Effect of Pd Precursor Salts on the Chemical State, Particle Size, and Performance of Activated Carbon-Supported Pd Catalysts for the Selective Hydrogenation of Palm Biodiesel
Abstract
1. Introduction
2. Results and Discussion
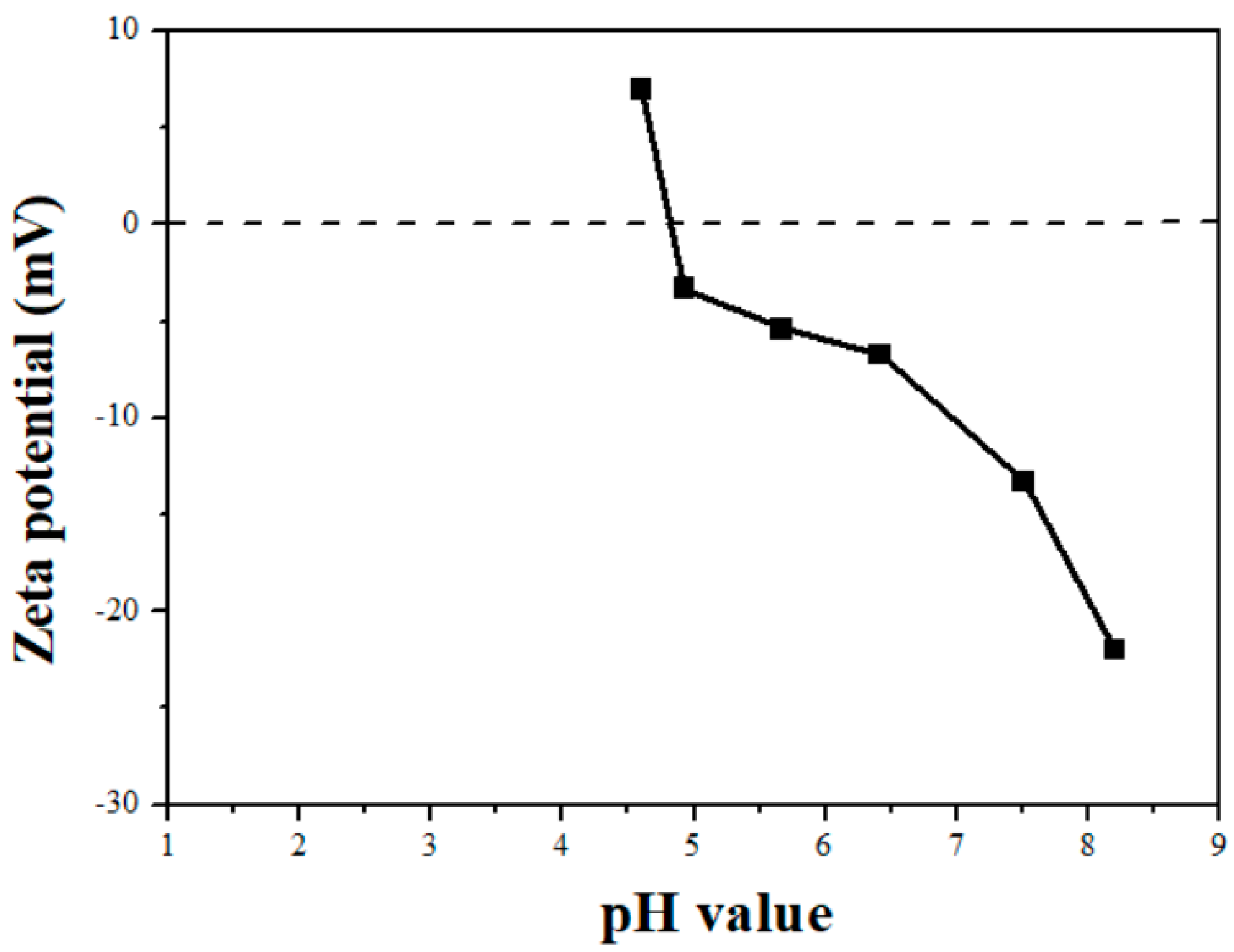
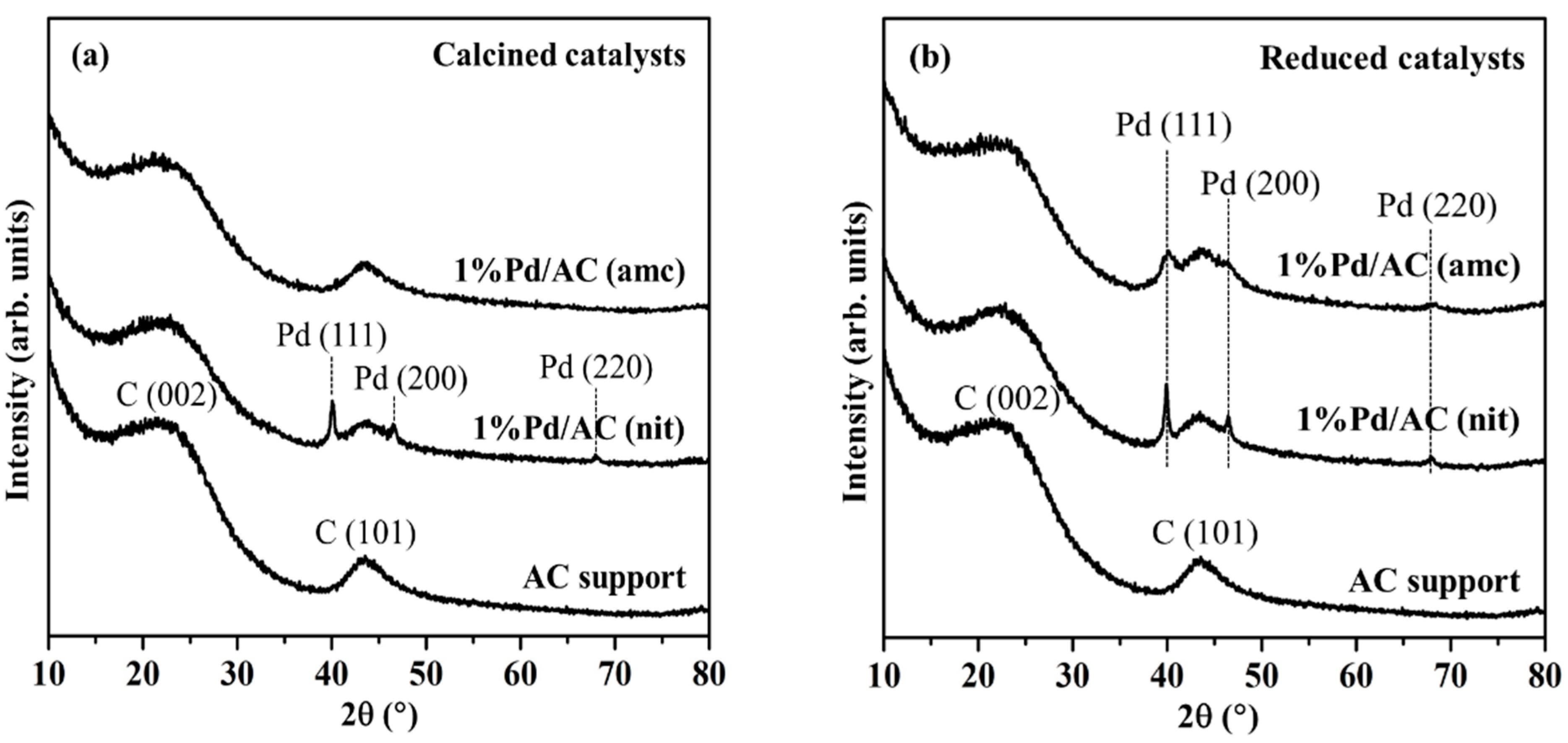
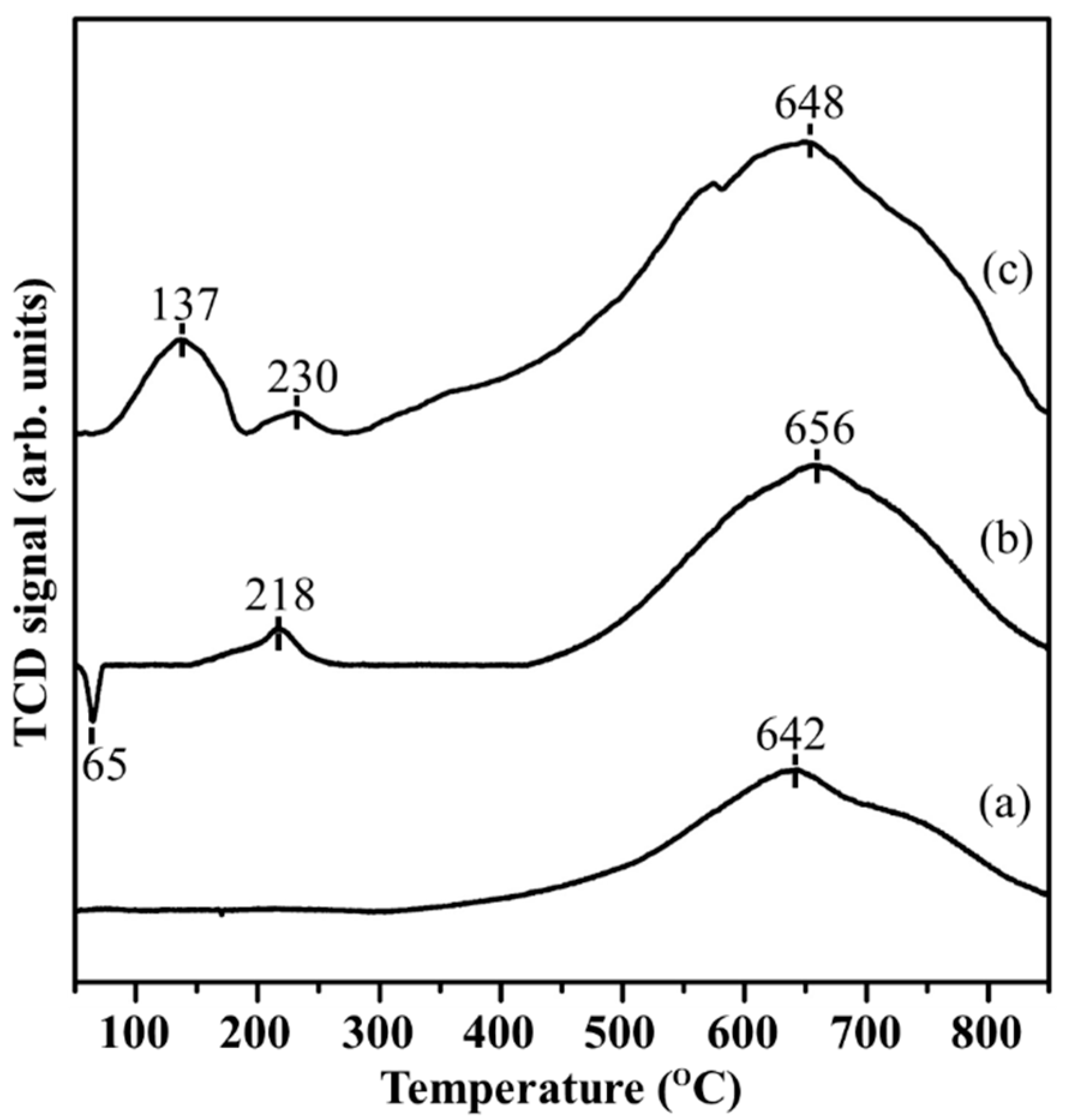
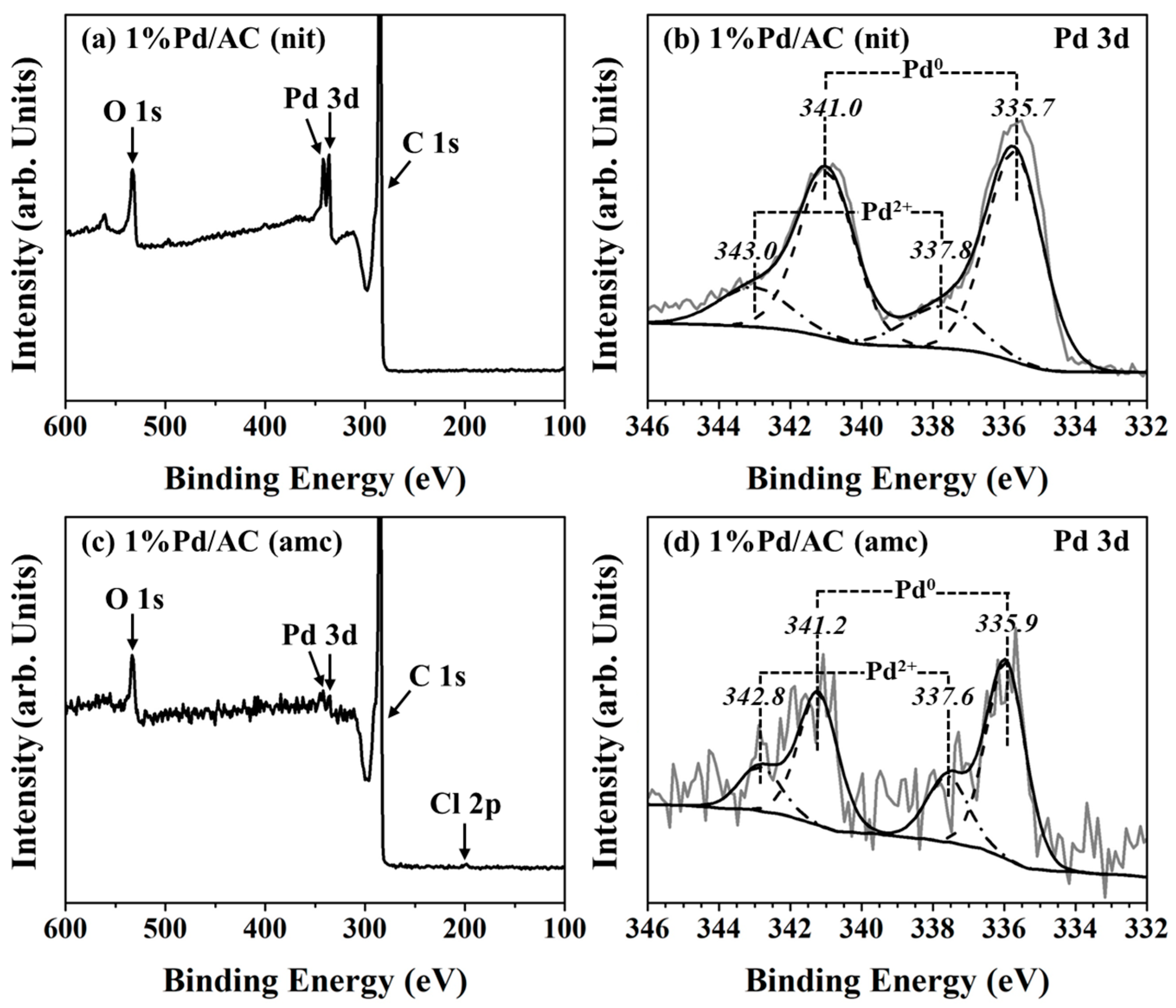
2.1. Characterizations
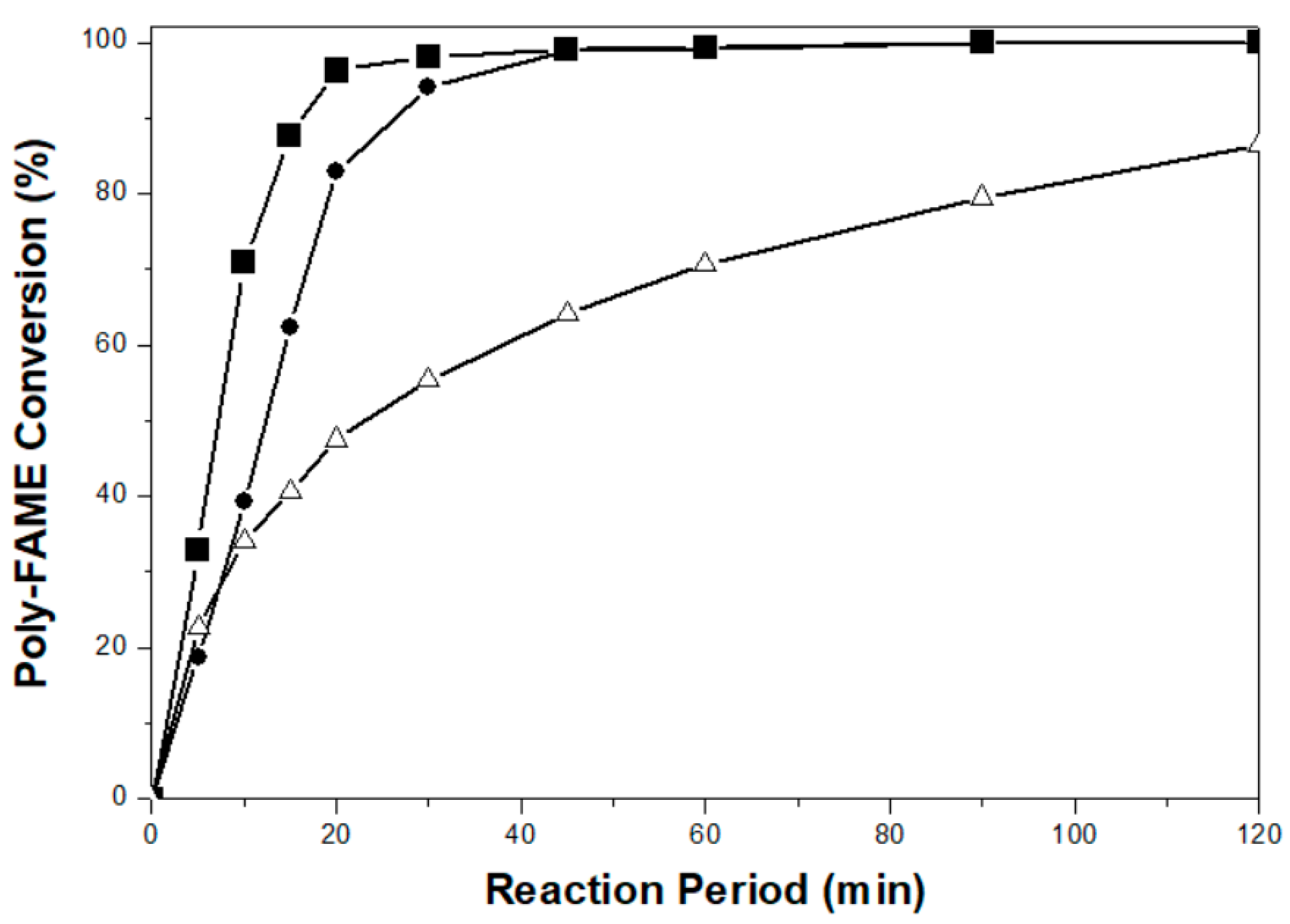
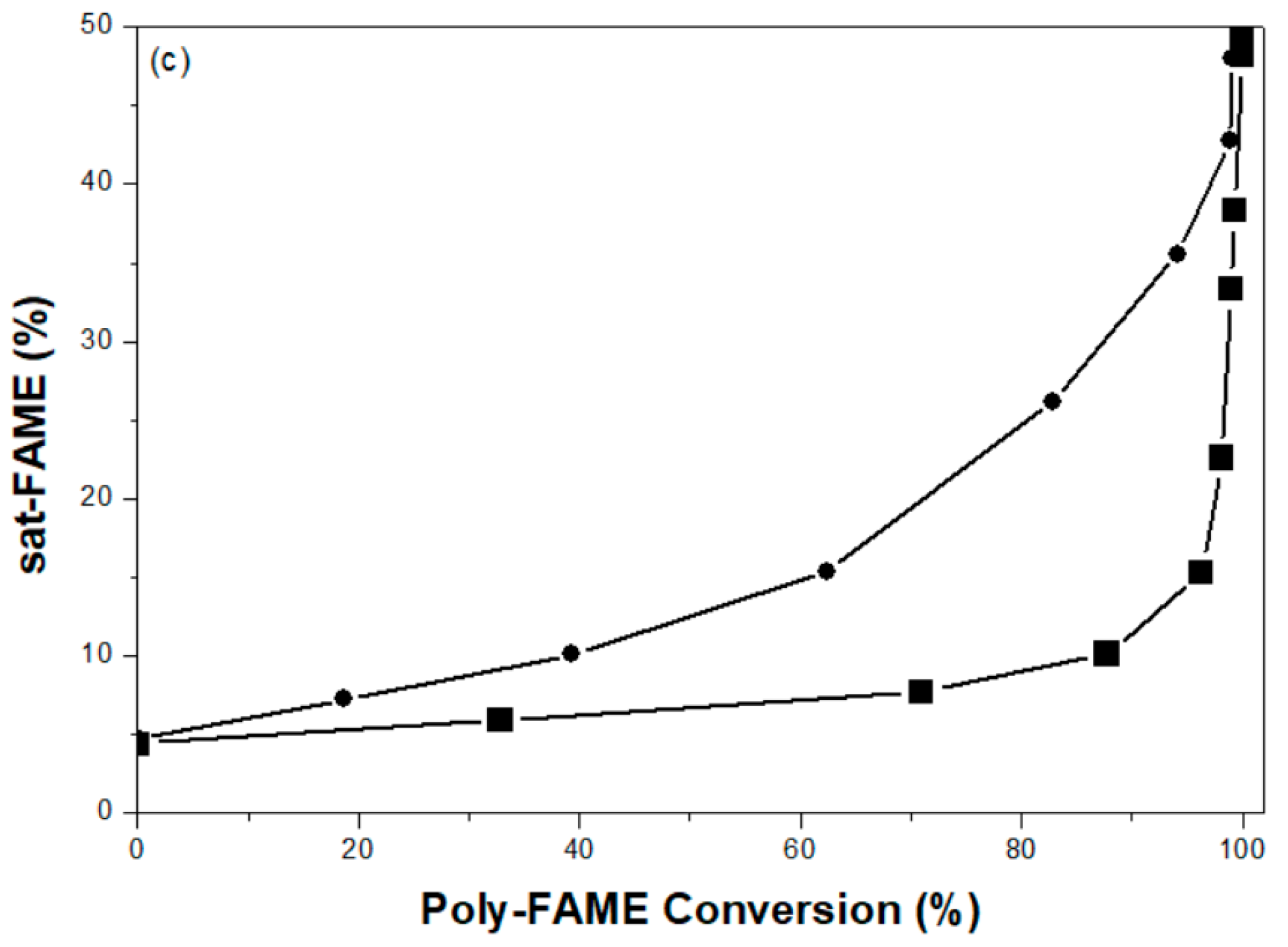
2.2. Partial Hydrogenation of Palm-BDF to Corresponding H-FAME


2.3. FAME Composition and Fuel Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Pd/AC Catalysts
3.3. Characterizations
3.4. Partial Hydrogenation of Palm-BDF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thailand Ministry of Energy. Alternative Energy Development Plan: AEDP2015. Ministry of Energy. Available online: http://www.eppo.go.th/images/POLICY/ENG/AEDP2015ENG.pdf (accessed on 17 September 2015).
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, G.; Sharma, M.P. Impact of cold flow properties of biodiesel on engine performance. Renew. Sustain. Energy Rev. 2014, 31, 650–656. [Google Scholar] [CrossRef]
- Numwong, N.; Luengnaruemitchai, A.; Chollacoop, N.; Yoshimura, Y. Effect of SiO2 pore size on partial hydrogenation of rapeseed oil-derived FAMEs. Appl. Catal. A Gen. 2012, 441–442, 72–78. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Attanatho, L.; Chang, A.; Laosombut, T.; Nishi, M.; Mochizuki, T.; Takagi, H.; Yang, C.-M.; Abe, Y.; Toba, M.; et al. Profiling and catalytic upgrading of commercial palm oil-derived biodiesel fuels for high-blend fuels. Catal. Today 2019, 332, 122–131. [Google Scholar] [CrossRef]
- Numwong, N.; Luengnaruemitchai, A.; Chollacoop, N.; Yoshimura, Y. Effect of Support Acidic Properties on Sulfur Tolerance of Pd Catalysts for Partial Hydrogenation of Rapeseed Oil-Derived FAME. J. Am. Oil Chem. Soc. 2012, 89, 2117–2120. [Google Scholar] [CrossRef]
- Thunyaratchatanon, C.; Luengnaruemitchai, A.; Chollacoop, N.; Chen, S.-Y.; Yoshimura, Y. Catalytic hydrogenation of soybean oil-derived fatty acid methyl esters over Pd supported on Zr-SBA-15 with various Zr loading levels for enhanced oxidative stability. Fuel Process. Technol. 2018, 179, 422–435. [Google Scholar] [CrossRef]
- Hongmanorom, P.; Luengnaruemitchai, A.; Chollacoop, N.; Yoshimura, Y. Effect of the Pd/MCM-41 Pore Size on the Catalytic Activity and cis–trans Selectivity for Partial Hydrogenation of Canola Biodiesel. Energy Fuels 2017, 31, 8202–8209. [Google Scholar] [CrossRef]
- Mochizuki, T.; Abe, Y.; Chen, S.-Y.; Toba, M.; Yoshimura, Y. Oxygen-Assisted Hydrogenation of Jatropha-Oil-Derived Biodiesel Fuel over an Alumina-Supported Palladium Catalyst to Produce Hydrotreated Fatty Acid Methyl Esters for High-Blend Fuels. ChemCatChem 2017, 9, 2633–2637. [Google Scholar] [CrossRef]
- Thunyaratchatanon, C.; Luengnaruemitchai, A.; Jitajamnong, J.; Chollacoop, N.; Chen, S.-Y.; Yoshimura, Y. Influence of Alkaline and Alkaline Earth Metal Promoters on the Catalytic Performance of Pd-M/SiO2 (M = Na, Ca, or Ba) Catalysts in the Partial Hydrogenation of Soybean Oil-derived Biodiesel for Oxidative Stability Improvement. Energy Fuels 2018, 32, 9744–9755. [Google Scholar] [CrossRef]
- Ito, K.; Ohshima, M.; Kurokawa, H.; Sugiyama, K.; Miura, H. Effect of residual Cl− derived from metal precursors on catalytic activity in the hydrogenation of naphthalene over supported Pd catalysts. Catal. Commun. 2002, 3, 527–531. [Google Scholar] [CrossRef]
- Gaspar, A.B.; Dieguez, L.C. Dispersion stability and methylcyclopentane hydrogenolysis in Pd/Al2O3 catalysts. Appl. Catal. A Gen. 2000, 201, 241–251. [Google Scholar] [CrossRef]
- Ali, S.H.; Goodwin, J.G., Jr. SSITKA Investigation of Palladium Precursor and Support Effects on CO Hydrogenation over Supported Pd Catalysts. J. Catal. 1998, 176, 3–13. [Google Scholar] [CrossRef]
- Panpranot, J.; Tangjitwattakorn, O.; Praserthdam, P.; Goodwin, J.G., Jr. Effects of Pd precursors on the catalytic activity and deactivation of silica-supported Pd catalysts in liquid phase hydrogenation. Appl. Catal. A Gen. 2005, 292, 322–327. [Google Scholar] [CrossRef]
- Redjel, A.; Boudjahem, A.-G.; Bettahar, M. Effect of palladium precursor and preparation method on the catalytic performance of Pd/SiO2 catalysts for benzene hydrogenation. Part. Sci. Technol. 2018, 36, 710–715. [Google Scholar] [CrossRef]
- Kankala, R.K.; Han, Y.-H.; Na, J.; Lee, C.-H.; Sun, Z.; Wang, S.-B.; Kimura, T.; Ok, Y.S.; Yamauchi, Y.; Chen, A.-Z.; et al. Nanoarchitectured Structure and Surface Biofunctionality of Mesoporous Silica Nanoparticles. Adv. Mater. 2020, 32, 1907035. [Google Scholar] [CrossRef]
- Yashnik, S.A.; Urzhuntsev, G.A.; Stadnichenko, A.I.; Svintsitskiy, D.A.; Ishchenko, A.V.; Boronin, A.I.; Ismagilov, Z.R. Effect of Pd- precursor and support acid properties on the Pd electronic state and the hydrodesulfurization activity of Pd-zeolite catalysts. Catal. Today 2019, 323, 257–270. [Google Scholar] [CrossRef]
- Catherin, N.; Blanco, E.; Laurenti, D.; Simonet, F.; Lorentz, C.; Leclerc, E.; Calemma, V.; Geantet, C. Selective ring opening of decalin over bifunctional RuS2/zeolite catalysts. Catal. Today 2019, 323, 105–111. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Likholobov, V.A. Approaches to the synthesis of Pd/C catalysts with controllable activity and selectivity in hydrogenation reactions. Catal. Today 2020, 357, 152–165. [Google Scholar] [CrossRef]
- Lederhos, C.R.; Badano, J.M.; Carrara, N.; Pascual, F.C.; Almansa, M.C.; Liprandi, D.; Quiroga, M. Metal and Precursor Effect during 1-Heptyne Selective Hydrogenation Using an Activated Carbon as Support. Sci. World J. 2013, 2013, 528453. [Google Scholar] [CrossRef]
- Cabiac, A.; Cacciaguerra, T.; Trens, P.; Durand, R.; Delahay, G.; Medevielle, A.; Plée, D.; Coq, B. Influence of textural properties of activated carbons on Pd/carbon catalysts synthesis for cinnamaldehyde hydrogenation. Appl. Catal. A Gen. 2008, 340, 229–235. [Google Scholar] [CrossRef]
- Han, W.; Zhang, G.; Lu, G.; Tang, Z. Influence of pore structures of a carbon support on the surface textures of a CO oxidation catalyst. RSC Adv. 2015, 5, 59666–59676. [Google Scholar] [CrossRef]
- Contreras, R.C.; Guicheret, B.; Machado, B.F.; Cárcamo, C.R.; Alvarez, M.A.C.; Salas, B.V.; Ruttert, M.; Placke, T.; Réguillon, A.F.; Vanoye, L.; et al. Effect of mesoporous carbon support nature and pretreatments on palladium loading, dispersion and apparent catalytic activity in hydrogenation of myrcene. J. Catal. 2019, 372, 226–244. [Google Scholar] [CrossRef]
- Park, J.; Regalbuto, J.R. A Simple, Accurate Determination of Oxide PZC and the Strong Buffering Effect of Oxide Surfaces at Incipient Wetness. J. Colloid Interface Sci. 1995, 175, 239–252. [Google Scholar] [CrossRef]
- Toebes, M.L.; van Dillen, A.J.; de Jong, K.P. Synthesis of supported palladium catalysts. J. Mol. Catal. A Chem. 2001, 173, 75–98. [Google Scholar] [CrossRef]
- Amorim, C.; Keane, M.A. Palladium supported on structured and nonstructured carbon: A consideration of Pd particle size and the nature of reactive hydrogen. J. Colloid Interface Sci. 2008, 322, 196–208. [Google Scholar] [CrossRef]
- Lambert, S.; Job, N.; D’Souza, L.; Pereira, M.F.R.; Pirard, R.; Heinrichs, B.; Figueiredo, L.; Pirard, J.-P.; Regalbuto, J.R. Synthesis of very highly dispersed platinum catalysts supported on carbon xerogels by the strong electrostatic adsorption method. J. Catal. 2009, 261, 23–33. [Google Scholar] [CrossRef]
- Hao, X.; Barnes, S.; Regalbuto, J.R. A fundamental study of Pt impregnation of carbon: Adsorption equilibrium and particle synthesis. J. Catal. 2011, 279, 48–65. [Google Scholar] [CrossRef]
- Auer, E.; Freund, A.; Pietsch, J.; Tacke, T. Carbons as supports for industrial precious metal catalysts. Appl. Catal. A Gen. 1998, 173, 259–271. [Google Scholar] [CrossRef]
- Rodríguez-reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Harada, T.; Ikeda, S.; Miyazaki, M.; Sakata, T.; Mori, H.; Matsumura, M. A simple method for preparing highly active palladium catalysts loaded on various carbon supports for liquid-phase oxidation and hydrogenation reactions. J. Mol. Catal. A Chem. 2007, 268, 59–64. [Google Scholar] [CrossRef]
- Ramos, A.L.D.; Alves, P.S.; Aranda, D.A.G.; Schmal, M. Characterization of carbon supported palladium catalysts: Inference of electronic and particle size effects using reaction probes. Appl. Catal. A Gen. 2004, 277, 71–81. [Google Scholar] [CrossRef]
- Numwong, N.; Thachuangtumle, N.; Luengnaruemitchai, A. Partial Hydrogenation of Palm Oil-Derived Biodiesel Over Pd/C Catalysts. Int. J. Adv. Sci. Eng. Technol. 2016, 4, 195–200. [Google Scholar]
- Numwong, N.; Luengnaruemitchai, A.; Chollacoop, N.; Yoshimura, Y. Partial hydrogenation of polyunsaturated fatty acid methyl esters over Pd/activated carbon: Effect of type of reactor. Chem. Eng. J. 2012, 210, 173–181. [Google Scholar] [CrossRef]
- Solar, J.M.; Leon, C.A.L.; Osseo-Asare, K.; Radovic, L.R. On the importance of the electrokinetic properties of carbons for their use as catalyst supports. Carbon 1990, 28, 369–375. [Google Scholar] [CrossRef]
- Banerjee, R.; Regalbuto, J.R. Rectifying the chemisorption—XRD discrepancy of carbon supported Pd: Residual chloride and/or carbon decoration. Appl. Catal. A Gen. 2020, 595, 117504. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lin, D.-Y.; Chen, B.-H. Metasilicate-based catalyst prepared from natural diatomaceous earth for biodiesel production. Renew. Energy 2019, 138, 1042–1050. [Google Scholar] [CrossRef]
- Prahas, D.; Kartika, Y.; Indraswati, N.; Ismadji, S. Activated carbon from jackfruit peel waste by H3PO4 chemical activation: Pore structure and surface chemistry characterization. Chem. Eng. J. 2008, 140, 32–42. [Google Scholar] [CrossRef]
- Nishi, M.; Chen, S.-Y.; Takagi, H. Mild Ammonia Synthesis over Ba-Promoted Ru/MPC Catalysts: Effects of the Ba/Ru Ratio and the Mesoporous Structure. Catalysts 2019, 9, 480. [Google Scholar] [CrossRef]
- Nishi, M.; Chen, S.-Y.; Takagi, H. Energy Efficient and Intermittently Variable Ammonia Synthesis over Mesoporous Carbon-Supported Cs-Ru Nanocatalysts. Catalyst 2019, 9, 406. [Google Scholar] [CrossRef]
- Nishi, M.; Chen, S.-Y.; Takagi, H. A Mesoporous Carbon-Supported and Cs-promoted Ru Catalyst with Enhanced Activity and Stability for Sustainable Ammonia Synthesis. ChemCatChem 2018, 10, 3411–3414. [Google Scholar] [CrossRef]
- Nishi, M.; Chen, S.-Y.; Takagi, H. X-ray absorption spectroscopy of Ba- and Cs-promoted Ru/mesoporous carbon catalysts for long-term ammonia synthesis under intermittent operation conditions. Sustain. Energy Fuels 2020, 4, 832–842. [Google Scholar] [CrossRef]
- Chang, T.-C.; Chen, J.-J.; Yeh, C.-T. Temperature-programmed reduction and temperature-resolved sorption studies of strong metal-support interaction in supported palladium catalysts. J. Catal. 1985, 96, 51–57. [Google Scholar] [CrossRef]
- Guerrero-Ruiz, A.; Badenes, P.; Rodríguez-Ramos, I. Study of some factors affecting the Ru and Pt dispersions over high surface area graphite-supported catalysts. Appl. Catal. A Gen. 1998, 173, 313–321. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Nishi, M.; Mochizuki, T.; Takagi, H.; Takatsuki, A.; Roschat, W.; Toba, M.; Yoshimura, Y. Co-Processing of Jatropha-Derived Bio-Oil with Petroleum Distillates over Mesoporous CoMo and NiMo Sulfide Catalysts. Catalysts 2018, 8, 59. [Google Scholar] [CrossRef]
- Murthy, J.K.; Shekar, S.C.; Kumar, V.S.; Rao, K.S.R. Highly selective zirconium oxychloride modified Pd/C catalyst in the hydrodechlorination of dichlorodifluoromethane to difluoromethane. Catal. Commun. 2002, 3, 145–149. [Google Scholar] [CrossRef]
- Thunyaratchatanon, C.; Luengnaruemitchai, A.; Chollacoop, N.; Yoshimura, Y. Catalytic upgrading of soybean oil methyl esters by partial hydrogenation using Pd catalysts. Fuel 2016, 163, 8–16. [Google Scholar] [CrossRef]
- Na Rungsi, A.; Luengnaruemitchai, A.; Wongkasemjit, S.; Chollachoop, N.; Chen, S.-Y.; Yoshimura, Y. Influence of silica sources on structural property and activity of Pd-supported on mesoporous MCM-41 synthesized with an aid of microwave heating for partial hydrogenation of soybean methyl esters. Appl. Catal. A Gen. 2018, 563, 80–90. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, W.; Li, Y.; Lefferts, L. Effect of chlorine on performance of Pd catalysts prepared via colloidal immobilization. Catal. Today 2017, 297, 308–315. [Google Scholar] [CrossRef]
- Na Rungsi, A.; Luengnaruemitchai, A.; Chollachoop, N.; Chen, S.-Y.; Mochizuki, T.; Takagi, H.; Yoshimura, Y. Preparation of MCM-41-supported Pd–Pt catalysts with enhanced activity and sulfur resistance for partial hydrogenation of soybean oil-derived biodiesel fuel. Appl. Catal. A Gen. 2020, 590, 117351. [Google Scholar] [CrossRef]
- Vilarrasa-García, E.; Infantes-Molina, A.; Moreno-Tost, R.; Rodríguez-Castellón, E.; Jiménez-López, A.; Cavalcante, C.L., Jr.; Azevedo, D.C.S. Thiophene Adsorption on Microporous Activated Carbons Impregnated with PdCl2. Energy Fuels 2010, 24, 3436–3442. [Google Scholar] [CrossRef]
- Rodrigues, A.K.O.; Ramos, J.E.T.; Cavalcante, C.L., Jr.; Rodríguez-Castellón, E.; Azevedo, D.C.S. Pd-loaded mesoporous silica as a robust adsorbent in adsorption/desorption desulfurization cycles. Fuel 2014, 126, 96–103. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Chang, A.; Na Rungsi, A.; Attanatho, L.; Chang, C.-L.; Pan, J.-H.; Suemanotham, A.; Mochizuki, T.; Takagi, H.; Yang, C.-M.; et al. Superficial Pd nanoparticles supported on carbonaceous SBA-15 as efficient hydrotreating catalyst for upgrading biodiesel fuel. Appl. Catal. A Gen. 2020, 602, 117707. [Google Scholar] [CrossRef]
- Pullen, J.; Saeed, K. An overview of biodiesel oxidation stability. Renew. Sustain. Energy Rev. 2012, 16, 5924–5950. [Google Scholar] [CrossRef]
- Numwong, N.; Prabnasak, P.; Prayoonpunratn, P.; Triphatthanaphong, P.; Thunyaratchatanon, C.; Mochizuki, T.; Chen, S.-Y.; Luengnaruemitchai, A.; Sooknoi, T. Effect of Pd particle size on activity and cis-trans selectivity in partial hydrogenation of soybean oil-derived FAMEs over Pd/SiO2 catalysts. Fuel Process. Technol. 2020, 203, 106393. [Google Scholar] [CrossRef]
- Eldib, I.A.; Albright, L.F. Operating Variables in Hydrogenating Cottonseed Oil. Ind. Eng. Chem. 1957, 49, 825–831. [Google Scholar] [CrossRef]
- Albright, L.F. Quantitative measure of selectivity of hydrogenation of triglycerides. J. Am. Oil Chem. Soc. 1965, 42, 250–253. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Mochizuki, T.; Nishi, M.; Takagi, H.; Yoshimura, Y.; Toba, M. Hydrotreating of Jatropha-derived Bio-oil over Mesoporous Sulfide Catalysts to Produce Drop-in Transportation Fuels. Catalysts 2019, 9, 392. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Attanatho, L.; Mochizuki, T.; Abe, Y.; Toba, M.; Yoshimura, Y.; Kumpidet, C.; Somwonhsa, P.; Lao-ubol, S. Upgrading of palm biodiesel fuel over supported palladium catalysts. Comptes Rendus Chim. 2016, 19, 1166–1173. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Attanatho, L.; Mochizuki, T.; Qingxin, Z.; Toba, M.; Yoshimura, Y.; Somwongsa, P.; Lao-ubol, S. Influences of the Support Property and Pd Loading on Activity of Mesoporous Silica-Supported Pd Catalysts in Partial Hydrogenation of Palm Biodiesel Fuel. Adv. Porous Mater. 2016, 4, 230–237. [Google Scholar] [CrossRef]
- Department of Energy Business, Thailand Ministry of Energy. Specification of Fatty Acid Methyl Ester (B100): 2009. Available online: https://www.dede.go.th/ewt_dl_link.php?nid=373 (accessed on 13 July 2009).
- Department of Energy Business, Thailand Ministry of Energy. Specification of Alternative Diesel (B7): 2014. Available online: https://www.dede.go.th/ewt_dl_link.php?nid=49989 (accessed on 2 May 2014).
- Serrano, M.; Oliveros, R.; Sánchez, M.; Moraschini, A.; Martínez, M.; Aracil, J. Influence of blending vegetable oil methyl esters on biodiesel fuel properties: Oxidative stability and cold flow properties. Energy 2014, 65, 109–115. [Google Scholar] [CrossRef]
- Nainwal, S.; Sharma, N.; Sharma, A.S.; Jain, S.; Jain, S. Cold flow properties improvement of Jatropha curcas biodiesel and waste cooking oil biodiesel using winterization and blending. Energy 2015, 89, 702–707. [Google Scholar] [CrossRef]


| Sample | Pd (wt.%) | Cl 1 (wt.%) | SBET (m2 g−1) | Dp (nm) | Vtotal (cm3 g−1) | Pd Dispersion 2 (%) | Pd Size (nm) | Acidity | Pd0/Pd2+ Ratio 5 | |
|---|---|---|---|---|---|---|---|---|---|---|
| CO Chem. 2 | TEM 3 | (mmol g−1) 4 | ||||||||
| AC | - | n.d. | 1782 | 0.87 | 0.70 | - | - | - | 0.01 | - |
| 1%Pd/AC (nit) | 0.92 | n.d. | 1746 | 0.80 | 0.63 | 5.8 | 8.3 | 8.4 ± 1.5 | 0.10 | 3.6 |
| 1%Pd/AC (amc) | 1.03 | 0.11 | 1777 | 0.80 | 0.70 | 59 | 0.8 | 2.7 ± 0.8 | 0.12 | 2.8 |
| 5%Pd/AC (com) | 4.82 | n.d. | 919 | 0.77 | 0.46 | 15 | 3.3 | - | 0.51 | - |
| 1%Pd/AC (nit) | 1%Pd/AC (amc) | 5%Pd/AC (com) | |
|---|---|---|---|
| Initial rate (mmol gpd−1 s−1) 2 | 544 | 258 | 307 |
| Turnover frequency (TOF) (s−1) 2 | 88 | 4.1 | 19 |
| Ratio of cis-/trans-C18:1 3 | 1.4 | 1.3 | n.d. 4 |
| Degree of complete hydrogenation (%) 3 | 16.1 | 157 | n.d. 4 |
| k1 (s−1 g−1) | 0.23 | 0.082 | 0.064 |
| k2 (s−1 g−1) | 0.091 | 0.136 | n.d. 4 |
| S = k1/k2 | 2.6 | 0.60 | n.d. 4 |
| Palm-BDF | H-FAME | ||||
|---|---|---|---|---|---|
| 1%Pd/AC (amc) 1 | 1%Pd/AC (nit) 1 | 2%Pd/CA 2 | 2%Pd/AC 2 | ||
| Pd size (nm) | - | 2.7 | 8.4 | 10 | 18 |
| FAME composition (wt.%) | |||||
| C18:3 | 0.1 | 0.0 | 0.0 | - | - |
| C18:2 | 8.5 | 0.9 | 0.9 | - | - |
| Total C18:1 | 36.2 | 17.1 | 37.8 | - | - |
| cis-C18:1 | 36.1 | 9.5 | 22.0 | - | - |
| trans-C18:1 | 0.1 | 7.6 | 15.8 | - | - |
| C18:0 | 4.8 | 31.6 | 10.9 | - | - |
| Poly-FAME | 8.6 | 0.9 | 0.9 | 0 | 0 |
| Mono-FAME | 36.2 | 17.1 | 38.0 | 4.26 | 28.1 |
| Sat-FAME | 52.6 | 81.4 | 60.9 | 95.7 | 71.9 |
| Fuel properties | |||||
| Oxidative stability (h) | 13 | 61 | 60 | 41 | 36 |
| Cloud point (°C) | 15 | 28 | 18 | 26 | 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udomsap, P.; Eiad-Ua, A.; Chen, S.-Y.; Mochizuki, T.; Chollacoop, N.; Yoshimura, Y.; Nishi, M.; Tateno, H.; Takagi, H. Effect of Pd Precursor Salts on the Chemical State, Particle Size, and Performance of Activated Carbon-Supported Pd Catalysts for the Selective Hydrogenation of Palm Biodiesel. Int. J. Mol. Sci. 2021, 22, 1256. https://doi.org/10.3390/ijms22031256
Udomsap P, Eiad-Ua A, Chen S-Y, Mochizuki T, Chollacoop N, Yoshimura Y, Nishi M, Tateno H, Takagi H. Effect of Pd Precursor Salts on the Chemical State, Particle Size, and Performance of Activated Carbon-Supported Pd Catalysts for the Selective Hydrogenation of Palm Biodiesel. International Journal of Molecular Sciences. 2021; 22(3):1256. https://doi.org/10.3390/ijms22031256
Chicago/Turabian StyleUdomsap, Parncheewa, Apiluck Eiad-Ua, Shih-Yuan Chen, Takehisa Mochizuki, Nuwong Chollacoop, Yuji Yoshimura, Masayasu Nishi, Hiroyuki Tateno, and Hideyuki Takagi. 2021. "Effect of Pd Precursor Salts on the Chemical State, Particle Size, and Performance of Activated Carbon-Supported Pd Catalysts for the Selective Hydrogenation of Palm Biodiesel" International Journal of Molecular Sciences 22, no. 3: 1256. https://doi.org/10.3390/ijms22031256
APA StyleUdomsap, P., Eiad-Ua, A., Chen, S.-Y., Mochizuki, T., Chollacoop, N., Yoshimura, Y., Nishi, M., Tateno, H., & Takagi, H. (2021). Effect of Pd Precursor Salts on the Chemical State, Particle Size, and Performance of Activated Carbon-Supported Pd Catalysts for the Selective Hydrogenation of Palm Biodiesel. International Journal of Molecular Sciences, 22(3), 1256. https://doi.org/10.3390/ijms22031256







