Expression of Recombinant Human Octamer-Binding Transcription Factor 4 in Rice Suspension Cells
Abstract
1. Introduction
2. Results and Discussion
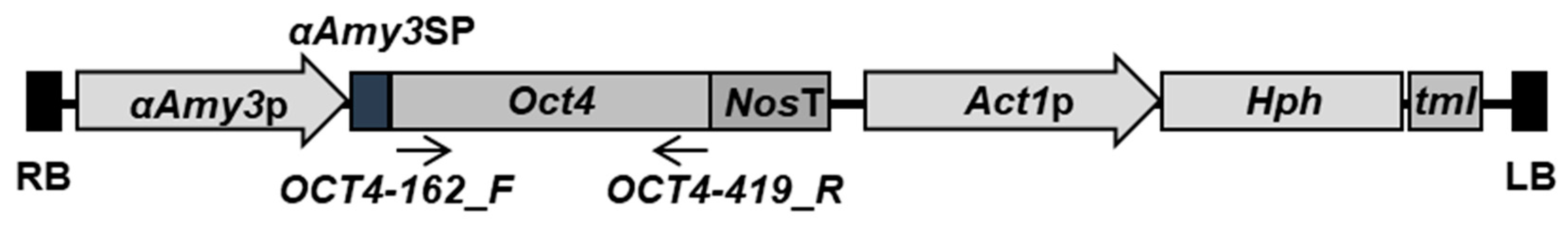
2.1. Generation of Transgenic Rice Cell Lines Harboring αAmy3p-SP-Oct4 Gene
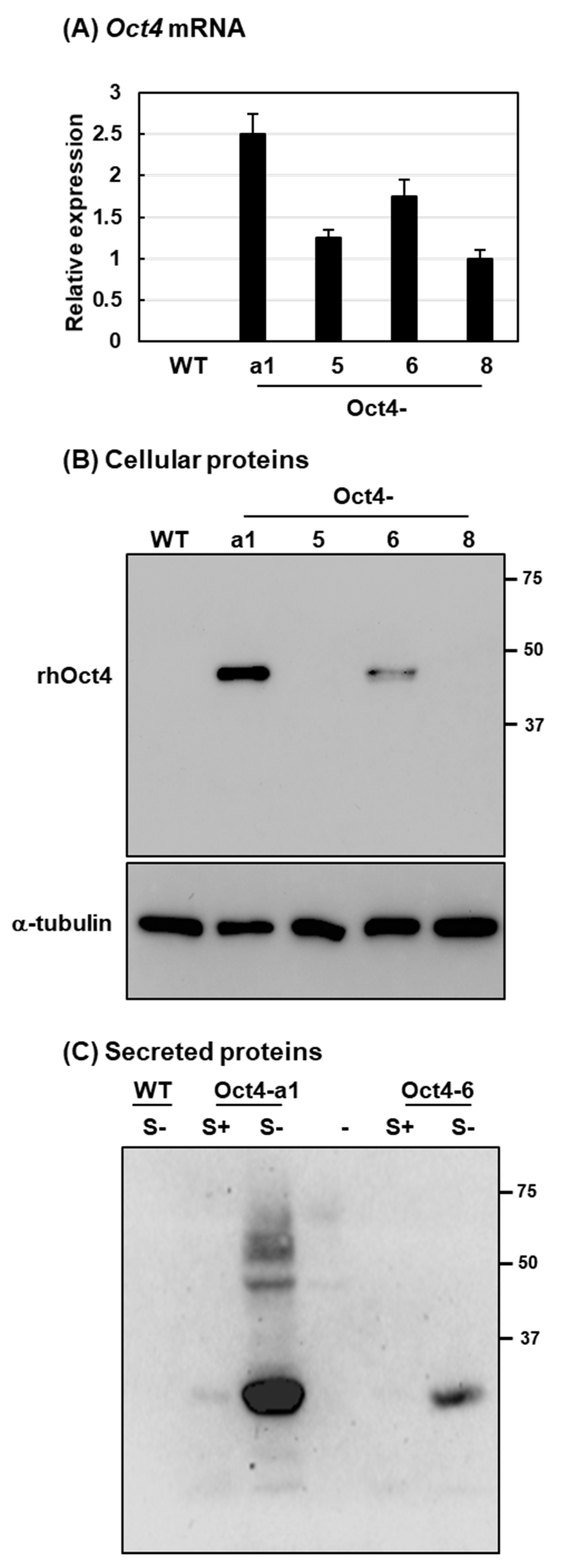
2.2. Recombinant Human Oct4 Proteins Were Produced by Transgenic Rice Suspension Cell Lines
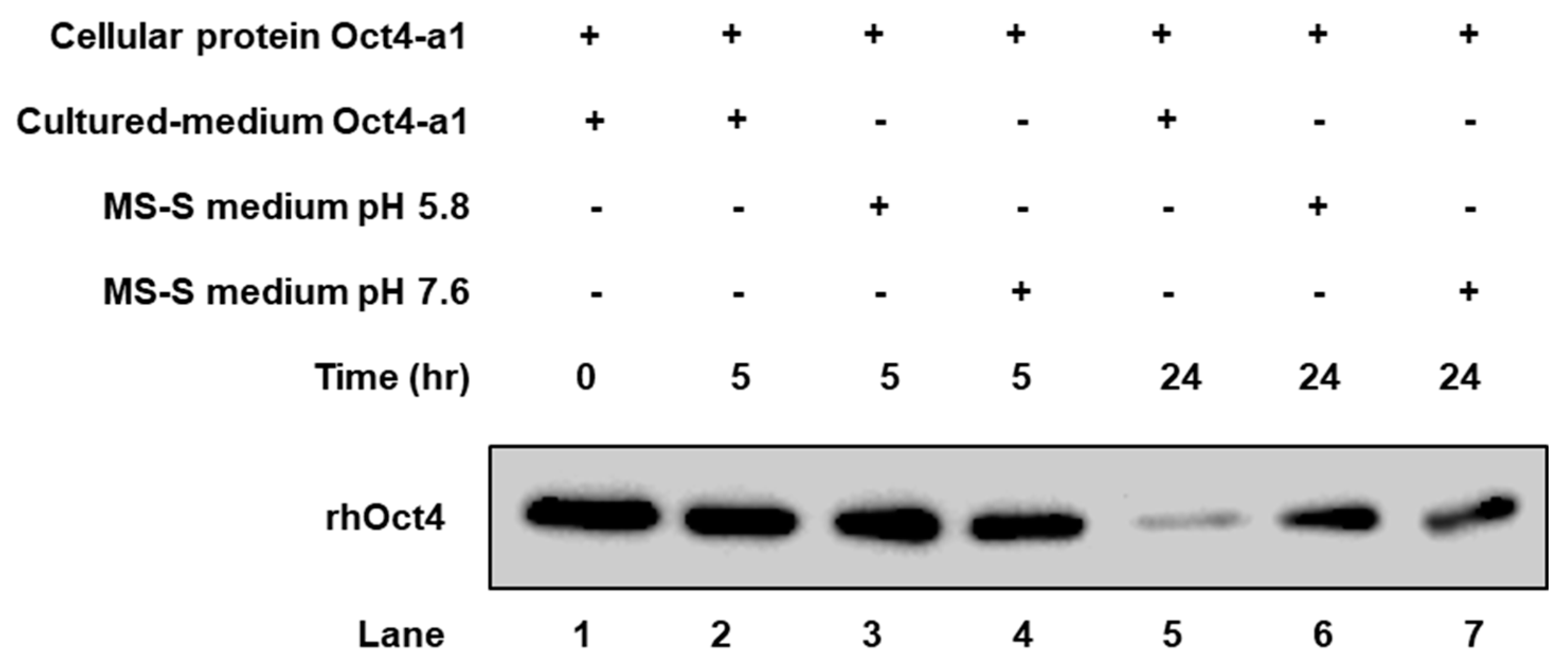
2.3. The rhOct4 Protein Is Unstable in Sugar-Free Culture Medium
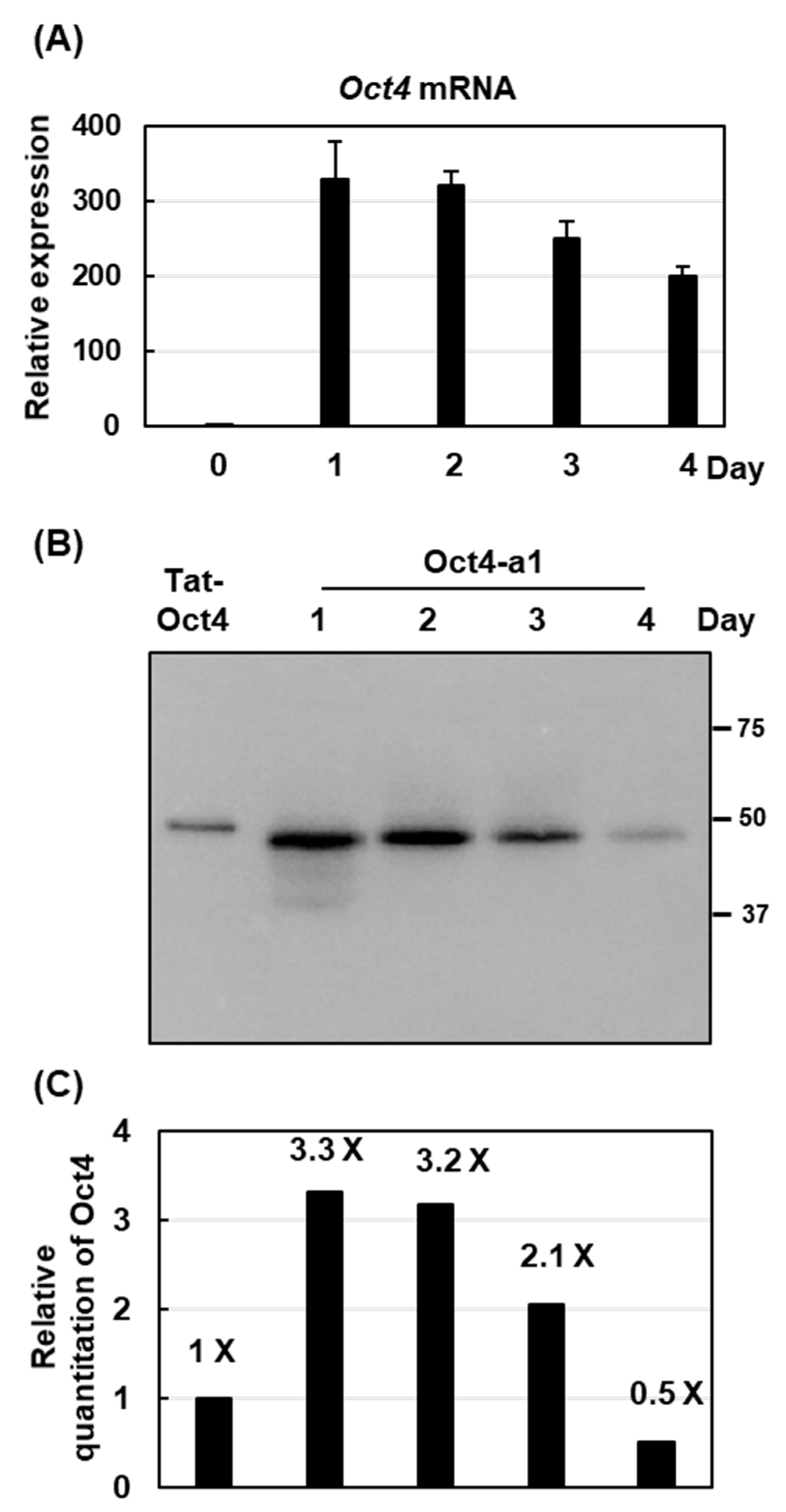
2.4. The Highest Production of Oct4 Protein Was 0.41% of Total Soluble Proteins
2.5. Rice Cells Produce Biologically Active rhOct4
3. Materials and Methods
3.1. Plant Materials and Growth Conditions
3.2. Plasmid Construction
3.3. Rice Transformation
3.4. PCR-Base Genotype Analysis
3.5. Quantitative RT-PCR
3.6. Western Blot Analysis
3.7. Oct4 Transcription Factor Binding Assay
3.8. In-Gel Protease Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Oct4 | Octamer-binding Transcription Factor 4 |
| iPSCs | induced pluripotent stem cells |
| qRT-PCR | quantitative real-time PCR |
| MS medium | Murashige and Skoog medium |
References
- Elitt, M.S.; Barbar, L.; Tesar, P.J. Drug screening for human genetic diseases using iPSC models. Hum. Mol. Genet. 2018, 27, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Blangero, J.; Curran, J.E. Induced pluripotent stem cells in disease modeling and gene identification. Methods Mol. Biol. 2018, 1706, 17–38. [Google Scholar] [PubMed]
- Chun, Y.S.; Chaudhari, P.; Jang, Y.Y. Applications of patient-specific induced pluripotent stem cells; focused on disease modeling, drug screening and therapeutic potentials for liver disease. Int. J. Biol. Sci. 2010, 6, 796–805. [Google Scholar] [CrossRef]
- Zaret, K.S.; Mango, S.E. Pioneer transcription factors, chromatin dynamics, and cell fate control. Curr. Opin. Genet. Dev. 2016, 37, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Schöler, H.R.; Hatzopoulos, A.K.; Balling, R.; Suzuki, N.; Gruss, P. A family of octamer-specific proteins present during mouse embryogenesis: Evidence for germline-specific expression of an Oct factor. EMBO J. 1989, 8, 2543–2550. [Google Scholar] [CrossRef] [PubMed]
- Schöler, H.R.; Balling, R.; Hatzopoulos, A.K.; Suzuki, N.; Gruss, P. Octamer binding proteins confer transcriptional activity in early mouse embryogenesis. EMBO J. 1989, 8, 2551–2557. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Karwacki-Neisius, V.; Göke, J.; Osorno, R.; Halbritter, F.; Ng, J.H.; Weiße, A.Y.; Wong, F.C.; Gagliardi, A.; Mullin, N.P.; Festuccia, N.; et al. Reduced Oct4 expression directs a robust pluripotent state with distinct signaling activity and increased enhancer occupancy by Oct4 and Nanog. Cell Stem Cell. 2013, 12, 531–545. [Google Scholar] [CrossRef]
- Niwa, H.; Miyazaki, J.; Smith, A.G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 2000, 24, 372–376. [Google Scholar] [CrossRef]
- Thier, M.; Münst, B.; Edenhofe, F. Exploring refined conditions for reprogramming cells by recombinant Oct4 protein. Int. J. Dev. Biol. 2010, 54, 1713–1721. [Google Scholar] [CrossRef]
- Chou, B.K.; Mali, P.; Huang, X.; Ye, Z.; Dowey, S.N.; Resar, L.M.S.; Zou, C.; Zhang, Y.A.; Tong., J.; Cheng, L. Efficient human iPS cell deri- vation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011, 21, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Woltjen, K.; Michael, I.P.; Mohseni, P.; Desai, R.; Mileikovsky, M.; Hamalainen, R.; Cowling, R.; Wang, W.; Liu, P.; Gertsenstein, M.; et al. PiggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 2009, 458, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Li, Y.; Zhang, X.; Liu, C.; Guan, J.; Li, H.; Zhao, T.; Ye, J.; Yang, W.; Liu, K.; et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 2013, 341, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Lee, C.S.; Kwon, Y.W.; Paek, J.S.; Lee, S.H.; Hur, J.; Lee, E.J.; Roh, T.Y.; Chu, I.S.; Leem, S.H.; et al. Induction of pluripotent stem cells from adult somatic cells by protein-based reprogramming without genetic manipulation. Blood 2010, 11, 386–395. [Google Scholar] [CrossRef]
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.H.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.K.; Smith, Z.D.; Meissner, A.; et al. Highly efficient reprogramming to pluripo- tency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010, 7, 618–630. [Google Scholar] [CrossRef]
- Sterneckert, J.; Höing, S.; Schöler, H.R. Concise review: Oct4 and more: The reprogramming expressway. Stem Cells 2012, 30, 15–21. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Y.; Gu, J.; Liao, B.; Li, J.; Wong, J.; Jin, Y. Reprogramming of somatic cells via TAT-mediated protein transduction of recombinant factors. Biomaterials 2012, 33, 5047–5055. [Google Scholar] [CrossRef]
- Kim, D.; Kim, C.H.; Moon, J.I.; Chung, Y.G.; Chang, M.Y.; Han, B.S.; Ko, S.; Yang, E.; Cha, K.Y.; Lanza, R.; et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 2009, 4, 472–476. [Google Scholar] [CrossRef]
- Pan, C.; Jia, W.; Lu, B.; Bishop, C.E. Expression of TAT recombinant Oct4, Sox2, Lin28, and Nanog proteins from baculovirus-infected Sf9 insect cells. Gene 2015, 556, 245–248. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Kong, N.; Wei, A.; Zhang, Y.; Ma, J.; Zhou, Y.; Yan, W. Expression, purification and characterization of a recombinant Tat47-57-Oct4 fusion protein in Pichia pastoris. Mol. Med. Rep. 2014, 9, 471–475. [Google Scholar] [CrossRef]
- Liu, G.D.; Zhou, S.F.; Ding, X.C.; Fang, C.L.; Mi, S.Y.; Gao, X.C.; Han, Q. Soluble expression of recombinant cMyc, Klf4, Oct4, and Sox2 proteins in bacteria and transduction into living cells. Int. J. Ophthalmol. 2017, 10, 560–566. [Google Scholar] [PubMed]
- Andrews, G.R.; Roberts, S.C. Bioprocess Engineering of Plant Cell Suspension Cultures. In Applied Bioengineering; Yoshida, T., Ed.; Wiley: New Jersey, NJ, USA, 2017; pp. 283–326. [Google Scholar]
- Nishimura, A.; Aichi, I.; Matsuoka, M. A protocol for Agrobacterium-mediated transformation in rice. Nat. Protoc. 2006, 1, 2796–2802. [Google Scholar] [CrossRef] [PubMed]
- Hiei, Y.; Ohta, S.; Komari, T.; Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994, 6, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Tan, C.C.; Ku, J.T.; Hsu, W.C.; Su, S.C.; Lu, C.A.; Huang, L.F. Improving pharmaceutical protein production in Oryza sativa. Int. J. Mol. Sci. 2013, 14, 8719–8739. [Google Scholar] [CrossRef]
- Huang, L.F.; Liu, Y.K.; Lu, C.A.; Hsieh, S.L.; Yu, S.M. Production of human serum albumin by sugar starvation induced promoter and rice cell culture. Transgenic Res. 2005, 14, 569–581. [Google Scholar] [CrossRef]
- Liu, Y.K.; Huang, L.F.; Ho, S.L.; Liao, C.Y.; Liu, H.Y.; Lai, Y.H.; Yu, S.M.; Lu, C.A. Production of mouse granulocyte-macrophage colony-stimulating factor by gateway technology and transgenic rice cell culture. Biotechnol. Bioeng. 2012, 109, 1239–1247. [Google Scholar] [CrossRef]
- Kim, T.G.; Baek, M.Y.; Lee, E.K.; Kwon, T.H.; Yang, M.S. Expression of human growth hormone in transgenic rice cell suspension culture. Plant Cell Rep. 2008, 27, 885–891. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, N.S.; Kwon, T.H.; Jang, Y.S.; Yang, M.S. Increased production of human granulocyte-macrophage colony stimulating factor (hGM-CSF) by the addition of stabilizing polymer in plant suspension cultures. J. Biotechnol. 2002, 96, 205–211. [Google Scholar] [CrossRef]
- Kim, B.G.; Kim, S.H.; Kim, N.S.; Huy, N.X.; Choi, Y.S.; Lee, J.Y.; Jang, Y.S.; Yang, M.S.; Kim, T.G. Production of monoclonal antibody against FimA protein from Porphyromonas gingivalis in rice cell suspension culture. Plant Cell Tiss. Org. 2014, 118, 293–304. [Google Scholar] [CrossRef]
- Van Giap, D.; Jung, J.W.; Kim, N.S. Production of functional recombinant cyclic citrullinated peptide monoclonal antibody in transgenic rice cell suspension culture. Transgenic Res. 2019, 28, 177–188. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Ding, L.W.; Lomonossoff, G.P.; Sun, Y.B.; Luo, M.; Li, C.Q.; Jiang, L.; Xu, Z.F. Improved expression and purification of recombinant human serum albumin from transgenic tobacco suspension culture. J. Biotechnol. 2011, 155, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.A.; Lim, E.K.; Yu, S.M. Sugar response sequence in the promoter of a rice alpha-amylase gene serves as a transcriptional enhancer. J. Biol. Chem. 1998, 273, 10120–10131. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Liu, L.F.; Chen, Y.R.; Wu, H.K.; Yu, S.M. Expression of alpha-amylases, carbohydrate metabolism, and autophagy in cultured rice cells is coordinately regulated by sugar nutrient. Plant J. 1994, 6, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.J.; Hong, S.Y.; Kwon, T.H.; Jang, Y.S.; Yang, M.S. High level of expression of recombinant human granulocyte-macrophage colony stimulating factor in transgenic rice cell suspension culture. Biotechnol. Bioeng. 2003, 82, 778–783. [Google Scholar] [CrossRef]
- Jang, H.; Kim, T.W.; Yoon, S.; Choi, S.Y.; Kang, T.W.; Kim, S.Y.; Kwon, Y.W.; Cho, E.J.; Youn, H.D. O-GlcNAc regulates pluripotency and reprogramming by directly acting on core components of the pluripotency network. Cell Stem Cell 2012, 11, 62–74. [Google Scholar] [CrossRef]
- De Muynck, B.; Navarre, C.; Nizet, Y.; Stadlmann, J.; Boutry, M. Different subcellular localization and glycosylation for a functional antibody expressed in Nicotiana tabacum plants and suspension cells. Transgenic Res. 2009, 18, 467–482. [Google Scholar] [CrossRef]
- Sharp, J.M.; Doran, P.M. Characterization of monoclonal antibody fragments produced by plant cells. Biotechnol. Bioeng. 2001, 73, 338–346. [Google Scholar] [CrossRef]
- Stevens, L.H.; Stoopen, G.M.; Elbers, I.J.; Molthoff, J.W.; Bakker, H.A.; Lommen, A.; Bosch, D.; Jordi, W. Effect of climate conditions and plant developmental stage on the stability of antibodies expressed in transgenic tobacco. Plant Physiol. 2000, 124, 173–182. [Google Scholar] [CrossRef][Green Version]
- Kim, N.S.; Kim, T.G.; Kim, O.H.; Ko, E.M.; Jang, Y.S.; Jung, E.S.; Kwon, T.H.; Yang, M.S. Improvement of recombinant hGM-CSF production by suppression of cysteine proteinase gene expression using RNA interference in a transgenic rice culture. Plant Mol. Biol. 2008, 68, 263–275. [Google Scholar] [CrossRef]
- Kim, N.S.; Jang, S.H.; Yu, H.Y.; Chung, N.D.; Kwon, T.H.; Yang, M.S.; Kim, T.G. Amylase and cysteine proteinase gene knockdown in rice cells using RNA interference for enhancing production of recombinant proteins. Plant Cell Tiss. Org. 2013, 114, 97–107. [Google Scholar] [CrossRef]
- Lin, M.T.; Lee, H.H.; Ho, S.L. Characterization of the Proteases Activities in Rice Suspension Cultured Cells and Medium during Sucrose Starvation. Crop. Environ. Bioinform. 2008, 5, 237–247. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Huang, L.F.; Tan, C.C.; Yeh, J.F.; Liu, H.Y.; Liu, Y.K.; Ho, S.L.; Lu, C.A. Efficient secretion of recombinant proteins from rice suspension-cultured cells modulated by the choice of signal peptide. PLoS ONE 2015, 10, e0140812. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.-F.; Sinaga, D.S.; Tan, C.-C.; Hsieh, S.-J.M.; Huang, C.-H. Expression of Recombinant Human Octamer-Binding Transcription Factor 4 in Rice Suspension Cells. Int. J. Mol. Sci. 2021, 22, 1409. https://doi.org/10.3390/ijms22031409
Huang L-F, Sinaga DS, Tan C-C, Hsieh S-JM, Huang C-H. Expression of Recombinant Human Octamer-Binding Transcription Factor 4 in Rice Suspension Cells. International Journal of Molecular Sciences. 2021; 22(3):1409. https://doi.org/10.3390/ijms22031409
Chicago/Turabian StyleHuang, Li-Fen, Desyanti Saulina Sinaga, Chia-Chun Tan, Shu-Ju Micky Hsieh, and Chi-Hung Huang. 2021. "Expression of Recombinant Human Octamer-Binding Transcription Factor 4 in Rice Suspension Cells" International Journal of Molecular Sciences 22, no. 3: 1409. https://doi.org/10.3390/ijms22031409
APA StyleHuang, L.-F., Sinaga, D. S., Tan, C.-C., Hsieh, S.-J. M., & Huang, C.-H. (2021). Expression of Recombinant Human Octamer-Binding Transcription Factor 4 in Rice Suspension Cells. International Journal of Molecular Sciences, 22(3), 1409. https://doi.org/10.3390/ijms22031409






