Changes in the Expression of TBP-2 in Response to Histone Deacetylase Inhibitor Treatment in Human Endometrial Cells
Abstract
1. Introduction
2. Results
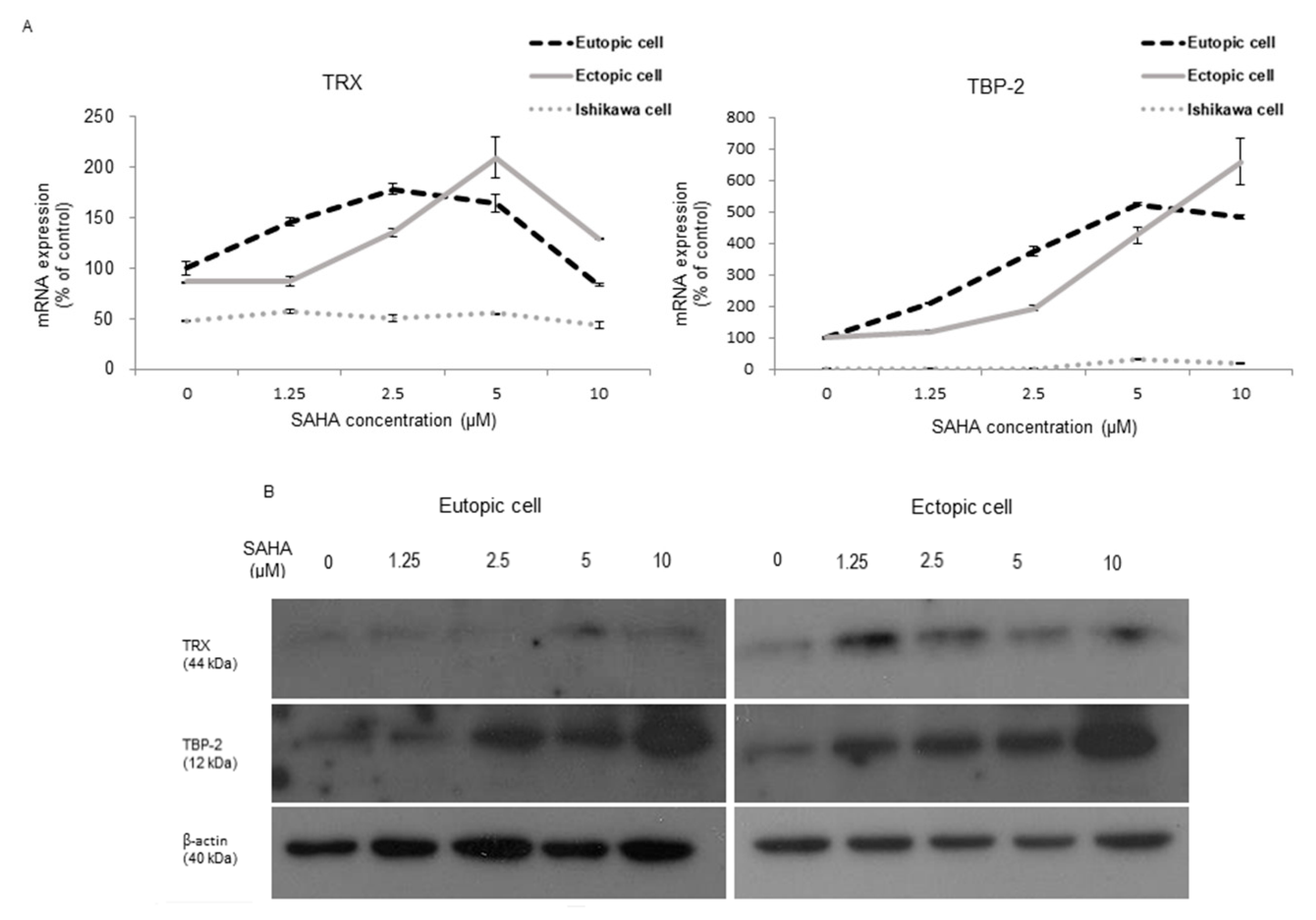
2.1. TRX and TBP-2 Changes to SAHA Treatment in HESCs and Ishikawa Cells
2.2. Changes after SAHA Treatment in the Oxidative Stress-Induced Altered Endometrial Stromal and Epithelial Cells
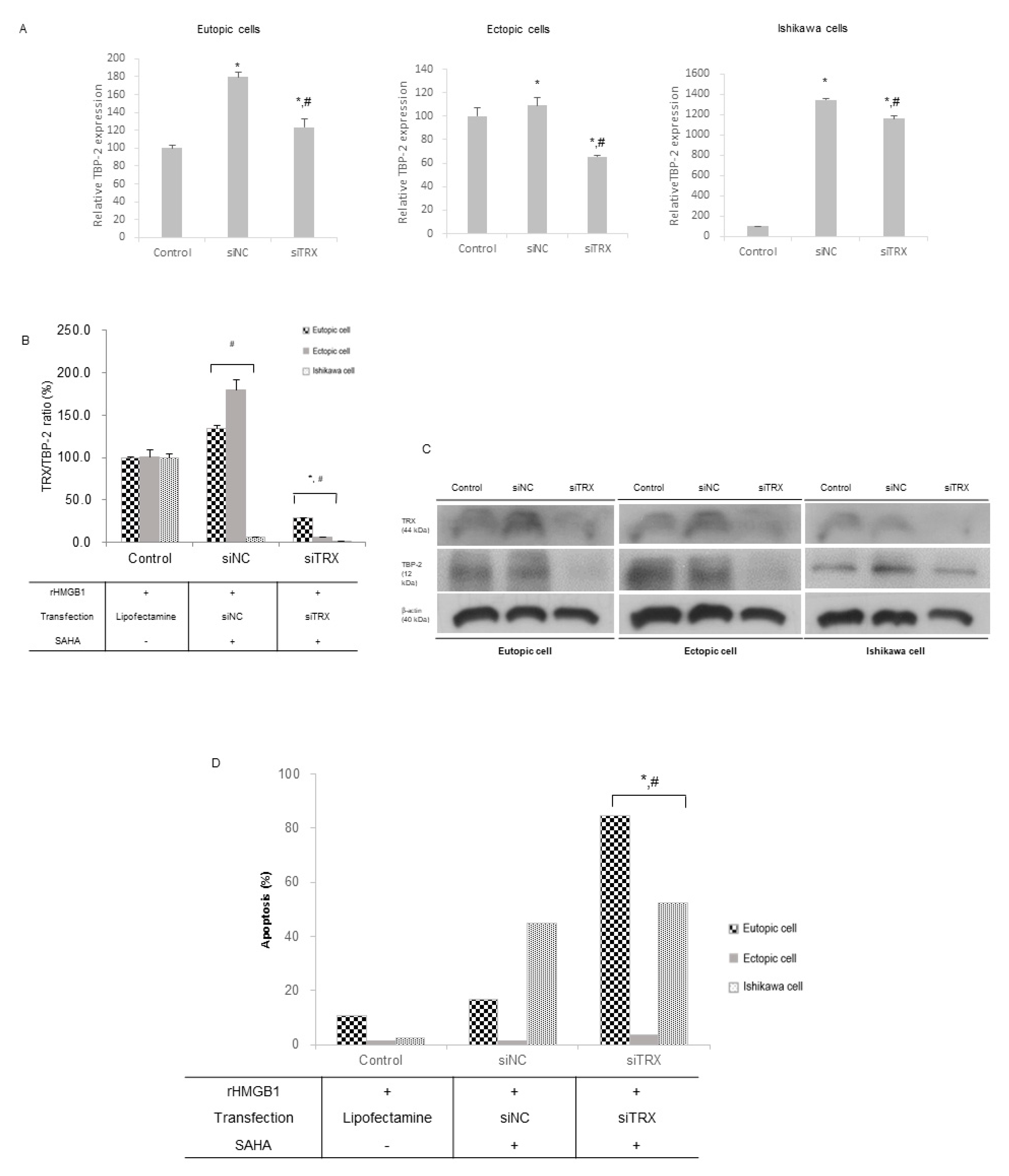
2.3. SAHA Treatment Inhibits TRX Gene Expression in the Altered Endometrial Cells
3. Discussion
Conclusions
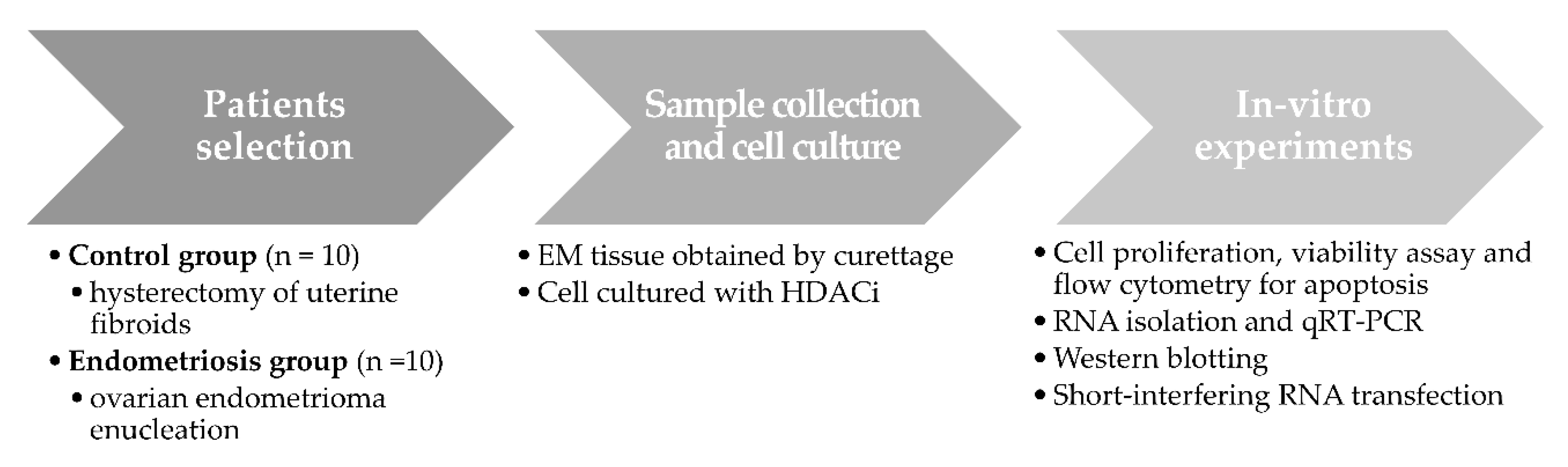
4. Materials and Methods
4.1. Participants
4.2. Sample Collection and Cell Culture
4.3. Drugs and Chemicals
4.4. Cell Proliferation and Viability, Analysis of Apoptosis
4.5. RNA Isolation and qRT-PCR
4.6. Western Blotting
4.7. Short-Interfering RNA Transfection
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmood, T.A.; Templeton, A. Prevalence and genesis of endometriosis. Hum. Reprod. 1991, 6, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; Harris, H.R.; Kvaskoff, M.; Zondervan, K.; Missmer, S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Surrey, E.; Bonafede, M.; Nelson, J.K.; Castelli-Haley, J. Real-World Evaluation of Direct and Indirect Economic Burden Among Endometriosis Patients in the United States. Adv. Ther. 2018, 35, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T.; World Endometriosis Research Foundation Global Study of Women’s Health, Consortium. Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2011, 96, 366–373.e8. [Google Scholar] [CrossRef] [PubMed]
- Hudelist, G.; Fritzer, N.; Thomas, A.; Niehues, C.; Oppelt, P.; Haas, D.; Tammaa, A.; Salzer, H. Diagnostic delay for endometriosis in Austria and Germany: Causes and possible consequences. Hum. Reprod. 2012, 27, 3412–3416. [Google Scholar] [CrossRef]
- Vinatier, D.; Orazi, G.; Cosson, M.; Dufour, P. Theories of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 96, 21–34. [Google Scholar] [CrossRef]
- Matsuura, K.; Ohtake, H.; Katabuchi, H.; Okamura, H. Coelomic metaplasia theory of endometriosis: Evidence from in vivo studies and an in vitro experimental model. Gynecol. Obstet. Investig. 1999, 47, 18–20. [Google Scholar] [CrossRef]
- Sourial, S.; Tempest, N.; Hapangama, D.K. Theories on the pathogenesis of endometriosis. Int. J. Reprod. Med. 2014, 2014, 179515. [Google Scholar] [CrossRef]
- Marks, P.A.; Richon, V.M.; Miller, T.; Kelly, W.K. Histone deacetylase inhibitors. Adv. Cancer Res. 2004, 91, 137–168. [Google Scholar]
- Prebet, T.; Delaunay, J.; Wattel, E.; Braun, T.; Cony-Makhoul, P.; Dimicoli, S.; Wickenhauser, S.; Lejeune, J.; Chevret, S.; Vey, N.; et al. Groupe Francophone des, M. Addition of suberoylanilide hydroxamic acid (Vorinostat) to azacitidine for patients with higher risk myelodysplastic syndromes and azacitidine failure: A phase II add-on study from the Groupe Francophone des Myelodysplasies. Br. J. Haematol. 2018, 180, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Kelly, W.K.; Richon, V.M.; O’Connor, O.; Curley, T.; MacGregor-Curtelli, B.; Tong, W.; Klang, M.; Schwartz, L.; Richardson, S.; Scher, H.; et al. Phase I clinical trial of histone deacetylase inhibitor: Suberoylanilide hydroxamic acid administered intravenously. Clin. Cancer Res. 2003, 9, 3578–3588. [Google Scholar] [PubMed]
- Luu, T.H.; Morgan, R.J.; Leong, L.; Lim, D.; McNamara, M.; Portnow, J.; Frankel, P.; Smith, D.D.; Doroshow, J.H.; Somlo, G.; et al. A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: A California Cancer Consortium study. Clin. Cancer Res. 2008, 14, 7138–7142. [Google Scholar] [CrossRef] [PubMed]
- Reguart, N.; Rosell, R.; Cardenal, F.; Cardona, A.F.; Isla, D.; Palmero, R.; Moran, T.; Rolfo, C.; Pallares, M.C.; Taron, M.; et al. Phase I/II trial of vorinostat (SAHA) and erlotinib for non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations after erlotinib progression. Lung Cancer 2014, 84, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Mikropoulos, C.; Samartzis, E.; Karihtala, P.; Moschetta, M.; Sheriff, M.; Karathanasi, A.; Sadauskaite, A.; Rassy, E.; Pavlidis, N. Wise Management of Ovarian Cancer: On the Cutting Edge. J. Pers. Med. 2020, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Oki, S.; Sone, K.; Oda, K.; Hamamoto, R.; Ikemura, M.; Maeda, D.; Takeuchi, M.; Tanikawa, M.; Mori-Uchino, M.; Fujii, T.; et al. Oncogenic histone methyltransferase EZH2: A novel prognostic marker with therapeutic potential in endometrial cancer. Oncotarget 2017, 8, 40402–40411. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.B.; Long, C.L.; Liu, X.Q.; Chen, X.M.; Guo, L.R.; Xia, Y.Y.; He, J.L.; Wang, Y.X. 5-aza-2′-deoxycytidine leads to reduced embryo implantation and reduced expression of DNA methyltransferases and essential endometrial genes. PLoS ONE 2012, 7, e45364. [Google Scholar] [CrossRef]
- Weberpals, J.I.; O’Brien, A.M.; Niknejad, N.; Garbuio, K.D.; Clark-Knowles, K.V.; Dimitroulakos, J. The effect of the histone deacetylase inhibitor M344 on BRCA1 expression in breast and ovarian cancer cells. Cancer Cell Int. 2011, 11, 29. [Google Scholar] [CrossRef]
- Ungerstedt, J.; Du, Y.; Zhang, H.; Nair, D.; Holmgren, A. In vivo redox state of human thioredoxin and redox shift by the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA). Free Radic. Biol. Med. 2012, 53, 2002–2007. [Google Scholar] [CrossRef]
- Yoshihara, E.; Chen, Z.; Matsuo, Y.; Masutani, H.; Yodoi, J. Thiol redox transitions by thioredoxin and thioredoxin-binding protein-2 in cell signaling. Methods Enzymol. 2010, 474, 67–82. [Google Scholar]
- Seo, S.K.; Yang, H.I.; Lee, K.E.; Kim, H.Y.; Cho, S.; Choi, Y.S.; Lee, B.S. The roles of thioredoxin and thioredoxin-binding protein-2 in endometriosis. Hum. Reprod. 2010, 25, 1251–1258. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patel, B.G.; Lenk, E.E.; Lebovic, D.I.; Shu, Y.; Yu, J.; Taylor, R.N. Pathogenesis of endometriosis: Interaction between Endocrine and inflammatory pathways. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.H.; Chon, S.J.; Choi, Y.S.; Cho, S.; Lee, B.S.; Seo, S.K. Pathophysiology of Endometriosis: Role of High Mobility Group Box-1 and Toll-Like Receptor 4 Developing Inflammation in Endometrium. PLoS ONE 2016, 11, e0148165. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Warner, M.L. Epidemiology of endometriosis. Obstet. Gynecol. Clin. N. Am. 1997, 24, 235–258. [Google Scholar] [CrossRef]
- Matsuoka, S.; Tsuchiya, H.; Sakabe, T.; Watanabe, Y.; Hoshikawa, Y.; Kurimasa, A.; Itamochi, H.; Harada, T.; Terakawa, N.; Shiota, G.; et al. Involvement of thioredoxin-binding protein 2 in the antitumor activity of CD437. Cancer Sci. 2008, 99, 2485–2490. [Google Scholar] [CrossRef]
- Shao, Y.; Gao, Z.; Marks, P.A.; Jiang, X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc. Natl. Acad. Sci. USA 2004, 101, 18030–18035. [Google Scholar] [CrossRef]
- Dmowski, W.P.; Ding, J.; Shen, J.; Rana, N.; Fernandez, B.B.; Braun, D.P. Apoptosis in endometrial glandular and stromal cells in women with and without endometriosis. Hum. Reprod. 2001, 16, 1802–1808. [Google Scholar] [CrossRef]
- Ungerstedt, J.S.; Sowa, Y.; Xu, W.S.; Shao, Y.; Dokmanovic, M.; Perez, G.; Ngo, L.; Holmgren, A.; Jiang, X.; Marks, P.A. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc. Natl. Acad. Sci. USA 2005, 102, 673–678. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Zhao, F.; Wang, M.; Swaby, R.F.; Sparano, J.A.; Meropol, N.J.; Bhalla, K.N.; Pellegrino, C.M.; Katherine Alpaugh, R.; Sledge, G.W.; et al. A Phase I/II study of suberoylanilide hydroxamic acid (SAHA) in combination with trastuzumab (Herceptin) in patients with advanced metastatic and/or local chest wall recurrent HER2-amplified breast cancer: A trial of the ECOG-ACRIN Cancer Research Group (E1104). Breast Cancer Res. Treat. 2017, 165, 375–382. [Google Scholar]
- Zhen, Z.; Yang, K.; Ye, L.; You, Z.; Chen, R.; Liu, Y.; He, Y. Suberoylanilide hydroxamic acid sensitizes neuroblastoma to paclitaxel by inhibiting thioredoxin-related protein 14-mediated autophagy. Cancer Sci. 2017, 108, 1485–1492. [Google Scholar] [CrossRef]
- Imesch, P.; Samartzis, E.P.; Dedes, K.J.; Fink, D.; Fedier, A. Histone deacetylase inhibitors down-regulate G-protein-coupled estrogen receptor and the GPER-antagonist G-15 inhibits proliferation in endometriotic cells. Fertil. Steril. 2013, 100, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Liu, Y.; Yang, S.; Zhai, D.; Zhang, D.; Bai, L.; Wang, Z.; Yu, J.; Yu, C.; Cai, Z. Puerarin suppresses proliferation of endometriotic stromal cells in part via differential recruitment of nuclear receptor coregulators to estrogen receptor-alpha. J. Steroid Biochem. Mol. Biol. 2013, 138, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Lac, V.; Huntsman, D.G. Distinct developmental trajectories of endometriotic epithelium and stroma: Implications for the origins of endometriosis. J. Pathol. 2018, 246, 257–260. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.I.; Seo, S.K.; Chon, S.J.; Kim, G.H.; Lee, I.; Yun, B.H. Changes in the Expression of TBP-2 in Response to Histone Deacetylase Inhibitor Treatment in Human Endometrial Cells. Int. J. Mol. Sci. 2021, 22, 1427. https://doi.org/10.3390/ijms22031427
Kim HI, Seo SK, Chon SJ, Kim GH, Lee I, Yun BH. Changes in the Expression of TBP-2 in Response to Histone Deacetylase Inhibitor Treatment in Human Endometrial Cells. International Journal of Molecular Sciences. 2021; 22(3):1427. https://doi.org/10.3390/ijms22031427
Chicago/Turabian StyleKim, Hye In, Seok Kyo Seo, Seung Joo Chon, Ga Hee Kim, Inha Lee, and Bo Hyon Yun. 2021. "Changes in the Expression of TBP-2 in Response to Histone Deacetylase Inhibitor Treatment in Human Endometrial Cells" International Journal of Molecular Sciences 22, no. 3: 1427. https://doi.org/10.3390/ijms22031427
APA StyleKim, H. I., Seo, S. K., Chon, S. J., Kim, G. H., Lee, I., & Yun, B. H. (2021). Changes in the Expression of TBP-2 in Response to Histone Deacetylase Inhibitor Treatment in Human Endometrial Cells. International Journal of Molecular Sciences, 22(3), 1427. https://doi.org/10.3390/ijms22031427





