Design and Synthesis of N-Substituted 3,4-Pyrroledicarboximides as Potential Anti-Inflammatory Agents
Abstract
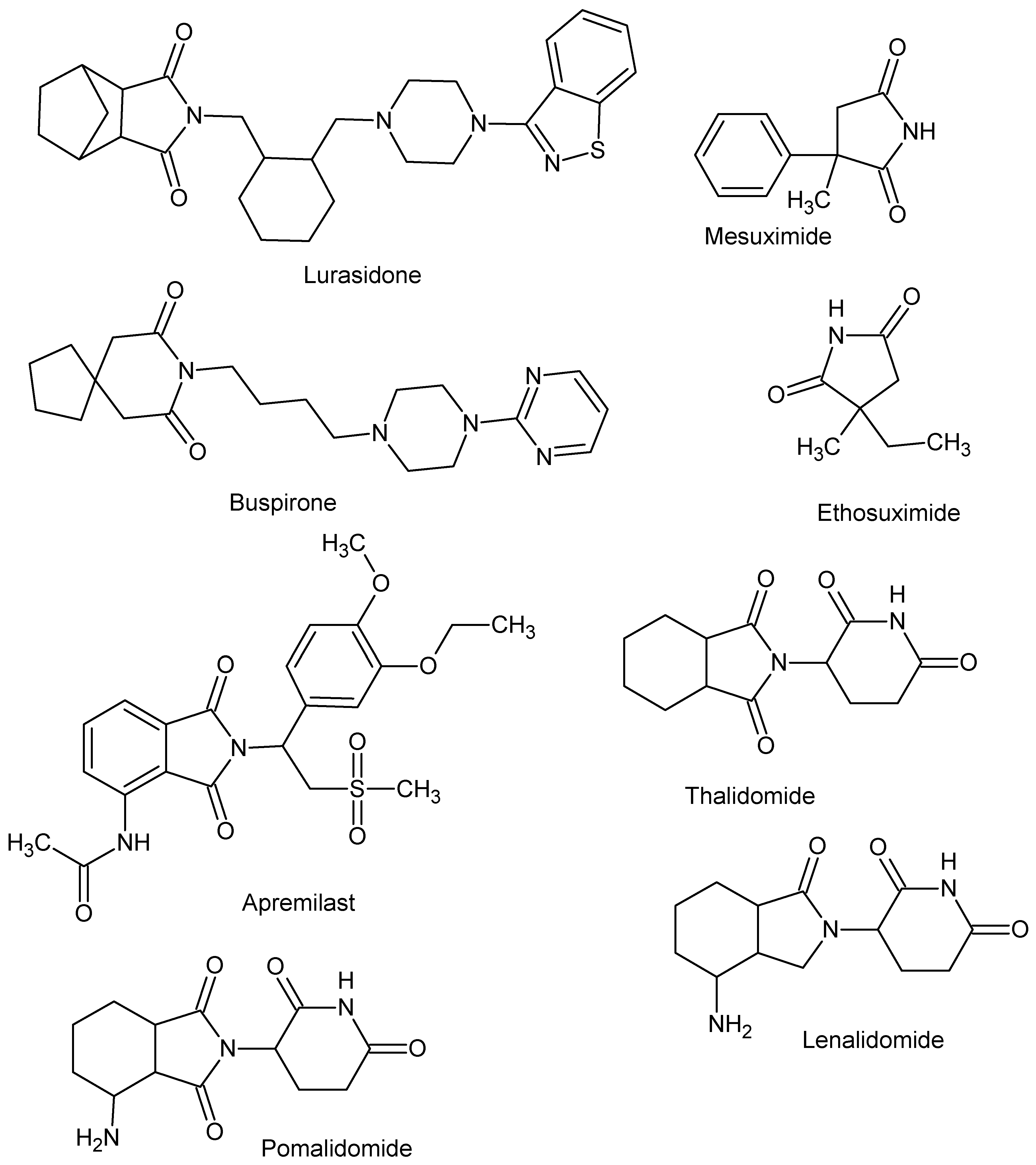
1. Introduction
2. Results and Discussion
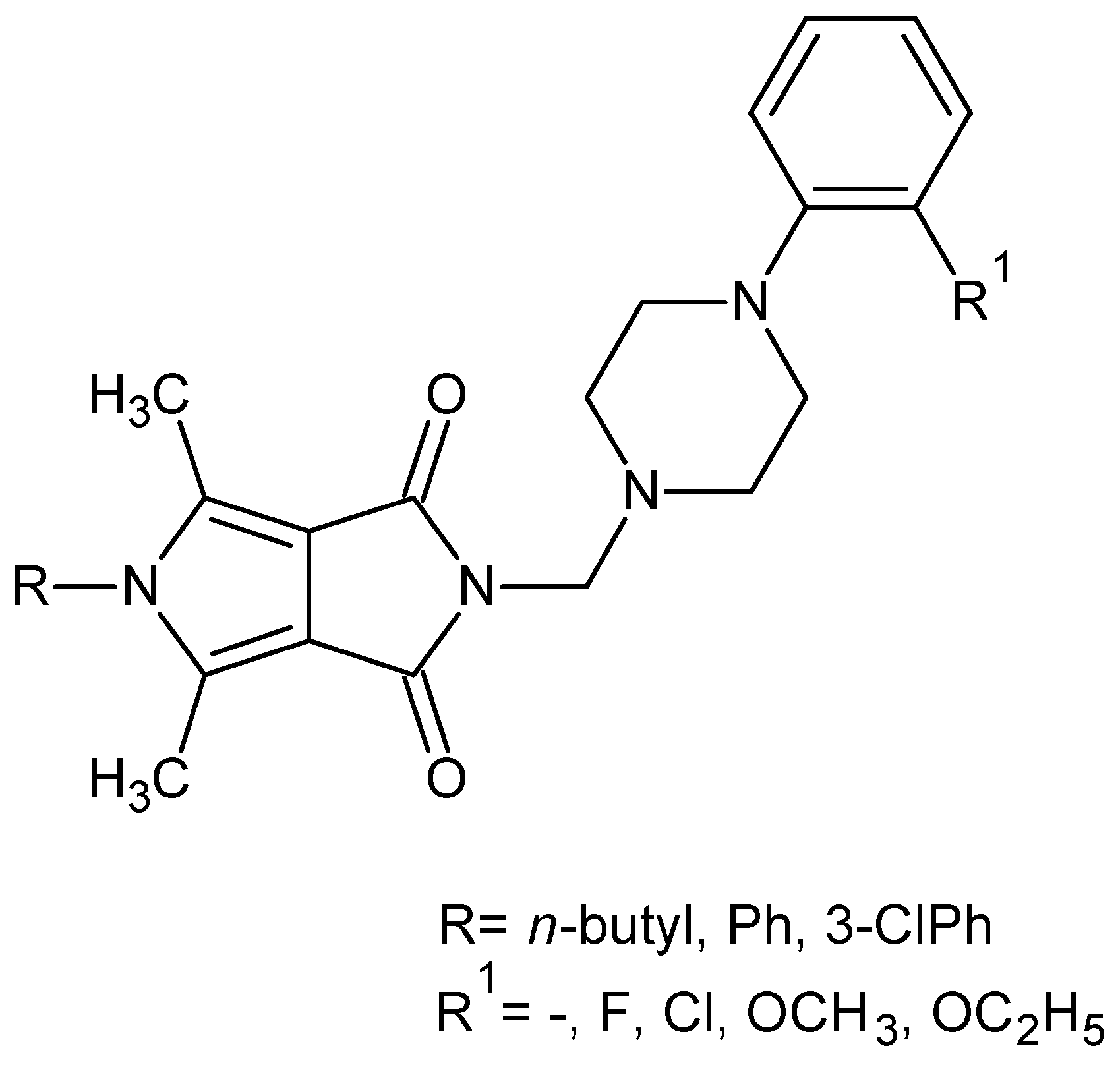
2.1. Chemistry
2.2. Biological Tests
2.2.1. Cyclooxygenase Inhibition
2.2.2. Viability of Cell Cultures
2.3. Computational Studies
2.3.1. The Analysis of Lipinski and Veber Rules
2.3.2. Structure–Activity Relationship (SAR) of N-Substituted 3,4-Pyrroledicarboximides 2a–2p
- All compounds 2a–2p inhibited COX-2 a stronger than meloxicam, which was used as a reference.
- Within the piperazine derivatives the best COX selectivity ratio IC50 (COX-2)/IC50 (COX-1) had compounds 2b and 2c. Compound 2b does not meet the Lipinski and Veber rules. However, it should be emphasized that these rules define only general properties that describe potential orally administered drugs. Therefore, the predictive ability of these rules may be limited in some cases, and one should be careful during interpretation such type of results.
- Replacement of aryl substituent in a series of piperazine derivatives with heteroaryl or cycloalkyl substituent (2g–2i) leads to a significant reduction in COX-1 inhibition while maintaining COX-2 inhibition higher than that of the reference drug. The 2h compound has the lowest COX-1 inhibition value.
- Introduction of hydrophilic hydroxyethyl substituent (2j) leads to a decrease of inhibitory activity in relation to both enzymes.
- Replacement of the 4-arylpiperazine moiety with 4-arylpiperidine leads to a loss of COX-2 inhibition.
- Introduction of group OH in a series of arylpiperidine derivatives significantly reduces the COX-1 inhibition (compare 2m to 2n–2p).
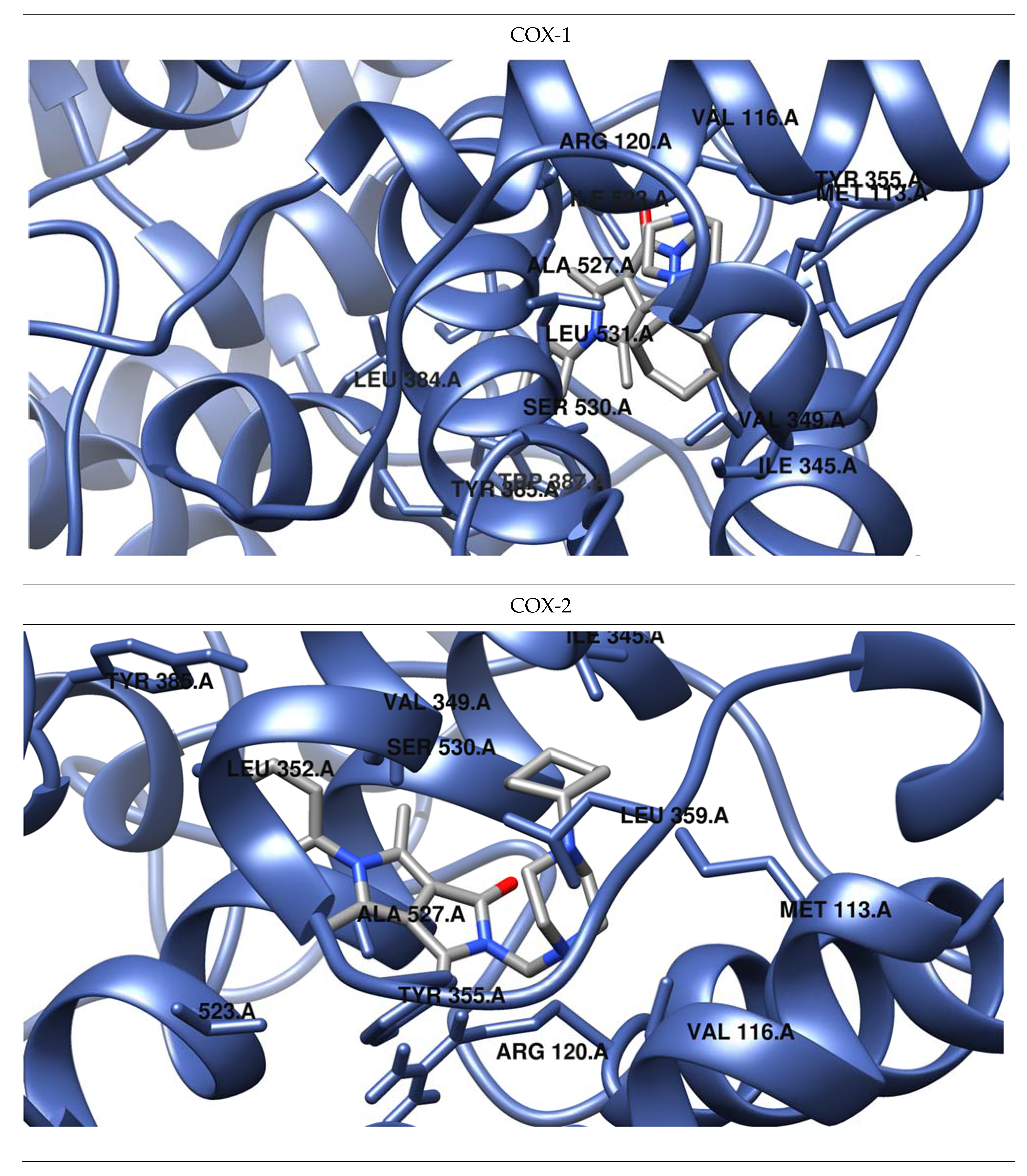
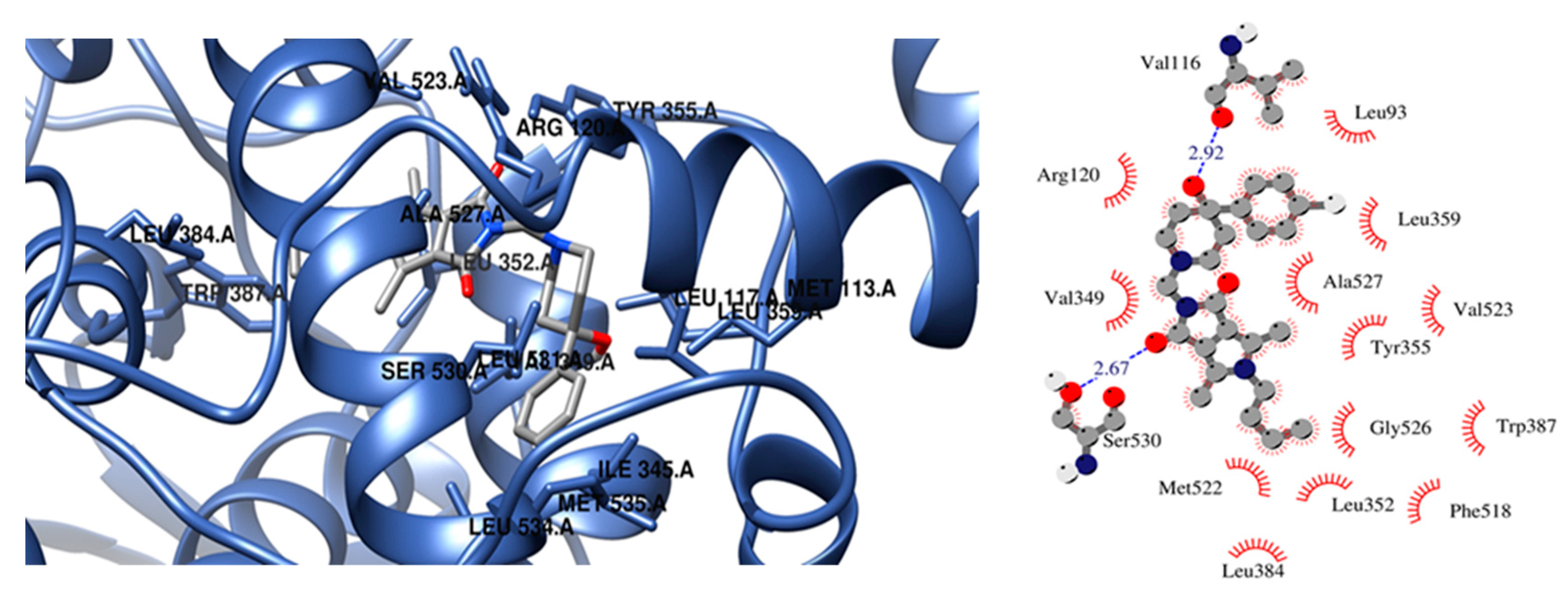
2.3.3. Molecular Docking Studies
2.3.4. QSAR Analysis of Biological Properties
3. Materials and Methods
3.1. Chemistry
3.1.1. Instrumentation and Chemicals
3.1.2. General Procedure for Preparation of N-Substituted 3,4-Pyrroledicarboximides 2a–2p
- 2a: from 1a and 1-(p-bromophenyl)piperazine. Yield 72%, m.p. 213–215 °C.
- 1H NMR (300 MHz, CDCl3) δ: 2.18, 2.20 (2x s, 6H, 4,6–CH3), 2.75–2.85 (m, 4H, 2xCH2-piperazine), 3.15–3.25 (m, 4H, 2xCH2-piperazine), 4.58 (s, 2H, CH2), 6.73 (d, 2H, ArH, J = 9 Hz), 7.20–7.23 (m, 2H, ArH), 7.30–7.33 (m, 2H, ArH), 7.52–7.54 (m, 3H, ArH)
- 13C NMR (75 MHz, CDCl3) δ: 166.05, 135.88, 131.84, 129.94, 129.64, 127.80, 117.75, 116.31, 58.31, 50.28, 48.99, 11.89
- FT-IR (selected lines, ϒmax, cm−1): 1688 (C=O), 1747 (C=O)
- ESI-MS (m/z): calcd. for C25H25BrN4O2 [M+H]+: 494.4034; found: 494.3202
- 2b: from 1c and 1-(p-bromophenyl)piperazine. Yield 78%, m.p. 230–232 °C.
- 1H NMR (300 MHz, CDCl3) δ: 2.19, 2.22 (2x s, 6H, 4,6–CH3), 2.80–2.90 (m, 4H, 2xCH2-piperazine), 3.15–3.30 (m, 4H,2xCH2-piperazine), 4.58 (s, 2H, CH2), 6.73–6.76 (m, 2H, ArH), 7.11–7.15 (m, 1H, ArH), 7.25–7.32 (m, 3H, ArH), 7.50–7.53 (m, 2H ArH)
- 13C NMR (75 MHz, CDCl3) δ: 165.82, 150.23, 137.00, 135.66, 131.84, 130.95, 130.04, 128.17, 126.17, 117.74, 116.62, 58.36, 50.28, 48.97, 11.87
- FT-IR (selected lines, ϒmax, cm−1): 1694 (C=O), 1752 (C=O)
- ESI-MS (m/z): calcd. for C25H24BrClN4O2 [M+H]+: 528.8485; found: 529.0797
- 2c: from 1a and 1-(o-cyanophenyl)piperazine. Yield 70%, m.p. 203–205 °C.
- 1H NMR (300 MHz, CDCl3) δ: 2.19, 2.25 (2x s, 6H, 4,6–CH3), 2.85–2.95 (m, 4H, 2xCH2-piperazine), 3.15–3.30 (m, 4H, 2xCH2-piperazine), 4.59 (s, 2H, CH2), 6.97–7.10 (m, 2H, ArH), 7.24–7.28 (m, 2H, ArH), 7.44–7.60 (m, 5H, ArH)
- 13C NMR (75 MHz, CDCl3) δ: 166.03, 155.90, 136.00, 134.73, 133.76, 130.07, 129.89, 129.54, 127.87, 121.79, 118.87, 118.38, 116.42, 106.17, 58.26, 51.78, 50.55, 11.90
- FT-IR (selected lines, ϒmax, cm−1): 1694 (C=O), 1752 (C=O)
- ESI-MS (m/z): calcd. for C26H25N5O2 [M+H]+: 440.5168; found: 440.2015
- 2d: from 1a and 1-(m-trifluoromethylphenyl)piperazine. Yield 82%, m.p. 232–235 °C.
- 1H NMR (300 MHz, CDCl3) δ: 2.19 (s, 6H, 4,6–CH3), 2.83–2.86 (m, 4H, 2xCH2-piperazine), 3.23–3.26 (m, 4H, 2xCH2-piperazine), 4.58 (s, 2H, CH2), 7.02–7.08 (m, 3H, ArH), 7.21–7.24 (m, 2H ArH), 7.28–7.35 (m, 1H, ArH), 7.54–7.57 (m, 3H, ArH)
- 13C NMR (75 MHz, CDCl3) δ: 166.07, 151.42, 135.92, 131.57, 131.16, 130.08, 129.92, 129.60, 129.49, 127.80, 126.12, 118.75, 116.37, 115.64, 115.59, 112.18, 112.13, 58.41, 50.36, 48.77, 11.86
- FT-IR (selected lines, ϒmax, cm−1): 1686 (C=O), 1745 (C=O)
- ESI-MS (m/z): calcd. for C26H25F3N4O2 [M+H]+ 483.5053; found: 483.1990
- 2e: from 1b and 1-(p-methylphenyl)piperazine. Yield 80%, m.p. 115–117 °C.
- 1H NMR (300 MHz, CDCl3) δ: 0.99 (t, 3H, CH3, J = 7.2 Hz), 1.37–1.44 (m, 2H, CH2), 1.62–1.68 (m, 2H, CH2), 2.26 (s, 3H, CH3), 2.41 (s, 6H, 4,6–CH3), 2.70–2.85 (m, 4H, 2xCH2-piperazine), 3.10–3.20 (m, 4H, 2xCH2-piperazine), 3.77 (t, 2H, CH2, J = 7.8 Hz), 4.54 (s, 2H, CH2), 6.80 (d, 2H, ArH, J = 9 Hz), 7.04 (d, 2H, ArH, J = 9 Hz)
- 13C NMR (75 MHz, CDCl3) δ: 166.11, 129.58, 128.72, 116.60, 116.13, 58.23, 50.40, 49.82, 43.88, 32.45, 20.40, 20.02, 13.70, 11.35
- FT-IR (selected lines, ϒmax, cm−1): 1694 (C=O), 1730 (C=O)
- ESI-MS (m/z): calcd. for C24H32N4O2 [M+H]+: 409.5443; found: 409.2565
- 2f: from 1b and 1-(2-pyrimidinyl)piperazine. Yield 71%, m.p. 203–205 °C.
- 1H NMR (300 MHz, CDCl3) δ: 0.97 (t, 3H, CH3, J = 7.5Hz), 1.24–1.44 (m, 2H, CH2), 1.57–1.67 (m, 2H, CH2), 2.38 (s, 6H, 4,6–CH3), 2.65–2.71 (m, 4H, 2xCH2-piperazine), 3.75 (t, 2H, CH2, J = 7.5Hz), 3.80–3.82 (m, 4H, 2xCH2-piperazine), 4.52 (s, 2H, CH2), 6.43 (t, 1H, 5-H pyrimidine, J = 4.5 Hz), 8.25 (d, 2H, 4,6-H pyrimidine, J = 4.8 Hz)
- 13C NMR (75 MHz, CDCl3) δ: 165.93, 161.56, 157.63, 128.72, 116.06, 109.65, 58.42, 50.46, 43.86, 43.50, 32.43, 19.99, 13.68, 11.34
- FT-IR (selected lines, ϒmax, cm−1): 1689 (C=O), 1738 (C=O)
- ESI-MS (m/z): calcd. for C21H28N6O2 [M+H]+ 397.4938; found: 397.2313
- 2g: from 1a and 1-cyclohexylpiperazine. Yield 75%, m.p. 140–142 °C.
- 1H NMR (300 MHz, CDCl3) δ: 1.21–1.26 (m, 6H, 3xCH2), 1.62–1.88 (m, 4H, 2xCH2), 2.16 (s, 6H, 4,6–CH3), 2.50–2.65 (m. 4H, 2xCH2-piperazine), 2.70–2.74 (m, 4H, 2xCH2-piperazine), 4.52 (s, 2H, CH2), 7.19–7.26 (m, 2H, ArH), 7.54–7.56 (m, 3H, ArH)
- 13C NMR (75 MHz, CDCl3) δ: 165.95, 137.09, 135.59, 130.91, 129.62, 128.19, 126.24, 116.83, 63.50, 58.27, 50.93, 48.89, 28.86, 26.23, 25.84, 11.78
- FT-IR (selected lines, ϒmax, cm−1): 1691 (C=O), 1746 (C=O)
- ESI-MS (m/z): calcd. for C25H32N4O2 [M+H]+ 421.5549; found: 421.2578
- 2h: from 1c and 1-cyclohexylpiperazine. Yield 72%, m.p. 199–201 °C.
- 1H NMR (300 MHz, CDCl3) δ: 0.99–1.24 (m, 4H, CH2), 1.59–1.63 (m, 2H, CH2), 1.76–1.87 (m, 4H, CH2), 2.18 (s, 6H, 4,6–CH3), 2.50–2.60 (m. 4H, 2xCH2-piperazine), 2.65–2.71 (m, 4H, 2xCH2-piperazine), 4.53 (s, 2H, CH2), 7.14–7.15 (m, 1H, ArH), 7.25–7.27 (m, 1H, ArH), 7.51–7.55 (m, 2H ArH)
- 13C NMR (75 MHz, CDCl3) δ: 165.96, 137.11, 135.59, 130.90, 129.95, 129.58, 128.19, 126.24, 116.85, 63.43, 58.33, 50.97, 48.92, 28.96, 26.31, 25.88, 11.77
- FT-IR (selected lines, ϒmax, cm−1): 1695 (C=O), 1756 (C=O)
- ESI-MS (m/z): calcd. for C25H31ClN4O2 [M+H]+: 455.9900; found: 455.2193
- 2i: from 1b and 1-cyclohexylpiperazine. Yield 70%, m.p. 103–105 °C.
- 1H NMR (300 MHz, CDCl3): δ: 0.98 (t, 3H, CH3, J = 7.2 Hz), 1.20–1.25 (m, 4H, 2xCH2), 1.35–1.42 (m, 2H, CH2), 1.60–1.63 (m, 4H, 2xCH2), 1.76–1.95 (m, 4H, 2xCH2), 2.37 (s, 6H, 4,6–CH3), 2.40–2.72 (m. 8H, 4xCH2-piperazine), 3.75 (t, 2H, CH2, J = 7.2 Hz), 4.47 (s, 2H, CH2)
- 13C NMR (75 MHz, CDCl3) δ: 166.17, 129.12, 116.18, 43.85, 32.43, 28.85, 25.76, 20.02, 13.69, 11.41, 11.32
- FT-IR (selected lines, ϒmax, cm−1): 1685 (C=O), 1743 (C=O)
- ESI-MS (m/z): calcd. for C23H36N4O2 [M+H]+: 401.5653; found: 401.2901
- 2j: from 1b and 1-(2-hydroxyethyl)piperazine. Yield 80%, m.p. 129–131 °C.
- 1H NMR (300 MHz, CDCl3) δ: 0.99 (t, 3H, CH3, J = 7.2 Hz), 1.34–1.41 (m, 2H, CH2), 1.60–1.65 (m, 2H, CH2), 2.41 (s, 6H, 4,6–CH3), 2.60–2.65 (m, 6H, 2xCH2-piperazine+ CH2), 2.70–2.77 (m, 4H, 2xCH2-piperazine), 3.65 (t, 2H, CH2, J = 5.4Hz), 3.74 (t, 2H, CH2, J = 7.8 Hz), 4.48 (s, 2H, CH2)
- 13C NMR (75 MHz, CDCl3) δ: 165.99, 128.89, 116.05, 59.90, 57.91, 56.96, 53.07, 49.27, 43.91, 32.46, 20.05, 13.69, 11.38
- FT-IR (selected lines, ϒmax, cm−1): 1685 (C=O), 1737 (C=O), 3199 (OH)
- ESI-MS (m/z): calcd. for C19H33N5O3 [M+H]+ 363.4743; found: 363.2362
- 2k: from 1a and piperazine. Yield 84%, m.p. 288–290 °C.
- 1H NMR (300 MHz, CDCl3) δ: 2.19 (s, 6H, 4,6–CH3), 2.60–2.75 (m. 4H, 2xCH2-piperazine), 3.60–3.75 (m, 4H, 2xCH2-piperazine), 4.50 (s, 2H, CH2), 7.21–7.26 (m, 2H, ArH), 7.54–7.57 (m, 3H, ArH)
- 13C NMR (75 MHz, CDCl3) δ: 166.02, 135.98, 129.89, 129.55, 127.85, 116.41, 58.27, 50.46, 11.84
- FT-IR (selected lines, ϒmax, cm−1): 1686 (C=O), 1747 (C=O)
- ESI-MS (m/z): calcd. for C19H22N4O2 [M+H]+ 339.4113; found: 339.4114
- 2l: from 1a and morpholine. Yield 73%, m.p. 133–135 °C.
- 1H NMR (300 MHz, CDCl3) δ: 2.18 (s, 6H, 4,6–CH3), 2.60–2.75 (m, 4H, 2xCH2-piperazine), 3.60–3.75 (m, 4H, 2xCH2-piperazine), 4.50 (s, 2H, CH2), 7.21–7.26 (m, 2H, ArH), 7.54–7.57 (m, 3H, ArH)
- 13C NMR (75 MHz, CDCl3) δ: 165.99, 136.00, 129.93, 129.61, 127.81, 116.35, 67.01, 58.71, 50.89, 11.88
- FT-IR (selected lines, ϒmax, cm−1): 1687 (C=O), 1746 (C=O)
- ESI-MS (m/z): calcd. for C19H21N3O3 [M+H]+: 340.3961; found: 340.1622
- 2m: from 1b and 4-phenyl-1-piperidine. Yield 80%, m.p. 232–235 °C.
- 1H NMR (300 MHz, CDCl3) δ: 0.99 (t, 3H, CH3, J = 7.5 Hz), 1.35–1.40 (m, 2H, CH2), 1.45–1.58 (m, 2H, CH2), 1.60–1.73 (m, 4H, CH2), 1.80–1.90 (m, 2H, CH2), 2.42 (s, 6H, 4,6–CH3), 3.10–3.21 (m, 4H, 2XCH2), 3.78 (t, 2H, CH2, J = 7.8 Hz), 4.51 (s, 2H, CH2), 7.10–7.39 (m, 5H, ArH)
- 13C NMR (75 MHz, CDCl3) δ: 166.17, 128.53, 116.24, 48.81, 43.83, 32.43, 25.75, 20.00, 13.68, 11.30
- FT-IR (selected lines, ϒmax, cm−1): 1686 (C=O), 1747 (C=O)
- ESI-MS (m/z): calcd. for C24H40N4O2 [M+H]+;394.8590 found: 394.2452
- 2n: from 1b and 4-(p-bromopheny)l-4-hydroxypiperidine. Yield 74%, m.p. 169–171 °C.
- 1H NMR (300 MHz, CDCl3) δ: 1.00 (t, 3H, CH3, J = 7.2 Hz), 1.38–1.48 (m, 2H, CH2), 1.62–1.75 (m, 4H, CH2), 2.00–2.20 (m, 2H, CH2), 2.42 (s, 3H, 4–CH3), 2.43 (s, 3H, 6–CH3), 2.65–2.75 (m. 2H, CH2), 2.85–3.10 (m, 2H, CH2), 3.79 (t, 2H, CH2, J = 7.8 Hz), 4.54 (s, 2H, CH2), 7.20–7.32 (m, 2H, ArH), 7.42–7.45 (m, 2H, ArH)
- 13C NMR (75 MHz, CDCl3) δ: 165.92, 146.57, 133.25, 128.38, 126.10, 46.88, 43.91, 32.48, 20.04, 13.69, 11.36
- FT-IR (selected lines, ϒmax, cm−1): 1680 (C=O), 1733 (C=O), 3524 (OH)
- ESI-MS (m/z): calcd. for C24H30BrN3O3 [M+H]+;489.4251 found: 489.1990
- 2o: from 1b and 4-(p-chlorophenyl)-4-hydroxypiperidine. Yield 78%, m.p. 170–172 °C.
- 1H NMR (300 MHz, CDCl3) δ: 0.98 (t, 3H, CH3, J = 7.2 Hz), 1.30–1.45 (m, 2H, CH2), 1.62–1.71 (m, 4H, 2xCH2), 2.00–2.20 (m, 2H, CH2), 2.38 (s, 6H, 4,6–CH3), 2.60–2.75 (m, 2H, CH2), 2.85–2.95 (m, 2H, CH2), 3.76 (t, 2H, CH2, J = 7.8 Hz), 4.49 (s, 2H, CH2), 7.33–7.36 (m, 2H, ArH), 7.42–7.46 (m, 2H, ArH)
- 13C NMR (75 MHz, CDCl3) δ: 166.08, 146.87, 132.75, 128.75, 128.36, 126.11, 116.14, 98.74, 70.67, 58.51, 46.87, 43.90, 38.30, 32.47, 20.04, 13.70, 11.37
- FT-IR (selected lines, ϒmax, cm−1): 1680 (C=O), 1733 (C=O), 3517 (OH)
- ESI-MS (m/z): calcd. for C24H30ClN3O3 [M+H]+: 444.9741; found: 444.2015
- 2p: from 1b and 4-benzyl-4-hydroxypiperidine, Yield 83%, m.p. 141–143 °C.
- 1H NMR (300 MHz, CDCl3) δ: 0.98 (t, 3H, CH3, J = 7.2 Hz), 1.35–1.40 (m, 2H, CH2), 1.45–1.58 (m, 2H CH2), 1.60–1.73 (m, 4H, 2xCH2), 2.38 (s, 6H, 4,6–CH3), 2.50–2.60 (m, 2H, 2XCH2), 2.71 (s, 2H, CH2), 2.71–2.78 (m, 2H, CH2), 3.75 (t, 2H, CH2, J = 7.8 Hz), 4.45 (s, 2H, CH2), 7.15–7.18 (m, 2H, ArH), 7.22–7.29 (m, 3H, ArH)
- 13C NMR (75 MHz, CDCl3) δ: 165.92, 136.37, 130.54, 128.75, 128.27, 126.63, 116.12, 68.72, 58.36, 48.88, 46.82, 43.88, 36.49, 32.46, 20.03, 13.69, 11.34
- FT-IR (selected lines, ϒmax, cm−1): 1682 (C=O), 1742 (C=O), 3504 (OH)
- ESI-MS (m/z): calcd. for C25H33N3O3 [M+H]+ 424.5556; found: 424.2565.
3.2. Crystallography
3.2.1. X-ray Structure Determinations of 2h
X-ray Experimental Details
3.3. Pharmacology
3.3.1. Determination of COX-1/COX-2-Inhibition
Material and Methods
3.3.2. Tested Compounds
3.3.3. SRB Assay
3.3.4. Cyclooxygenase Inhibition Assay
3.3.5. Statistical Analysis
3.4. Molecular Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COX | cyclooxygenase |
| CNS | central nervous system |
| IMIDs | Immunomodulatory imide drugs |
| Ph | phenyl |
| Bzl | benzyl |
| RT | room temperature |
| SRB | sulforhodamine B |
| QSAR | Quantitative structure–activity relationship |
| LOX | lipoxygenase |
| MS | mass spectra |
| DMSO | dimethyl sulfoxide |
| NHDF | normal human dermal fibroblasts |
References
- Lamie, P.F.; Philoppes, J.N.; El-Gendy, A.O.; Rarova, L.; Gruz, J. Design, Synthesis and Evaluation of Novel Phthalimide Derivatives as in Vitro Anti-Microbial, Anti-Oxidant and Anti-Inflammatory Agents. Molecules 2015, 20, 16620–16642. [Google Scholar] [CrossRef] [PubMed]
- Pareshkumar, P.; Gohel, V.; Purohit, D.; Patolia, V.N. Synthesis and Biological Evaluation of Some New Chalcone and Isoxazole Derivatives. Int. J. Sci. Technoledge 2014, 2, 138–141. [Google Scholar]
- Dhivare, R.S.; Rajput, S.S. Microwave Assisted Solvent Free Synthesis and Antifungal Evaluation Of3, 5-Bis-(4-Hydroxy-3-Methoxybenzylidene)-N-phenylpiperidine-2, 6-Dione derived from N-Phenyl Glutarimides. Int. J. ChemTech Res. 2016, 9, 325–331. [Google Scholar]
- Al-Azzawi, A.M. Synthesis, Characterization and Evaluation of Antibacterial Activity of Several New 3,4-Dimethyl Maleimides and 1,8-Naphthalimides Containing 1,3,4-Oxadiazole Ring. Al-Nahrain J. Sci. 2011, 14, 15–29. [Google Scholar] [CrossRef]
- Khalil, A.E.G.M.; Berghot, M.A.; Gouda, M.A. Synthesis and Study of Some New N-Substituted Imide Derivatives as Potential Antibacterial Agents. Chem. Pap. 2010, 64, 637–644. [Google Scholar] [CrossRef]
- Azzawi, A.; Al-Obiadi, K.K. Synthesis and anti-microbial screening of new bisschiff bases and their acetyl oxadiazole azetidinone derivatives derived from pyromelliticdiimide. Int. J. Res. Pharm. Chem. 2016, 6, 1–8. [Google Scholar]
- Dhivare, R.; Rajput, S. Synthesis and anti-microbial activity of five membered cyclic imide derivatives of mono, di and tri substituted aromatic amines and naphtyl amine. World J. Pharm. Res. 2015, 4, 1650–1658. [Google Scholar]
- Ahmed, H.E.A.; Abdel-Salam, H.A.; Shaker, M.A. Synthesis, Characterization, Molecular Modeling, and Potential Antimicrobial and Anticancer Activities of Novel 2-Aminoisoindoline-1,3-Dione Derivatives. Bioorg. Chem. 2016, 66, 1–11. [Google Scholar] [CrossRef]
- Bansode, T.N.; Shelke, J.V.; Dongre, V.G. Synthesis and Antimicrobial Activity of Some New N-Acyl Substituted Phenothiazines. Eur. J. Med. Chem. 2009, 44, 5094–5098. [Google Scholar] [CrossRef]
- Navakoski De Oliveira, K.; Chiaradia, L.D.; Alves Martins, P.G.; Mascarello, A.; Sechini Cordeiro, M.N.; Carvalho Guido, R.V.; Andricopulo, A.D.; Yunes, R.A.; Nunes, R.J.; Vernal, J.; et al. Sulfonyl-Hydrazones of Cyclic Imides Derivatives as Potent Inhibitors of the Mycobacterium Tuberculosis Protein Tyrosine Phosphatase B (PtpB). MedChemComm 2011, 2, 500–504. [Google Scholar] [CrossRef]
- Kim, B.S.; Moon, S.S.; Hwang, B.K. Isolation, Antifungal Activity, and Structure Elucidation of the Glutarimide Antibiotic, Streptimidone, Produced by Micromonospora Coerulea. J. Agric. Food Chem. 1999, 47, 3372–3380. [Google Scholar] [CrossRef] [PubMed]
- Lahore, S.; Aiwale, S.T.; Sardi, P.; Dallavalle, S. Synthesis of Natural Maleimides Farinomaleins C-E and Evaluation of Their Antifungal Activity. Tetrahedron Lett. 2014, 55, 4196–4198. [Google Scholar] [CrossRef]
- Akgün, H.; Karamelekoglu, I.; Berk, B.; Kurnaz, I.; Saribiyik, G.; Öktem, S.; Kocagöz, T. Synthesis and Antimycobacterial Activity of Some Phthalimide Derivatives. Bioorg. Med. Chem. 2012, 20, 4149–4154. [Google Scholar] [CrossRef]
- Gayoso, C.W.; Lima, E.D.O.; De Souza, E.L.; Cechinel Filho, V.; Trajano, V.N.; Pereira, F.D.O.; Lima, I.O. Antimicrobial Effectiveness of Maleimides on Fungal Strains Isolated from Onychomycosis. Braz. Arch. Biol. Technol. 2006, 49, 661–664. [Google Scholar] [CrossRef]
- Sortino, M.; Cechinel Filho, V.; Corrêa, R.; Zacchino, S. N-Phenyl and N-Phenylalkyl-Maleimides Acting against Candida spp.: Time-to-Kill, Stability, Interaction with Maleamic Acids. Bioorg. Med. Chem. 2008, 16, 560–568. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.M. Novel and Versatile Methodology for Synthesis of Cyclic Imides and Evaluation of Their Cytotoxic, DNA Binding, Apoptotic Inducing Activities and Molecular Modeling Study. Eur. J. Med. Chem. 2007, 42, 614–626. [Google Scholar] [CrossRef]
- Collin, X.; Robert, J.M.; Wielgosz, G.; Le Baut, G.; Bobin-Dubigeon, C.; Grimaud, N.; Petit, J.Y. New Anti-Inflammatory N-Pyridinyl(Alkyl)Phthalimides Acting as Tumor Necrosis Factor-α Production Inhibitors. Eur. J. Med. Chem. 2001, 36, 639–649. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Alanazi, A.M.; Abdel-Aziz, N.I.; Al-Suwaidan, I.A.; El-Sayed, M.A.A.; El-Sherbeny, M.A.; Abdel-Aziz, A.A.M. Synthesis, Molecular Modeling Study, Preliminary Antibacterial, and Antitumor Evaluation of N-Substituted Naphthalimides and Their Structural Analogues. Med. Chem. Res. 2013, 22, 2360–2375. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, N.; Roy, P.; Sondhi, S.M.; Sharma, A. Synthesis of Acridine Cyclic Imide Hybrid Molecules and Their Evaluation for Anticancer Activity. Med. Chem. Res. 2015, 24, 3272–3282. [Google Scholar] [CrossRef]
- Tumiatti, V.; Milelli, A.; Minarini, A.; Micco, M.; Gasperi Campani, A.; Roncuzzi, L.; Baiocchi, D.; Marinello, J.; Capranico, G.; Zini, M.; et al. Design, Synthesis, and Biological Evaluation of Substituted Naphthalene Imides and Diimides as Anticancer Agent ∞. J. Med. Chem. 2009, 52, 7873–7877. [Google Scholar] [CrossRef]
- Jindal, D.P.; Bedi, V.; Jit, B.; Karkra, N.; Guleria, S.; Bansal, R.; Palusczak, A.; Hartmann, R.W. Synthesis and Study of Some New N-Substituted Imide Derivatives as Potential Anticancer Agents. Il Farmaco 2005, 60, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Radice, T.; Antonino, M.; Beccalli, E.M.; Tinelli, S.; Zunino, F.; Corsini, A.; Pratesi, G.; Ragg, E.M.; Gelmi, M.L.; et al. Synthesis, Structural, and Biological Evaluation of Bis-Heteroarylmaleimides and Bis-Heterofused Imides. Bioorg. Med. Chem. 2011, 19, 5291–5299. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, S.M.; Rani, R.; Roy, P.; Agrawal, S.K.; Saxena, A.K. Microwave-Assisted Synthesis of N-Substituted Cyclic Imides and Their Evaluation for Anticancer and Anti-Inflammatory Activities. Bioorg. Med. Chem. Lett. 2009, 19, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, A.A.M.; El-Azab, A.S.; Attia, S.M.; Al-Obaid, A.M.; Al-Omar, M.A.; El-Subbagh, H.I. Synthesis and Biological Evaluation of Some Novel Cyclic-Imides as Hypoglycaemic, Anti-Hyperlipidemic Agents. Eur. J. Med. Chem. 2011, 46, 4324–4329. [Google Scholar] [CrossRef]
- Reddy, D.S.; Kongot, M.; Singh, V.; Maurya, N.; Patel, R.; Kumar Singhal, N.; Avecilla, F.; Kumar, A. Coumarin Tethered Cyclic Imides as Efficacious Glucose Uptake Agents and Investigation of Hit Candidate to Probe Its Binding Mechanism with Human Serum Albumin. Bioorg. Chem. 2019, 92, 103212. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Abdel-Aziz, A.A.M.; Sakr, H.M.; El-Azab, A.S.; Bua, S.; Supuran, C.T. Synthesis and Human/Bacterial Carbonic Anhydrase Inhibition with a Series of Sulfonamides Incorporating Phthalimido Moieties. Bioorg. Med. Chem. 2017, 25, 2524–2529. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Abdel-Aziz, A.A.M.; Ayyad, R.R.; Ceruso, M.; Supuran, C.T. Inhibition of Carbonic Anhydrase Isoforms I, II, IV, VII and XII with Carboxylates and Sulfonamides Incorporating Phthalimide/Phthalic Anhydride Scaffolds. Bioorg. Med. Chem. 2016, 24, 20–25. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.M.; El-Azab, A.S.; Ceruso, M.; Supuran, C.T. Carbonic Anhydrase Inhibitory Activity of Sulfonamides and Carboxylic Acids Incorporating Cyclic Imide Scaffolds. Bioorg. Med. Chem. Lett. 2014, 24, 5185–5189. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.M.; El-Azab, A.S.; Abu El-Enin, M.A.; Almehizia, A.A.; Supuran, C.T.; Nocentini, A. Synthesis of Novel Isoindoline-1,3-Dione-Based Oximes and Benzenesulfonamide Hydrazones as Selective Inhibitors of the Tumor-Associated Carbonic Anhydrase IX. Bioorg. Chem. 2018, 80, 706–713. [Google Scholar] [CrossRef]
- Sethi, K.K.; Verma, S.M.; Tanç, M.; Purper, G.; Calafato, G.; Carta, F.; Supuran, C.T. Carbonic Anhydrase Inhibitors: Synthesis and Inhibition of the Human Carbonic Anhydrase Isoforms I, II, IX and XII with Benzene Sulfonamides Incorporating 4- and 3-Nitrophthalimide Moieties. Bioorg. Med. Chem. 2014, 22, 1586–1595. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.M.; El-Azab, A.S.; Ghiaty, A.H.; Gratteri, P.; Supuran, C.T.; Nocentini, A. 4-Substituted Benzenesulfonamides Featuring Cyclic Imides Moieties Exhibit Potent and Isoform-Selective Carbonic Anhydrase II/IX Inhibition. Bioorg. Chem. 2019, 83, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Malinka, W.; Sieklucka-Dziuba, M.; Rajtar, G.; Rejdak, R.; Rejdak, K.; Kleinrok, Z. Synthesis of some N-substituted 3,4-pyrroledicarboximides as potential CNS depressive agents. Pharmazie 2000, 55, 9–16. [Google Scholar] [PubMed]
- Norman, M.H.; Minick, D.J.; Rigdon, G.C. Effect of Linking Bridge Modifications on the Antipsychotic Profile of Some Phthalimide and Isoindolinone Derivatives. J. Med. Chem. 1996, 39, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, Y.; Yang, F.; Wu, C.; Wang, Z.; Ye, B.; Jiang, X.; Zhao, Q.; Li, J.; Liu, Y.; et al. Synthesis and Biological Evaluation of a Series of Multi-Target N-Substituted Cyclic Imide Derivatives with Potential Antipsychotic Effect. Eur. J. Med. Chem. 2018, 145, 74–85. [Google Scholar] [CrossRef]
- Kossakowski, J.; Jarocka, M. Synthesis of New N-Substituted Cyclic Imides with an Expected Anxiolytic Activity. XVII. Derivatives of 1-ethoxybicyclo[2.2.2]-oct-5-one-2,3-dicarboximide. Il Farmaco 2001, 56, 785–789. [Google Scholar] [CrossRef]
- Kamiński, K.; Obniska, J.; Wiklik, B.; Atamanyuk, D. Synthesis and Anticonvulsant Properties of New Acetamide Derivatives of Phthalimide, and Its Saturated Cyclohexane and Norbornene Analogs. Eur. J. Med. Chem. 2011, 46, 4634–4641. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.M.; El-Azab, A.S.; AlSaif, N.A.; Alanazi, M.M.; El-Gendy, M.A.; Obaidullah, A.J.; Alkahtani, H.M.; Almehizia, A.A.; Al-Suwaidan, I.A. Synthesis, Anti-Inflammatory, Cytotoxic, and COX-1/2 Inhibitory Activities of Cyclic Imides Bearing 3-Benzenesulfonamide, Oxime, and β-Phenylalanine Scaffolds: A Molecular Docking Study. J. Enzym. Inhib. Med. Chem. 2020, 35, 610–621. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.M.; Eltahir, K.E.H.; Asiri, Y.A. Synthesis, Anti-Inflammatory Activity and COX-1/COX-2 Inhibition of Novel Substituted Cyclic Imides. Part 1: Molecular Docking Study. Eur. J. Med. Chem. 2011, 46, 1648–1655. [Google Scholar] [CrossRef]
- Redzicka, A.; Szczukowski, Ł.; Kochel, A.; Wiatrak, B.; Gębczak, K.; Czyżnikowska, Ż. COX-1/COX-2 Inhibition Activities and Molecular Docking Study of Newly Designed and Synthesized Pyrrolo[3,4-c]Pyrrole Mannich Bases. Bioorg. Med. Chem. 2019, 27, 3918–3928. [Google Scholar] [CrossRef]
- Alanazi, A.M.; El-Azab, A.S.; Al-Suwaidan, I.A.; Eltahir, K.E.H.; Asiri, Y.A.; Abdel-Aziz, N.I.; Abdel-Aziz, A.A.M. Structure-Based Design of Phthalimide Derivatives as Potential Cyclooxygenase-2 (COX-2) Inhibitors: Anti-Inflammatory and Analgesic Activities. Eur. J. Med. Chem. 2015, 92, 115–123. [Google Scholar] [CrossRef]
- Pophale, R.A.; Deodhar, M. Synthesis and Evaluation of Novel Phthalimide Derivatives as Analgesic and Antiinflammatory Agents. Pharma Chem. 2010, 2, 185–193. [Google Scholar]
- Abu-Hashem, A.A.; Gouda, M.A. Synthesis, Anti-Inflammatory and Analgesic Evaluation of Certain New 3a,4,9,9a-Tetrahydro-4,9-Benzenobenz(f)Isoindole-1,3-Diones. Arch. Pharm. 2011, 344, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Al-Suwaidan, I.A.; Alanazi, A.M.; El-Azab, A.S.; Al-Obaid, A.M.; Eltahir, K.E.H.; Maarouf, A.R.; Abu El-Enin, M.A.; Abdel-Aziz, A.A.M. Molecular Design, Synthesis and Biological Evaluation of Cyclic Imides Bearing Benzenesulfonamide Fragment as Potential COX-2 Inhibitors. Part 2. Bioorg. Med. Chem. Lett. 2013, 23, 2601–2605. [Google Scholar] [CrossRef] [PubMed]
- De Campos, F.; Corrêa, R.; De Souza, M.M.; Yunes, R.A.; Nunes, R.J.; Cechinel-Filho, V. Studies on New Cyclic Imides Obtained from Aminophenazone with Analgesic Properties/Potent Effects of a 3,4-Dichloromaleimide Derivative. ArzneimittelForschung/Drug Res. 2002, 52, 455–461. [Google Scholar] [CrossRef]
- Sano, H.; Noguchi, T.; Tanatani, A.; Hashimoto, Y.; Miyachi, H. Design and Synthesis of Subtype-Selective Cyclooxygenase (COX) Inhibitors Derived from Thalidomide. Bioorg. Med. Chem. 2005, 13, 3079–3091. [Google Scholar] [CrossRef] [PubMed]
- Andricopulo, A.; Borchhardt, D.; Castilho, M. Classical and Fragment-Based Hologram Structure-Activity Relationships for a Series of Analgesic Cyclic Imides. Lett. Drug Des. Discov. 2008, 5, 57–64. [Google Scholar] [CrossRef]
- Said, S.A.; Amr, A.E.G.E.; Sabry, N.M.; Abdalla, M.M. Analgesic, Anticonvulsant and Anti-Inflammatory Activities of Some Synthesized Benzodiazipine, Triazolopyrimidine and Bis-Imide Derivatives. Eur. J. Med. Chem. 2009, 44, 4787–4792. [Google Scholar] [CrossRef]
- Lopchuk, J.M. Imide Natural Products. In Imides: Medicinal, Agricultural, Synthetic Applications and Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 255–334. [Google Scholar] [CrossRef]
- Tempesta, M.S.; Corley, D.G.; Beutler, J.A.; Metral, C.J.; Yunes, R.A.; Giacomozzi, C.A.; Calixto, J.B. Phyllanthimide, a New Alkaloid from Phyllanthus Sellowianus. J. Nat. Prod. 1988, 51, 617–618. [Google Scholar] [CrossRef]
- Prado, S.R.T.; Cechinel-Filho, V.; Campos-Buzzi, F.; Corrêa, R.; Cadena, S.M.C.S.; De Oliveira, M.B.M. Biological Evaluation of Some Selected Cyclic Imides: Mitochondrial Effects and in Vitro Cytotoxicity. Z. Naturforsch. C 2004, 59, 663–672. [Google Scholar] [CrossRef][Green Version]
- Sultana, K.; Khan, H.; Shahid, K. Synthesis, Characterization and In Vitro Antibacterial Evaluation of Sn, Sb, and Zn Coordination Complexes of 2-(2-Methoxyphenyl)-1H-Isoindole-1, 3(2h)-Dione. Int. J. Pharm. Sci. Rev. Res. 2014, 28, 1–5. [Google Scholar]
- Marulasiddaiah, R.; Kalkhambkar, R.G.; Kulkarni, M.V. Synthesis and Biological Evaluation of Cyclic Imides with Coumarins and Azacoumarins. Open J. Med. Chem. 2012, 2, 89–97. [Google Scholar] [CrossRef][Green Version]
- Hargreaves, M.K.; Pritchard, J.G.; Dave, H.R. Cyclic Carboxylic Monoimides. Chem. Rev. 1970, 70, 439–469. [Google Scholar] [CrossRef]
- Szczȩśniak-Siega, B.; Gȩbczak, K.; Gȩbarowski, T.; Maniewska, J. Synthesis, COX-1/2 Inhibition and Antioxidant Activities of New Oxicam Analogues Designed as Potential Chemopreventive Agents. Acta Biochim. Pol. 2018, 65, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Malinka, W.; Bodalski, T. Synthesis of some 1-substituted-2,5-dimethylpyrrole-3,4-dicarboxyimides from α,β-diacetylsuccinate. Pol. J. Chem. 1994, 68, 297–307. [Google Scholar] [CrossRef]
- Redzicka, A.; Szczukowski, Ł.; Kochel, A. Crystal Structure of 4,6-Dimethyl-5-(3-Chlorophenyl)-2-{[4-(3,4-Dichlorophenyl)-1-Piperazinyl]Methyl}-Pyrrolo[3,4-c]Pyrrole-1,3(2H,5H)-Dione. X-ray Struct. Anal. Online 2018, 34, 55–56. [Google Scholar] [CrossRef]
- SwissADME. Available online: https://www.swissadme.ch/ (accessed on 18 December 2020).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- CrysAlis PRO. Rigaku; Oxford Diffraction Ltd.: Yarnton, UK, 2017. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Diamond—Crystal and Molecular Structure Visualization, Crystal Impact—Dr. H. Putz & Dr. K. Brandenburg GbR, Germany. Available online: https://www.crystalimpact.com/diamond/ (accessed on 18 December 2020).
- Świątek, P.; Strzelecka, M.; Urniaz, R.; Gębczak, K.; Gębarowski, T.; Gąsiorowski, K.; Malinka, W. Synthesis, COX-1/2 Inhibition Activities and Molecular Docking Study of Isothiazolopyridine Derivatives. Bioorg. Med. Chem. 2017, 25, 316–326. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Cancès, E.; Mennucci, B.; Tomasi, J. A New Integral Equation Formalism for the Polarizable Continuum Model: Theoretical Background and Applications to Isotropic and Anisotropic Dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange-Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09; Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hermanson, D.J.; Banerjee, S.; Ghebreselasie, K.; Clayton, G.M.; Garavito, R.M.; Marnett, L.J. Oxicams Bind in a Novel Mode to the Cyclooxygenase Active Site via a Two-Water-Mediated h-Bonding Network. J. Biol. Chem. 2014, 289, 6799–6808. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand-Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Potemkin, V.; Grishina, M. Principles for 3D/4D QSAR Classification of Drugs. Drug Discov. Today 2008, 13, 952–959. [Google Scholar] [CrossRef]
- Potemkin, V.A.; Grishina, M.A.; Bartashevich, E.V. Modeling of Drug Molecule Orientation within a Receptor Cavity in the BiS Algorithm Framework. J. Struct. Chem. 2007, 48, 155–160. [Google Scholar] [CrossRef][Green Version]

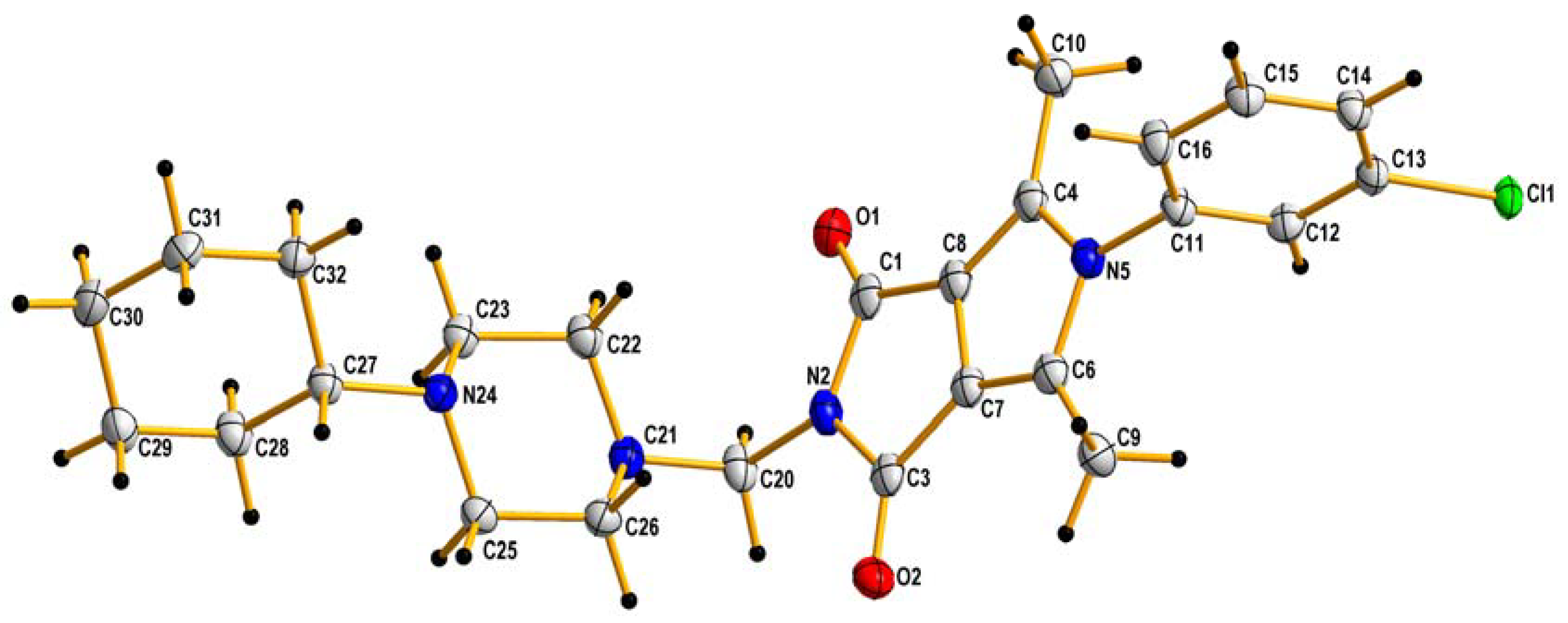

| Chemical formula: C25 H31 Cl N4 O2 |
| Formula weight = 454.99 g mol−1 |
| Crystal system: triclinic Space group P1 |
| Unit cell parameters |
| a = 8.9619(3) Å |
| b = 10.2613(3) Å |
| c = 14.5659(4) Å |
| α = 88.746(3 |
| β = 73.072(2)° |
| γ = 64.597(3)° |
| V = 1149.30(7) Å3 |
| Molecular multiplicity Z = 2 |
| Dcalc = 1.315 g cm−3 |
| Crystal color and shape: colorless plates |
| Crystal size: 0.15 × 0.14 × 0.12 mm |
| F(000) = 484 |
| μ = 1.707 mm−1 |
| 7595 measured reflections |
| 4065 independent reflections |
| 3909 reflections with I > 2σ(I) |
| 289 parameters (0 restraints) |
| R[F2 > 2σ(F2)] = 0.0577, |
| wR(F2) = 0.1640, |
| largest diff. peak and hole = 0.634 e Å−3/−0.890 e Å−3. |
| Compd. | Cyclooxygenase Inhibition Assay IC50 [µM] | COX Selectivity Ratio IC50(COX-2)/IC50(COX-1) | SRB Assay IC50 [µM] | |
|---|---|---|---|---|
| COX-1 | COX-2 | |||
| 2a | 87.90 ± (0.03) | 60.85 ± (0.02) | 0.69 | 480.88 ± (0.12) |
| 2b | 94.17 ± (0.04) | 61.46 ± (0.02) | 0.65 | 460.54 ± (0.10) |
| 2c | 94.56 ± (0.02) | 61.53 ± (0.09) | 0.65 | 360.01 ± (0.15) |
| 2d | 77.46 ± (0.06) | 60.24 ± (0.03) | 0.78 | 143.23 ± (0.02) |
| 2e | 78.73 ± (0.06) | 60.75 ± (0.02) | 0.77 | 198.96 ± (0.16) |
| 2f | 80.51 ± (0.06) | 58.87 ± (0.08) | 0.73 | 282.33 ± (0.16) |
| 2g | 80.25 ± (0.06) | 59.92 ± (0.05) | 0.75 | 96.57 ± (0.17) |
| 2h | 76.54 ± (0.06) | 59.37 ± (0.05) | 0.78 | 110.95 ± (0.08) |
| 2i | 79.87 ± (0.07) | 60.96 ± (0.04) | 0.76 | 251.53 ± (0.03) |
| 2j | 84.90 ± (0.05) | 59.77 ± (0.11) | 0.70 | 299.36 ± (0.10) |
| 2k | 83.47 ± (0.05) | 58.43 ± (0.06) | 0.70 | 171.52 ± (0.08) |
| 2l | 82.72 ± (0.04) | 59.33 ± (0.09) | 0.72 | 171.52 ± (0.13) |
| 2m | 88.15 ± (0.02) | 60.39 ± (0.02) | 0.69 | 270.17 ± (0.09) |
| 2n | 77.59 ± (0.09) | 57.65 ± (0.04) | 0.74 | 136.35 ± (0.09) |
| 2o | 78.25 ± (0.10) | 58.38 ± (0.16) | 0.75 | 268.42 ± (0.19) |
| 2p | 82.94 ± (0.04) | 59.37 ± (0.07) | 0.72 | 258.51 ± (0.10) |
| Meloxicam | 83.68 ± (0.03) | 57.14 ± (0.05) | 0.68 | 174.23 ± (0.09) |
| Comp. | Lipinski Rules | Veber Rules | |||||
|---|---|---|---|---|---|---|---|
| MW ≤500 | LogP ≤5 | NHD a ≤5 | NHA b ≤10 | Violations of Rules | NBR c ≤10 | TPSA d ≤140 | |
| 2a | 493.40 | 4.33 | 0 | 3 | 0 | 4 | 50.48 |
| 2b | 527.84 | 4.41 | 0 | 3 | 2 | 4 | 50.48 |
| 2c | 439.51 | 3.85 | 0 | 4 | 0 | 4 | 74.27 |
| 2d | 482.50 | 3.98 | 0 | 6 | 1 | 5 | 50.48 |
| 2e | 408.54 | 4.06 | 0 | 3 | 0 | 6 | 50.48 |
| 2f | 396.49 | 3.57 | 0 | 5 | 0 | 6 | 76.26 |
| 2g | 420.55 | 4.29 | 0 | 4 | 0 | 4 | 50.48 |
| 2h | 454.99 | 4.73 | 0 | 4 | 0 | 4 | 50.48 |
| 2i | 400.56 | 4.43 | 0 | 4 | 0 | 6 | 50.48 |
| 2j | 362.47 | 3.47 | 1 | 5 | 0 | 7 | 70.71 |
| 2k | 338.40 | 3.08 | 1 | 4 | 0 | 3 | 59.27 |
| 2l | 339.39 | 3.24 | 0 | 4 | 0 | 3 | 56.47 |
| 2m | 393.52 | 4.16 | 0 | 3 | 0 | 6 | 47.24 |
| 2n | 488.42 | 4.26 | 1 | 4 | 0 | 6 | 67.47 |
| 2o | 443.97 | 4.15 | 1 | 4 | 0 | 6 | 67.47 |
| 2p | 423.55 | 4.07 | 1 | 4 | 0 | 7 | 67.47 |
| Compd. | Free Energy of Binding COX-1 [kcal/mol] | Hydrogen Bonds | Hydrophobic Interactions |
|---|---|---|---|
| 2a | −9.33 | Ser530 | Leu93, Met113, Val116, Val349, Leu352, Tyr355, Leu357, Leu359, Leu384, Trp387, Phe518, Met522, Ile523, Gly526, Ala527, Ser530 |
| 2b | −7.43 | - | Ile89, Leu93, Met113, Val116, Arg120, Val349, Leu352, Tyr355, Leu357, Leu359, Ala527, Glu526, Ser530, Leu531, Ile532 |
| 2c | −9.01 | - | Ile89, Leu93, Met113, Val116, Arg120, Val349, Leu352, Tyr355, Leu357, Leu359, Ile523, Gly526, Ala527, Ser530, Leu531 |
| 2d | −8.29 | Lys360 Asp362 Leu531 | Met113, Leu117, Ile345, Val349, Leu352, Ser353, Trp387, Phe518, Ile522, Ile523, Gly526, Ala527, Ser530, Leu535 |
| 2e | −9.32 | - | Met113, Leu117, Ile345, Val349, Leu352, Ser353, Leu359, Tyr355, Leu384, Tyr385, Trp387, Ile523, Gly526, Ala527, Ser530, Leu531, Leu534, Leu535 |
| 2f | −9.21 | Ser530 | Ser93, Met113, Val116, Leu117, Arg120, Val349, Leu352, Tyr355, Leu357, Leu384, Trp387, Leu359, Met522, Ile523, Gly526, Ala527, Ser530, Leu531, Leu534 |
| 2g | −9.75 | Arg120 Tyr355 | Met113, Val116, Arg120, Ile345, Val349, Leu352, Tyr355, Phe381, Tyr385, Leu384, Tyr385, Ile523, Gly526, Ala527, Ser530, Leu531, Leu534, Leu535 |
| 2h | −10.53 | Arg120 Tyr355 | Met113, Val116, Arg120, Ile345, Val349, Leu352, Tyr355, Val359, Tyr385, Trp387, Ile523, Gly526, Ala527, Ser530, Leu531, Leu534, |
| 2i | −9.05 | Ser530 | Leu93, Met113, Val116, Val349, Leu352, Tyr355, Leu357, Leu359, Phe381, Leu384, Tyr385, Trp387, Ile523, Gly526, Ala527, Ser530, |
| 2j | −8.89 | Lys360 Asp362 | Met113, Leu117, Ile345, Val349, Leu352, Lys360, Phe361, Asp362, Leu384, Trp387, Phe518, Met522, Ile523, Gly526, Ala527, Ser530, Leu531, Leu535 |
| 2k | −8.22 | Ser530 | Met113, Ile345, Val349, Leu352, Leu359, Leu384, Trp387, Phe518, Met522, Ile523, Gly526, Ala527, Ser530, Leu531, Leu535 |
| 2l | −8.88 | Ser530 | Met113, Ile345, Val349, Leu352, Leu359, Leu384, Trp387, Phe518, Met522, Ile523, Gly526, Ala527, Ser530, Leu531, Leu534, Leu535 |
| 2m | −10.04 | Arg120 Tyr355 | Met113, Val116, Leu117, Arg120, Ile345, Val349, Leu352, Tyr355, Leu359, Phe381, Leu384, Trp387, Gly526, Tyr385, Ile523, Ala527, Ser530, Leu531, Leu534, Leu535 |
| 2n | −7.38 | Arg120 Tyr355 | Ile89, Leu93, Met113, Val116, Arg120, Val349, Leu352, Tyr355, Leu357, Leu359, Trp387, Gly526, Ala527, Ser530 |
| 2o | −8.94 | Arg120 Val349 | Met113, Val116, Leu117, Arg120, Ile345, Val349, Leu352, Ser353, Tyr355, Phe518, Ile523, Gly526, Ala527, Leu531, Leu534, Leu535, Leu539 |
| 2p | −8.9 | Arg120 | Met113, Val116, Leu117, Arg120, Ile345, Val349, Leu352, Tyr385, Trp387, Ile523, Gly526, Ala527, Ser530, Leu531, Leu535 |
| Compd. | Free Energy of Binding COX-2 [kcal/mol] | Hydrogen Bonds | Hydrophobic Interactions |
|---|---|---|---|
| 2a | −10.39 | Ser530 | Leu93, Val116, Arg120, Val349, Leu352, Trp387, Phe518, Met522, Val523, Gly526, Ala527, Ser530 |
| 2b | −10.95 | Arg120 Tyr355 | Val89, Leu93, Val116, Arg120, Val349, Leu352, Tyr355, Leu359, Val523, Trp387, Phe518, Met522, Gly526, Ala527, Ser530 |
| 2c | −10.84 | Arg120 Tyr355 | Val89, Leu93, Val116, Arg120, Val349, Leu352, Tyr355, Leu359, Trp387, Phe518, Met522, Val523, Gly526, Ala527, Ser530 |
| 2d | −8.79 | Ile345 Ser530 Leu531 | Ile345, Val349, Leu352, Leu384, Tyr385, Trp387, Met522, Val523, Gly526, Ala527, Ser530, Leu531, Leu534, |
| 2e | −9.74 | Ser530 | Leu93, Val116, Arg120, Val189, Val349, Leu352, Tyr355, Leu359, Leu384, Tyr385, Trp387, Phe518, Met522, Val523, Gly526, Ala527, Ser530 |
| 2f | −11.26 | Tyr355 | Leu117, Arg120, Ile345, Val349, Leu352, Tyr355, Lru384, Trp387, Phe518, Met522, Val523, Gly526, Ala527, Ser530, Leu531, Leu534, Met535 |
| 2g | −10.98 | Arg120 Tyr355 | Met113, Val116, Val117, Arg120, Ile345, Val349, Leu352, Tyr355, Leu359, Trp387, Val523, Ala527, Ser530, Leu534 |
| 2h | −11.92 | Arg120 Tyr355 | Met113, Val116, Leu117, Arg120, Ile345, Val349, Leu352, Tyr355, Leu359, Trp387, Val523, Ala527, Ser530, Leu531 |
| 2i | −10.46 | Arg120 Tyr355 | Val116, Leu117, Arg120, Ile345, Val349, Leu352, Ser353, Tyr355, Leu359, Met522, Val523, Ala527, Ser530, Leu531, Leu534, Met535 |
| 2j | −8.88 | Ser530 Leu531 Met535 | Ile345, Val349, Leu352, Leu384, Tyr385, Trp387, Met522, Val523, Gly526, Ala527, Ser530, Leu531, Leu534, Met535 |
| 2k | −8.79 | Ser530 | Met113, Leu117, Ile345, Val349, Leu352, Leu384, Trp387, Phe518, Met522, Val523, Gly526, Ala527, Ser530, Leu531 |
| 2l | −8.64 | Ser520 | Met113, Leu117, Ile345, Val349, Leu352, Leu384, Trp387, Phe518, Met522, Val523, Gly526, Ala527, Ser530, Leu531 |
| 2m | −11.03 | Arg120 Tyr355 | Met113, Val116, Leu117, Arg120, Ile345, Val349, Leu352, Tyr355, Phe381, Tyr385, Trp387, Val523, Gly526, Ala527, Ser530, Leu531, Leu534, Met535 |
| 2n | −10.33 | Arg120 Tyr355 | Val89, Val116, Arg120, Val349, Leu352, Ser353, Tyr355, Leu359, Trp387, Val523, Gly526, Ala527, Ser530, |
| 2o | −9.81 | Val116 Ser530 | Leu93, Val116, Arg120, Val349, Leu352, Tyr355, Leu359, Leu384, Trp387, Phe518, Met522, Val523, Gly526, Ala527, Ser530 |
| 2p | −10.02 | Tyr355 | Leu117, Ile345, Val349, Leu352, Tyr355, Leu359, Leu384, Tyr385, Trp387, Met522, Val523, Gly526, Ala527, Leu531, Leu534, Met535, Ser539 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redzicka, A.; Czyżnikowska, Ż.; Wiatrak, B.; Gębczak, K.; Kochel, A. Design and Synthesis of N-Substituted 3,4-Pyrroledicarboximides as Potential Anti-Inflammatory Agents. Int. J. Mol. Sci. 2021, 22, 1410. https://doi.org/10.3390/ijms22031410
Redzicka A, Czyżnikowska Ż, Wiatrak B, Gębczak K, Kochel A. Design and Synthesis of N-Substituted 3,4-Pyrroledicarboximides as Potential Anti-Inflammatory Agents. International Journal of Molecular Sciences. 2021; 22(3):1410. https://doi.org/10.3390/ijms22031410
Chicago/Turabian StyleRedzicka, Aleksandra, Żaneta Czyżnikowska, Benita Wiatrak, Katarzyna Gębczak, and Andrzej Kochel. 2021. "Design and Synthesis of N-Substituted 3,4-Pyrroledicarboximides as Potential Anti-Inflammatory Agents" International Journal of Molecular Sciences 22, no. 3: 1410. https://doi.org/10.3390/ijms22031410
APA StyleRedzicka, A., Czyżnikowska, Ż., Wiatrak, B., Gębczak, K., & Kochel, A. (2021). Design and Synthesis of N-Substituted 3,4-Pyrroledicarboximides as Potential Anti-Inflammatory Agents. International Journal of Molecular Sciences, 22(3), 1410. https://doi.org/10.3390/ijms22031410








