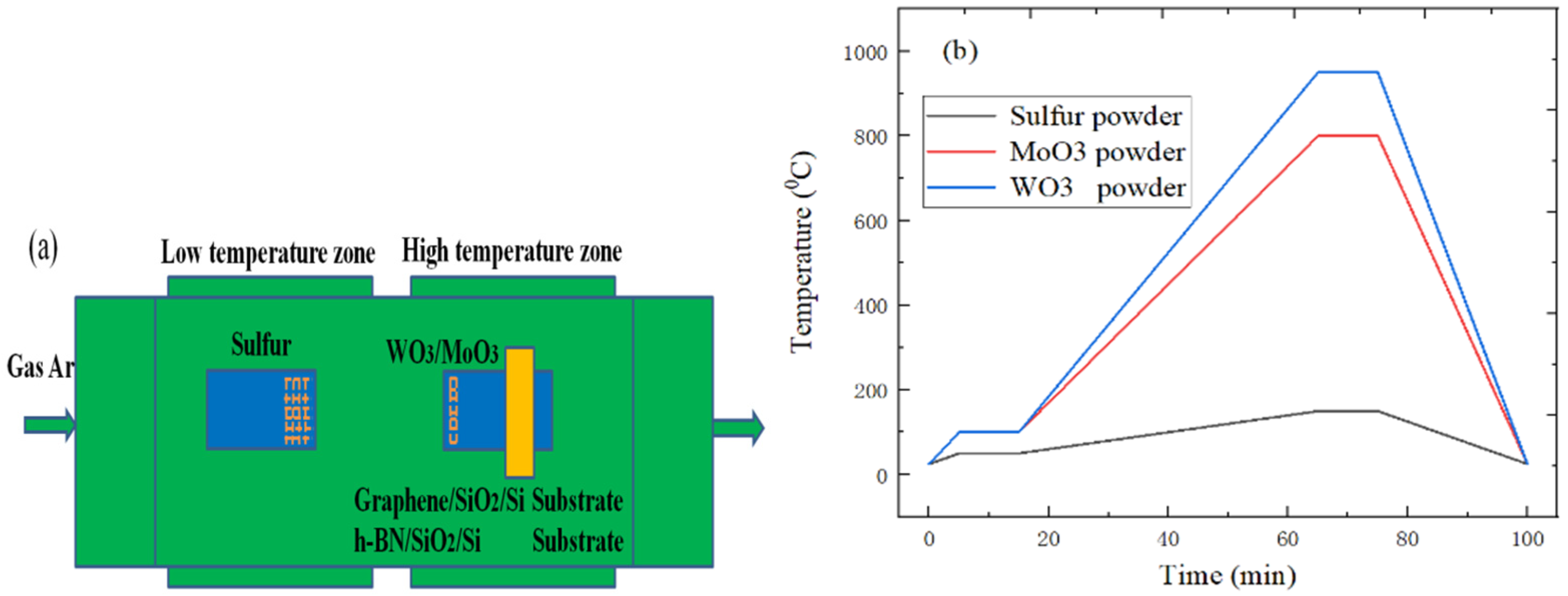
3.3. The Preparation of WS2 or MoS2 Material
3.4. The Test Characterization of Heterojunction Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.J.; Zhang, X.W.; Wang, C.Z.; Zhao, Y.Z.; Zhou, H.P.; Li, J.B.; Jin, H.B. The synthesis of hierarchical nanostructured MoS2/Graphene composites with enhanced visible-light photo-degradation property. Appl. Surf. Sci. 2017, 412, 207–213. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Jia, Y.X.; Cheng, J.Y.; Chen, S.M.; Chai, J.X.; Yang, X.Y.; Wu, Z.Y.; Wang, H.; Zhang, W.J.; Zhao, Z.L.; et al. High-performance microwave absorption materials based on MoS2-graphene isomorphic hetero-structures. J. Alloy. Compd. 2018, 758, 62–71. [Google Scholar] [CrossRef]
- Liu, M.X.; Shi, J.P.; Li, Y.C.; Zhou, X.B.; Ma, D.L.; Qi, Y.; Zhang, Y.F.; Liu, Z.F. Temperature-Triggered Sulfur Vacancy Evolution in Monolayer MoS2/Graphene Heterostructures. Small 2017, 13, 1602967. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Wang, B.; Zhou, Q.; Xiao, J.X.; Jia, X.H. Preparation and tribological properties of MoS2/graphene oxide composites. Appl. Surf. Sci. 2017, 419, 24–34. [Google Scholar] [CrossRef]
- Xie, Y.N.; Chou, T.M.; Yang, W.F.; He, M.H.; Zhao, Y.R.; Li, N.; Lin, Z.H. Flexible thermoelectric nanogenerator based on the MoS2/graphene nanocomposite and its application for a self-powered temperature sensor. Semicond. Sci. Technol. 2017, 32, 044003. [Google Scholar] [CrossRef]
- Liu, X.J.; Gao, J.F.; Zhang, G.; Zhang, Y.W. MoS2-graphene in-plane contact for high interfacial thermal conduction. Nano Res. 2017, 10, 2944–2953. [Google Scholar] [CrossRef]
- Biroju, R.K.; Pal, S.; Sharma, R.; Giri, P.K.; Narayanan, T.N. Stacking sequence dependent photo-electrocatalytic performance of CVD grown MoS2/graphene van der Waals solids. Nanotechnology 2017, 28, 085101. [Google Scholar] [CrossRef]
- Ding, L.; Ukhtary, M.S.; Chubarov, M.; Choudhury, T.H.; Zhang, F.; Yang, R.; Zhang, A.; Fan, J.A.; Terrones, M.; Redwing, J.M.; et al. Understanding interlayer coupling in TMD-HBN heterostructure by Raman spectroscopy. IEEE Trans. Electron Devices 2018, 65, 4059–4067. [Google Scholar] [CrossRef]
- Xu, W.S.; Kozawa, D.; Zhou, Y.Q.; Wang, Y.Z.; Sheng, Y.W.; Jiang, T.; Strano, M.S.; Warner, J.H. Controlling Photoluminescence Enhancement and Energy Transfer in WS2:hBN:WS2 Vertical Stacks by Precise Interlayer Distances. Small 2020, 16, 1905985. [Google Scholar] [CrossRef]
- Zollner, K.; Junior, P.E.F.; Fabian, J. Giant proximity exchange and valley splitting in transition metal dichalcogenide/h-BN/(Co, Ni) heterostructures. Phys. Rev. B 2020, 101, 085112. [Google Scholar] [CrossRef]
- Okada, M.; Kutana, A.; Kureishi, Y.; Kobayashi, Y.; Saito, Y.; Saito, T.; Watanabe, K.; Taniguchi, T.; Gupta, S.; Miyata, Y.; et al. Direct and indirect interlayer excitons in a van der Waals heterostructure of hBN/WS2/MoS2/Hbn. ACS Nano 2018, 12, 2498–2505. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.X.; Erb, C.; Wang, K.; Moradifar, P.; Crespi, V.H.; Alem, N. Full orientation control of epitaxial MoS2 on hBN assisted by substrate defects. Phys. Rev. B 2019, 99, 155430. [Google Scholar] [CrossRef]
- Athreya, N.; Leburton, J.P. Electronic Detection of Nucleotides in Multi-Layered MoS2-hBN Nanopore FET Devices. Biophys. J. 2020, 118, 157a. [Google Scholar] [CrossRef]
- Yosuke, U.; Alex, K.; Watanabe, K.; Taniguchi, T.; Kana, K.; Takahiko, E.; Yasumitsu, M.; Hisanori, S.; Ryo, K. Momentum-forbidden dark excitons in hBN-encapsulated monolayer MoS2. NPJ 2D Mater. Appl. 2019, 3. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.B.; Wu, X.M.; Zhang, W.Z.; Luo, C.Y.; Li, J.H. Fabrication of MoS2-graphene modified with Fe3O4 particles and its enhanced microwave absorption performance. Adv. Powder Technol. 2018, 29, 744–750. [Google Scholar] [CrossRef]
- Sun, B.; Shi, T.L.; Liu, Z.Y.; Wu, Y.N.; Zhou, J.X.; Liao, G.L. Large-area flexible photodetector based on atomically thin MoS2/graphene film. Mater. Des. 2018, 154, 1–7. [Google Scholar] [CrossRef]
- Deng, Y.K.; Ding, L.X.; Liu, Q.X.; Zhan, L.; Wang, Y.L.; Yang, S.B. Two-dimensional MoS2-graphene hybrid nanosheets for high gravimetric and volumetric lithium storage. Appl. Surf. Sci. 2018, 437, 384–389. [Google Scholar] [CrossRef]
- Alamri, M.; Sakidja, R.; Goul, R.; Ghopry, S.; Wu, J.Z. Plasmonic Au nanoparticles on 2D MoS2/Graphene van der Waals heterostructures for high-sensitivity surface-enhanced Raman spectroscopy. ACS Appl. Nano Mater. 2019, 2, 1412–1420. [Google Scholar] [CrossRef]
- Kim, H.U.; Kim, M.; Jin, Y.H.; Hyeon, Y.; Kim, K.S.; An, B.S.; Yang, C.W.; Kanade, V.; Moon, J.Y.; Yeom, G.Y.; et al. Low-temperature wafer-scale growth of MoS2-graphene heterostructures. Appl. Surf. Sci. 2019, 470, 129–134. [Google Scholar] [CrossRef]
- Pham, T.; Ramnani, P.; Villarreal, C.C.; Lopez, J.; Das, P.; Lee, I.; Neupane, M.R.; Rheem, Y.; Mulchandani, A. MoS2-graphene heterostructures as efficient organic compounds sensing 2D materials. Carbon 2019, 142, 504–512. [Google Scholar] [CrossRef]
- Song, X.Y.; Lu, L.H.; Wei, M.J.; Dai, Z.Y.; Wang, S.S. Molecular dynamics simulations on the water flux in different two-dimension materials. Mol. Simul. 2018, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Ye, F.; Xu, J.; Zhang, W.; Feng, P.X.L.; Zhang, X. Gate-Tuned Temperature in a Hexagonal Boron Nitride-Encapsulated 2-D Semiconductor Device. IEEE Trans. Electron. Devices 2018, 65, 4068–4072. [Google Scholar] [CrossRef]
- Calman, E.V.; Fogler, M.M.; Butov, L.V.; Hu, S.; Mishchenko, A.; Geim, A.K. Indirect excitons in van der Waals heterostructures at room temperature. Nat. Commun. 2018, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.; Chae, S.H.; Ribeiro-Palau, R.; Hone, J. Disorder in van der Waals heterostructures of 2D materials. Nat. Mater. 2019, 18, 541. [Google Scholar] [CrossRef]
- Henriques, J.C.G.; Catarina, G.; Costa, A.T.; Fernández-Rossier, J.; Peres, N.M.R. Excitonic magneto-optical Kerr effect in two-dimensional transition metal dichalcogenides induced by spin proximity. Phys. Rev. B 2020, 101, 045408. [Google Scholar] [CrossRef]
- Ma, X.Z.; Liu, Q.S.; Xu, D.; Zhu, Y.Z.; Kim, S.G.; Cui, Y.T.; Zhong, L.L.; Liu, M. Capillary-force-assisted clean-stamp transfer of two-dimensional materials. Nano Lett. 2017, 17, 6961–6967. [Google Scholar] [CrossRef]
- Christopher, J.W.; Goldberg, B.B.; Swan, A.K. Long tailed trions in monolayer MoS2: Temperature dependent asymmetry and resulting red-shift of trion photoluminescence spectra. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Hu, Y.W.; Zhang, F.; Titze, M.; Deng, B.W.; Li, H.B.; Cheng, G.J. Straining effects in MoS2 monolayer on nanostructured substrates: Temperature-dependent photoluminescence and exciton dynamics. Nanoscale 2018, 10, 5717–5724. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Siddique, S. Ultraviolet-light-driven enhanced hysteresis effect in graphene-tungsten disulfide heterostructures. Carbon 2017, 123, 168–173. [Google Scholar] [CrossRef]
- Omar, S.; van Wees, B.J. Graphene-WS2 heterostructures for tunable spin injection and spin transport. Phys. Rev. B 2017, 95, 081404. [Google Scholar] [CrossRef]
- O’Farrell, E.C.T.; Avsar, A.; Tan, J.Y.; Eda, G.; Ozyilmaz, B. Quantum Transport Detected by Strong Proximity Interaction at a Graphene–WS2 van der Waals Interface. Nano Lett. 2015, 15, 5682–5688. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, T.; Liu, H.; Wang, S.; Chen, S.; Yang, K.; Li, Z. Synthesis and Spectral Characteristics Investigation of the 2D-2D vdWs Heterostructure Materials. Int. J. Mol. Sci. 2021, 22, 1246. https://doi.org/10.3390/ijms22031246
Han T, Liu H, Wang S, Chen S, Yang K, Li Z. Synthesis and Spectral Characteristics Investigation of the 2D-2D vdWs Heterostructure Materials. International Journal of Molecular Sciences. 2021; 22(3):1246. https://doi.org/10.3390/ijms22031246
Chicago/Turabian StyleHan, Tao, Hongxia Liu, Shulong Wang, Shupeng Chen, Kun Yang, and Zhandong Li. 2021. "Synthesis and Spectral Characteristics Investigation of the 2D-2D vdWs Heterostructure Materials" International Journal of Molecular Sciences 22, no. 3: 1246. https://doi.org/10.3390/ijms22031246
APA StyleHan, T., Liu, H., Wang, S., Chen, S., Yang, K., & Li, Z. (2021). Synthesis and Spectral Characteristics Investigation of the 2D-2D vdWs Heterostructure Materials. International Journal of Molecular Sciences, 22(3), 1246. https://doi.org/10.3390/ijms22031246






