Asciminib Mitigates DNA Damage Stress Signaling Induced by Cyclophosphamide in the Ovary
Abstract
:1. Introduction
2. Results
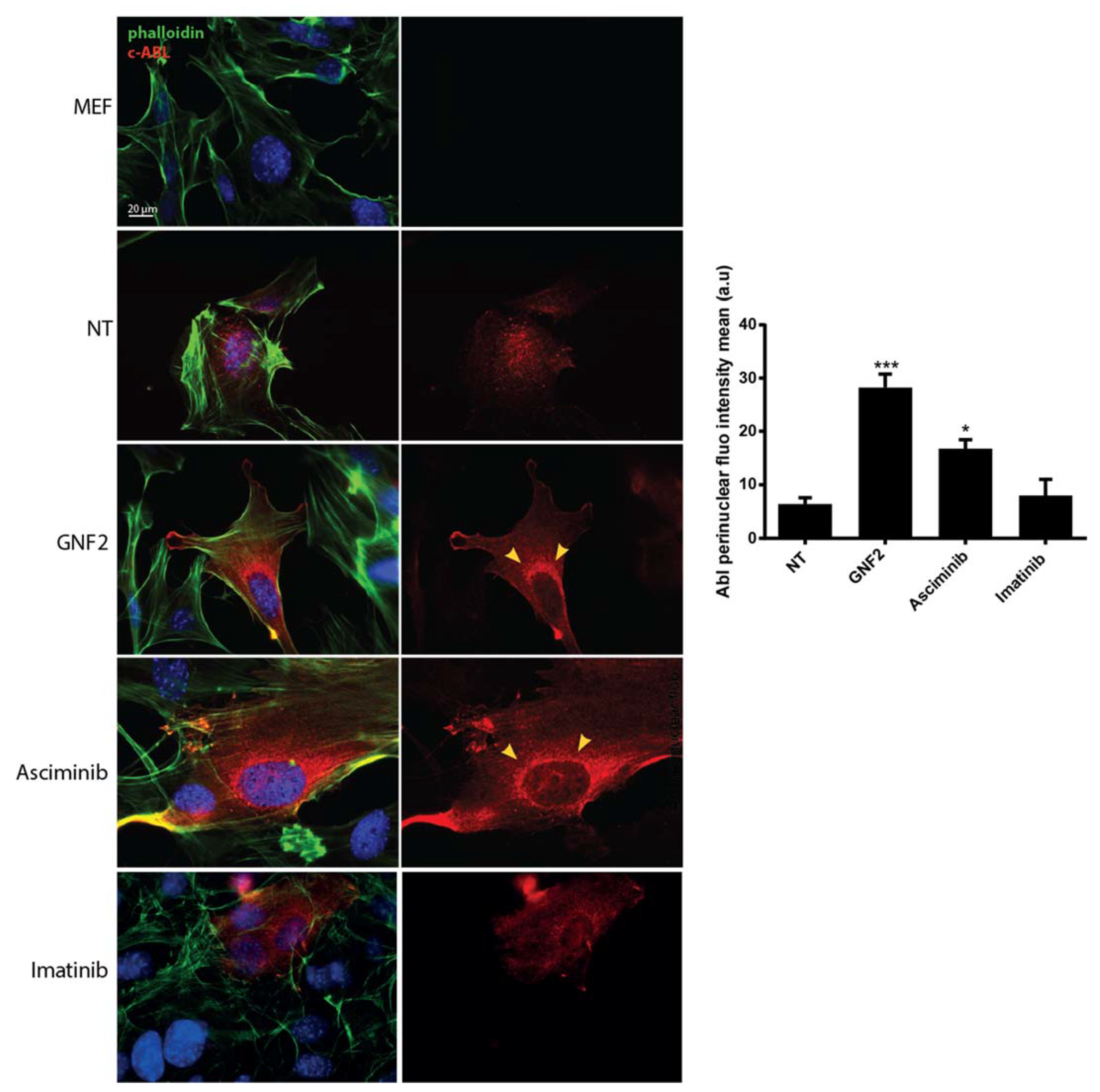
2.1. Asciminib Induced a Re-Localization of the C-Abl Tyrosine Kinase to the Perinuclear Zone
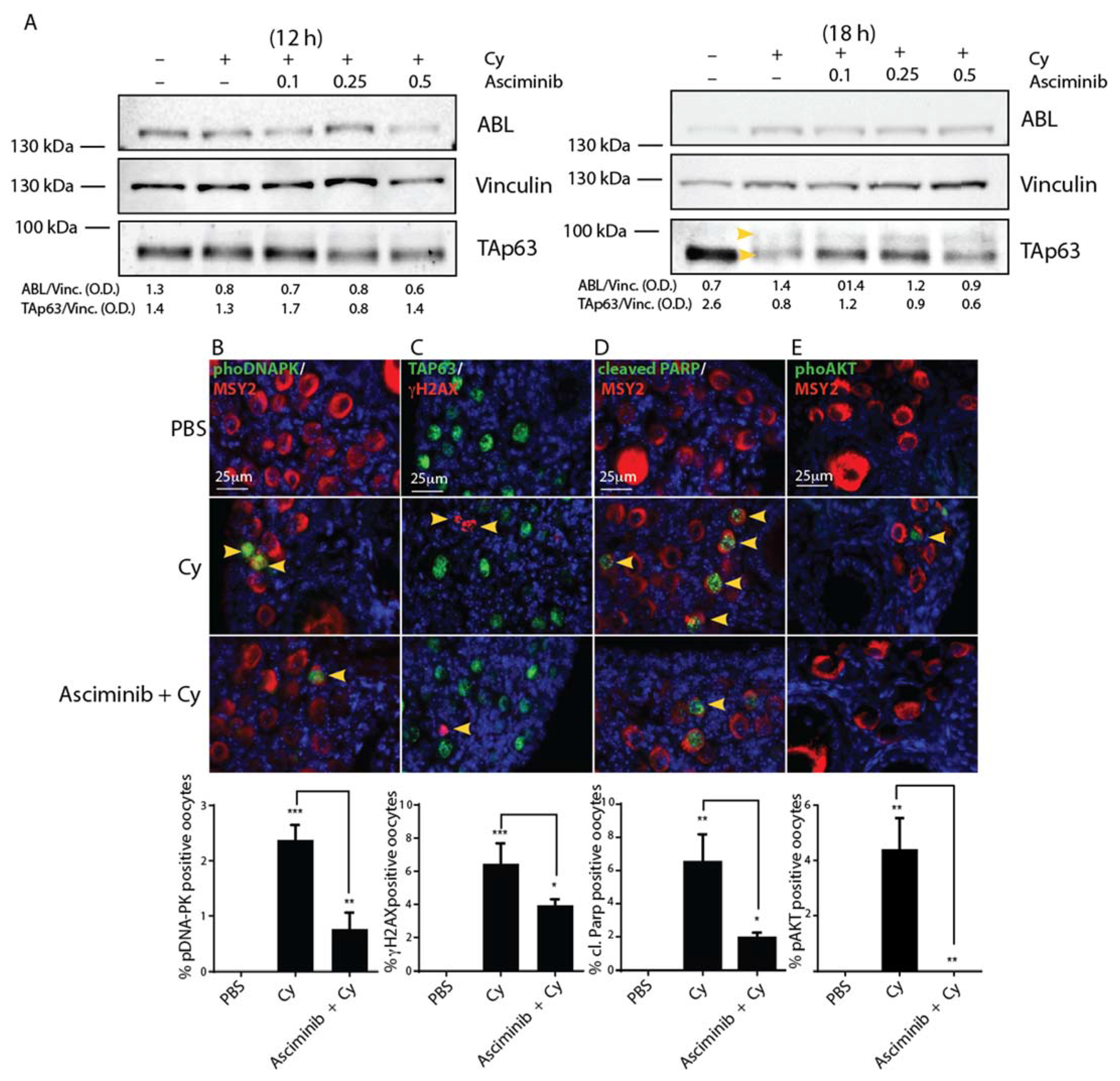
2.2. Asciminib Modulated the DDR and the Follicle Activation Induced by Cy Exposure In Vivo
2.3. Asciminib Protected the Ovarian Reserve Following Cy Treatment
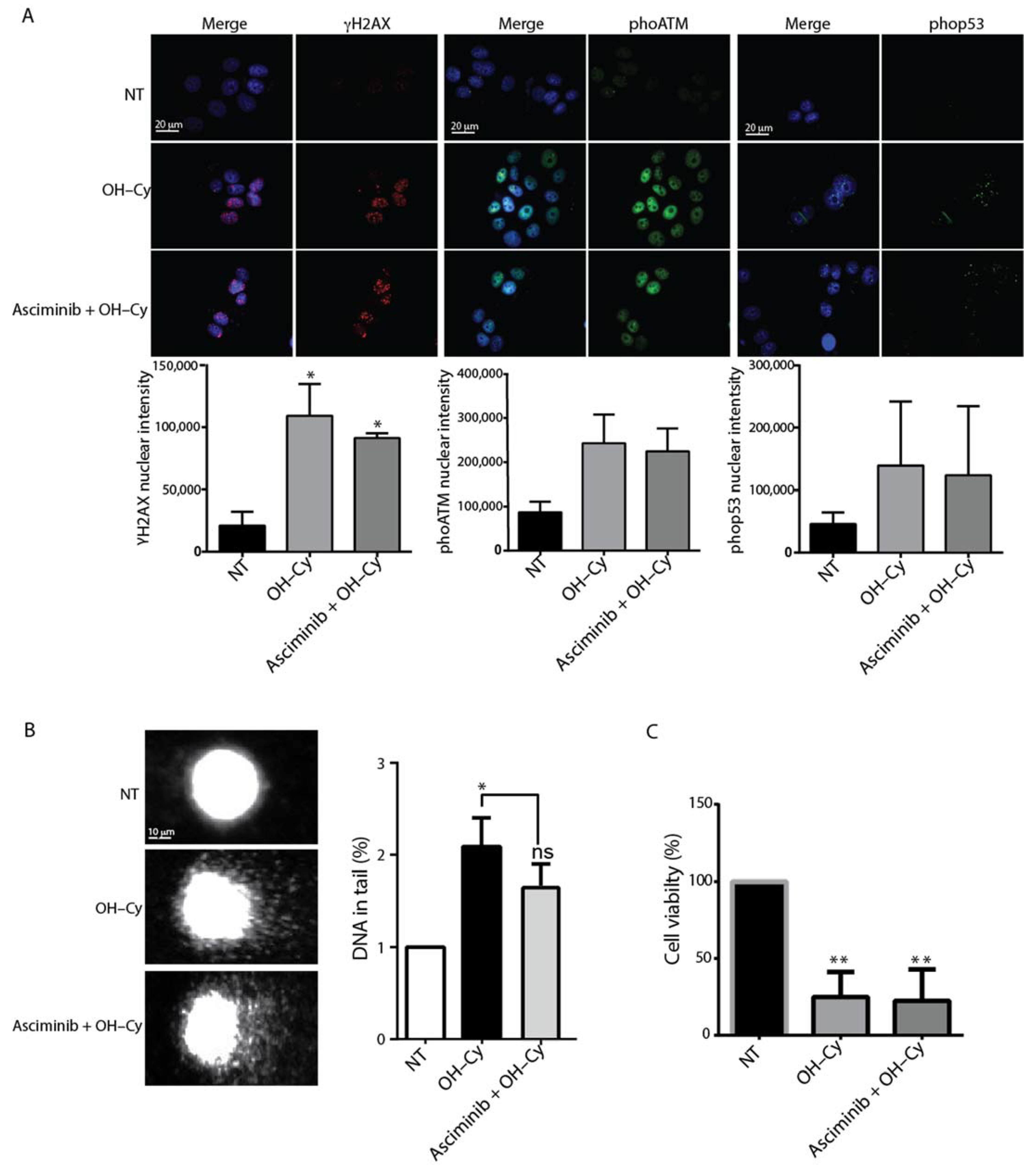
2.4. Asciminib Did Not Prevent the DNA Damage Induced by Cy in MCF7
3. Discussion
4. Materials and Methods
4.1. Animals and Injection
4.2. Immunohistochemistry, Follicle Counting, and Statistical Analysis
4.3. Immunofluorescence and Immunoblot Analysis
4.4. Cell Culture
4.5. Cell Transfection and Immunofluorescence Assay
4.6. Single-Cell Gel Electrophoresis Assay (Comet Assay)
4.7. MTS Assay
4.8. Reagents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Cy | cyclosphosphamide |
| DDR | DNA damage response |
| AKT | protein kinase B |
| FOXO3a | forkhead box O3 |
| PBS | phosphate-buffered saline |
| 4-OH-Cy | 4-hydroperoxy-cyclophosphamide |
| IF | immunofluorescence |
| MCF7 | Michigan Cancer Foundation-7 |
| MEF | mouse embryonic fibroblasts |
| γH2AX | phosphorylated (Ser319) of histone 2AX |
| DNA-PK | DNA-dependent protein kinase, catalytic subunit |
| PARP | poly (ADP-ribose) polymerase |
| POF | premature ovarian failure |
| DSBS | double strand breaks |
| ATP | adenosine triphosphate |
| CML | chronic myeloid leukemia |
| IHC | immunohistochemistry |
| DMSO | dimethyl sulfoxide |
| WB | Western blotting |
References
- Oktem, O.; Oktay, K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 2007, 110, 2222–2229. [Google Scholar] [CrossRef]
- Meirow, D.; Biederman, H.; Anderson, R.A.; Wallace, W.H.B. Toxicity of Chemotherapy and Radiation on Female Reproduction. Clin. Obstet. Gynecol. 2010, 53, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.K.; Finlayson, C.; Rowell, E.E.; Gosiengfiao, Y.; Pavone, M.E.; Lockart, B.; Orwig, K.E.; Brannigan, R.E.; Woodruff, T.K. Fertility Preservation for Pediatric Patients: Current State and Future Possibilities. J. Urol. 2017, 198, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Sonmezer, M.; Oktay, K. Fertility preservation in female patients. Hum. Reprod. Update 2004, 10, 251–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalich-Philosoph, L.; Roness, H.; Carmely, A.; Fishel-Bartal, M.; Ligumsky, H.; Paglin, S.; Wolf, I.; Kanety, H.; Sredni, B.; Meirow, D. Cyclophosphamide Triggers Follicle Activation and "Burnout"; AS101 Prevents Follicle Loss and Preserves Fertility. Sci. Transl. Med. 2013, 5, 185ra62. [Google Scholar] [CrossRef] [PubMed]
- Bellusci, G.; Mattiello, L.; Iannizzotto, V.; Ciccone, S.; Maiani, E.; Villani, V.; Diederich, M.; Gonfloni, S. Kinase-independent inhibition of cyclophosphamide-induced pathways protects the ovarian reserve and prolongs fertility. Cell Death Dis. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Luan, Y.; Edmonds, M.E.; Woodruff, T.K.; Kim, S.-Y. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. J. Endocrinol. 2019, 240, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, Q.N.; Zerafa, N.; Liew, S.H.; Findlay, J.K.; Hickey, M.; Hutt, K.J. Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Mol. Hum. Reprod. 2019, 25, 433–444. [Google Scholar] [CrossRef]
- Roness, H.; Kashi, O.; Meirow, D. Prevention of chemotherapy-induced ovarian damage. Fertil. Steril. 2016, 105, 20–29. [Google Scholar] [CrossRef]
- Roness, H.; Kalich-Philosoph, L.; Meirow, D. Prevention of chemotherapy-induced ovarian damage: Possible roles for hormonal and non-hormonal attenuating agents. Hum. Reprod. Update 2014, 20, 759–774. [Google Scholar] [CrossRef] [Green Version]
- Kano, M.; Sosulski, A.E.; Zhang, L.; Saatcioglu, H.D.; Wang, D.; Nagykery, N.; Sabatini, M.E.; Gao, G.; Donahoe, P.K.; Pépin, D. AMH/MIS as a contraceptive that protects the ovarian reserve during chemotherapy. Proc. Natl. Acad. Sci. USA 2017, 114, E1688–E1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonigo, C.; Beau, I.; Grynberg, M.; Binart, N. AMH prevents primordial ovarian follicle loss and fertility alteration in cyclophosphamide-treated mice. FASEB J. 2018, 33, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Seeliger, M.A.; Panjarian, S.B.; Kim, H.; Deng, X.; Sim, T.; Couch, B.; Koleske, A.J.; Smithgall, T.E.; Gray, N.S. N-Myristoylated c-Abl Tyrosine Kinase Localizes to the Endoplasmic Reticulum upon Binding to an Allosteric Inhibitor. J. Biol. Chem. 2009, 284, 29005–29014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedoschi, G.; Navarro, P.A.; Oktay, K. Chemotherapy-induced damage to ovary: Mechanisms and clinical impact. Future Oncol. 2016, 12, 2333–2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, S.; Anderson, R.; Gourley, C.; Wallace, W.; Spears, N. How do chemotherapeutic agents damage the ovary? Hum. Reprod. Update 2012, 18, 525–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spears, N.; Lopes, F.; Stefansdottir, A.; Rossi, V.; De Felici, M.; Anderson, R.A.; Klinger, F.G. Ovarian damage from chemotherapy and current approaches to its protection. Hum. Reprod. Update 2019, 25, 673–693. [Google Scholar] [CrossRef]
- Szymanska, K.J.; Tan, X.; Oktay, K. Unraveling the mechanisms of chemotherapy-induced damage to human primordial follicle reserve: Road to developing therapeutics for fertility preservation and reversing ovarian aging. Mol. Hum. Reprod. 2020, 26, 553–566. [Google Scholar] [CrossRef]
- Suh, E.-K.; Yang, A.; Kettenbach, A.N.; Bamberger, C.; Michaelis, A.H.; Zhu, Z.; Elvin, J.A.; Bronson, R.T.; Crum, C.P.; McKeon, F. p63 protects the female germ line during meiotic arrest. Nat. Cell Biol. 2006, 444, 624–628. [Google Scholar] [CrossRef]
- Livera, G.; Petre-Lazar, B.; Guerquin, M.-J.; Trautmann, E.; Coffigny, H.; Habert, R. p63 null mutation protects mouse oocytes from radio-induced apoptosis. Reproduction 2007, 135, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Kerr, J.B.; Hutt, K.J.; Michalak, E.M.; Cook, M.; Vandenberg, C.J.; Liew, S.H.; Bouillet, P.; Mills, A.; Scott, C.L.; Findlay, J.K.; et al. DNA Damage-Induced Primordial Follicle Oocyte Apoptosis and Loss of Fertility Require TAp63-Mediated Induction of Puma and Noxa. Mol. Cell 2012, 48, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Bolcun-Filas, E.; Rinaldi, V.D.; White, M.E.; Schimenti, J.C. Reversal of Female Infertility by Chk2 Ablation Reveals the Oocyte DNA Damage Checkpoint Pathway. Science 2014, 343, 533–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, Q.-N.; Zerafa, N.; Liew, S.H.; Morgan, F.H.; Strasser, A.; Scott, C.L.; Findlay, J.K.; Hickey, M.; Hutt, K.J. Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Cell Death Dis. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Nair, D.M.; Romero, M.; Serna, V.A.; Koleske, A.J.; Woodruff, T.K.; Kurita, T. Transient inhibition of p53 homologs protects ovarian function from two distinct apoptotic pathways triggered by anticancer therapies. Cell Death Differ. 2019, 26, 502–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonfloni, S.; Di Tella, L.; Caldarola, S.; Cannata, S.M.; Klinger, F.G.; Di Bartolomeo, C.; Mattei, M.; Candi, E.; De Felici, M.; Melino, G.; et al. Inhibition of the c-Abl–TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat. Med. 2009, 15, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Cordeiro, M.H.; Serna, V.A.; Ebbert, K.; Butler, L.M.; Sinha, S.C.; Mills, A.A.; Woodruff, T.K.; Kurita, T. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 2013, 20, 987–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinaldi, V.D.; Hsieh, K.; Munroe, R.; Bolcun-Filas, E.; Schimenti, J.C. Pharmacological Inhibition of the DNA Damage Checkpoint Prevents Radiation-Induced Oocyte Death. Genetics 2017, 206, 1823–1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodruff, T.K. Preserving fertility during cancer treatment. Nat. Med. 2009, 15, 1124–1125. [Google Scholar] [CrossRef] [PubMed]
- Gonfloni, S. Modulating c-Abl nuclear activity as a strategy to preserve female fertility. Cell Cycle 2010, 9, 217–218. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, V.D.; Bloom, J.C.; Schimenti, J.C. Oocyte Elimination Through DNA Damage Signaling from CHK1/CHK2 to p53 and p63. Genetics 2020, 215, 373–378. [Google Scholar] [CrossRef] [Green Version]
- McGinnis, L.; Carroll, D.J.; Kinsey, W.H. Protein tyrosine kinase signaling during oocyte maturation and fertilization. Mol. Reprod. Dev. 2011, 78, 831–845. [Google Scholar] [CrossRef] [Green Version]
- Gonfloni, S.; Maiani, E.; Di Bartolomeo, C.; Diederich, M.; Cesareni, G. Oxidative Stress, DNA Damage, and c-Abl Signaling: At the Crossroad in Neurodegenerative Diseases? Int. J. Cell Biol. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Wang, J.; Pendergast, A.M. Mutifunctional Abl kinases in health and disease. J. Cell Sci. 2016, 129, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiani, E.; Di Bartolomeo, C.; Klinger, F.G.; Cannata, S.M.; Bernardini, S.; Chateauvieux, S.; Mack, F.; Mattei, M.; De Felici, M.; Diederich, M.; et al. Reply to: Cisplatin-induced primordial follicle oocyte killing and loss of fertility are not prevented by imatinib. Nat. Med. 2012, 18, 1172–1174. [Google Scholar] [CrossRef] [PubMed]
- Vandormaelpournin, S.; Guigon, C.J.; Ishaq, M.; Coudouel, N.; Ave, P.; Huerre, M.; Magre, S.; Cohentannoudji, J. Oocyte-specific inactivation of Omcg1 leads to DNA damage and c-Abl/TAp63-dependent oocyte death associated with dramatic remodeling of ovarian somatic cells. Cell Death Differ. 2014, 22, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Manley, P.W.; Barys, L.; Cowan-Jacob, S.W. The specificity of asciminib, a potential treatment for chronic myeloid leukemia, as a myristate-pocket binding ABL inhibitor and analysis of its interactions with mutant forms of BCR-ABL1 kinase. Leuk. Res. 2020, 98, 106458. [Google Scholar] [CrossRef]
- Reddy, E.P.; Aggarwal, A.K. The Ins and Outs of Bcr-Abl Inhibition. Genes Cancer 2012, 3, 447–454. [Google Scholar] [CrossRef]
- Adrián, F.J.; Ding, Q.; Sim, T.; Velentza, A.V.; Sloan, C.; Liu, Y.; Zhang, G.; Hur, W.; Ding, S.; Manley, P.W.; et al. Allosteric inhibitors of Bcr-abl–dependent cell proliferation. Nat. Chem. Biol. 2006, 2, 95–102. [Google Scholar] [CrossRef]
- Zhan, J.-Y.; Ma, J.; Zheng, Q. Molecular dynamics investigation on the Asciminib resistance mechanism of I502L and V468F mutations in BCR-ABL. J. Mol. Graph. Model. 2019, 89, 242–249. [Google Scholar] [CrossRef]
- Schoepfer, J.; Jahnke, W.; Berellini, G.; Buonamici, S.; Cotesta, S.; Cowan-Jacob, S.W.; Dodd, S.; Drueckes, P.; Fabbro, D.; Gabriel, T.; et al. Discovery of Asciminib (ABL001), an Allosteric Inhibitor of the Tyrosine Kinase Activity of BCR-ABL1. J. Med. Chem. 2018, 61, 8120–8135. [Google Scholar] [CrossRef] [Green Version]
- Wylie, A.A.; Schoepfer, J.; Jahnke, W.; Cowan-Jacob, S.W.; Loo, A.; Furet, P.; Marzinzik, A.L.; Pelle, X.; Donovan, J.; Zhu, W.; et al. The allosteric inhibitor ABL001 enables dual targeting of BCR–ABL1. Nat. Cell Biol. 2017, 543, 733–737. [Google Scholar] [CrossRef]
- Hughes, T.P.; Mauro, M.J.; Cortes, J.E.; Minami, H.; Rea, D.; DeAngelo, D.J.; Breccia, M.; Goh, Y.-T.; Talpaz, M.; Hochhaus, A.; et al. Asciminib in Chronic Myeloid Leukemia after ABL Kinase Inhibitor Failure. N. Engl. J. Med. 2019, 381, 2315–2326. [Google Scholar] [CrossRef] [PubMed]
- Voronin, M.V.; Kadnikov, I.A. Contribution of Sigma-1 receptor to cytoprotective effect of afobazole. Pharmacol. Res. Perspect. 2016, 4, e00273. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattiello, L.; Pucci, G.; Marchetti, F.; Diederich, M.; Gonfloni, S. Asciminib Mitigates DNA Damage Stress Signaling Induced by Cyclophosphamide in the Ovary. Int. J. Mol. Sci. 2021, 22, 1395. https://doi.org/10.3390/ijms22031395
Mattiello L, Pucci G, Marchetti F, Diederich M, Gonfloni S. Asciminib Mitigates DNA Damage Stress Signaling Induced by Cyclophosphamide in the Ovary. International Journal of Molecular Sciences. 2021; 22(3):1395. https://doi.org/10.3390/ijms22031395
Chicago/Turabian StyleMattiello, Luca, Giulia Pucci, Francesco Marchetti, Marc Diederich, and Stefania Gonfloni. 2021. "Asciminib Mitigates DNA Damage Stress Signaling Induced by Cyclophosphamide in the Ovary" International Journal of Molecular Sciences 22, no. 3: 1395. https://doi.org/10.3390/ijms22031395







